The Inc FII Plasmid and its Contribution in the Transmission of blaNDM-1 and blaKPC-2 in Klebsiella pneumoniae in Egypt
Abstract
1. Introduction
2. Results
2.1. Cabapenemase Production by K. pneumoniae
2.2. Hypermucoviscocity Phenotyping
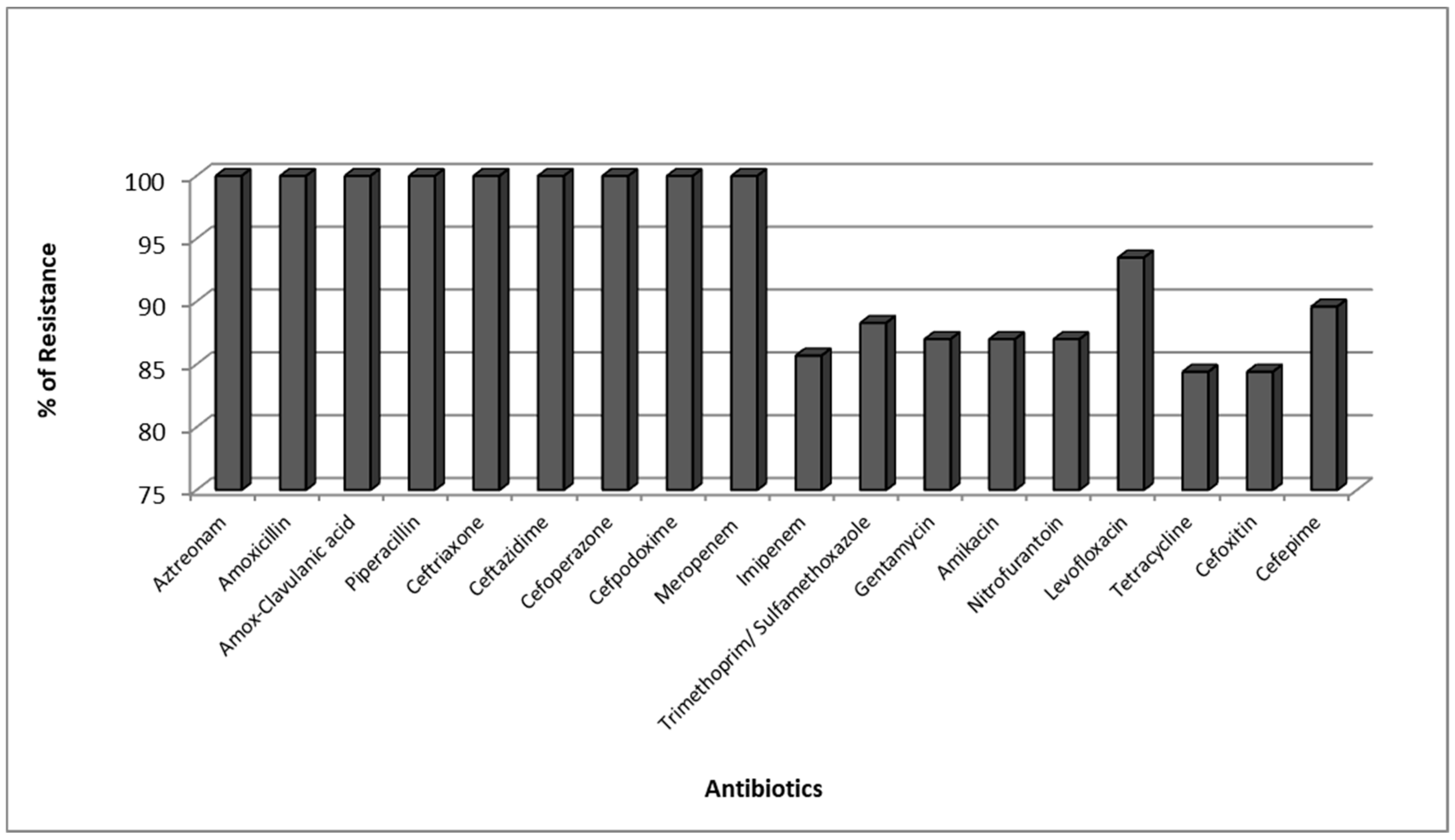
2.3. Antimicrobial Resistance Pattern of Carbapenemase Producing K. pneumoniae (CPKP)
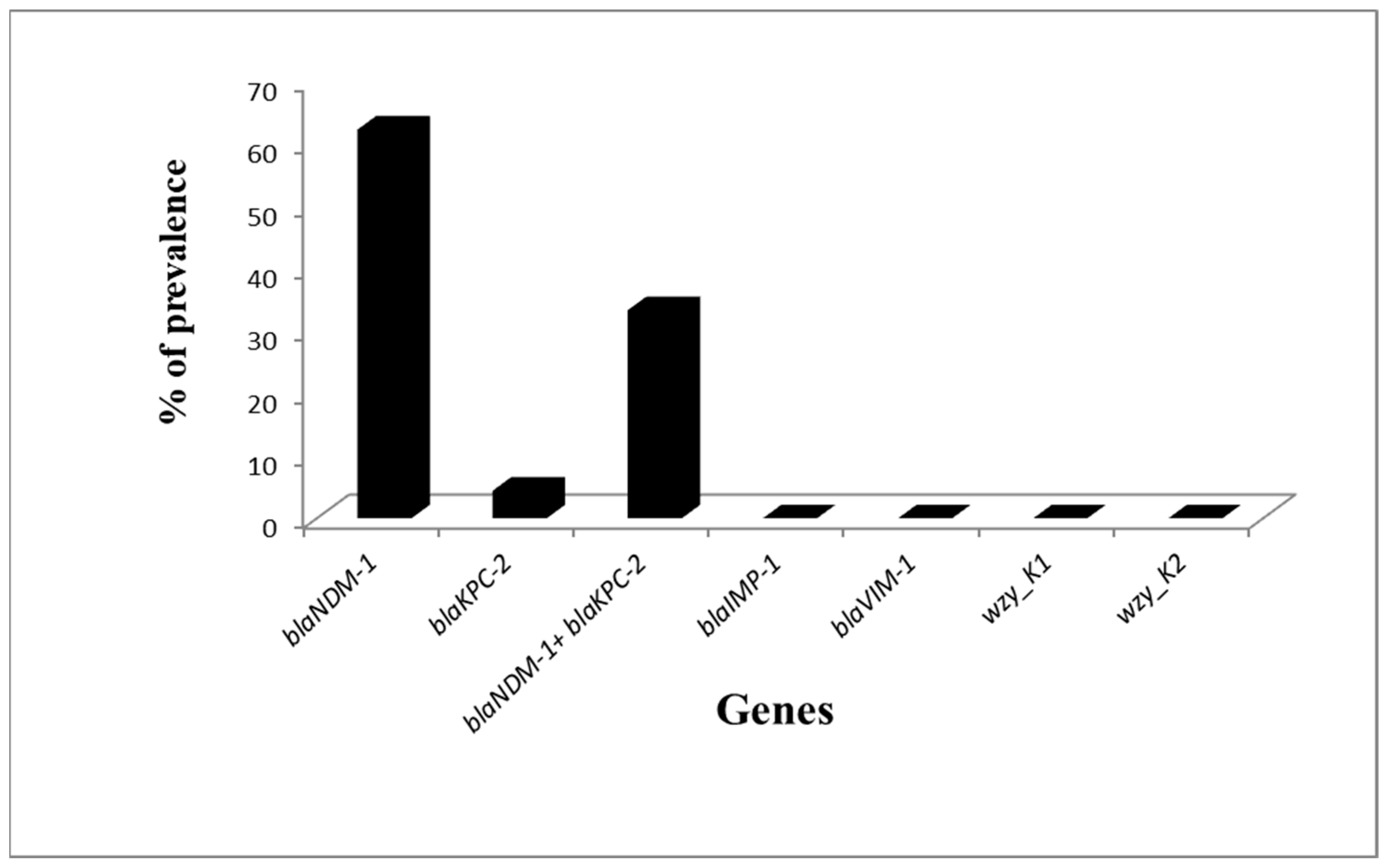
2.4. PCR for Carbapenemase Genes and KI and K2 Capsular Genes
2.5. Comparison of Phenotypic and Genotypic Methods for Detection of CPKP
2.6. Plasmid Transfers
2.7. PCR-Based Replicon Typing of blaKPC2 and/or blaNDM1 Encoding K. pneumoniae
2.8. Transfer of Plasmids with a Specific Replicon Type to the Transconjugants
3. Discussion
4. Methods
4.1. Klebsiella pneumoniae Isolation and Identification
4.2. Antimicrobial Susceptibility Testing
4.3. Detection of Carbapenemase-Producing Isolates
4.3.1. Modified Carbapenem Inactivation Methods (mCIM) for the Suspected Carbapenemase Producing Isolates
4.3.2. EDTA-Modified Carbapenem Inactivation Method (eCIM) for Detection of MβL Enzymes
4.4. Detection of Hypermucoviscocity Phenotyping
4.5. PCR for Carbapenemase Genes and KI and K2 Genes
4.6. Plasmid Transfers
4.7. PCR-Based Replicon Typing (Inc/Rep PCR) for Major Plasmid Incompatibility Groups among Klebseilla Isolates and Transconjugant DNA Lysates
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Decre, D.; Verdet, C.; Emirian, A.; Le Gourrierec, T.; Petit, J.C.; Offenstadt, G.; Maury, E.; Brisse, S.; Arlet, G. Emerging severe and fatal infections due to Klebsiella pneumoniae in two university hospitals in France. J. Clin. Microbiol. 2011, 49, 3012–3014. [Google Scholar] [CrossRef] [PubMed]
- Deng, C.; Li, X.; Zou, Y.; Wang, J.; Wang, J.; Namba, F.; Hiroyuki, Y.; Yu, J.; Yamauchi, Y.; Guo, C. Risk factors and pathogen profile of ventilator-associated pneumonia in a neonatal intensive care unit in China. Pediatrics Int. Off. J. Jpn. Pediatric Soc. 2011, 53, 332–337. [Google Scholar] [CrossRef] [PubMed]
- Foglia, E.; Meier, M.D.; Elward, A. Ventilator-associated pneumonia in neonatal and pediatric intensive care unit patients. Clin. Microbiol. Rev. 2007, 20, 409–425, table of contents. [Google Scholar] [CrossRef] [PubMed]
- Everest, P. The Enterobacteria, 2nd Edition. Gut 2007, 56, 1331. [Google Scholar] [CrossRef][Green Version]
