Vitamin C in the Presence of Sub-Inhibitory Concentration of Aminoglycosides and Fluoroquinolones Alters Proteus mirabilis Biofilm Inhibitory Rate
Abstract
:1. Introduction
2. Results
2.1. Minimal Inhibitory Concentration of Antibiotics and Ascorbic Acid
2.2. Biofilm Formation
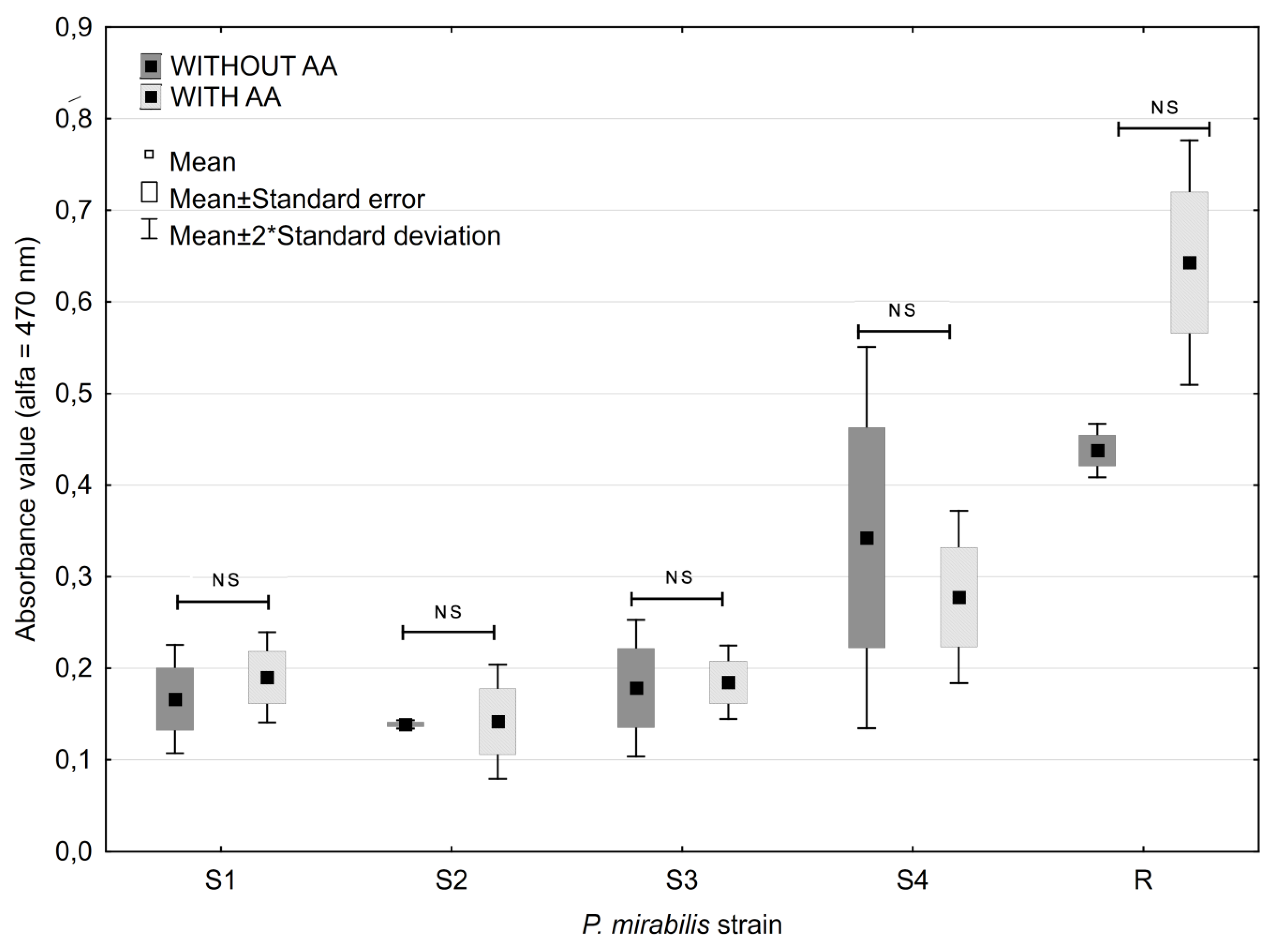
2.3. Ascorbic Acid Impact on Biofilm Formation
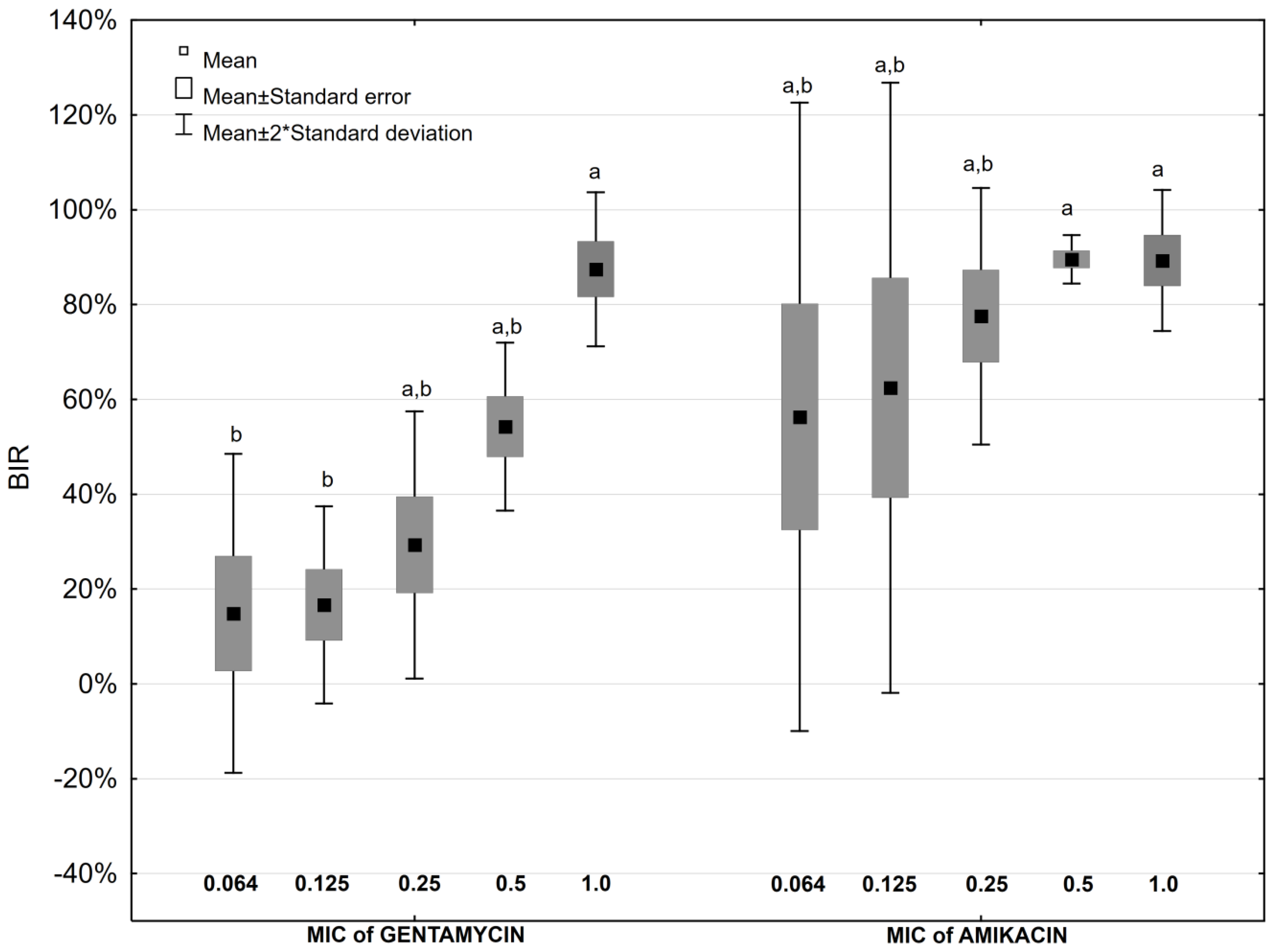
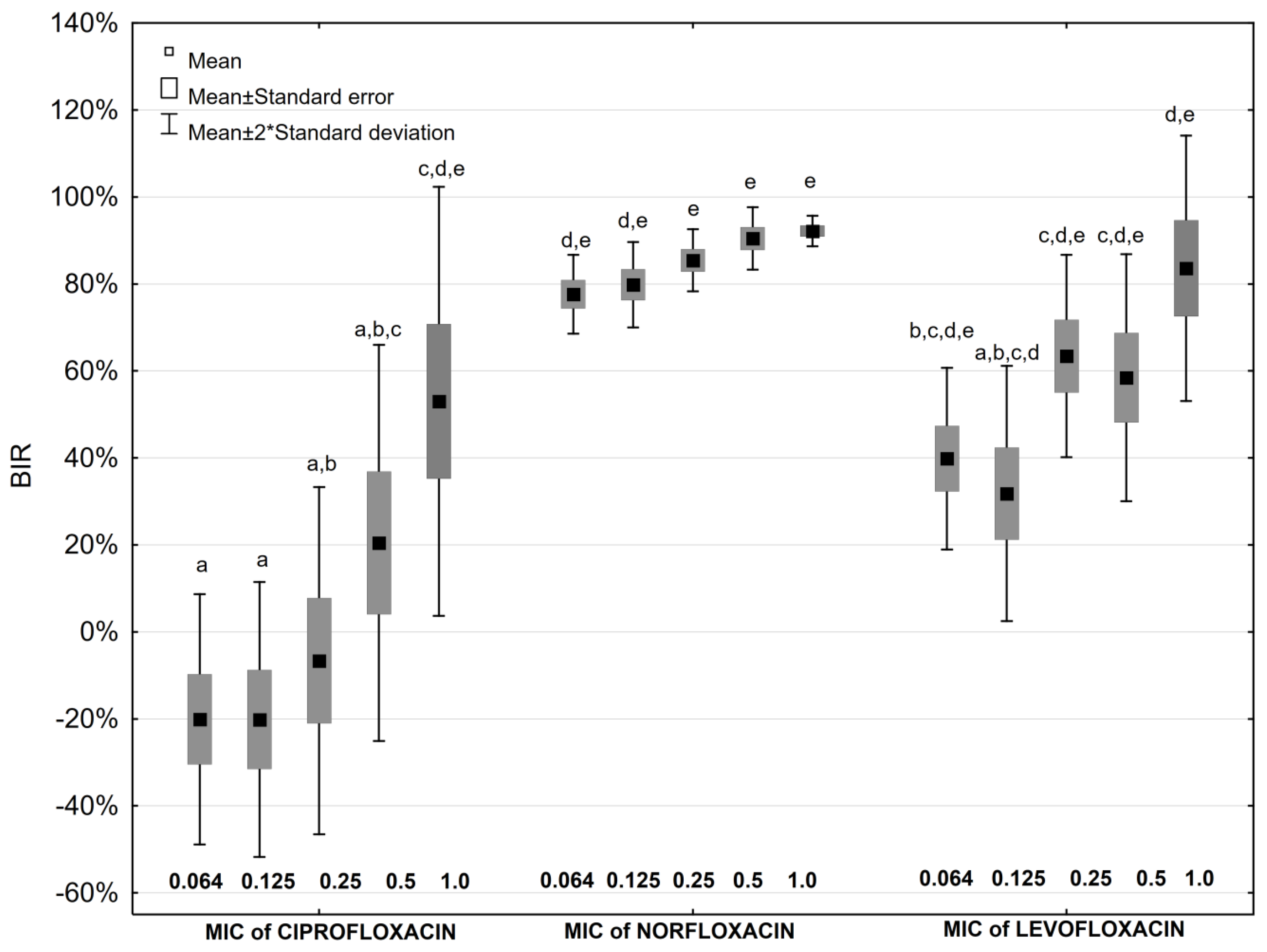
2.4. Antibiotic Impact on Biofilm Formation
2.5. Antibiotic and Ascorbic Acid Impact on Biofilm Formation
3. Discussion
4. Materials and Methods
4.1. Strains Origin
4.2. Minimal Inhibitory Concentration of Antibiotics and Ascorbic Acid
4.3. Biofilm Formation
4.4. Ascorbic Acid Impact on Biofilm Formation
4.5. Antibiotic Impact on Biofilm Formation
4.6. Antibiotic and Ascorbic Acid Impact on Biofilm Formation
4.7. Biofilm Inhibition Rate
4.8. Statistical Analysis
Author Contributions
Funding
Conflicts of Interest
References
- Pacier, C.; Martirosyan, D.M. Vitamin C: Optimal dosages, supplementation and use in disease prevention. FFHD 2015, 5, 89–107. [Google Scholar] [CrossRef]
- Padayatty, S.J.; Levine, M. Vitamin C: The known and the unknown and Goldilocks. Oral Dis. 2016, 22, 463–493. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.; Kim, D.Y.; Choue, R.; Lim, H. Effects of vitamin C supplementation on plasma and urinary vitamin C concentration in Korean women. Clin. Nutr. Res. 2017, 6, 198–205. [Google Scholar] [CrossRef] [PubMed]
