Novel Small-molecule Antibacterials against Gram-positive Pathogens of Staphylococcus and Enterococcus Species
Abstract
1. Introduction
2. Results and Discussion
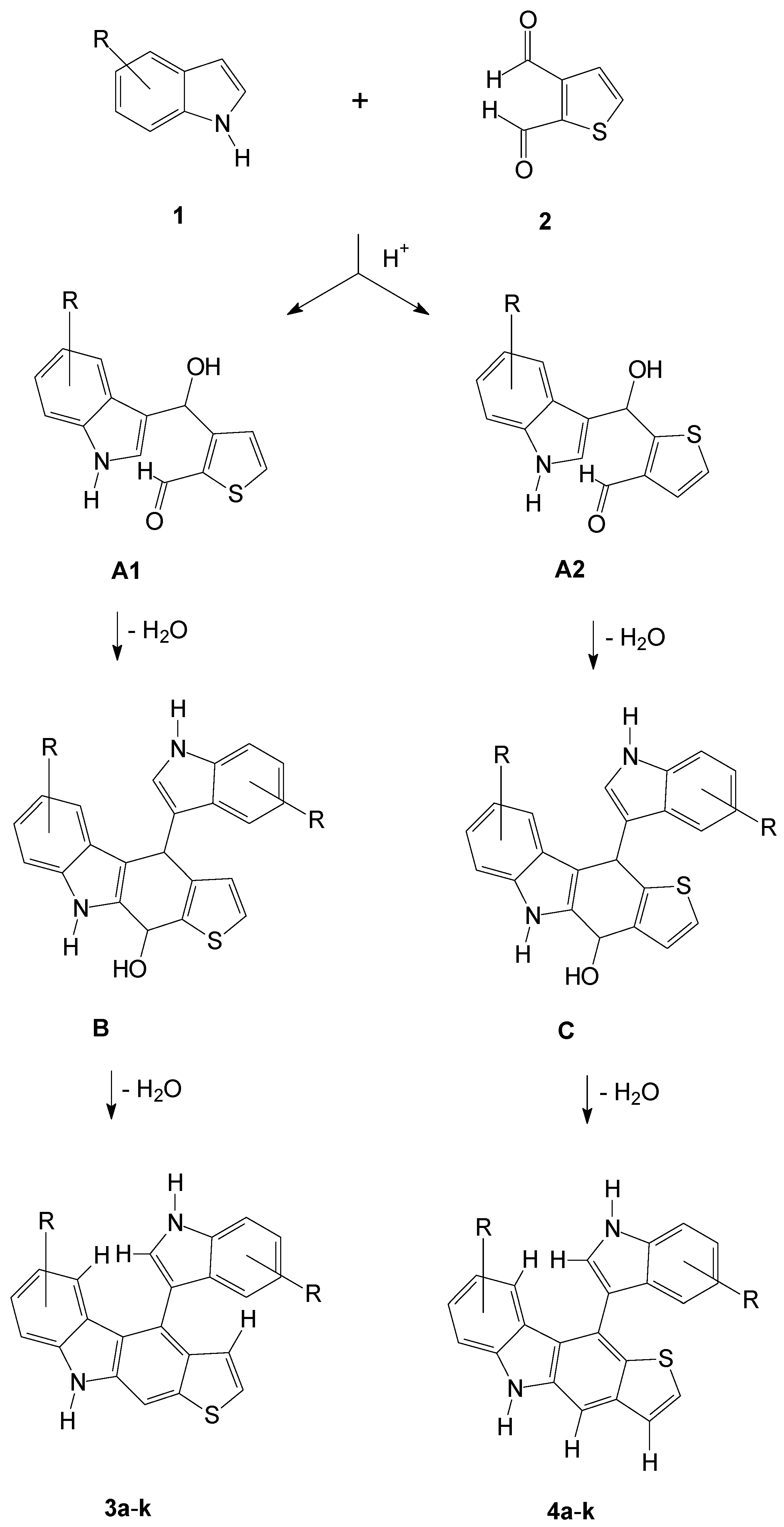
2.1. Synthesis of the Thienocarbazoles
2.2. In Vitro Antibacterial Activity of the Thienocarbazoles
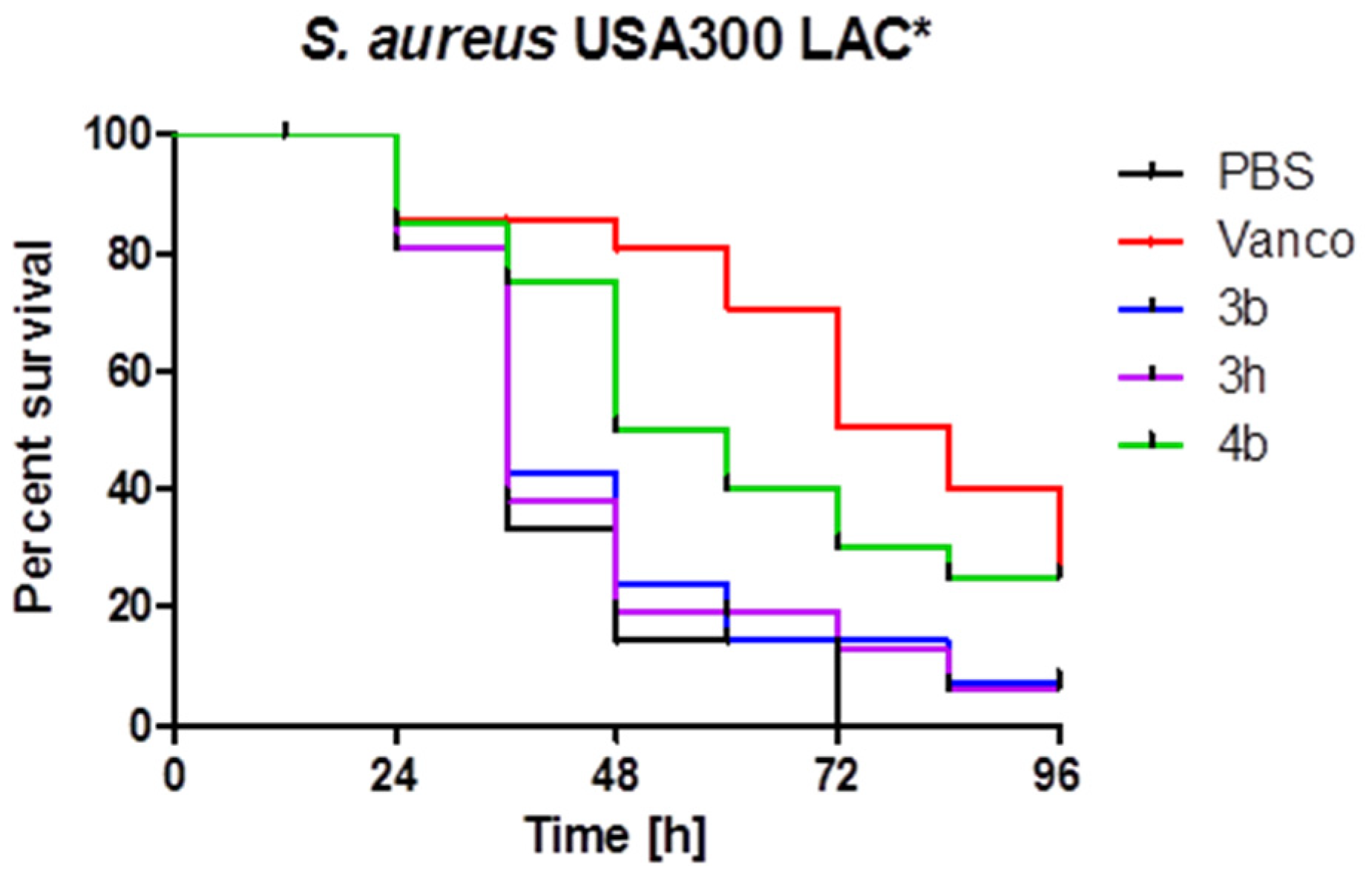
2.3. In Vivo Antibacterial Activity of Thieno[b]carbazoles
3. Materials and Methods
3.1. Chemical Reagents and Instruments
3.2. Procedure for the Synthesis of Compound Classes 3 and 4
3.3. In Vitro Antibacterial Activity
3.4. In Vivo Antibacterial Assay
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Mantravadi, P.K.; Karunakaran, A.K.; Renwick, C.J.; Hudson, A.O.; Parthasarathy, A. The Quest for Novel Antimicrobial Compounds: Emerging Trends in Research, Development, and Technologies. Antibiotics 2019, 8, 8. [Google Scholar] [CrossRef] [PubMed]
- Balloux, F.; van Dorp, L. Q & A: What are pathogens, and what have they done to and for us? BMC Biol. 2017, 15, 91. [Google Scholar]
- Ventola, C.L. The Antibiotic Crisis. Pharm. Ther. 2015, 40, 277–283. [Google Scholar]
- Eades, C.; Highes, S.; Heard, K.; Moore, L. Antimicrobial therapies for Gram-positive infections. Clin. Pharm. 2017, 9, 9. [Google Scholar]
- Kluytmans, J.; van Belkum, A.; Verbrugh, H. Nasal carriage of Staphylococcus aureus: Epidemiology, underlying mechanisms, and associated risks. Clin. Microbiol. Rev. 1997, 10, 505–520. [Google Scholar] [CrossRef]
- Agyare, C.; Boamah, V.E.; Zumbi, C.N.; Osei, F.B. Antibiotic Use in Poultry Production and Its Effect on Bacterial Resistance. In Antimicrobial Resistance—A Global Threat; Kumar, Y., Ed.; IntechOpen: London, UK, 2018. [Google Scholar] [CrossRef]
- Roth, C. Drug-Filled Rivers Aiding Resistance to Antibiotics. 2019. Available online: http://p.dw.com/p/3JDQZ (accessed on 28 May 2019).
