Present and Future of Carbapenem-resistant Enterobacteriaceae (CRE) Infections
Abstract
1. Introduction
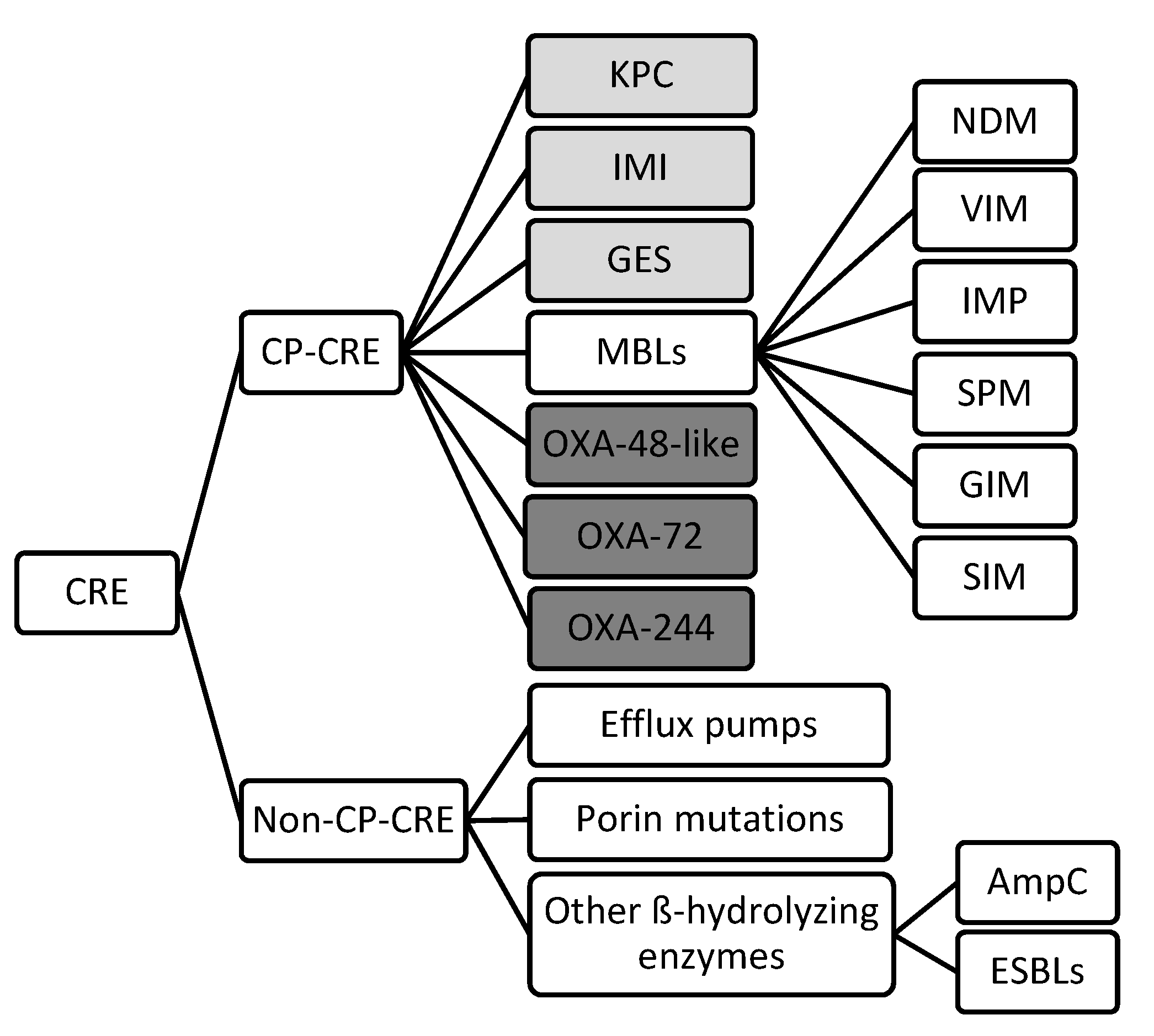
2. Mechanisms of Drug Resistance
2.1. Carbapenemase-producing CRE
2.2. Non-Carbapenemase-producing CRE
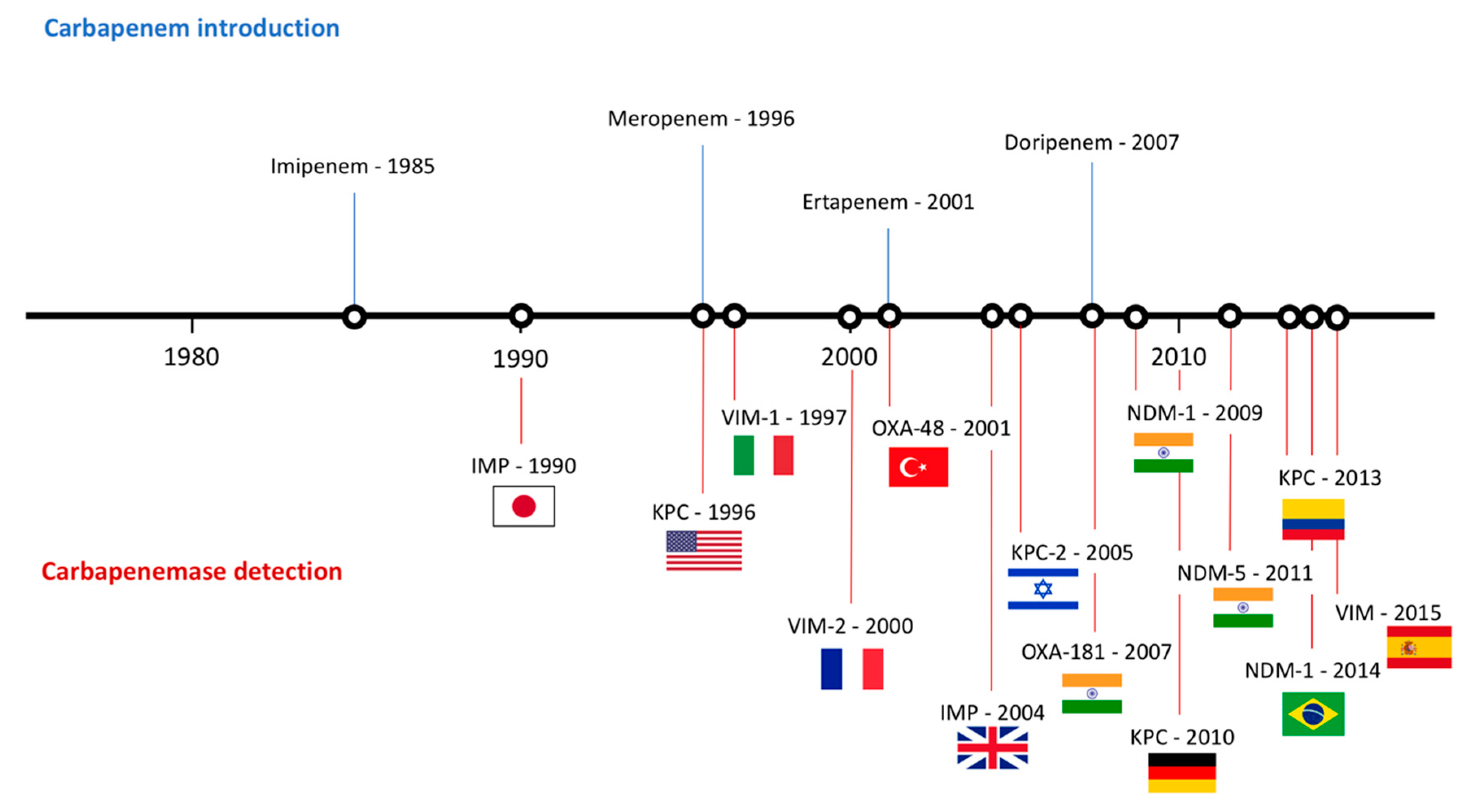
3. Current Resistance Status
4. Treatment Options
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Centers for Disease Control and Prevention. About Antimicrobial Resistance. 2018. Available online: https://www.cdc.gov/drugresistance/about.html (accessed on 20 July 2019).
- Niu, G.; Li, W. Next-Generation Drug Discovery to Combat Antimicrobial Resistance. Trends Biochem. Sci. 2019, in press. [Google Scholar] [CrossRef] [PubMed]
- O’Neill, J. Antimicrobial Resistance: Tackling a Crisis for the Health and Wealth of Nations (HM Government and Wellcome Trust). 2014. Available online: https://amr-review.org/sites/default/files/AMR%20Review%20Paper%20-%20Tackling%20a%20crisis%20for%20the%20health%20and%20wealth%20of%20nations_1.pdf (accessed on 20 July 2019).
- World Health Organization. WHO Priority Pathogens List for R&D of New Antibiotics. 2017. Available online: http://www.who.int/bulletin/volumes/94/9/16-020916.pdf (accessed on 20 July 2019).
- Lee, C.; Lai, C.C.; Chiang, H.T.; Lu, M.C.; Wang, L.F.; Tsai, T.L.; Kang, M.Y.; Jan, Y.N.; Lo, Y.T.; Ko, W.C.; et al. Presence of multidrug-resistant organisms in the residents and environments of long-term care facilities in Taiwan. J. Microbiol. Immunol. Infect. 