- Shibata, K.; Fukuwatari, T.; Ohta, M.; Okamoto, H.; Watanabe, T.; Fukui, T.; Nishimuta, M.; Totani, M.; Kimura, M.; Nakashima, M.; et al. Values of water-soluble vitamins in blood and urine of Japanese young men and women consuming a semi-purified diet based on the Japanese Dietary Reference Intakes. J. Nutr. Sci. Vitaminol. (Tokyo) 2005, 51, 319–328. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Ren, Z.; Zhang, J.; Chuang, C.C.; Kandaswamy, E.; Zhou, T.; Zuo, L. Role of ROS and nutritional antioxidants in human diseases. Front Physiol. 2018, 9, 477. [Google Scholar] [CrossRef] [PubMed]
- Hemilä, H. Vitamin C and infections. Nutrients 2017, 9, 339. [Google Scholar] [CrossRef] [PubMed]
- Verghese, R.J.; Mathew, S.K.; David, A. Antimicrobial activity of vitamin C demonstrated on pathogenic Escherichia coli and Klebsiella pneumoniae. J. Curr. Res. Sci. Med. 2017, 3, 88–93. [Google Scholar] [CrossRef]
- Shahzad, S.; Ashraf, M.A.; Sajid, M.; Shahzad, A.; Rafigue, A.; Mohmood, M.S. Evaluation of synergistic antimicrobial effect of vitamins (A, B1, B2, B6, B12, C, D, E and K) with antibiotics against resistant bacterial strains. J. Glob. Antimicrob. Resist. 2018, 13, 231–236. [Google Scholar] [CrossRef]
- Carlsson, S.; Weitzberg, E.; Wiklund, P.; Lundberg, J.O. Intravesical nitric oxide delivery for prevention of catheter-associated urinary tract infections. Antimicrob. Agents Chemother. 2005, 49, 2352–2355. [Google Scholar] [CrossRef]
