Qualitative Analysis of Primary Care Provider Prescribing Decisions for Urinary Tract Infections
Abstract
1. Introduction
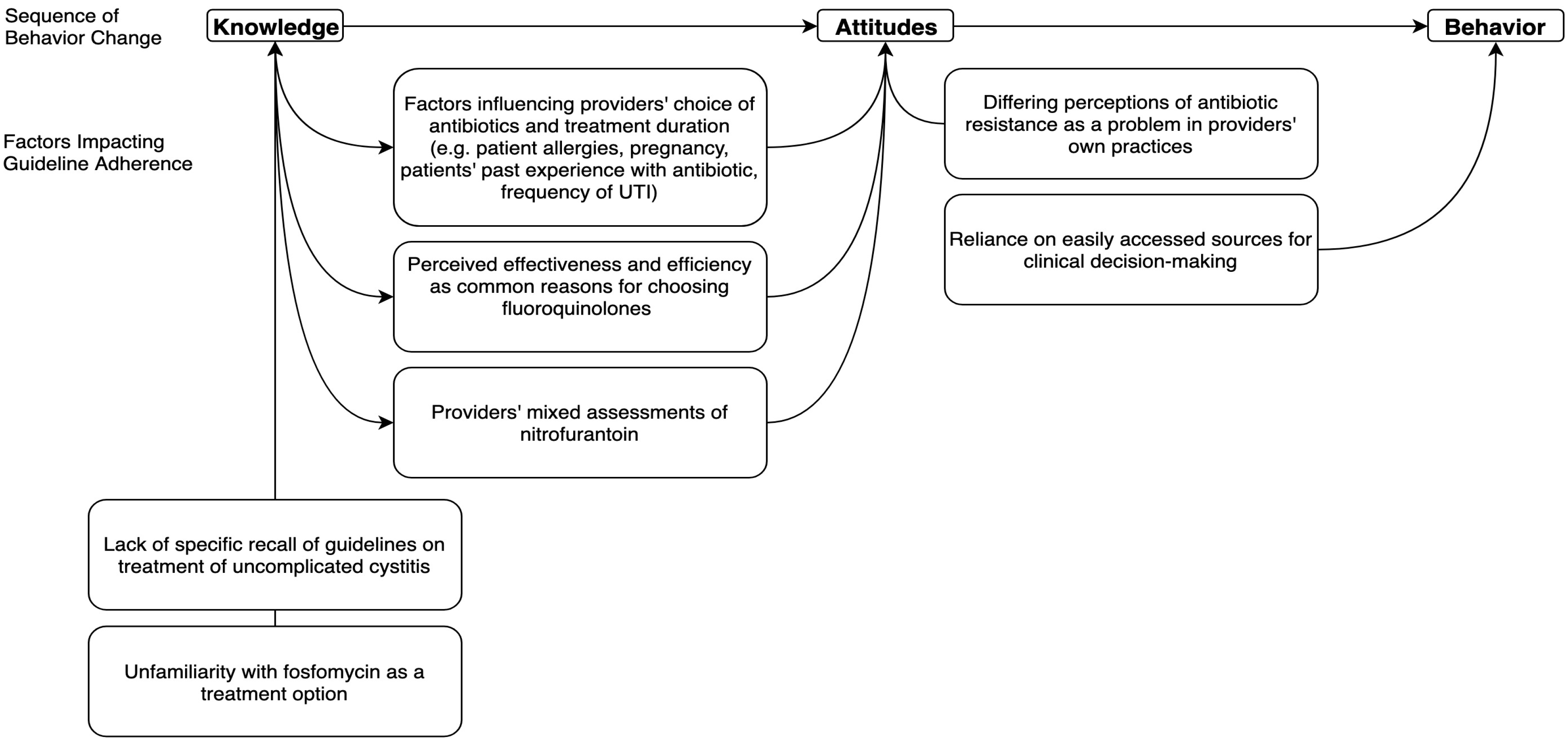
2. Results
2.1. Theme 1: Perceived Effectiveness and Efficiency were Common Reasons for Choosing Fluoroquinolones
2.2. Theme 2: Factors Influencing Providers’ Choice of Antibiotics and Treatment Duration
2.3. Theme 3: Providers’ Mixed Assessments of Nitrofurantoin
2.4. Theme 4: Unfamiliarity with Fosfomycin as a Treatment Option
2.5. Theme 5: Reliance on Easily Accessed Sources for Clinical Decision-making
2.6. Theme 6: Lack of Specific Recall of Guidelines on Treatment of Uncomplicated Cystitis
2.7. Theme 7: Differing Perceptions of Antibiotic Resistance as a Problem in Providers’ Own Practices
3. Discussion
4. Materials and Methods
4.1. Ethics
4.2. Setting and Participants
4.3. Interview Guide Development
4.4. Interview Procedures
4.5. Data Collection and Analysis
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Centers for Disease Control and Prevention. Antibiotic/Antimicrobial Resistance. Biggest Threats. Available online: https://www.cdc.gov/drugresistance/biggest_threats.html (accessed on 17 June 2019).