2017, 50, 133–144. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Bano, J.; Gutierrez-Gutierrez, B.; Machuca, I.; Pascual, A. Treatment of infections caused by extended-spectrum-beta-lactamase-, AmpC-, and carbapenemase-producing Enterobacteriaceae. Clin. Microbiol. Rev. 2018, 31, e00079-17. [Google Scholar] [CrossRef]
- Pana, Z.D.; Zaoutis, T. Treatment of extended-spectrum ß-lactamase-producing Enterobacteriaceae (ESBLs) infections: What have we learned until now? F1000Res 2018, 7. [Google Scholar] [CrossRef]
- D’Angelo, R.G.; Johnson, J.K.; Bork, J.T.; Heil, E.L. Treatment options for extended-spectrum beta-lactamase (ESBL) and AmpC-producing bacteria. Expert Opin. Pharmacother. 2016, 17, 953–967. [Google Scholar] [CrossRef] [PubMed]
- Sheu, C.C.; Lin, S.Y.; Chang, Y.T.; Lee, C.Y.; Chen, Y.H.; Hsueh, P.R. Management of infections caused by extended-spectrum beta-lactamase-producing Enterobacteriaceae: Current evidence and future prospects. Expert Rev. Anti Infect. Ther. 2018, 16, 205–218. [Google Scholar] [CrossRef] [PubMed]
- Durante-Mangoni, E.; Andini, R.; Zampino, R. Management of carbapenem-resistant Enterobacteriaceae infections. Clin. Microbiol. Infect. 2019, 25, 943–950. [Google Scholar] [CrossRef] [PubMed]
- Centers for Disease Control and Prevention. Facility Guidance for Control of Carbapenem-Resistant Enterobacteriaceae (CRE)—November 2015 Update CRE Toolkit. Available online: https://www.cdc.gov/hai/organisms/cre/Cre-toolkit/index.html (accessed on 22 July 2019).