- Sydnor, E.R.M.; Perl, T.M. Hospital epidemiology and infection control in acute-care settings. Clin. Microbiol. Rev. 2011, 24, 141–173. [Google Scholar] [CrossRef]
- Hawkey, P.M.; Warren, R.E.; Livermore, D.M.; McNulty, C.A.M.; Enoch, D.A.; Otter, J.A.; Wilson, A.P.R. Treatment of infections caused by multidrug-resistant Gram-negative bacteria: Report of the British Society for Antimicrobial Chemotherapy/Healthcare Infection Society/British Infection Association Joint Working Party. J. Antimicrob. Chemother. 2018, 73, iii2–iii78. [Google Scholar] [CrossRef]
- Wang, X.; Chen, G.; Wu, X.; Wang, L.; Cai, J.; Chan, E.W.; Chen, S.; Zhang, R. Increased prevalence of carbapenem resistant Enterobacteriaceae in hospital setting due to cross-species transmission of the blaNDM-1 element and clonal spread of progenitor resistant strains. Front. Microbiol. 2015, 6, 595. [Google Scholar] [CrossRef]
- Yan, J.; Pu, S.; Jia, X.; Xu, X.; Yang, S.; Shi, J.; Sun, S.; Zhang, L. Multidrug resistance mechanisms of carbapenem resistant Klebsiella pneumoniae strains isolated in Chongqing, China. Ann. Lab. Med. 2017, 37, 398–407. [Google Scholar] [CrossRef][Green Version]
- Miriagou, V.; Cornaglia, G.; Edelstein, M.; Galani, I.; Giske, C.; Gniadkowski, M.; Malamou-Lada, E.; Martinez-Martinez, L.; Navarro, F.; Nordmann, P. Acquired carbapenemases in Gram-negative bacterial pathogens: Detection and surveillance issues. Clin. Microbiol. Infect. 2010, 16, 112–122. [Google Scholar] [CrossRef]
- El-Sweify, M.; Gomaa, N.; El-Maraghy, N.; Mohamed, H. Phenotypic Detection of Carbapenem Resistance among Klebsiella pneumoniae in Suez Canal University Hospitals, Ismailiya, Egypt. Int. J. Curr. Microbiol. Appl. Sci. 2015, 4, 10–18. [Google Scholar]
- Gamal, D.; Fernández-Martínez, M.; Salem, D.; El-Defrawy, I.; Montes, L.Á.; Ocampo-Sosa, A.A.; Martínez-Martínez, L. Carbapenem-resistant Klebsiella pneumoniae isolates from Egypt containing blaNDM-1 on IncR plasmids and its association with rmtF. Int. J. Infect. Dis. 2016, 43, 17–20. [Google Scholar] [CrossRef] [PubMed]
- Morsi, S.S. Comparative Evaluation of Phenotypic and Genotypic Methods for Detection of Carbapenemases in Clinically Significant Klebsiella pneumoniae Isolates. Egypt. J. Med Microbiol. 2016, 38, 1–8. [Google Scholar] [CrossRef]
