Solution-Mediated Modulation of Pseudomonas aeruginosa Biofilm Formation by a Cationic Synthetic Polymer
Abstract
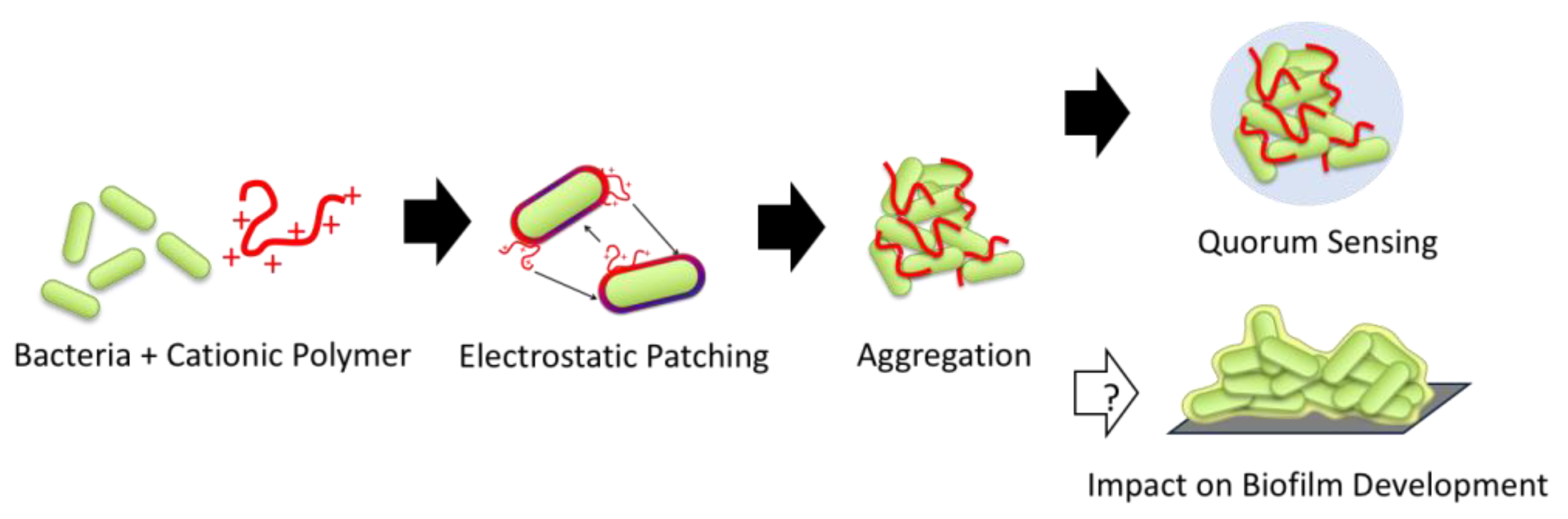
1. Introduction
2. Results
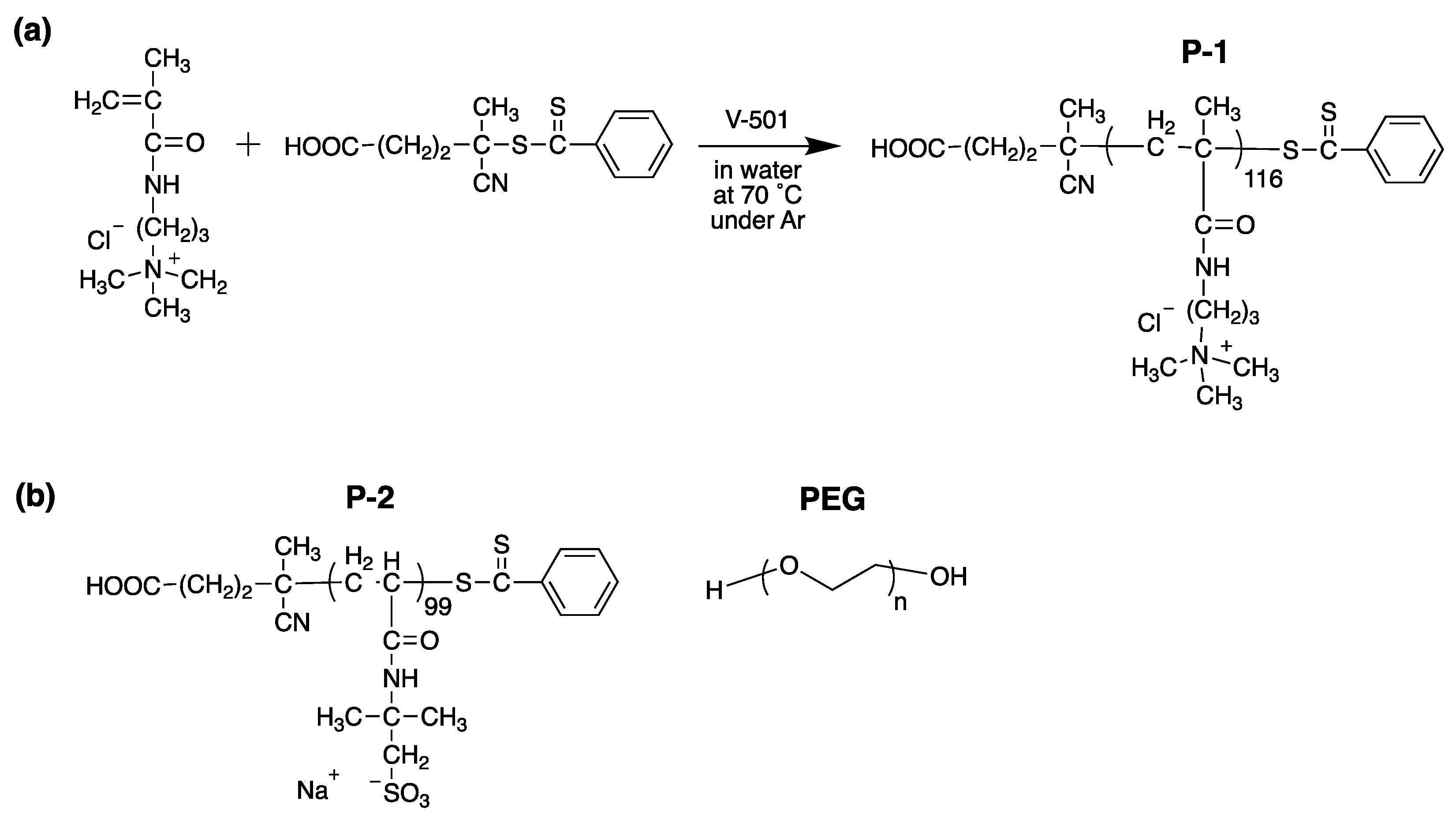
2.1. Polymer Design, Synthesis, and Characterization
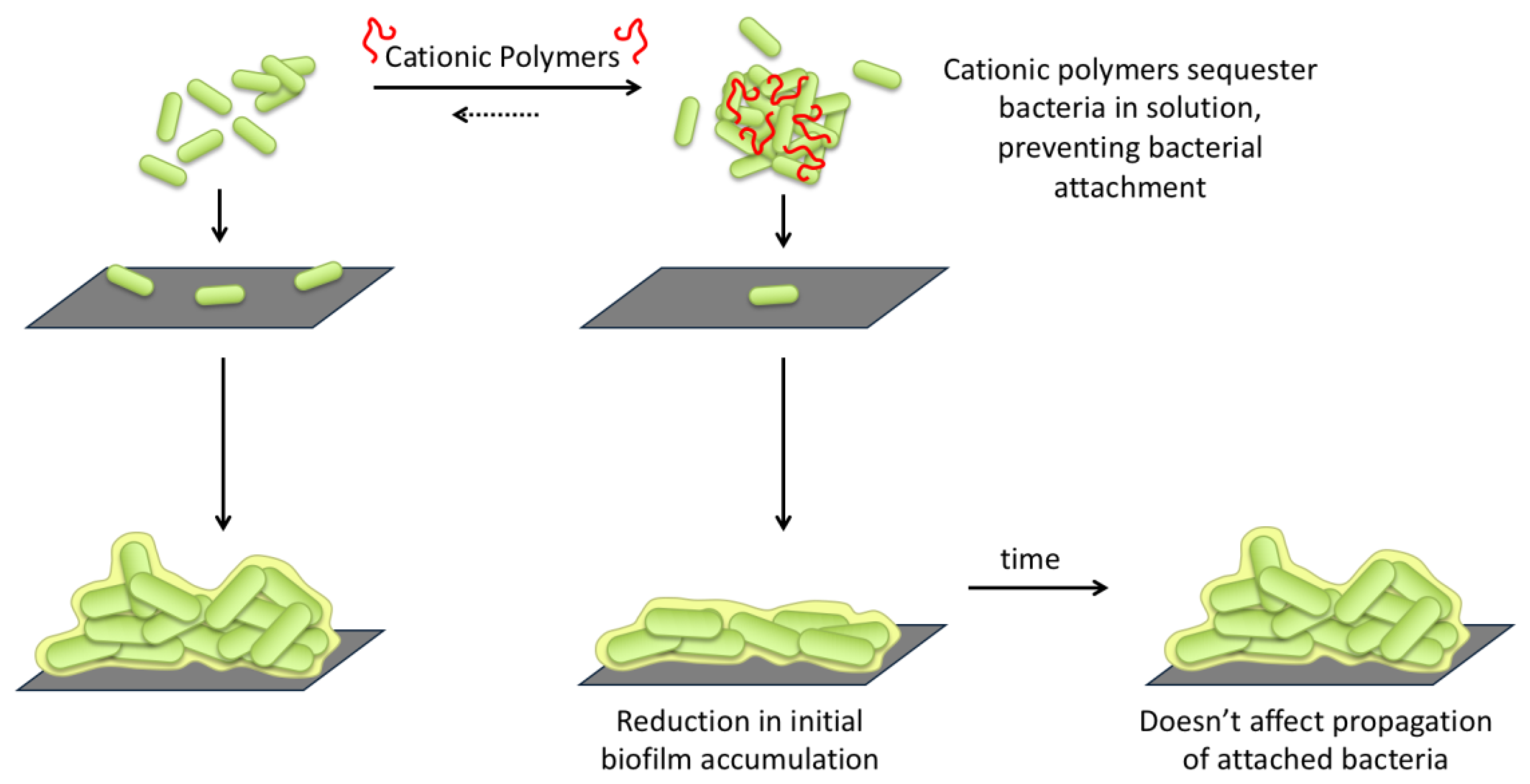