- Haidar, G.; Clancy, C.J.; Chen, L.; Samanta, P.; Shields, R.K.; Kreiswirth, B.N.; Nguyen, M.H. Identifying specra of activity and therapeutic niches for ceftazidime-avibactam and imipenem-relebactam against carbapenem-resistant Enterobacteriaceae. Antimicrob. Agents Chemother. 2017, 61. [Google Scholar] [CrossRef]
- Tooke, C.L.; Hinchliffe, P.; Bragginton, E.C.; Colenso, C.K.; Hirvonen, V.H.A.; Takebayashi, Y.; Spencer, J. ß-Lactamases and ß-Lactamase Inhibitors in the 21st Century. J. Mol. Biol. 2019. [Google Scholar] [CrossRef]
- Codjoe, F.S.; Donkor, E.S. Carbapenem Resistance: A review. Med. Sci. 2018, 6, 1. [Google Scholar] [CrossRef]
- Lutgring, J.D.; Limbago, B.M. The Problem of Carbapenemase-Producing-Carbapenem-Resistant-Enterobacteriaceae Detection. J. Clin. Microbiol. 2016, 54, 529–534. [Google Scholar] [CrossRef]
- Ambler, R.P. The structure of ß-lactamases. Philos. Trans. R. Soc. Lond. B 1980, 289, 321–331. [Google Scholar] [CrossRef]
- Queenan, A.M.; Bush, K. Carbapenemases: The versatile ß-lactamases. Clin. Microbiol. Rev. 2007, 20, 440–458. [Google Scholar] [CrossRef] [PubMed]
- Yigit, H.; Queenan, A.M.; Anderson, G.J.; Domenech-Sanchez, A.; Biddle, J.W.; Steward, C.D.; Alberti, S.; Bush, K.; Tenover, F.C. Novel carbapenem-hydrolyzing beta-lactamase, KPC-1, from a carbapenem-resistant strain of Klebsiella pneumoniae. Antimicrob. Agents Chemother. 2001, 45, 1151–1161. [Google Scholar] [CrossRef] [PubMed]
- Ji, S.; Lv, F.; Du, X.; Wei, Z.; Fu, Y.; Mu, X.; Jiang, Y.; Yu, Y. Cefepime combined with amoxicillin/clavulanic acid: A new choice for the KPC-producing K. pneumoniae infection. Int. J. Infect. Dis. 2015, 38, 108–114. [Google Scholar] [CrossRef] [PubMed]
- Porreca, A.M.; Sullivan, K.V.; Gallagher, J.C. The Epidemiology, Evolution, and Treatment of KPC-Producing Organisms. Curr. Infect. Dis. Rep. 2018, 20, 13. [Google Scholar] [CrossRef] [PubMed]
- Nordmann, P.; Cuzon, G.; Naas, T. The real threat of Klebsiella pneumoniae carbapenemase-producing bacteria. Lancet Infect. Dis. 2009, 9, 228–233. [Google Scholar] [CrossRef]
- Miriagou, V.; Cornaglia, G.; Edelstein, M.; Galani, I.; Giske, G. Acquired carbapenemases in Gram-negative bacterial pathogens: Detection and surveillance issues. Clin. Microbiol. Infect. 2010, 16, 112–122. [Google Scholar] [CrossRef] [PubMed]
- Okoche, D.; Asiimwe, B.B.; Katabazi, F.A.; Kato, L.; Najjuka, C.F. Prevalence and Characterization of Carbapenem-Resistant Enterobacteriaceae Isolated from Mulago National Referral Hospital, Uganda. PLoS ONE 2015, 10, e0135745. [Google Scholar] [CrossRef]
- Boutal, H.; Vogel, A.; Bernabeu, S.; Devilliers, K.; Creton, E.; Cotellon, G.; Plaisance, M.; Oueslati, S.; Dortet, L.; Jousset, A.; et al. A multiplex lateral flow immunoassay for the rapid identification of NDM-, KPC-, IMP- and VIM-type and OXA-48-like carbapenemase-producing Enterobacteriaceae. J. Antimicrob. Chemother. 2018, 73, 909–915. [Google Scholar] [CrossRef]
- Fernández, J.; Guerra, B.; Rodicio, M.R. Resistance to Carbapenems in Non-Typhoidal Salmonella enterica Serovars from Humans, Animals and Food. Vet. Sci. 2018, 5, 40. [Google Scholar] [CrossRef] [PubMed]
- Abdallah, M.; Balshi, A. First literature review of carbapenem-resistant Providencia. New Microb. New Infect. 2018, 25, 16–23. [Google Scholar] [CrossRef] [PubMed]
- Perez, F.; van Duin, D. Carbapenem-resistant Enterobacteriaceae: A menace to our most vulnerable patients. Clevel Clin. J. Med. 2013, 80, 225–233. [Google Scholar] [CrossRef] [PubMed]
- Walsh, T.R.; Toleman, M.A.; Piorel, L.; Nordman, P. Metallo-b-lactamases: The quiet before the storm? Clin. Microbiol. Rev. 2005, 18, 306–325. [Google Scholar] [CrossRef] [PubMed]
- Van Duin, D.; Doi, Y. The global epidemiology of carbapenemase-producing Enterobacteriaceae. Virulence 2017, 8, 460–469. [Google Scholar] [CrossRef] [PubMed]
- Livermore, D.M.; Woodford, N. Carbapenemases: A problem in waiting? Curr. Opin. Microbiol. 2000, 3, 489–495. [Google Scholar] [CrossRef]
- Gupta, V. Metallo-b-lactamases in Pseudomonas aeruginosa and Acinetobacter species. Expert Opin. Investig. Drugs 2008, 17, 131–143. [Google Scholar] [CrossRef]
- Bush, K. Past and Present Perspectives on ß-Lactamases. Antimicrob. Agents Chemother. 2018, 62, e01076-18. [Google Scholar] [CrossRef]
- Walsh, T.R.; Weeks, J.; Livermore, D.M.; Toleman, M.A. Dissemination of NDM-1 positive bacteria in the New Delhi environment and its implications for human health: An environmental point prevalence study. Lancet Infect. Dis. 2011, 11, 355–362. [Google Scholar] [CrossRef]
- Poirel, L.; Hombrouck-Alet, C.; Freneaux, C.; Bernabeu, S.; Nordmann, P. Global spread of New Delhi metallo-ß-lactamase 1. Lancet Infect. Dis. 2010, 10, 832. [Google Scholar] [CrossRef]
- Ivanov, I.; Sabtcheva, S.; Dobreva, E.; Todorova, B.; Velinov, T.Z.; Borissova, V.; Kantardijev, T. Prevalence of carbapenemase genes among 16S rRNA methyltransferase-producing Enterobacteriaceae isolated for cancer patients. Probl. Infect. Parasit. Dis. 2014, 42, 10–13. [Google Scholar]
- Poirel, L.; Potron, A.; Nordmann, P. OXA-48-like carbapenemases: The phantom menace. J. Antimicrob. Chemother. 2012, 67, 1597–1606. [Google Scholar] [CrossRef] [PubMed]
- Rasmussen, J.W.; Hoiby, N. OXA-type carbapenemases. J. Antimicrob. Chemother. 2006, 57, 373–383. [Google Scholar] [CrossRef] [PubMed]
- Stewart, A.; Harris, P.; Henderson, A.; Paterson, D. Treatment of Infections by OXA-48-Producing Enterobacteriaceae. Antimicrob. Agents Chemother. 2018, 62, e01195-18. [Google Scholar] [CrossRef] [PubMed]
- Logan, L.K.; Weinstein, R.A. The Epidemiology of Carbapenem-Resistant Enterobacteriaceae: The Impact and Evolution of a Global Menace. J. Infect. Dis. 2017, 215 (Suppl. 1), S28–S36. [Google Scholar] [CrossRef]