- Afzal, S.; Ashraf, M.; Buksh, A.; Akhtar, A.; Rasheed, A.D. Efficacy of anti-microbial agents with ascorbic acid in catheter associated urinary tract infection. J. Infect. Dis. Prev. Med. 2017, 5, 1–6. [Google Scholar] [CrossRef]
- Habash, M.B.; Van der Mei, H.C.; Busscher, H.J.; Reid, G. The effect of water, ascorbic acid, and cranberry derived supplementation on human urine and uropathogen adhesion to silicone rubber. Can. J. Microbiol. 1999, 45, 691–694. [Google Scholar] [CrossRef] [PubMed]
- Tambyah, P.A.; Knasinski, V.; Maki, D.G. The direct costs of nosocomial catheter-associated urinary tract infection in the era of managed care. Infect. Control Hosp. Epidemiol. 2002, 23, 27–31. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.K.; Chiu, C.S.; Chow, V.C.; Lam, R.K.; Lai, R.W. Prevalence of hospital infection and antibiotic use at a university medical center in Hong Kong. J. Hosp. Infect. 2007, 65, 341–347. [Google Scholar] [CrossRef] [PubMed]
- Barford, J.M.T.; Coates, A.R.M. The pathogenesis of catheter-associated urinary tract infection. J. Infect. Prev. 2009, 10, 50–56. [Google Scholar] [CrossRef]
- Coker, C.; Poore, C.A.; Li, X.; Mobley, H.L.T. Pathogenesis of Proteus mirabilis urinary tract infection. Microbes Infect. 2000, 2, 1497–1505. [Google Scholar] [CrossRef]
- Chen, C.Y.; Chen, Y.H.; Lu, P.; Lin, W.R.; Chen, T.C.; Lin, C.Y. Proteus mirabilis urinary tract infection and bacteremia: Risk factors, clinical presentation, and outcomes. J. Microbiol. Immunol. Infect. 2012, 45, 228–236. [Google Scholar] [CrossRef] [PubMed]