- Lerminiaux, N.A.; Cameron, A.D.S. Horizontal transfer antibiotic resistance genes in clinical environments. Can. J. Microbiol. 2019, 65, 34–44. [Google Scholar] [CrossRef]
- Bertrand, S.; Bohni, N.; Schnee, S.; Schumpp, O.; Gindro, K.; Wolfender, J.L. Metabolite Induction Via Microorganism Co-Cultues: A Potential Way to Enhance Chemical Diversity for Drug Discovery. Biotechnol. Adv. 2014, 32, 1180–1204. [Google Scholar] [CrossRef]
- Tyc, O.; De Jager, V.C.L.; Van Den Berg, M.; Gerards, S.; Janssens, T.K.S.; Zaagman, N.; Kai, M.; Svatos, A.; Zweers, H.; Hordijk, C.; et al. Exploring Bacterial Interspecific Interactions for Discovery of Novel Antimicrobial Compounds. Microb. Biotechnol. 2017, 10, 910–925. [Google Scholar] [CrossRef]
- Tyc, O.; Van Den Berg, M.; Gerads, S.; Van Veen, J.A.; Raaijmakers, J.M.; De Boer, W.; Garbeva, P. Impact of Interspecific Interactions on Antimicrobial Activity among Soil Bacteria. Front. Microbiol. 2014, 5, 567. [Google Scholar] [CrossRef]
- Abdallah, E.M. Plants: An alternative source for antimicrobials. J. Appl. Pharm. Sci. 2011, 1, 16–20. [Google Scholar]
- Choudray, A.; Naughton, L.M.; Montánchez, I.; Dobson, D.W.; Rai, D.K. Current Status and Future Prospects of Marine Natural Products (MNPs) as Antimicrobials. Mar. Drugs 2017, 15, 272. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.; Patocka, J.; Kuca, K. Insect Antimicrobial Peptides, a Mini Review. Toxins 2018, 10, 461. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Y.; Liu, C.; Lai, R. Antimicrobial peptides from amphibians. Biomol. Concepts 2011, 2, 27–38. [Google Scholar] [CrossRef] [PubMed]
- Klahn, P.; Brönstrup, M. Bifunctional antimicrobial conjugates and hybrid antimicrobials. Nat. Prod. Rep. 2017, 34, 832–885. [Google Scholar] [CrossRef]