- Tzouvelekis, L.S.; Marjogiannakis, A.; Psichogiou, M.; Tassios, P.T.; Daikos, G.L. Carbapenemases in Klebsiella pneumoniae and other Enterobacteriaceae: An evolving crisis of global dimensions. Clin. Microbiol. Rev. 2012, 25, 682–707. [Google Scholar] [CrossRef]
- Queenan, A.M.; Shang, W.; Flamm, R.; Bush, K. Hydrolysis and inhibition profiles of ß-lactamases from molecular classes A to D with doripenem, imipenem, and meropenem. Antimicrob. Agents Chemother. 2010, 54, 565–569. [Google Scholar] [CrossRef]
- Goessens, W.H.F.; van der Bij, A.K.; van Boxtel, R.; Pitout, J.D.D.; van Ulsen, P.; Melles, D.C.; Tommassen, J. Antibiotic trapping by plasmid-encoded CMY-2 ß-lactamase combined with reduced outer membrane permeability as a mechanism of carbapenem resistance in Escherichia coli. Antimicrob. Agents Chemother. 2013, 57, 3941–3949. [Google Scholar] [CrossRef]
- Philippon, A.; Arlet, G.; Jacoby, G.A. Plasmid-determined AmpC-type ß-lactamases. Antimicrob. Agents Chemother. 2002, 46, 1–11. [Google Scholar] [CrossRef]
- Weston, N.; Sharma, P.; Ricci, V.; Piddock, L.J.V. Regulation of the AcrAB-TolC efflux pump in Enterobacteriaceae. Res. Microbiol. 2018, 169, 425–431. [Google Scholar] [CrossRef]
- Routh, M.D.; Zalucki, Y.; Su, C.C.; Zhang, Q.; Shager, W.M.; Yu, E.W. Efflux pumps of the resistance-nodulation-division family: A perspective of their structure, function, and regulation in gram-negative bacteria. Adv. Enzymol. Relat. Areas Mol. Biol. 2011, 77, 109–146. [Google Scholar] [PubMed]
- Courvalin, P. Transfer of antibiotic resistance genes between gram-positive and gram-negative bacteria. Antimicrob. Agents Chemother. 1994, 38, 1447–1451. [Google Scholar] [CrossRef] [PubMed]
- Bialek-Davenet, S.; Mayer, N.; Vergalli, J.; Duprilot, M.; Brisse, S.; Pagès, J.M.; Nicolas-Chanoine, M.H. In-vivo loss of carbapenem resistance by extensively drug-resistant Klebsiella pneumoniae during treatment via porin expression modification. Sci. Rep. 2017, 7, 6722. [Google Scholar] [CrossRef] [PubMed]
- Masi, M.; Réfrigiers, M.; Pos, K.M.; Pagès, J.M. Mechanisms of envelope permeability and antibiotic influx and efflux in Gram-negative bacteria. Nat. Microbiol. 2017, 2, 17001. [Google Scholar] [CrossRef] [PubMed]
- Walsh, C. Molecular mechanisms that confer antibacterial drug resistance. Nature 2000, 406, 775–781. [Google Scholar] [CrossRef] [PubMed]
- Ito, H.; Arakawa, Y.; Oshuka, S.; Wacharotayankun, R.; Kato, N.; Ohta, M. Plasmid-mediated dissemination of the metallo-beta-lactamase gene blaIMP among clinically isolated strains of Serratia marcescens. J. Antimicrob. Chemother. 1995, 50, 503–511. [Google Scholar] [CrossRef] [PubMed]
- Nordmann, P.; Poirel, L. The difficult-to-control spread of carbapenemase producers among Enterobacteriaceae worldwide. Clin. Microbiol. Infect. 2014, 20, 821–830. [Google Scholar] [CrossRef]
- Nordmann, P.; Naas, T.; Poirel, L. Global spread of carbapenemase-producing Enterobacteriaceae. Emerg. Infect. Dis. 2011, 17, 1791–1798. [Google Scholar] [CrossRef]
- Lauretti, L.; Riccio, M.K.; Mazzariol, A.; Cornaglia, G.; Amicosante, G.; Fontana, R.; Rossolini, G.M. Cloning and characterization of blaVIM, a new integron-borne metallo-beta-lactamase gene from a Pseudomonas aeruginosa clinical isolate. Antimicrob. Agents Chemother. 1999, 43, 1584–1590. [Google Scholar] [CrossRef]
- Yong, D.; Toleman, M.A.; Giske, C.G.; Cho, H.S.; Sundman, K.; Lee, K.; Walsh, T.R. Characterization of new metallo-beta-lactamase gene, bla(NDM-1), and a novel erythromycin esterase gene carried on a unique genetic structure in Klebsiella pneumoniae sequence type 14 from India. Antimicrob. Agents Chemother. 2009, 53, 5046–5054. [Google Scholar] [CrossRef]
- Dortet, L.; Poirel, L.; Nordmann, P. Worldwide dissemination of the NDM-type carbapenemases in Gram-negative bacteria. Biomed. Res. Int. 2014, 2014, 249856. [Google Scholar] [CrossRef] [PubMed]
- Poirel, L.; Savov, E.; Nazli, A.; Trifonova, A.; Todorova, I.; Gergova, I.; Nordmann, P. Outbreak caused by NDM-1- and RmtB- producing Escherichia coli in Bulgaria. Antimicrob. Agents Chemother. 2012, 58, 2472–2474. [Google Scholar] [CrossRef] [PubMed]
- Patel, G.; Bonomo, R.A. Stormy waters ahead: Global emergence of carbapenemases. Front. Microbiol. 2013, 4, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Bradford, P.A.; Bratu, S.; Urban, C.; Visalli, M.; Mariano, N.; Landman, D.; Rahal, J.J.; Brooks, S.; Cebular, S.; Quale, J. Emergence of carbapenem-resistant Klebsiella species possessing the class a carbapenem-hydrolyzing KPC-2 and inhibitor-resistant TEM-30 ß-lactamases in New York City. Clin. Infect. Dis. 2004, 39, 55–60. [Google Scholar] [CrossRef] [PubMed]
- Munoz-Price, L.S.; Poirel, L.; Bonomo, R.A.; Schwaber, M.J.; Daikos, G.L.; Cormican, M.; Cornaglia, G.; Garau, J.; Gniadkowski, M.; Hayden, M.K. Clinical epidemiology of the global expansion of Klebsiella pneumoniae carbapenemases. Lancet Infect. Dis. 2013, 13, 785–796. [Google Scholar] [CrossRef]
- Maya, J.J.; Ruiz, S.J.; Blanco, V.M.; Gotuzzo, E.; Guzman-Blanco, M.; Labarca, J.; Salles, M.; Quinn, J.P.; Villegas, M.V. Current status of carbapenemases in Latin America. Expert Rev. Anti Infect. Ther. 2013, 11, 657–667. [Google Scholar] [CrossRef]
- Poirel, L.; Bonnin, R.A.; Nordmann, P. Genetic features of the widespread plasmid coding for the carbapenemase OXA-48. Antimicrob. Agents Chemother. 2012, 56, 559–562. [Google Scholar] [CrossRef]
- The European Committee on Antimicrobial Susceptibility Testing. Breakpoint Tables for Interpretation of MICs and Zone Diameters, Version 9.0; 2019. Available online: http//www.eucast.org (accessed on 25 July 2019).