- National Center for Biotechnology Information. Available online: https://www.ncbi.nlm.nih.gov/ (accessed on 18 October 1996).
- Różalski, A.; Kwil, I.; Torzewska, A.; Baranowska, M.; Strączek, P. Proteus bacilli: Features and virulence factors. Post. Hig. Med. Dośw. 2007, 61, 204–219. [Google Scholar]
- Jones, S.M.; Yerly, J.; Hu, Y.; Ceri, H.; Martinuzzi, R. Structure of Proteus mirabilis biofilms grown in artificial urine and standard laboratory media. FEMS Microbiol. Lett. 2007, 268, 16–21. [Google Scholar] [CrossRef] [PubMed]
- Hawthorn, L.; Reid, G. The effect of protein and urine on uropathogen adhesion to polymer substrata. J. Biomed. Mater. Res. 1990, 24, 1325–1332. [Google Scholar] [CrossRef]
- Stewart, P.S. Mechanisms of antibiotic resistance in bacterial biofilms. Int. J. Med. Microbiol. 2002, 292, 107–113. [Google Scholar] [CrossRef]
- Morris, N.S.; Stickler, D.J.; Winters, C. Which indwelling urethral catheters resist encrustation by Proteus mirabilis biofilms? Br. J. Urol. 1997, 80, 58–63. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, K.; Khan, A.; Khattak, M.M.A.K. Biological Significance of Ascorbic Acid (Vitamin C) in Human Health—A Review. Pak. J. Nutr. 2004, 3, 5–13. [Google Scholar] [CrossRef]
- El-Gebaly, E.; Essam, T.; Hashem, S.; El-Baky, R.A. Effect of levofloxacin and vitamin C on bacterial adherence and preformed biofilm on urethral catheter surfaces. J. MicrobBiochem. Technol. 2012, 4, 131–136. [Google Scholar] [CrossRef]