- Carattoli, A. Plasmids and the spread of resistance. Int. J. Med Microbiol. 2013, 303, 298–304. [Google Scholar] [CrossRef] [PubMed]
- Hawkey, P.M.; Jones, A.M. The changing epidemiology of resistance. J. Antimicrob. Chemother. 2009, 64, i3–i10. [Google Scholar] [CrossRef]
- Carattoli, A. Plasmids in Gram negatives: Molecular typing of resistance plasmids. Int. J. Med Microbiol. 2011, 301, 654–658. [Google Scholar] [CrossRef]
- García-Fernández, A.; Villa, L.; Carta, C.; Venditti, C.; Giordano, A.; Venditti, M.; Mancini, C.; Carattoli, A. Klebsiella pneumoniae ST258 producing KPC-3 identified in Italy carries novel plasmids and OmpK36/OmpK35 porin variants. Antimicrob. Agents Chemother. 2012, 56, 2143–2145. [Google Scholar] [CrossRef]
- Hardiman, C.; Weingarten, R.; Conlan, S.; Khil, P.; Dekker, J.; Mathers, A.; Sheppard, A.; Segre, J.; Frank, K. Horizontal transfer of carbapenemase-encoding plasmids and comparison with hospital epidemiology data. Antimicrob. Agents Chemother. 2016, 60, 4910–4919. [Google Scholar] [CrossRef]
- Yigit, H.; Queenan, A.M.; Anderson, G.J.; Domenech-Sanchez, A.; Biddle, J.W.; Steward, C.D.; Alberti, S.; Bush, K.; Tenover, F.C. Novel carbapenem-hydrolyzing β-lactamase, KPC-1, from a carbapenem-resistant strain of Klebsiella pneumoniae. Antimicrob. Agents Chemother. 2001, 45, 1151–1161. [Google Scholar] [CrossRef]
- Nordmann, P.; Poirel, L.; Walsh, T.R.; Livermore, D.M. The emerging NDM carbapenemases. Trends Microbiol. 2011, 19, 588–595. [Google Scholar] [CrossRef]
- Bottery, M.J.; Wood, A.J.; Brockhurst, M.A. Selective Conditions for a Multidrug Resistance Plasmid Depend on the Sociality of Antibiotic Resistance. Antimicrob. Agents Chemother. 2016, 60, 2524–2527. [Google Scholar] [CrossRef]
- Raymond, B. Five rules for resistance management in the antibiotic apocalypse, a road map for integrated microbial management. Evol. Appl. 2019. [Google Scholar] [CrossRef] [PubMed]
- Kamruzzaman, M.; Shoma, S.; Thomas, C.M.; Partridge, S.R.; Iredell, J.R. Plasmid interference for curing antibiotic resistance plasmids in vivo. PLoS ONE 2017, 12, e0172913. [Google Scholar] [CrossRef] [PubMed]
- Moemen, D.; Masallat, D.T. Prevalence and characterization of carbapenem-resistant Klebsiella pneumoniae isolated from intensive care units of Mansoura University hospitals. Egypt. J. Basic Appl. Sci. 2017, 4, 37–41. [Google Scholar] [CrossRef][Green Version]