- CLSI. Performance Standards for Antimicrobial Susceptibility Testing, 29th ed.; CLSI supplement M100; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2019. [Google Scholar]
- The European Committee on Antimicrobial Susceptibility Testing. Breakpoint Tables for Interpretation of MICs and Zone Diameters, Version 8.1; 2018. Available online: http//www.eucast.org (accessed on 25 July 2019).
- Karaiskos, I.; Lagou, S.; Pontikis, K.; Rapti, V.; Poulakou, G. The “Old” and the “New” antibiotics for MDR gram-negative pathogens: For whom, when and how. Front. Public Health 2019, 7, 151. [Google Scholar] [CrossRef]
- Vardakas, K.Z.; Legakis, N.J.; Triarides, N.; Falagas, M.E. Susceptibility of contemporary isolates to Fosfomycin: A systematic review of the literature. Int. J. Antimicrob. Agents 2016, 47, 269–285. [Google Scholar] [CrossRef]
- Satlin, M.J.; Kubin, C.J.; Blumental, J.S.; Cohen, A.B.; Furuya, E.Y.; Wilson, S.J.; Jenkins, S.G.; Calfee, D.P. Comparative effectiveness of aminoglycosides, polymyxin B and tigecycline for clearance of carbapenem resistant Klebsiella pneumoniae from urine. Antimicrob. Agents Chemother. 2011, 55, 2528–2531. [Google Scholar] [CrossRef]
- Sader, H.S.; Castanheira, M.; Duncan, L.R.; Flamm, R.K. Antimicrobial susceptibility of Enterobacteriaceae and Pseudomonas aeruginosa isolates from United States medical centers stratified by infection type: Results from the international network for optimal resistance monitoring (INFORM) surveillance program, 2015–2016. Diagn. Microbiol. Infect. Dis. 2018, 92, 69–74. [Google Scholar] [CrossRef] [PubMed]
- Daikos, G.L.; Tsaousi, S.; Tzouvelekis, L.S.; Anyfantis, I.; Psichogiou, M.; Argyropoulou, A.; Stefanou, I.; Sypsa, V.; Miriagou, V.; Nepka, M. Carbapenemase-producing Klebsiella pneumoniae bloodstream infections: Lowering mortality by antibiotic combination schemes and the role of carbapenems. Antimicrob. Agents Chemother. 2014, 58, 2322–2328. [Google Scholar] [CrossRef] [PubMed]
- Ni, W.; Han, Y.; Liu, J.; Wei, C.; Zhao, J.; Cui, J.; Wang, R.; Liu, Y. Tigecycline treatment for carbapenem-resistant Enterobacteriaceae infections: A systematic review and meta-analysis. Medicine 2016, 95, e3126. [Google Scholar] [CrossRef] [PubMed]
- Giamarellou, H.; Poulakou, G. Pharmakokinetic and pharmacodynamic evaluation of tigecycline. Expert Opin. Drug Metab. Toxicol. 2011, 7, 1459–1470. [Google Scholar] [CrossRef] [PubMed]
- De Pascale, G.; Montini, L.; Pennisi, M.; Bernini, V.; Maviglia, R.; Bello, G.; Spanu, T.; Tumbarello, M.; Antonelli, M. High dose tigecycline in critically ill patients with severe infections due to multidrug-resistant bacteria. Crit. Care 2014, 18, R90. [Google Scholar] [CrossRef] [PubMed]
- Bulik, C.C.; Nicolau, D.P. Double-carbapenem therapy for carbapenemase-producing Klebsiella pneumoniae. Antimicrob. Agents Chemother. 2011, 55, 3002–3004. [Google Scholar] [CrossRef] [PubMed]
- Anderson, K.F.; Lonsway, D.R.; Rasheed, J.K.; Biddle, J.; Jensen, B.; McDougal, L.K.; Carey, R.B.; Thompson, A.; Stocker, S.; Limbago, B.; et al. Evaluation of methods to identify the Klebsiella pneumoniae carbapenemase in Enterobacteriaceae. J. Clin. Microbiol. 2007, 45, 2723–2725. [Google Scholar] [CrossRef] [PubMed]
- Oliva, A.; Scorzolini, L.; Castaldi, D.; Gizzi, F.; De Angelis, M.; Sotrto, M.; D’Abramo, A.; Aloj, F.; Mascellino, M.T.; Mastroianni, C.M.; et al. Double-carbapenem regimen, alone or in combination with colistin, in the treatment of infections caused by carbapenem-resistent Klebsiella pneumoniae (CR-Kp). J. Infect. 2017, 74, 103–106. [Google Scholar] [CrossRef]
- Venugopalan, V.; Nogid, B.; Le, T.N.; Rahman, S.M.; Bias, T.E. Double carbapenem therapy (DCT) for bacteremia due to carbapenem-resistant Klebsiella pneumoniae (CRKP): From the test tube to clinical practice. Infect. Dis. 2017, 49, 867–870. [Google Scholar] [CrossRef]
- De Jonge, B.L.; Karlowsky, J.A.; Kazmierczak, K.M.; Biedenbach, D.J.; Sahm, D.F.; Nichols, W.W. In vitro susceptibility to ceftazidime-avibactam of carbapenem-nonsusceptible Enterobacteriaceae isolates collected during the INFORM Global Surveillance Study (2012 to 2014). Antimicrob. Agents Chemother. 2016, 60, 3163–3169. [Google Scholar] [CrossRef]