2.2. Planktonic Bacteria-Polymer Interactions and Consequences
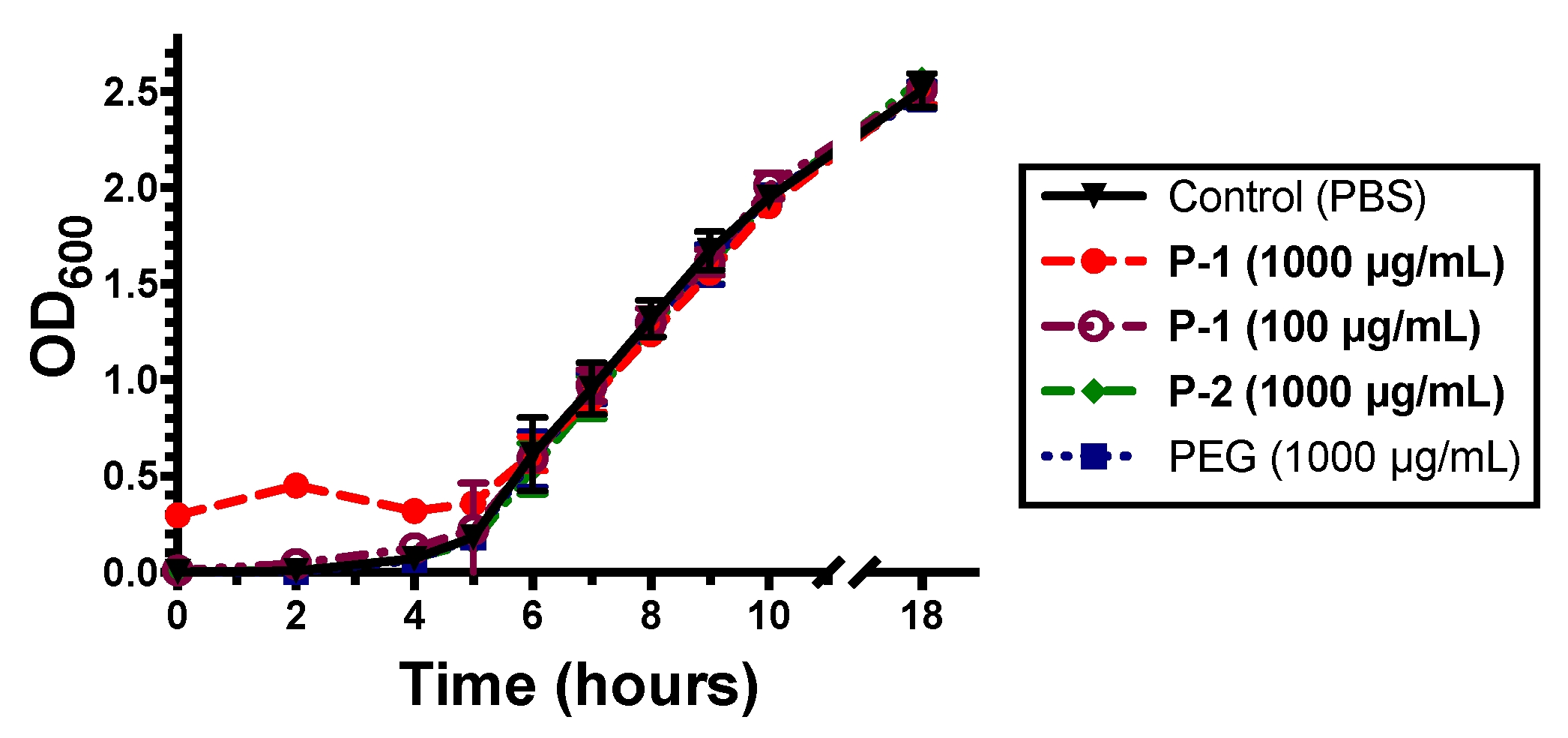
2.2.1. Antimicrobial Activity of Polymers
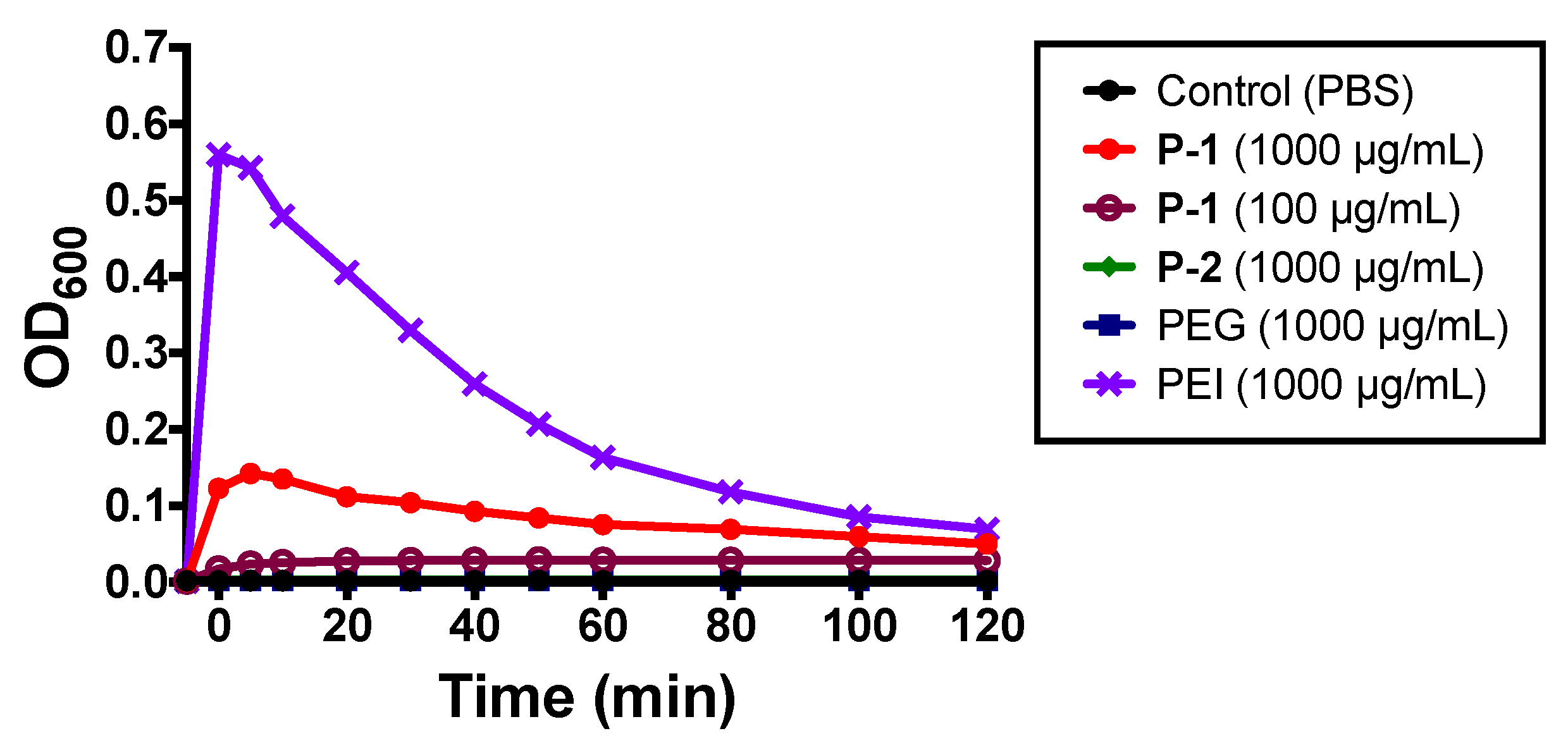
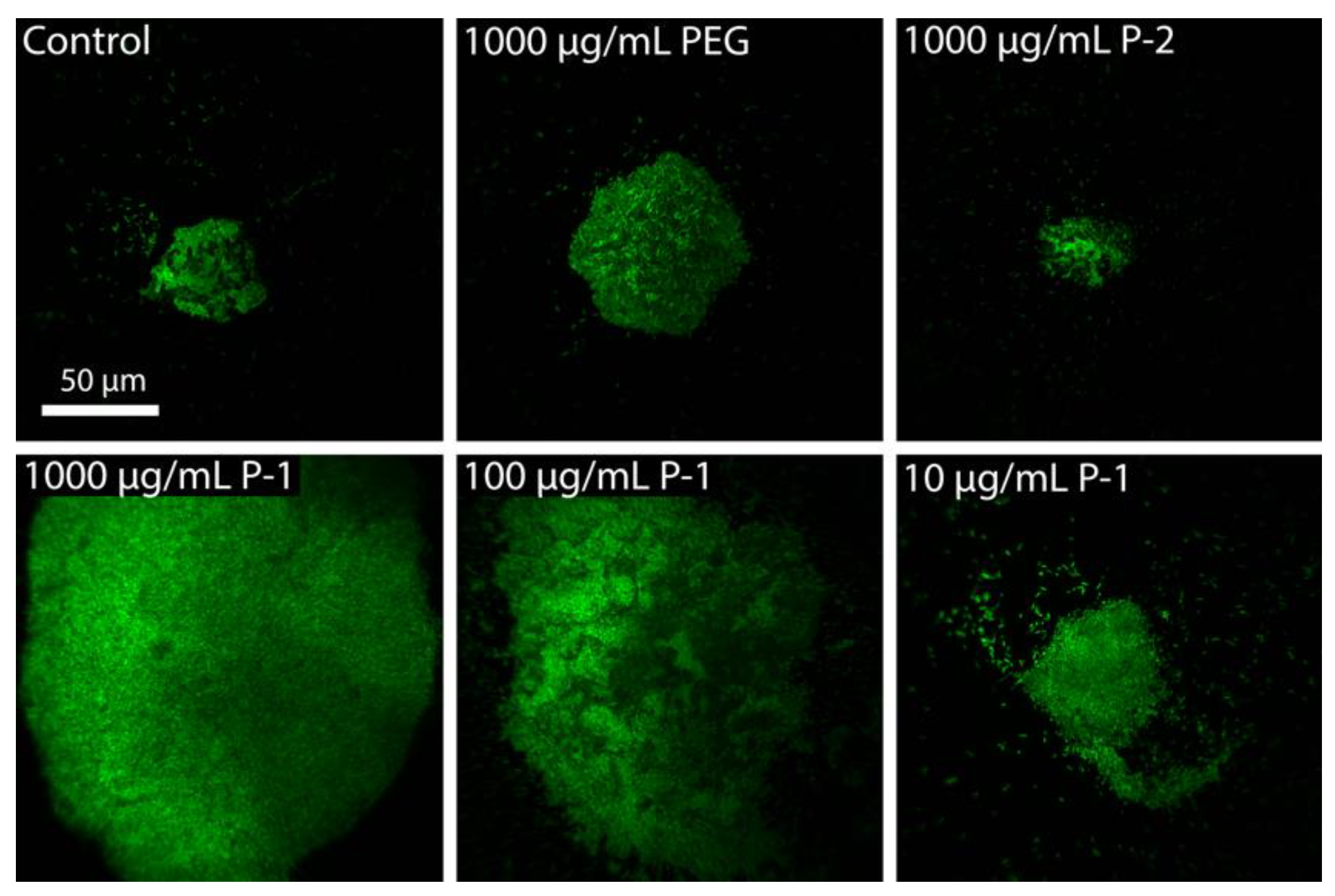
2.2.2. Bacterial Aggregation and Flocculation
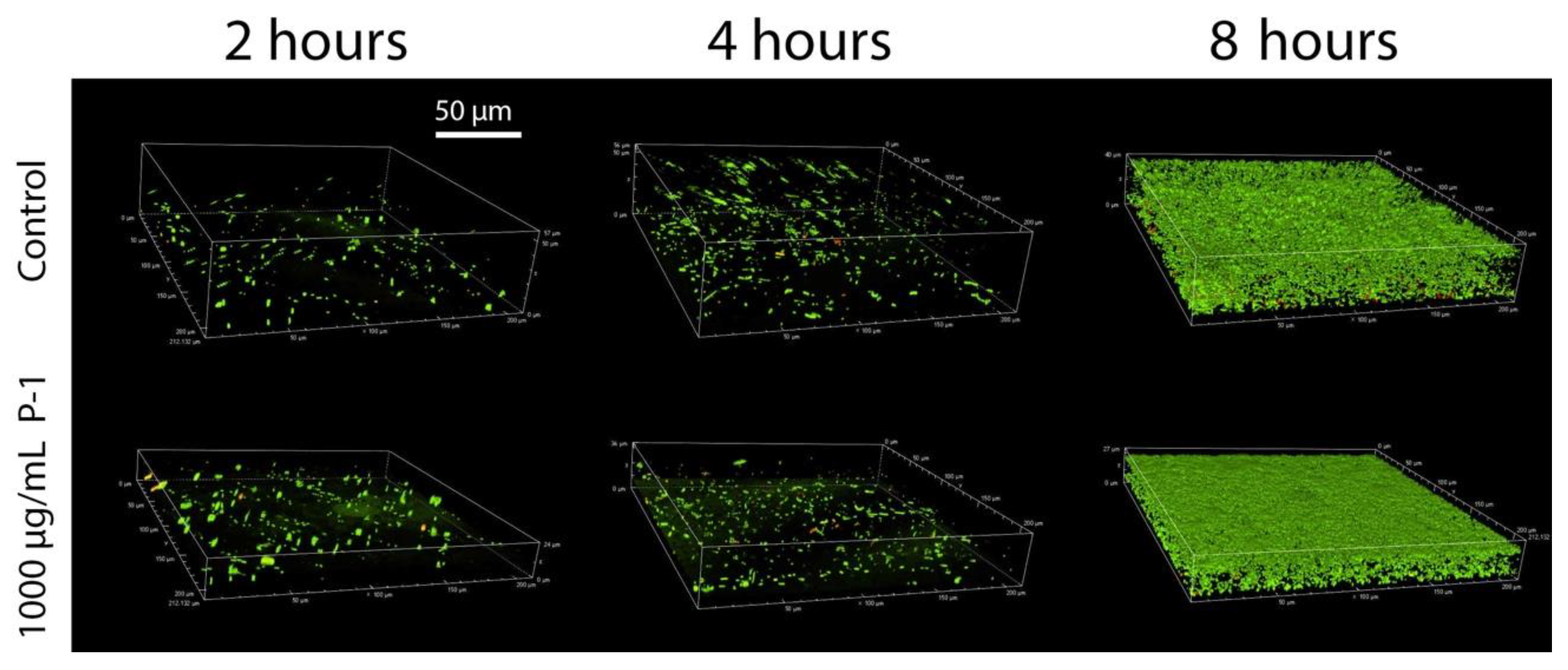
2.3. Development of Bacterial Biofilms