- Wasfi, R.; Abd El-Rahman, O.A.; Mansour, L.E.; Hanora, A.S.; Hashem, A.M.; Ashour, M.S. Antimicrobial activities against biofilm formed by Proteus mirabilis isolates from wound and urinary tract infections. Indian J. Med. Microbiol. 2012, 30, 76–80. [Google Scholar] [CrossRef] [PubMed]
- Masadeh, M.M.; Mhaidat, N.M.; Alzoubi, K.H.; Al-Azzam, S.I.; Shaweesh, A.I. Ciprofloxacin-induced antibacterial activity is reversed by vitamin E and vitamin C. Curr. Microbiol. 2012, 64, 457–462. [Google Scholar] [CrossRef] [PubMed]
- Aiassa, V.; Barnes, A.I.; Smania, A.M.; Albesa, I. Sublethal ciprofloxacin treatment leads to resistance via antioxidant systems in Proteus mirabilis. FEMS Microbiol. Lett. 2012, 327, 25–32. [Google Scholar] [CrossRef] [PubMed]
- Awofisayo, S.O.; Umoren, F.J.; Uwanta, E.J. The pharmacokinetic and biopharmaceutical effect of ascorbic acid (vitamin C) on pefloxacin on concurrent administration in human. J. Appl. Pharm. Sci. 2012, 2, 107–110. [Google Scholar] [CrossRef]
- Pandit, S.; Ravikumar, V.; Adbel-Haleem, A.M.; Derouiche, A.; Mokkapati, V.R.S.S.; Shibom, C.; Mineta, K.; Gojobori, T.; Gao, X.; Westerlund, F.; et al. Low concentration of vitamin C reduce the synthesis of extracellular polymers and destabilize bacterial biofilms. Front. Microbiol. 2017, 8, 2599. [Google Scholar] [CrossRef]