- Van Duin, D.; Lok, J.J.; Earley, M.; Cober, E.; Richter, S.S.; Perez, F.; Salata, R.A.; Kalayjian, R.C.; Watkins, R.R.; Doi, Y.; et al. Colistin Versus Ceftazidime-Avibactam in the Treatment of Infections Due to Carbapenem-Resistant Enterobacteriaceae. Clin. Infect. Dis. 2018, 66, 163–171. [Google Scholar] [CrossRef] [PubMed]
- Nelson, K.; Hemarajata, P.; Sun, D.; Rubio-Aparicio, D.; Tsivokovski, R.; Yang, S.; Sebra, R.; Kasarskis, A.M.; Nguyen, H.; Hanson, B.M.; et al. Resistance to Ceftazidime-Avibactam Is Due to Transposition of KPC in a Porin-Deficient Strain of Klebsiella pneumoniae with Increased Efflux Activity. Antimicrob. Agents Chemother. 2017, 61, e00989-17. [Google Scholar] [CrossRef] [PubMed]
- Venditti, C.; Nisii, C.; D’Arezzo, S.; Vulcano, A.; Capone, A.; Antonini, M.; Ippolito, G.; Di Caro, A. Molecular and phenotypical characterization of two cases of antibiotic-driven ceftazidime-avibactam resistance in blaKPC-3-harboring Klebsiella pneumoniae. Infect. Drug Resist. 2019, 12, 1935–1940. [Google Scholar] [CrossRef] [PubMed]
- Humphries, R.M. Mechanisms of Resistance to Ceftazidime-Avibactam, 28th ed.; European Congress of Clinical Microbiology & Infectious Diseases (ECCMID): Madrid, Spain, 2018. [Google Scholar]
- Petty, L.A.; Henig, O.; Patel, T.S.; Pogue, J.M.; Kaye, K.S. Overview of meropenem-vaborbactam and newer antimicrobial agents for the treatment of carbapenem-resistant Enterobacteriaceae. Infect. Drug Resist. 2018, 11, 1461–1472. [Google Scholar] [CrossRef] [PubMed]
- Lomovskaya, O.; Sun, D.; Rubio-Aparicio, D.; Nelson, K.; Tsivkovski, R.; Griffith, D.C.; Dudley, M.N. Vaborbactam: Spectrum of beta-lactamase inhibition and impact of resistance mechanisms on activity in Enterobacteriaceae. Antimicrob. Agents Chemother. 2017, 61, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Wunderink, R.G.; Giamarellos-Bourboulis, E.J.; Rahav, G.; Mathers, A.J.; Bassetti, M.; Vazquez, J.; Cornely, O.A.; Solomkin, J.; Bhowmick, T.; Bishara, J.; et al. Effect and safety of meropenem-vaborbactam versus best-available therapy in patients with carbapenem-resistant Enterobacteriaceae infections: The TANGO II randomized clinical trial. Infect. Dis. Ther. 2018, 7, 439–455. [Google Scholar] [CrossRef]
- Landman, D.; Babu, E.; Shah, N.; Kelly, P.; Backer, M.; Bratu, S.; Quale, J. Activity of a novel aminoglycoside, ACHN-490, against clinical isolates of Escherichia coli and Klebsiella pneumoniae from New York City. J. Antimicrob. Chemother. 2010, 65, 2123–2127. [Google Scholar] [CrossRef]
- Endimiani, A.; Hujer, K.M.; Hujer, A.M.; Armstrong, E.S.; Choudhary, Y.; Aggen, J.B.; Bonomo, R.A. ACHN-490, a neoglycoside with potent in vitro activity against multidrug-resistant Klebsiella pneumoniae isolates. Antimicrob. Agents Chemother. 2009, 53, 4504–4507. [Google Scholar] [CrossRef]
- Walkty, A.; Adam, H.; Baxter, M.; Denisuik, A.; Lagace-Wiens, P.; Karlowsky, J.A.; Hoban, D.J.; Zhanel, G.G. In vitro activity of plazomicin against 5,015 Gram-negative and Gram-positive clinical isolates obtained from patients in Canadian hospitals as part of the CANWARD study, 2011–2012. Antimicrob. Agents Chemother. 2014, 58, 2554–2563. [Google Scholar] [CrossRef]
- Castanheira, M.; Deshpande, L.M.; Woosley, L.N.; Serio, A.W.; Krause, K.M.; Flamm, R.K. Activity of plazomicin compared with other aminoglycosides against isolates from European and adjacent countries, including Enterobacteriaceae molecularly characterized for aminoglycoside-modifying enzymes and other resistance mechanisms. J. Antimicrob. Chemother. 2018, 73, 3346–3354. [Google Scholar] [CrossRef]
- Zhanel, G.G.; Cheung, D.; Adam, H.; Zelenitsky, S.; Golden, A.; Schweizer, F.; Gorityala, B.; Lagacé-Wiens, P.R.; Walkty, A.; Gin, A.S.; et al. Review of eravacycline, a novel fluorocycline antibacterial agent. Drugs 2016, 76, 567–588. [Google Scholar] [CrossRef] [PubMed]
- Bassetti, M.; Righi, E. Eravacycline for the treatment of intra-abdominal infections. Expert Opin. Investig. Drugs 2014, 23, 1575–1584. [Google Scholar] [CrossRef] [PubMed]
- XERAVA (Eravacycline) for Injection. XERAVA (Eravacycline) IGNITE1 and IGNITE4 Trial Results. 2019. Available online: https://www.xerava.com/efficacy (accessed on 25 July 2019).