- National Action Plan for Combating Antibiotic-Resistant Bacteria. Available online: https://aspe.hhs.gov/system/files/pdf/258516/ProgressYears1and2CARBNationalActionPlan.pdf (accessed on 17 June 2019).
- The Pew Charitable Trusts. Antibiotic Use in Outpatient Settings. Available online: http://www.pewtrusts.org/~/media/assets/2016/05/antibioticuseinoutpatientsettings.pdf (accessed on 17 June 2019).
- Meeker, D.; Linder, J.A.; Fox, C.R.; Friedberg, M.W.; Persell, S.D.; Goldstein, N.J.; Knight, T.K.; Hay, J.W.; Doctor, J.N. Effect of Behavioral Interventions on Inappropriate Antibiotic Prescribing Among Primary Care Practices: A Randomized Clinical Trial. JAMA 2016, 315, 562–570. [Google Scholar] [CrossRef] [PubMed]
- Drekonja, D.M.; Filice, G.A.; Greer, N.; Olson, A.; MacDonald, R.; Rutks, I.; Wilt, T.J. Antimicrobial stewardship in outpatient settings: A systematic review. Infect. Control Hosp. Epidemiol. 2015, 36, 142–152. [Google Scholar] [CrossRef] [PubMed]
- Hecker, M.T.; Fox, C.J.; Son, A.H.; Cydulka, R.K.; Siff, J.E.; Emerman, C.L.; Sethi, A.K.; Muganda, C.P.; Donskey, C.J. Effect of a stewardship intervention on adherence to uncomplicated cystitis and pyelonephritis guidelines in an emergency department setting. PLoS ONE 2014, 9, e87899. [Google Scholar] [CrossRef] [PubMed]
- Fleming-Dutra, K.E.; Hersh, A.L.; Shapiro, D.J.; Bartoces, M.; Enns, E.A.; File, T.M.; Finkelstein, J.A., Jr.; Gerber, J.S.; Hyun, D.Y.; Linder, J.A.; et al. Prevalence of Inappropriate Antibiotic Prescriptions Among US Ambulatory Care Visits, 2010–2011. JAMA 2016, 315, 1864–1873. [Google Scholar] [CrossRef] [PubMed]
- Gupta, K.; Hooton, T.M.; Naber, K.G.; Wullt, B.; Colgan, R.; Miller, L.G.; Moran, G.J.; Nicolle, L.E.; Raz, R.; Schaeffer, A.J.; et al. International clinical practice guidelines for the treatment of acute uncomplicated cystitis and pyelonephritis in women: A 2010 update by the Infectious Diseases Society of America and the European Society for Microbiology and Infectious Diseases. Clin. Infect. Dis. 2011, 52, e103–e120. [Google Scholar] [CrossRef]
- Kobayashi, M.; Shapiro, D.J.; Hersh, A.L.; Sanchez, G.V.; Hicks, L.A. Outpatient Antibiotic Prescribing Practices for Uncomplicated Urinary Tract Infection in Women in the United States, 2002–2011. Open Forum Infect. Dis. 2016, 3, ofw159. [Google Scholar] [CrossRef] [PubMed]
- Grigoryan, L.; Zoorob, R.; Wang, H.; Trautner, B.W. Low Concordance With Guidelines for Treatment of Acute Cystitis in Primary Care. Open Forum Infect. Dis. 2015, 2, ofv159. [Google Scholar] [CrossRef]
- Shively, N.R.; Buehrle, D.J.; Clancy, C.J.; Decker, B.K. Prevalence of Inappropriate Antibiotic Prescribing in Primary Care Clinics within a Veterans Affairs Health Care System. Antimicrob. Agents Chemother. 2018, 62. [Google Scholar] [CrossRef]
- Petty, N.K.; Ben Zakour, N.L.; Stanton-Cook, M.; Skippington, E.; Totsika, M.; Forde, B.M.; Phan, M.D.; Gomes Moriel, D.; Peters, K.M.; Davies, M.; et al. Global dissemination of a multidrug resistant Escherichia coli clone. Proc. Natl. Acad. Sci. USA 2014, 111, 5694–5699. [Google Scholar] [CrossRef] [PubMed]
- U.S Food and Drug Administration. FDA Advises Restricting Fluoroquinolone Antibiotic Use for Certain Uncomplicated Infections; Warns about Disabling Side Effects that Can Occur Together. 2016. Available online: https://www.fda.gov/downloads/Drugs/DrugSafety/UCM500591.pdf (accessed on 17 June 2019).