- Anusionwu, C.G.; Aderibigbe, B.A.; Mbianda, X.Y. Hybrid Molecules Development: A Versatile Landscape for the Control of Antifungal Drug Resistance: A Review. Mini-Rev. Med. Chem. 2019, 19, 450–464. [Google Scholar] [CrossRef]
- Irfan, M.; Aneja, B.; Yadava, U.; Khan, S.I.; Manzoor, N.; Daniliuc, C.G.; Abid, M. Synthesis, QSAR and anticandidal evaluation of 1,2,3-triazoles derived from naturally bioactive scoffolds. Eur. J. Med. Chem. 2015, 93, 246–254. [Google Scholar] [CrossRef]
- Miniyar, P.B.; Mahajan, A.A.; Mokale, S.N.; Shah, M.U.; Kumar, A.S.; Chaturbhuj, G.U. Triazole hybrids as new type of anti-fungal agents. Arab. J. Chem. 2017, 10, 295–299. [Google Scholar] [CrossRef]
- Keri, R.S.; Chand, K.; Budagumpi, S.; Somappa, S.B.; Patil, S.A.; Nagaraja, B.M. An overview of benzo[b]thiophene-based medicinal chemistry. Eur. J. Med. Chem. 2017, 138, 1002–1033. [Google Scholar] [CrossRef]
- Suresha Kumar, T.H.; Mahadevan, K.M.; Hariskumar, H.N.; Padmashali, B.; Naganadowda, G. Synthesis of benzo[b]thiophene substituted carbamates, ureas, semicarbazides, and pyrazoles and their antimicrobial and analgesic activity. Phosphorus Sulfur Silicon Relat. Elem. 2009, 184, 1856–1879. [Google Scholar]
- Nagesh, H.K.; Padmashali, B.; Sandeep, C.; Yuvaraj, T.C.M.; Siddesh, M.B.; Mallikarjuna, S.M. Synthesis and antimicrobial activity of benzothiophene substituted coumarines, pyrimidines and pyrazole as new scaffold. Int. J. Pharm. Sci. Rev. Res. 2014, 28, 6–10. [Google Scholar]
- Aboulwafaa, U.M.; Berto, F.A.C. Benzo[b]thiophenes, part 1: Synthesis and antimicrobial activity of benzo[b]thienyl-1,3,4-oxadiazole, -1,2,3-triazolinea, and thiazoline derivatives. Arch. Pharm. 1992, 325, 123–127. [Google Scholar] [CrossRef] [PubMed]
- Chawia, R.; Arora, A.; Prameswaran, M.; Shama, P.C.; Michael, S.; Ravi, T.K. Synthesis of novel 1,3,4-oxadiazole derivatives as potential antimicrobial agents. Acta Pol. Pharma. Drug Res. 2010, 67, 247–253. [Google Scholar]