- Marquez, P.; Terashita, D.; Dassey, D.; Mascola, L. Population-based incidence of carbapenem-resistant Klebsiella pneumoniae along the continuum of care, Los Angeles County. Infect. Control Hosp. Epidemiol. 2013, 34, 144–150. [Google Scholar] [CrossRef][Green Version]
- Ashour, H.M.; El-Sharif, A. Species distribution and antimicrobial susceptibility of gram-negative aerobic bacteria in hospitalized cancer patients. J. Transl. Med. 2009, 7, 14. [Google Scholar] [CrossRef]
- Mainardi, J.-L.; Villet, R.; Bugg, T.D.; Mayer, C.; Arthur, M. Evolution of peptidoglycan biosynthesis under the selective pressure of antibiotics in Gram-positive bacteria. FEMS Microbiol. Rev. 2008, 32, 386–408. [Google Scholar] [CrossRef]
- CLSI. Performance Standards for Antimicrobial Susceptibility Testing, 28th ed.; Clsi Suppl. M100; Clinical and Laboratory Standards Institute: Waynepa, PA, USA, 2018. [Google Scholar]
- Yu, W.-L.; Ko, W.-C.; Cheng, K.-C.; Lee, C.-C.; Lai, C.-C.; Chuang, Y.-C. Comparison of prevalence of virulence factors for Klebsiella pneumoniae liver abscesses between isolates with capsular K1/K2 and non-K1/K2 serotypes. Diagn. Microbiol. Infect. Dis. 2008, 62, 1–6. [Google Scholar] [CrossRef]
- Bialek-Davenet, S.; Criscuolo, A.; Ailloud, F.; Passet, V.; Jones, L.; Delannoy-Vieillard, A.-S.; Garin, B.; Le Hello, S.; Arlet, G.; Nicolas-Chanoine, M.-H. Genomic definition of hypervirulent and multidrug-resistant Klebsiella pneumoniae clonal groups. Emerg. Infect. Dis. 2014, 20, 1812. [Google Scholar] [CrossRef]
- Turton, J.F.; Perry, C.; Elgohari, S.; Hampton, C.V. PCR characterization and typing of Klebsiella pneumoniae using capsular type-specific, variable number tandem repeat and virulence gene targets. J. Med Microbiol. 2010, 59, 541–547. [Google Scholar] [CrossRef]
- Lin, Y.-T.; Wang, Y.-P.; Wang, F.-D.; Fung, C.-P. Community-onset Klebsiella pneumoniae pneumonia in Taiwan: Clinical features of the disease and associated microbiological characteristics of isolates from pneumonia and nasopharynx. Front. Microbiol. 2015, 6, 122. [Google Scholar] [CrossRef]
- Abdelaziz, M.O.; Bonura, C.; Aleo, A.; Fasciana, T.; Mammina, C. NDM-1-and OXA-163-producing Klebsiella pneumoniae isolates in Cairo, Egypt, 2012. J. Glob. Antimicrob. Resist. 2013, 1, 213–215. [Google Scholar] [CrossRef] [PubMed]