- U.S Food and Drug Administration. FDA Updates Warnings for Fluoroquinolone Antibiotics on Risks of Mental Health and Low Blood Sugar Adverse Reactions. Available online: https://www.fda.gov/newsevents/newsroom/pressannouncements/ucm612995.htm (accessed on 17 June 2019).
- U.S. Food and Drug Administration. FDA Warns about Increased Risk of Ruptures or Tears in the Aorta Blood Vessel with Fluoroquinolone Antibiotics in Certain Patients. 2018. Available online: https://www.fda.gov/Drugs/DrugSafety/ucm628753.htm (accessed on 17 June 2019).
- Stephenson, A.L.; Wu, W.; Cortes, D.; Rochon, P.A. Tendon Injury and Fluoroquinolone Use: A Systematic Review. Drug Saf. 2013, 36, 709–721. [Google Scholar] [CrossRef]
- Wise, B.L.; Peloquin, C.; Choi, H.; Lane, N.E.; Zhang, Y. Impact of age, sex, obesity, and steroid use on quinolone-associated tendon disorders. Am. J. Med. 2012, 125, e1223–e1228. [Google Scholar] [CrossRef] [PubMed]
- Briasoulis, A.; Agarwal, V.; Pierce, W.J. QT prolongation and torsade de pointes induced by fluoroquinolones: Infrequent side effects from commonly used medications. Cardiology 2011, 120, 103–110. [Google Scholar] [CrossRef] [PubMed]
- Aspinall, S.L.; Good, C.B.; Jiang, R.; McCarren, M.; Dong, D.; Cunningham, F.E. Severe dysglycemia with the fluoroquinolones: A class effect? Clin. Infect. Dis. 2009, 49, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Chou, H.W.; Wang, J.L.; Chang, C.H.; Lee, J.J.; Shau, W.Y.; Lai, M.S. Risk of severe dysglycemia among diabetic patients receiving levofloxacin, ciprofloxacin, or moxifloxacin in Taiwan. Clin. Infect. Dis. 2013, 57, 971–980. [Google Scholar] [CrossRef] [PubMed]
- Ali, A.K. Peripheral neuropathy and Guillain-Barre syndrome risks associated with exposure to systemic fluoroquinolones: A pharmacovigilance analysis. Ann. Epidemiol. 2014, 24, 279–285. [Google Scholar] [CrossRef] [PubMed]
- Aoun, M.; Jacquy, C.; Debusscher, L.; Bron, D.; Lehert, M.; Noel, P.; van der Auwera, P. Peripheral neuropathy associated with fluoroquinolones. Lancet 1992, 340, 127. [Google Scholar] [CrossRef]
- Etminan, M.; Brophy, J.M.; Samii, A. Oral fluoroquinolone use and risk of peripheral neuropathy: A pharmacoepidemiologic study. Neurology 2014, 83, 1261–1263. [Google Scholar] [CrossRef]
- Durkin, M.J.; Keller, M.; Butler, A.M.; Kwon, J.H.; Dubberke, E.R.; Miller, A.C.; Polgreen, P.M.; Olsen, M.A. An Assessment of Inappropriate Antibiotic Use and Guideline Adherence for Uncomplicated Urinary Tract Infections. Open Forum Infect. Dis. 2018, 5, ofy198. [Google Scholar] [CrossRef]
- Saunders, B.; Sim, J.; Kingstone, T.; Baker, S.; Waterfield, J.; Bartlam, B.; Burroughs, H.; Jinks, C. Saturation in qualitative research: Exploring its conceptualization and operationalization. Qual. Quant. 2018, 52, 1893–1907. [Google Scholar] [CrossRef]
- Guest, G.; Bunce, A.; Johnson, L. How Many Interviews Are Enough? Field Methods 2006, 18, 59–82. [Google Scholar] [CrossRef]
- Cabana, M.D.; Rand, C.S.; Powe, N.R.; Wu, A.W.; Wilson, M.H.; Abboud, P.A.; Rubin, H.R. Why don’t physicians follow clinical practice guidelines? A framework for improvement. JAMA 1999, 282, 1458–1465. [Google Scholar] [CrossRef] [PubMed]