- Solomkin, J.S.; Ramesh, M.K.; Cesnauskas, G.; Novikovs, N.; Stefanova, P.; Sutcliffe, J.A.; Walpole, S.M.; Horn, P.T. Phase 2, randomized, double-blind study of the efficacy and safety of two dose regimens of eravacycline versus ertapenem for adult community-acquired complicated intra-abdominal infections. Antimicrob. Agents Chemother. 2014, 58, 1847–1854. [Google Scholar] [CrossRef]
- Blizzard, T.A.; Chen, H.; Kim, S.; Wu, J.; Bodner, R.; Gude, C.; Imbriglio, J.; Young, K.; Park, Y.W.; Ogawa, A.; et al. Discovery of MK-7655, a beta-lactamase inhibitor for combination with Primaxin®. Bioorg. Med. Chem. Lett. 2014, 24, 780–785. [Google Scholar] [CrossRef] [PubMed]
- Zhanel, G.G.; Lawrence, C.K.; Adam, H.; Schweizer, F.; Zelenitsky, S.; Zhanel, M.; Lagacé-Wiens, P.R.S.; Walkty, A.; Denisuik, A.; Golden, A.; et al. Imipenem-Relebactam and Meropenem-Vaborbactam: Two Novel Carbapenem-ß-Lactamase Inhibitor Combinations. Drugs 2018, 78, 65–98. [Google Scholar] [CrossRef] [PubMed]
- Motsch, J.; Oliveira, C.; Stus, V.; Koksal, I.; Lyulko, O.; Boucher, H.W.; Kaye, K.S.; File, T.M.; Brown, M.L.; Khan, I.; et al. RESTORE-IMI 1: A Multicenter, Randomized, Double-Blind, Comparator-Controlled Trial Comparing the Efficacy and Safety of Imipenem/Relebactam Versus Colistin Plus Imipenem in Patients with Imipenem-Non-Susceptible Bacterial Infections, 28th ed.; ECCMID: Madrid, Spain, 2018. [Google Scholar]
- Saisho, Y.; Katsube, T.; White, S.; Fukase, H.; Shimada, J. Pharmacokinetics, safety, and tolerability of cefiderocol, a novel siderophore cephalosporin for Gram-negative bacteria, in healthy subjects. Antimicrob. Agents Chemother. 2018, 62, e-02163-17. [Google Scholar] [CrossRef] [PubMed]
- Hackel, M.A.; Tsuji, M.; Yamano, Y.; Echols, R.; Karlowsky, J.A.; Sahm, D.F. In Vitro Activity of the Siderophore Cephalosporin, Cediferocol, against Carbapenem-Nonsusceptible and Multidrug-Resistant Isolates of Gram-Negative Bacilli Collected Worldwide in 2014 to 2016. Antimicrob. Agents Chemother. 2018, 62, e01968-17. [Google Scholar] [CrossRef]
- Corbett, D.; Wise, A.; Langley, T.; Skinner, K.; Trimby, E.; Birchall, S.; Dorali, A.; Sandiford, S.; Williams, J.; Warn, P.; et al. Potentiation of Antibiotic Activity by a Novel Cationic Peptide: Potency and Spectrum of Activity of SPR741. Antimicrob. Agents Chemother. 2017, 61. [Google Scholar] [CrossRef]
- Karaiskos, I.; Galani, I.; Souli, M.; Giamarellou, H. Novel ß-lactam-ß-lactamase inhibitor combinations: Expectations for the treatment of carbapenem-resistant Gram-negative pathogens. Expert Opin. Drug Metab. Toxicol. 2019, 15, 133–149. [Google Scholar] [CrossRef]
- Papp-Wallace, K.M.; Nguyen, N.Q.; Jacobs, M.R.; Bethel, C.R.; Barnes, M.D.; Kumar, V.; Bajaksouzian, S.; Rudin, S.D.; Bhavsar, S.; et al. Strategic Approaches to Overcome Resistance against Gram-Negative Pathogens Using ß-lactamase Inhibitors and ß-Lactam Enhancers: Activity of Three Novel Diazabicyclooctanes WCK 5153, Zidebactam (WCK 5107), and WCK 4234. J. Med. Chem. 2018, 61, 4067–4086. [Google Scholar] [CrossRef]
- Sader, H.S.; Rhomberg, P.R.; Flamm, R.K.; Jones, R.N.; Castanheira, M. WCK 5222 (cefepime/zidebactam) antimicrobial activity tested against Gram-negative organisms producing clinically relevant ß-lactamases. J. Antimicrob. Chemother. 2017, 72, 1696–1703. [Google Scholar] [CrossRef] [PubMed]
- Monogue, M.L.; Giovagnoli, S.; Bissantz, C.; Zampaloni, C.; Nicolau, D.P. In Vivo Efficacy of Meropenem with a Novel Non-ß-Lactam-ß-Lactamase Inhibitor, Nacubactam, against Gram-Negative Organisms Exhibiting Various Resistance Mechanisms in a Murine Complicated Urinary Tract Infection Model. Antimicrob. Agents Chemother. 2018, 62, e02596-17. [Google Scholar] [CrossRef] [PubMed]
- Daigle, D.; Hamrick, J.; Chatwin, C.; Kurepina, N.; Kreiswirth, B.N.; Shields, R.K.; Oliver, A.; Clancy, C.J.; Nguyen, M.H.; Pevear, D.; et al. Cefepime/VNRX-5133 Borad-Spectrum Activity Is Mantained Against Emerging KPC- and PDC-Variants in Multidrug-Resistant K. pneumoniae and P. aeruginosa. Open Forum Infect. Dis. 2018, 5 (Suppl. 1), S419–S420. [Google Scholar] [CrossRef]
| Species | Class A | Class B (MBLs) | Class D | Ref. |
|---|---|---|---|---|
| Klebsiella pneumoniae | KPC-3 | NDM-1, VIM-1 | OXA-48 | Okoche et al. Boutal et al. |
| Klebsiella oxytoca | OXA-48, OXA-181 | Okoche et al. Boutal et al. | ||
