The Oral Pheneticillin Absorption Test: An Accurate Method to Identify Patients with Inadequate Oral Pheneticillin Absorption
Abstract
:1. Background
2. Patients and Methods
Pheneticillin Oral Absorption Tests
3. Pheneticillin Assay
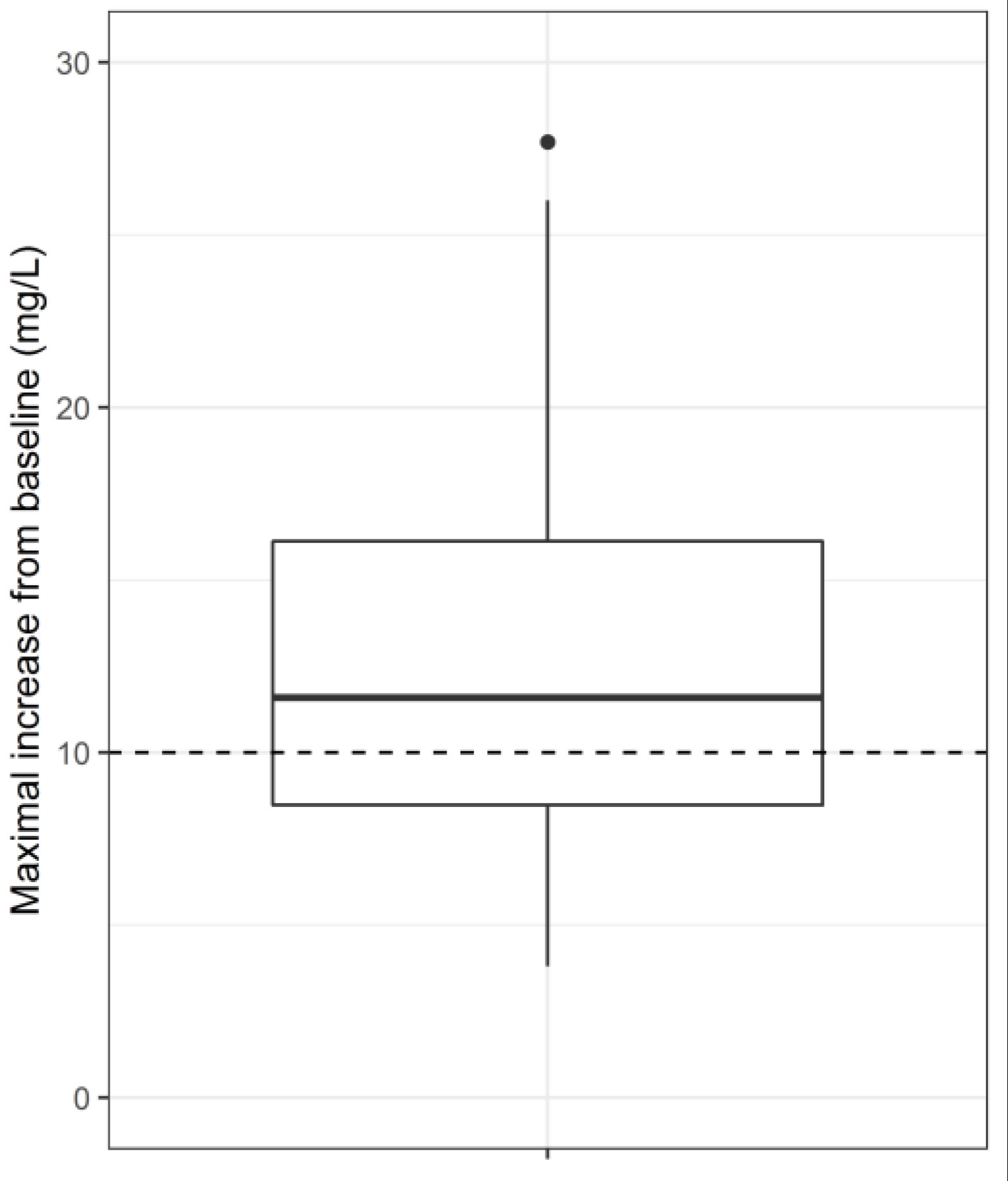
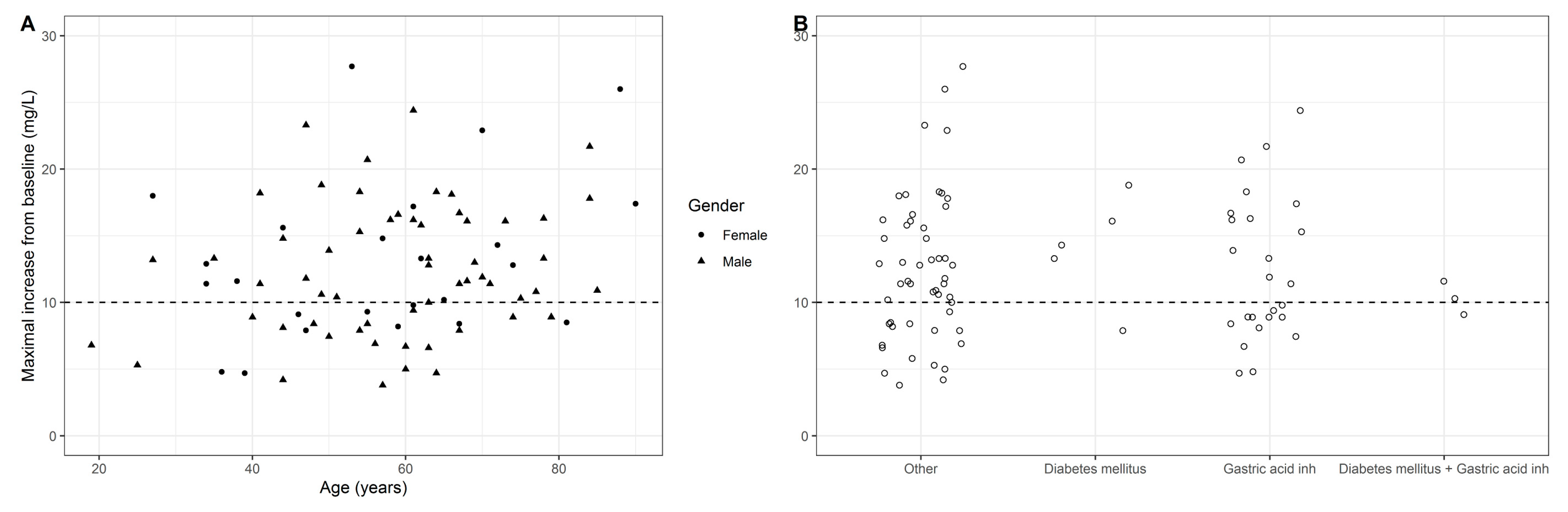
4. Results
5. Conclusions and Discussion
Author Contributions
Funding
Conflicts of Interest
References
- Bond, J.M.; Barber, M.; Lightbown, J.W.; Waterworth, P.M. A comparison of four phenoxypenicillins. BMJ 1963, 2, 956–961. [Google Scholar] [CrossRef] [PubMed]
- Grayson, L.M.; Cosgrove, S.E.; Crowe, S.; Hope, W.; McCarthy, J.S.; Mills, J.; Mouton, J.W.; Paterson, D.L. Kucers’ The Use of Antibiotics: A Clinical Review of Antibacterial, Antifungal, Antiparasitic, and Antiviral Drugs, 7th ed.; CRC: Boca Raton, FL, USA, 2017; p. 60. [Google Scholar]
- Dijkmans, A.C.; Hartigh, J.; van Dissel, J.T.; Burggraaf, J. A simplified oral flucloxacillin absorption test for patients requiring long-term treatment. Ther. Drug Monit. 2012, 34, 356–358. [Google Scholar] [CrossRef] [PubMed]
- Verbist, L. Triple crossover study on absorption and excretion of ampicillin, talampicillin, and amoxycillin. Antimicrob. Agents Chemother. 1976, 10, 173–175. [Google Scholar] [CrossRef] [PubMed]