- Singh, T.P.; Singh, O.M. Recent Progress in Biological Activities of Indole and Indole Alkaloids. Mini-Rev. Med. Chem. 2018, 18, 9–25. [Google Scholar] [CrossRef] [PubMed]
- Damit, E.F.; Nordin, N.; Ariffin, A.; Sulaiman, K. Synthesis of Novel Derivatives of Carbazole-Thiophene, Their Electronic Properties, and Computational Studies. J. Chem. 2016, 2016, 9360230. [Google Scholar] [CrossRef][Green Version]
- De Perio, M.A.; Yarnold, P.R.; Warren, J.; Noskin, G.A. Risk factors and outcomes associated with non-Enterococcus faecalis, non-Enterococcus faecium enterococcal bacterimia. Infect. Control Hosp. Epidemiol. 2006, 27, 28–33. [Google Scholar] [CrossRef]
- Schmidt-Hieber, M.; Blau, I.W.; Schwartz, S.; Uharek, L.; Weist, K.; Eckmanns, T.; Jonas, D.; Rüden, H.; Thiel, E.; Brandt, C. Intensified strategies to control vancomycin-resistant enterococci in immunocompromised patients. Int. J. Hematol. 2007, 86, 158–162. [Google Scholar] [CrossRef]
- Ahmed, M.O.; Baptiste, K.E. Vancomycin-Resistant Enterococci: A Review of Antimocrobial Resistance Mechanisms and Perspectives of Human and Animal Health. Microb. Drug Resist. 2018, 24. [Google Scholar] [CrossRef]
- Casper, Y.; Jeanty, M.; Blu, J.; Burchak, O.; Le Pihive, E.; Maigre, L.; Schneider, D.; Jolivalt, C.; Paris, J.-M.; Hequet, A.; et al. Novel synthetic bis-indolic derivatives with antistaphylococcal activity, including against MRDA and VISA strains. J. Antimicrob. Chemother. 2015, 70, 1727–1737. [Google Scholar]
| MIC [µg/mL] a | |||||
|---|---|---|---|---|---|
| Cpd. | R | Staphylococcus aureus | E. faecium | E. faecalis | E. casseliflavus |
| 3a | H | 8 | 128 | 16 | >128 |
| 3b | 5-Cl | 4 | 8 | 16 | 8 |
| 3c | 6-Cl | 8 | 32 | 32 | 16 |
| 3d | 5-Br | 8 | 8 | 16 | 16 |
| 3e | 6-Br | - | 64 | 64 | 16 |
| 3f | 5-CN | 8 | >128 | 32 | 128 |