2.4. Effect of Polymer Incubation on Biofilm Development
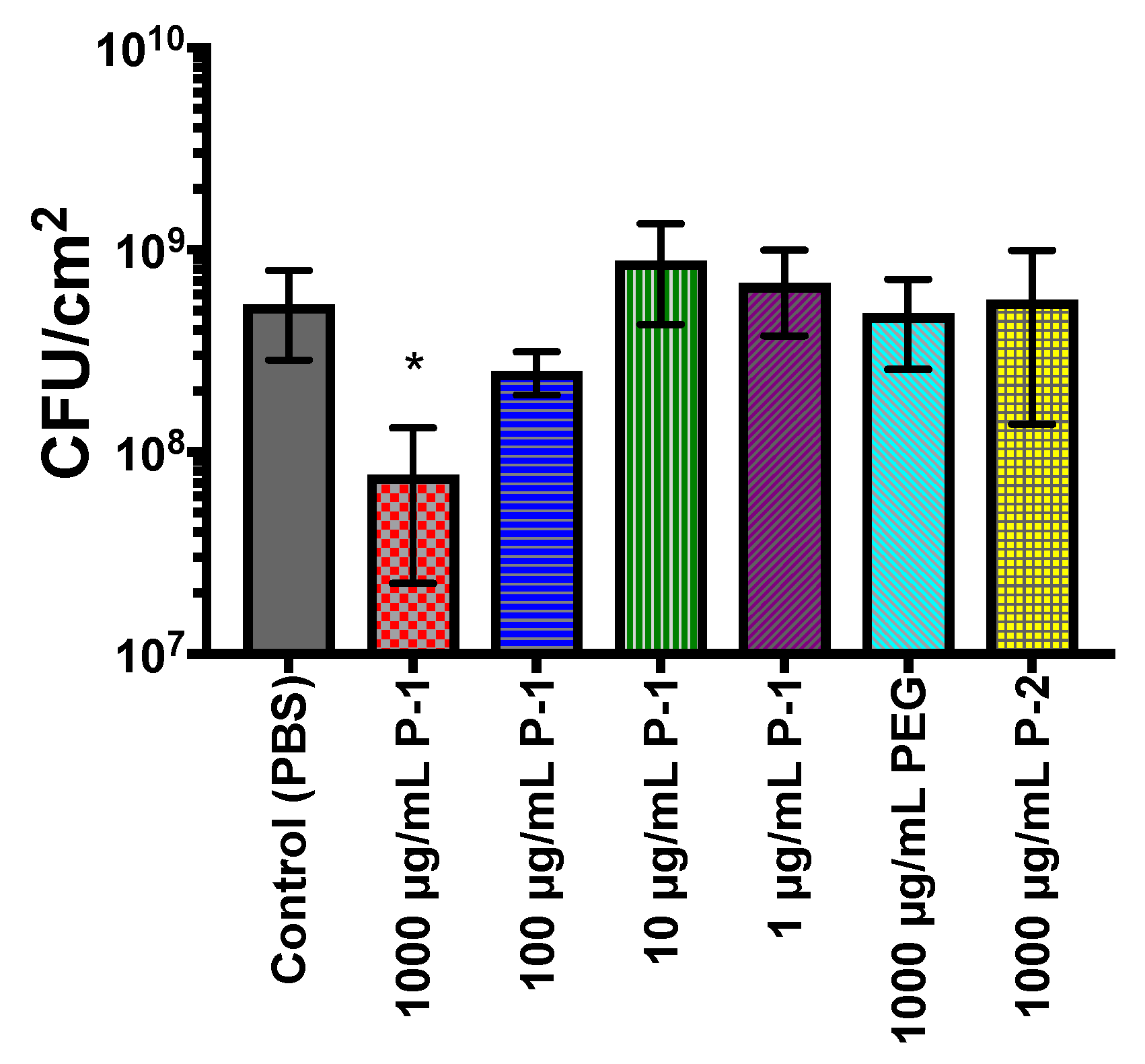
2.4.1. Accumulation of Total Biomass
2.4.2. Bacterial Biofilm Viability
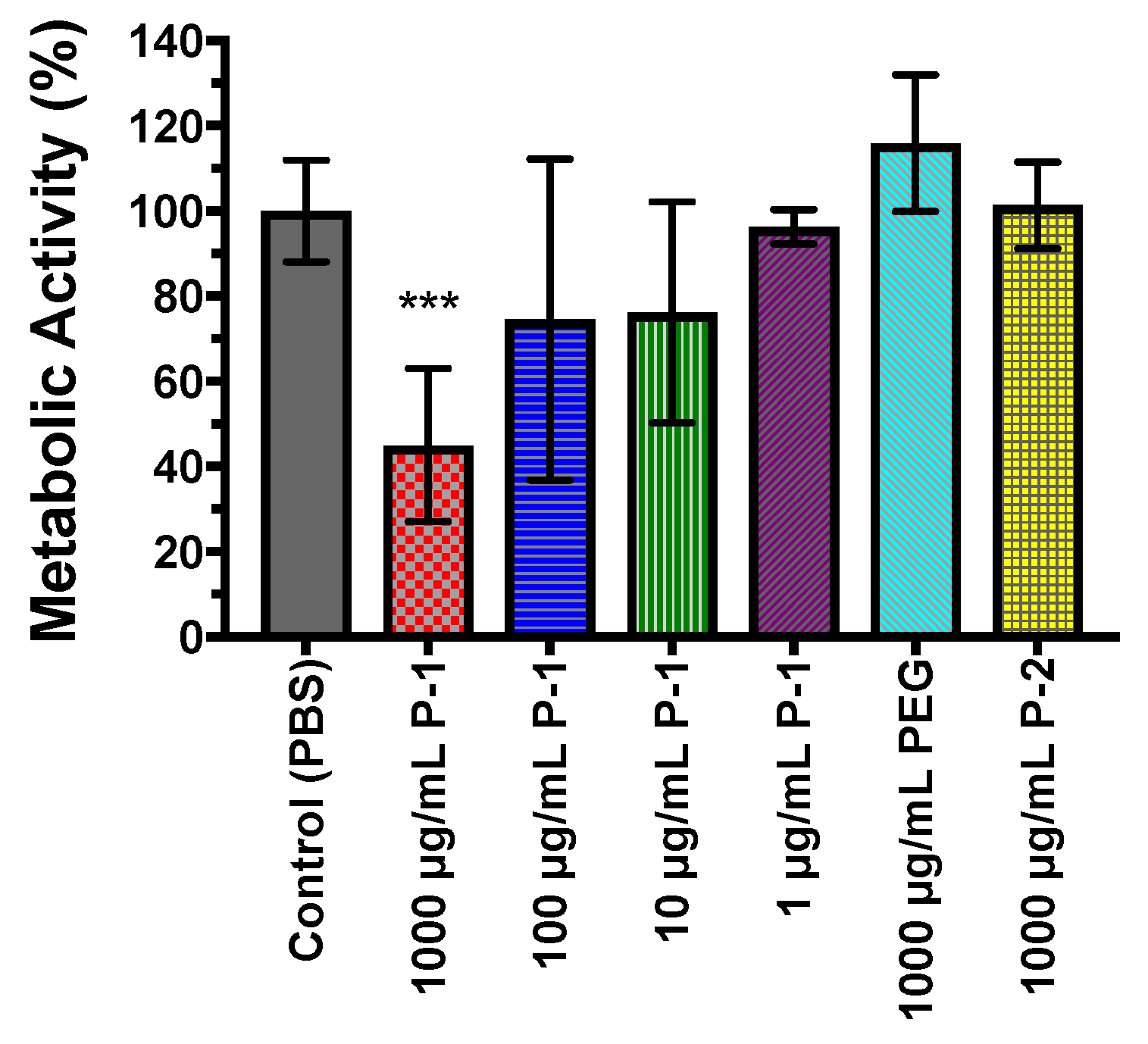
2.4.3. Metabolic Activity of Bacterial Biofilms
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Antimicrobial Activity Assessed by Minimum Inhibitory Concentration (MIC) Assay