- Zafer, M.M.; Amin, M.; El Mahallawy, H.; Ashour, M.S.E.-D.; Al Agamy, M. First report of NDM-1-producing Pseudomonas aeruginosa in Egypt. Int. J. Infect. Dis. 2014, 29, 80–81. [Google Scholar] [CrossRef] [PubMed]
- El-Sayed, M.A.E.-G.; Amin, M.A.; Tawakol, W.M.; Loucif, L.; Bakour, S.; Rolain, J.-M. High prevalence of blaNDM-1 carbapenemase-encoding gene and 16S rRNA armA methyltransferase gene among Acinetobacter baumannii clinical isolates in Egypt. Antimicrob. Agents Chemother. 2015, 59, 3602–3605. [Google Scholar] [CrossRef] [PubMed]
- Poirel, L.; Dortet, L.; Bernabeu, S.; Nordmann, P. Genetic features of blaNDM-1-positive Enterobacteriaceae. Antimicrobial agents and chemotherapy 2011, 55, 5403–5407. [Google Scholar] [CrossRef] [PubMed]
- Poirel, L.; Benouda, A.; Hays, C.; Nordmann, P. Emergence of NDM-1-producing Klebsiella pneumoniae in Morocco. J. Antimicrob. Chemother. 2011, 66, 2781–2783. [Google Scholar] [CrossRef]
- Shen, P.; Zhang, Y.; Li, G.; Jiang, X. Characterization of the genetic environment of the bla KPC-2 gene among Klebsiella pneumoniae isolates from a Chinese hospital. Braz. J. Infect. Dis. 2016, 20, 384–388. [Google Scholar] [CrossRef]
- Jin, Y.; Shao, C.; Li, J.; Fan, H.; Bai, Y.; Wang, Y. Outbreak of multidrug resistant NDM-1-producing Klebsiella pneumoniae from a neonatal unit in Shandong Province, China. PLoS ONE 2015, 10, e0119571. [Google Scholar] [CrossRef]
- Agyekum, A.; Fajardo-Lubián, A.; Ai, X.; Ginn, A.N.; Zong, Z.; Guo, X.; Turnidge, J.; Partridge, S.R.; Iredell, J.R. Predictability of phenotype in relation to common β-lactam resistance mechanisms in Escherichia coli and Klebsiella pneumoniae. J. Clin. Microbiol. 2016, 54, 1243–1250. [Google Scholar] [CrossRef]
- Al-Marzooq, F.; Mohd Yusof, M.Y.; Tay, S.T. Molecular Analysis of Antibiotic Resistance Determinants and Plasmids in Malaysian Isolates of Multidrug Resistant Klebsiella pneumoniae. PLoS ONE 2015, 10, e0133654. [Google Scholar] [CrossRef]
- uz Zaman, T.; Alrodayyan, M.; Albladi, M.; Aldrees, M.; Siddique, M.I.; Aljohani, S.; Balkhy, H.H. Clonal diversity and genetic profiling of antibiotic resistance among multidrug/carbapenem-resistant Klebsiella pneumoniae isolates from a tertiary care hospital in Saudi Arabia. BMC Infect. Dis. 2018, 18, 205. [Google Scholar] [CrossRef]