| Escherichia coli | KPC | NDM-1, NDM-5, NDM-9, VIM | OXA-48, OXA-181, OXA-244 | Okoche et al. Boutal et al. |
| Proteus mirabilis | KPC | OXA-48 | Okoche et al. Boutal et al. | |
| Serratia marcescens | KPC | VIM | Okoche et al. Boutal et al. | |
| Enterobacter cloacae | KPC, IMI-1 | VIM-4 | OXA-48 | Okoche et al. Boutal et al. |
| Enterobacter aerogenes | KPC | OXA-48 | Okoche et al. Boutal et al. | |
| Citrobacter freundii | VIM | OXA-48 | Okoche et al. Boutal et al. | |
| Citrobacter koseri | OXA-48 | Okoche et al. Boutal et al. | ||
| Salmonella enterica | KPC-2 | NMD-1, NMD-5, VIM-1, VIM-2, IMP-4 | OXA-48 | Fernández et al. |
| Morganella morganii | NDM-1 | OXA-48 | Boutal et al. | |
| Providencia stuartii | KPC-2 | VIM-1 | Abdallah et al. | |
| Providencia rettgeri | IMP-1 | OXA-72 | Abdallah et al. |
| Antibiotic | Guidelines | Disk Content (µg) | Disk Diffusion (mm) | Dilution (µg/mL) | ||||
|---|---|---|---|---|---|---|---|---|
| S | I | R | S | I | R | |||
| Ertapenem | EUCAST 1 CLSI 2 | 10 | ≥25 ≥23 | - 19–21 | ≤25 ≤18 | ≤0.5 ≤0.5 | - 1 | 0.5 ≥2 |
| Imipenem | EUCAST 1 CLSI 2 | 10 | 22 ≥23 | 21–18 20–22 | ≤17 ≤19 | ≤2 ≤1 | 3 2 | 4 ≥4 |
| Meropenem | EUCAST 1 CLSI 2 | 10 | 22 ≥23 | 21–17 20–22 | 16 ≤19 | ≤2 ≤1 | 3–7 2 | 8 ≥4 |
| Doripenem | EUCAST 3 CLSI 2 | 10 10 | 22 ≥23 | 21–17 20–22 | ≤16 ≤19 | ≤1 ≤1 | 2–3 2 | 4 ≥4 |
| Drug (Pharmaceutical Company) | Action Mechanism | Structure | Limitations | Ref. | |
|---|---|---|---|---|---|
| “Old Antibiotics” | Fosfomycin (Merck) | Cell wall synthesis inhibitor | 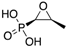 | Appearance of resistance | Vardakas et al. |
| Aminoglycosides | Protein synthesis inhibitor | 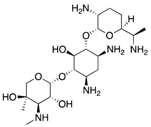 | Appearance of resistance | Rodriguez-Bano et al. Satlin et al. | |
| Colistin (Kobayashi Bacteriological Laboratory) | Cell membrane disruptor | 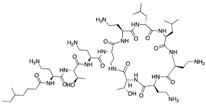 | Nephrotoxicity and other severe adverse effects | Karaiskos et al. Daikos et al. | |
| Tigecycline (Pfizer) | Protein synthesis inhibitor | 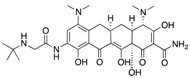 | Low concentration in tissue | Ni et al. | |
| Dual Therapies | Ertapenem + Meropenem/Doripenem | Cell wall synthesis inhibitor | 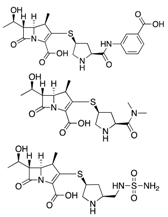 | - | Bulik et al. |
| Ceftazidime/Avibactam (Allergan) | Cell wall synthesis inhibitor/ß-lactamase inhibitor |  | Appearance of resistance | De Jonge et al. | |
| Meropenem/Vaborbactam (Melinta) | Cell wall synthesis inhibitor/ß-lactamase inhibitor | 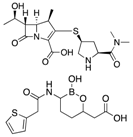 | Insufficient clinical data | Karaiskos et al. | |
| Novel Drugs | Plazomicin (Achaogen) | Protein synthesis inhibitor | 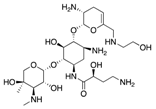 | Ineffective against MBL-producers | Landman et al. |
| Eravacycline (Tetraphase) | Protein synthesis inhibitor | 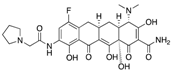 | Currently in clinical trials | Zhanel et al. | |
| Imipenem/Relebactam (Merck) | Cell wall synthesis inhibitor/ß-lactamase inhibitor |  | Currently in clinical trials | Blizzard et al. | |
| Cefiderocol (Shionogi) | Cell wall synthesis inhibitor | 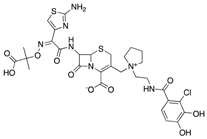 | Currently in clinical trials | Saisho et al. | |
| Zidebactam (Wockhardt) | ß-lactamase inhibitor | 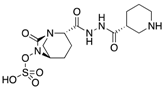 | Currently in clinical trials | Karaiskos et al. | |
| Nacubactam (Roche) | ß-lactamase inhibitor | 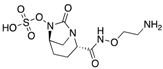 | Currently in clinical trials | Papp-Wallace et al. |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Suay-García, B.; Pérez-Gracia, M.T. Present and Future of Carbapenem-resistant Enterobacteriaceae (CRE) Infections. Antibiotics 2019, 8, 122. https://doi.org/10.3390/antibiotics8030122
Suay-García B, Pérez-Gracia MT. Present and Future of Carbapenem-resistant Enterobacteriaceae (CRE) Infections. Antibiotics. 2019; 8(3):122. https://doi.org/10.3390/antibiotics8030122
Chicago/Turabian StyleSuay-García, Beatriz, and María Teresa Pérez-Gracia. 2019. "Present and Future of Carbapenem-resistant Enterobacteriaceae (CRE) Infections" Antibiotics 8, no. 3: 122. https://doi.org/10.3390/antibiotics8030122
APA StyleSuay-García, B., & Pérez-Gracia, M. T. (2019). Present and Future of Carbapenem-resistant Enterobacteriaceae (CRE) Infections. Antibiotics, 8(3), 122. https://doi.org/10.3390/antibiotics8030122