- Yassein, M.A.M. Studies on the Prevention of Adherence of Biofilm Associated Bacteria to Human Cells and Implanted Medical Devices. Available online: https://dsr.kau.edu.sa/Show_Res.aspx?Site_ID=305&LNG=EN&RN=53934 (accessed on 10 August 2019).
- Novak, J.S.; Fratamico, P.M. Evaluation of ascorbic acid as a quorum-sensing analogue to control growth, sporulation, and enterotoxin production in Clostridium perfringens. J. Food Sci. 2004, 69, 72–78. [Google Scholar] [CrossRef]
- Cursino, L.; Chartone-Souza, E.; Nascimento, A.M.A. Synergic interaction between ascorbic acid and antibiotics against Pseudomnas aeruginosa. Braz. Arch. Biol. Technol. 2005, 48, 379–384. [Google Scholar] [CrossRef]
- Goswami, M.; Mangoli, S.H.; Jawali, N. Effects of glutathione and ascorbic acid on streptomycin sensitivity of Escherichia coli. Antimicrob. Agents Chemother. 2007, 51, 1119–1122. [Google Scholar] [CrossRef]
- Andrade, J.C.; Morais-Braga, M.F.B.; Guedes, G.M.M.; Tintino, S.R.; Freitas, M.A.; Menezes, I.R.A.; Coutinho, H.D.M. Enhancement of the antibiotic activity of aminoglycosides by alpha-tocopherol and other cholesterol derivates. Biomed. Pharmacother. 2014, 68, 1065–1069. [Google Scholar] [CrossRef]
- European Committee on Antimicrobial Susceptibility Testing. Breakpoint Tables for Interpretation of MICs and Zone Diameters. Version 8.0. Available online: http://www.eucast.org (accessed on 3 September 2018).
- Clinical Laboratory Standards Institute. Available online: https://clsi.org/ (accessed on 30 November 2001).