- Ailes, E.C.; Gilboa, S.M.; Gill, S.K.; Broussard, C.S.; Crider, K.S.; Berry, R.J.; Carter, T.C.; Hobbs, C.A.; Interrante, J.D.; Reefhuis, J.; et al. Association between antibiotic use among pregnant women with urinary tract infections in the first trimester and birth defects, National Birth Defects Prevention Study 1997 to 2011. Birth Defects Res. A Clin. Mol. Teratol. 2016, 106, 940–949. [Google Scholar] [CrossRef] [PubMed]
- Crider, K.S.; Cleves, M.A.; Reefhuis, J.; Berry, R.J.; Hobbs, C.A.; Hu, D.J. Antibacterial medication use during pregnancy and risk of birth defects: National Birth Defects Prevention Study. Arch. Pediatr. Adolesc. Med. 2009, 163, 978–985. [Google Scholar] [CrossRef] [PubMed]
- Warren, J.W.; Abrutyn, E.; Hebel, J.R.; Johnson, J.R.; Schaeffer, A.J.; Stamm, W.E. Guidelines for antimicrobial treatment of uncomplicated acute bacterial cystitis and acute pyelonephritis in women. Infectious Diseases Society of America (IDSA). Clin. Infect. Dis. 1999, 29, 745–758. [Google Scholar] [CrossRef] [PubMed]
- Sanchez, G.V.; Roberts, R.M.; Albert, A.P.; Johnson, D.D.; Hicks, L.A. Effects of knowledge, attitudes, and practices of primary care providers on antibiotic selection, United States. Emerg. Infect. Dis. 2014, 20, 2041–2047. [Google Scholar] [CrossRef] [PubMed]
- Grigoryan, L.; Trautner, B.W.; Gupta, K. Diagnosis and management of urinary tract infections in the outpatient setting: A review. JAMA 2014, 312, 1677–1684. [Google Scholar] [CrossRef]
- Spellberg, B.; Doi, Y. The Rise of Fluoroquinolone-Resistant Escherichia coli in the Community: Scarier Than We Thought. J. Infect. Dis. 2015, 212, 1853–1855. [Google Scholar] [CrossRef]
- Mody, L.; Juthani-Mehta, M. Urinary tract infections in older women: A clinical review. JAMA 2014, 311, 844–854. [Google Scholar] [CrossRef]
- Grigoryan, L.; Zoorob, R.; Wang, H.; Horsfield, M.; Gupta, K.; Trautner, B.W. Less workup, longer treatment, but no clinical benefit observed in women with diabetes and acute cystitis. Diabetes Res. Clin. Pract. 2017, 129, 197–202. [Google Scholar] [CrossRef]
- Schneeberger, C.; Stolk, R.P.; Devries, J.H.; Schneeberger, P.M.; Herings, R.M.; Geerlings, S.E. Differences in the pattern of antibiotic prescription profile and recurrence rate for possible urinary tract infections in women with and without diabetes. Diabetes Care 2008, 31, 1380–1385. [Google Scholar] [CrossRef]
- Smith, A.L.; Brown, J.; Wyman, J.F.; Berry, A.; Newman, D.K.; Stapleton, A.E. Treatment and Prevention of Recurrent Lower Urinary Tract Infections in Women: A Rapid Review with Practice Recommendations. J. Urol. 2018, 200, 1174–1191. [Google Scholar] [CrossRef] [PubMed]
- Muanda, F.T.; Sheehy, O.; Berard, A. Use of antibiotics during pregnancy and the risk of major congenital malformations: A population based cohort study. Br. J. Clin. Pharmacol. 2017, 83, 2557–2571. [Google Scholar] [CrossRef] [PubMed]
- Markowitz, M.A.; Wood, L.N.; Raz, S.; Miller, L.G.; Haake, D.A.; Kim, J.H. Lack of uniformity among United States recommendations for diagnosis and management of acute, uncomplicated cystitis. Int. Urogynecol. J. 2018. [Google Scholar] [CrossRef] [PubMed]
- Wolff, M.; Bower, D.J.; Marbella, A.M.; Casanova, J.E. US family physicians’ experiences with practice guidelines. Fam. Med. Kans. City 1998, 30, 117–121. [Google Scholar]