- Sutherland, R.; Croydon, E.A.; Rolinson, G.N. Amoxycillin: a new semi-synthetic penicillin. BMJ 1972, 3, 13–16. [Google Scholar] [CrossRef] [PubMed]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dijkmans, A.C.; Kweekel, D.M.; van Dissel, J.T.; van Esdonk, M.J.; Kamerling, I.M.C.; Burggraaf, J. The Oral Pheneticillin Absorption Test: An Accurate Method to Identify Patients with Inadequate Oral Pheneticillin Absorption. Antibiotics 2019, 8, 119. https://doi.org/10.3390/antibiotics8030119
Dijkmans AC, Kweekel DM, van Dissel JT, van Esdonk MJ, Kamerling IMC, Burggraaf J. The Oral Pheneticillin Absorption Test: An Accurate Method to Identify Patients with Inadequate Oral Pheneticillin Absorption. Antibiotics. 2019; 8(3):119. https://doi.org/10.3390/antibiotics8030119
Chicago/Turabian StyleDijkmans, Anneke C., Dinemarie M. Kweekel, Jaap T. van Dissel, Michiel J. van Esdonk, Ingrid M. C. Kamerling, and Jacobus Burggraaf. 2019. "The Oral Pheneticillin Absorption Test: An Accurate Method to Identify Patients with Inadequate Oral Pheneticillin Absorption" Antibiotics 8, no. 3: 119. https://doi.org/10.3390/antibiotics8030119