| 3g | 6-CN | 4 | >128 | 64 | >128 |
| 3h | 5-OH | 2 | 16 | 16 | 64 |
| 3i | 6-OH | 2 | 8 | 16 | 32 |
| 3j | 5-OBn | 64 | >128 | 128 | 64 |
| 3k | 6-OBn | 64 | >128 | 128 | 128 |
| 4a | H | 8 | >128 | 16 | >128 |
| 4b | 5-Cl | 4 | 8 | 8 | 8 |
| 4c | 6-Cl | 8 | 8 | 32 | 32 |
| 4e | 6-Br | 16 | 64 | 32 | 64 |
| 4f | 5-CN | 4 | >128 | 32 | >128 |
| 4g | 6-CN | 4 | >128 | 128 | >128 |
| 4h | 5-OH | 8 | 16 | 32 | 64 |
| 4i | 6-OH | 2 | 8 | 16 | 32 |
| 4j | 5-OBn | 64 | >128 | 128 | 64 |
| 4k | 6-OBn | 32 | >128 | 128 | 128 |
| Oxacillin | 1 | - | - | - | |
| Ciprofloxacin | - | - | 4 | - | |
| Ampicillin | - | >128 | 2 | - | |
| Vancomycin | 4 | >128 | 128 | 32 | |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Seethaler, M.; Hertlein, T.; Wecklein, B.; Ymeraj, A.; Ohlsen, K.; Lalk, M.; Hilgeroth, A. Novel Small-molecule Antibacterials against Gram-positive Pathogens of Staphylococcus and Enterococcus Species. Antibiotics 2019, 8, 210. https://doi.org/10.3390/antibiotics8040210
Seethaler M, Hertlein T, Wecklein B, Ymeraj A, Ohlsen K, Lalk M, Hilgeroth A. Novel Small-molecule Antibacterials against Gram-positive Pathogens of Staphylococcus and Enterococcus Species. Antibiotics. 2019; 8(4):210. https://doi.org/10.3390/antibiotics8040210
Chicago/Turabian StyleSeethaler, Marius, Tobias Hertlein, Björn Wecklein, Alba Ymeraj, Knut Ohlsen, Michael Lalk, and Andreas Hilgeroth. 2019. "Novel Small-molecule Antibacterials against Gram-positive Pathogens of Staphylococcus and Enterococcus Species" Antibiotics 8, no. 4: 210. https://doi.org/10.3390/antibiotics8040210
APA StyleSeethaler, M., Hertlein, T., Wecklein, B., Ymeraj, A., Ohlsen, K., Lalk, M., & Hilgeroth, A. (2019). Novel Small-molecule Antibacterials against Gram-positive Pathogens of Staphylococcus and Enterococcus Species. Antibiotics, 8(4), 210. https://doi.org/10.3390/antibiotics8040210