- Rozwandowicz, M.; Brouwer, M.; Fischer, J.; Wagenaar, J.; Gonzalez-Zorn, B.; Guerra, B.; Mevius, D.; Hordijk, J. Plasmids carrying antimicrobial resistance genes in Enterobacteriaceae. J. Antimicrob. Chemother. 2018, 73, 1121–1137. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, C.; Machado, E.; Ramos, H.; Peixe, L.; Novais, Â. Expansion of ESBL-producing Klebsiella pneumoniae in hospitalized patients: A successful story of international clones (ST15, ST147, ST336) and epidemic plasmids (IncR, IncFIIK). Int. J. Med Microbiol. 2014, 304, 1100–1108. [Google Scholar] [CrossRef] [PubMed]
- Pierce, V.M.; Simner, P.J.; Lonsway, D.R.; Roe-Carpenter, D.E.; Johnson, J.K.; Brasso, W.B.; Bobenchik, A.M.; Lockett, Z.C.; Charnot-Katsikas, A.; Ferraro, M.J.; et al. Modified Carbapenem Inactivation Method for Phenotypic Detection of Carbapenemase Production among Enterobacteriaceae. J. Clin. Microbiol. 2017, 55, 2321–2333. [Google Scholar] [CrossRef] [PubMed]
- Sfeir, M.M.; Hayden, J.A.; Fauntleroy, K.A.; Mazur, C.; Johnson, J.K.; Simner, P.J.; Das, S.; Satlin, M.J.; Jenkins, S.G.; Westblade, L.F. EDTA-Modified Carbapenem Inactivation Method: A Phenotypic Method for Detecting Metallo-β-Lactamase-Producing Enterobacteriaceae. J. Clin. Microbiol. 2019, 57, e01757-18. [Google Scholar] [CrossRef] [PubMed]
- Shon, A.S.; Bajwa, R.P.; Russo, T.A. Hypervirulent (hypermucoviscous) Klebsiella pneumoniae: A new and dangerous breed. Virulence 2013, 4, 107–118. [Google Scholar] [CrossRef] [PubMed]
- Fang, C.-T.; Lai, S.-Y.; Yi, W.-C.; Hsueh, P.-R.; Liu, K.-L.; Chang, S.-C. Klebsiella pneumoniae genotype K1: An emerging pathogen that causes septic ocular or central nervous system complications from pyogenic liver abscess. Clin. Infect. Dis. 2007, 45, 284–293. [Google Scholar] [CrossRef]
- Shibata, N.; Doi, Y.; Yamane, K.; Yagi, T.; Kurokawa, H.; Shibayama, K.; Kato, H.; Kai, K.; Arakawa, Y. PCR typing of genetic determinants for metallo-β-lactamases and integrases carried by gram-negative bacteria isolated in Japan, with focus on the class 3 integron. J. Clin. Microbiol. 2003, 41, 5407–5413. [Google Scholar] [CrossRef]
- Carattoli, A.; Bertini, A.; Villa, L.; Falbo, V.; Hopkins, K.L.; Threlfall, E.J. Identification of plasmids by PCR-based replicon typing. J. Microbiol. Methods 2005, 63, 219–228. [Google Scholar] [CrossRef]