- Bjorkman, I.; Berg, J.; Viberg, N.; Stalsby Lundborg, C. Awareness of antibiotic resistance and antibiotic prescribing in UTI treatment: A qualitative study among primary care physicians in Sweden. Scand. J. Prim. Health Care 2013, 31, 50–55. [Google Scholar] [CrossRef] [PubMed]
- Rank, E.L.; Lodise, T.; Avery, L.; Bankert, E.; Dobson, E.; Dumyati, G.; Hassett, S.; Keller, M.; Pearsall, M.; Lubowski, T.; et al. Antimicrobial Susceptibility Trends Observed in Urinary Pathogens Obtained From New York State. Open Forum Infect. Dis. 2018, 5, ofy297. [Google Scholar] [CrossRef]
- Delisle, G.; Quach, C.; Domingo, M.C.; Boudreault, A.A.; Gourdeau, M.; Bernatchez, H.; Lavallee, C. Escherichia coli antimicrobial susceptibility profile and cumulative antibiogram to guide empirical treatment of uncomplicated urinary tract infections in women in the province of Quebec, 2010–2015. J. Antimicrob. Chemother. 2016, 71, 3562–3567. [Google Scholar] [CrossRef]
- King, N. Using templates in the thematic analysis of text. In Essential Guide to Qualitative Methods in Organizational Research; Cassell, C., Symon, G., Eds.; Sage Publications Ltd.: Thousand Oaks, CA, USA, 2004; pp. 257–270. [Google Scholar]
- Rowley, J. Conducting research interviews. Manag. Res. Rev. 2012, 35, 260–271. [Google Scholar] [CrossRef]
- Riessman, C.K. Narrative Methods for the Human Sciences; Sage Publications Inc.: Thousand Oaks, CA, USA, 2008. [Google Scholar]
- Braun, V.; Clarke, V. Using thematic analysis in psychology. Qual. Res. Psychol. 2006, 3, 77–101. [Google Scholar] [CrossRef]

| Characteristic | n (%) |
|---|---|
| Female | 9 (50) |
| Provider type | |
| Physician | 15 (83) |
| Physician assistant | 3 (17) |
| Years in practice | |
| Fewer than ten years | 9 (50) |
| 10–20 years | 3 |
| Over 21 years | 6 |
| Board certified in Family Medicine | 14 |
| Board certified in other specialty | 1 a |
| Race/ethnicity | |
| White | 6 |
| Hispanic/Latino | 3 |
| Black | 1 |
| Asian | 8 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Grigoryan, L.; Nash, S.; Zoorob, R.; Germanos, G.J.; Horsfield, M.S.; Khan, F.M.; Martin, L.; Trautner, B.W. Qualitative Analysis of Primary Care Provider Prescribing Decisions for Urinary Tract Infections. Antibiotics 2019, 8, 84. https://doi.org/10.3390/antibiotics8020084
Grigoryan L, Nash S, Zoorob R, Germanos GJ, Horsfield MS, Khan FM, Martin L, Trautner BW. Qualitative Analysis of Primary Care Provider Prescribing Decisions for Urinary Tract Infections. Antibiotics. 2019; 8(2):84. https://doi.org/10.3390/antibiotics8020084
Chicago/Turabian StyleGrigoryan, Larissa, Susan Nash, Roger Zoorob, George J. Germanos, Matthew S. Horsfield, Fareed M. Khan, Lindsey Martin, and Barbara W. Trautner. 2019. "Qualitative Analysis of Primary Care Provider Prescribing Decisions for Urinary Tract Infections" Antibiotics 8, no. 2: 84. https://doi.org/10.3390/antibiotics8020084
APA StyleGrigoryan, L., Nash, S., Zoorob, R., Germanos, G. J., Horsfield, M. S., Khan, F. M., Martin, L., & Trautner, B. W. (2019). Qualitative Analysis of Primary Care Provider Prescribing Decisions for Urinary Tract Infections. Antibiotics, 8(2), 84. https://doi.org/10.3390/antibiotics8020084