- Kwiecińska-Piróg, J.; Skowron, K.; Zniszczol, K.; Gospodarek, E. The assessment of Proteus mirabilis susceptibility to ceftazidime and ciprofloxacin and the impact of these antibiotics at subinhibitory concentrations on Proteus mirabilis biofilms. BioMed Res. Int. 2013, 2013, 930876. [Google Scholar] [CrossRef]
| Strain No. | GEN [µg × mL−1] | AMK [µg × mL−1] | CIP [µg × mL−1] | NOR [µg × mL−1] | LEV [µg × mL−1] | AA [µg × mL−1] |
|---|---|---|---|---|---|---|
| S1 | 0.500 | 0.016 | 0.001 | 0.004 | 0.016 | 10.000 |
| S2 | 0.250 | 0.016 | 0.001 | 0.002 | 0.008 | 10.000 |
| S3 | 0.004 | 0.002 | 0.001 | 0.002 | 0.008 | 10.000 |
| S4 | 0.500 | 0.008 | 0.001 | 0.008 | 0.016 | 10.000 |
| R | 0.250 | 0.016 | 0.001 | 0.004 | 0.016 | 10.000 |
| Antibiotic Concentration | Biofilm Inhibition Rate [%] | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Aminoglycosides | Fluoroquinolones | ||||||||||||||
| GEN | p * | GEN + AA | AMK | p * | AMK + AA | CIP | p * | CIP + AA | NOR | p * | NOR + AA | LEV | p * | LEV+AA | |
| 0.064 MIC | 14.9 | NS | −94.7 | 56.3 | 0.028 | −63.7 | −20.1 | NS | −9.5 | 77.6 | NS | 68.4 | 39.8 | NS | 40.6 |
| 0.125 MIC | 16.7 | NS | −50.5 | 62.5 | 0.047 | −6.0 | −20.2 | NS | −11.1 | 79.8 | NS | 77.4 | 31.8 | NS | 42.3 |
| 0.25 MIC | 29.3 | NS | 0.7 | 77.6 | 0.016 | 9.5 | −6.6 | NS | −5.8 | 85.4 | 0.047 | 73.1 | 63.4 | NS | 50.5 |
| 0.5 MIC | 54.3 | NS | 5.6 | 89.4 | 0.009 | 8.8 | 20.5 | NS | 7.6 | 90.4 | 0.047 | 75.7 | 58.5 | NS | 48.3 |
| 1 MIC | 87.5 | 0.028 | 41.6 | 89.3 | 0.028 | 54.3 | 53.0 | NS | 32.8 | 92.2 | NS | 91.1 | 83.6 | NS | 66.5 |
| Strain No | Disease Diagnosis | Sex | Patients Age | Antibiotic Treatment |
|---|---|---|---|---|
| S1 | UTI | Female | 85 Years | Amoxicillin/clavulanic acid |
| S2 | Abdominal hernia surgery | Female | 59 Years | None |
| S3 | UTI (urinary stones) | Female | 86 Years | Ciprofloxacin |
| S4 | UTI | Male | 18 Months | None |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kwiecińska-Piróg, J.; Skowron, K.; Bogiel, T.; Białucha, A.; Przekwas, J.; Gospodarek-Komkowska, E. Vitamin C in the Presence of Sub-Inhibitory Concentration of Aminoglycosides and Fluoroquinolones Alters Proteus mirabilis Biofilm Inhibitory Rate. Antibiotics 2019, 8, 116. https://doi.org/10.3390/antibiotics8030116
Kwiecińska-Piróg J, Skowron K, Bogiel T, Białucha A, Przekwas J, Gospodarek-Komkowska E. Vitamin C in the Presence of Sub-Inhibitory Concentration of Aminoglycosides and Fluoroquinolones Alters Proteus mirabilis Biofilm Inhibitory Rate. Antibiotics. 2019; 8(3):116. https://doi.org/10.3390/antibiotics8030116
Chicago/Turabian StyleKwiecińska-Piróg, Joanna, Krzysztof Skowron, Tomasz Bogiel, Agata Białucha, Jana Przekwas, and Eugenia Gospodarek-Komkowska. 2019. "Vitamin C in the Presence of Sub-Inhibitory Concentration of Aminoglycosides and Fluoroquinolones Alters Proteus mirabilis Biofilm Inhibitory Rate" Antibiotics 8, no. 3: 116. https://doi.org/10.3390/antibiotics8030116
APA StyleKwiecińska-Piróg, J., Skowron, K., Bogiel, T., Białucha, A., Przekwas, J., & Gospodarek-Komkowska, E. (2019). Vitamin C in the Presence of Sub-Inhibitory Concentration of Aminoglycosides and Fluoroquinolones Alters Proteus mirabilis Biofilm Inhibitory Rate. Antibiotics, 8(3), 116. https://doi.org/10.3390/antibiotics8030116