- Carattoli, A.; Seiffert, S.N.; Schwendener, S.; Perreten, V.; Endimiani, A. Differentiation of IncL and IncM Plasmids Associated with the Spread of Clinically Relevant Antimicrobial Resistance. PLoS ONE 2015, 10, e0123063. [Google Scholar] [CrossRef]
- Villa, L.; Garcia-Fernandez, A.; Fortini, D.; Carattoli, A. Replicon sequence typing of IncF plasmids carrying virulence and resistance determinants. J. Antimicrob. Chemother. 2010, 65, 2518–2529. [Google Scholar] [CrossRef]
- Johnson, T.J.; Bielak, E.M.; Fortini, D.; Hansen, L.H.; Hasman, H.; Debroy, C.; Nolan, L.K.; Carattoli, A. Expansion of the IncX plasmid family for improved identification and typing of novel plasmids in drug-resistant Enterobacteriaceae. Plasmid 2012, 68, 43–50. [Google Scholar] [CrossRef] [PubMed]
- Carloni, E.; Andreoni, F.; Omiccioli, E.; Villa, L.; Magnani, M.; Carattoli, A. Comparative analysis of the standard PCR-Based Replicon Typing (PBRT) with the commercial PBRT-KIT. Plasmid 2017, 90, 10–14. [Google Scholar] [CrossRef] [PubMed]
| Genotypic Method | No. of Isolates | Phenotypic Detection of Carbapenemase Producing Isolates | |
|---|---|---|---|
| mCIM No. (%) * | eCIM No. (%) * | ||
| Group 1 | 32 | 0 (0) | NT |
| Group 2 | 28 | 28 (100) | 9 (32.1) |
| Group 3 | 2 | 2 (100) | 2 (100) |
| Group 4 | 15 | 15 (100) | 5 (33.3) |
| Antibiotics | Tetracycline | Gentamicin | Sulfamethoxazole/Trimethoprim | Levofloxacin | |
|---|---|---|---|---|---|
| Strains | |||||
| KP3/transconjugate | R/S | R/S | R/S | R/R | |
| KP4/transconjugate | R/S | R/S | R/R | R/R | |
| KP5/transconjugate | R/R | R/S | R/S | R/R | |
| KP7/transconjugate | R/S | R/S | R/R | R/R | |
| KP9/transconjugate | R/S | R/S | R/R | R/R | |
| KP10/transconjugate | R/S | R/S | R/R | R/R | |
| KP15/transconjugate | R/S | R/R | S/S | R/R | |
| KP16/transconjugate | R/R | R/R | R/S | R/R | |
| KP19/transconjugate | R/S | R/R | R/R | R/S | |
| KP25/transconjugate | R/R | S/S | R/S | R/S | |
| KP28/transconjugate | R/R | R/S | R/S | R/R | |
| KP29/transconjugate | R/R | R/S | R/R | R/R | |
| Strain Code | Carbapenemase Gene | PBRT of Isolates | PBRT of Transconjugant |
|---|---|---|---|
| Kp1 | NDM-1, KPC-2 | FII | FII |
| KP2 | NDM1 | FIIK | FIIK |
| KP3 | NDM-1 | FII, FIIK, L | FIIK |
| KP4 | NDM-1 | M, L | - |
| KP5 | NDM-1 | - | - |
| KP6 | NDM-1, KPC-2 | - | - |
| KP7 | NDM-1 | FIB, M | - |
| KP8 | KPC-2 | FII, FIIK, FIB | FII, FIIK |
| KP9 | NDM-1 | FIIK, FIB | FIIK, FIB |
| KP10 | NDM-1 | FIIK, M | FIIK |
| KP11 | NDM-1 | FIC | - |
| KP12 | NDM-1 | FIIK, FIB, FII, M | FIIK, FIB |
| KP13 | NDM-1, KPC-2 | FIC | - |
| KP14 | NDM-1 | FIB, FIIK, FII, FIC | FIIK |
| KP15 | NDM-1 | FIC | - |
| KP16 | NDM-1, KPC-2 | FII, FIIK, FIB | FII, FIIK, FIB |
| KP17 | KPC-2 | FII, FIIK | - |
| KP18 | NDM-1, KPC-2 | FII, FIIK, FIB | FIIK |
| KP19 | NDM-1 | L | - |
| KP20 | NDM-1 | FIB | - |
| KP21 | NDM-1, KPC-2 | FII, FIIK, FIB | FII, FIIK, FIB |
| KP22 | NDM-1 | - | - |
| KP23 | NDM-1, KPC-2 | FII, FIIK, FIB, M | FII, FIIK, FIB |
| KP24 | NDM-1, KPC-2 | FIIK, FII, FIB, | FIIK |
| KP25 | NDM-1, KPC-2 | FII, FIIK | FII, FIIK |
| KP26 | NDM-1 | FIIK, FIB, L, M | FIB |
| KP27 | NDM-1 | L, M | - |
| KP28 | NDM-1 | FIC, FII, FIIK | FII |
| KP29 | NDM-1, KPC-2 | FIIK, FII | - |
| KP30 | NDM-1, KPC-2 | FIIK, FII, FIB | FIIK |
| KP31 | NDM-1, KPC-2 | FIIK, FII, M | FIIK |
| KP32 | NDM-1 | FIIK, FIC, FIB | FIIK |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ramadan Mohamed, E.; Ali, M.Y.; Waly, N.G.F.M.; Halby, H.M.; Abd El-Baky, R.M. The Inc FII Plasmid and its Contribution in the Transmission of blaNDM-1 and blaKPC-2 in Klebsiella pneumoniae in Egypt. Antibiotics 2019, 8, 266. https://doi.org/10.3390/antibiotics8040266
Ramadan Mohamed E, Ali MY, Waly NGFM, Halby HM, Abd El-Baky RM. The Inc FII Plasmid and its Contribution in the Transmission of blaNDM-1 and blaKPC-2 in Klebsiella pneumoniae in Egypt. Antibiotics. 2019; 8(4):266. https://doi.org/10.3390/antibiotics8040266
Chicago/Turabian StyleRamadan Mohamed, Eman, Mamdouh Yones Ali, Nancy G F M Waly, Hamada Mohamed Halby, and Rehab Mahmoud Abd El-Baky. 2019. "The Inc FII Plasmid and its Contribution in the Transmission of blaNDM-1 and blaKPC-2 in Klebsiella pneumoniae in Egypt" Antibiotics 8, no. 4: 266. https://doi.org/10.3390/antibiotics8040266
APA StyleRamadan Mohamed, E., Ali, M. Y., Waly, N. G. F. M., Halby, H. M., & Abd El-Baky, R. M. (2019). The Inc FII Plasmid and its Contribution in the Transmission of blaNDM-1 and blaKPC-2 in Klebsiella pneumoniae in Egypt. Antibiotics, 8(4), 266. https://doi.org/10.3390/antibiotics8040266