4.3. Bacterial Growth Curves by Optical Density
4.4. Bacterial Aggregation/Flocculation Assessment by Optical Density
4.5. Bacterial Aggregate Observation: Confocal Microscopy
4.6. Bacterial Formation Over Time: Confocal Microscopy
4.7. Biofilm Formation with Polymer Co-incubation
4.8. Evaluation of Total Biomass by Crystal Violet Staining Assay
4.9. Evaluation of Viable Bacteria by Direct Enumeration
4.10. Evaluation of Metabolic Activity by MTT Assay
4.11. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Teschler, J.K.; Zamorano-Sanchez, D.; Utada, A.S.; Warner, C.J.A.; Wong, G.C.L.; Linington, R.G.; Yildiz, F.H. Living in the matrix: Assembly and control of Vibrio cholerae biofilms. Nat. Rev. Microbiol. 2015, 13, 255–268. [Google Scholar] [CrossRef] [PubMed]
- Vlamakis, H.; Chai, Y.R.; Beauregard, P.; Losick, R.; Kolter, R. Sticking together: Building a biofilm the Bacillus subtilis way. Nat. Rev. Microbiol. 2013, 11, 157–168. [Google Scholar] [CrossRef] [PubMed]
- Joo, H.S.; Otto, M. Molecular Basis of In Vivo Biofilm Formation by Bacterial Pathogens. Chem. Biol. 2012, 19, 1503–1513. [Google Scholar] [CrossRef] [PubMed]
- Costerton, J.W.; Stewart, P.S.; Greenberg, E.P. Bacterial biofilms: A common cause of persistent infections. Science 1999, 284, 1318–1322. [Google Scholar] [CrossRef] [PubMed]
- Bryers, J.D.; Ratner, B.D. Bioinspired implant materials befuddle bacteria. Asm. News 2004, 70, 232–237. [Google Scholar]
- Bryers, J.D. Medical Biofilms. Biotechnol. Bioeng. 2008, 100, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Hoiby, N.; Bjarnsholt, T.; Givskov, M.; Molin, S.; Ciofu, O. Antibiotic resistance of bacterial biofilms. Int. J. Antimicrob. Ag. 2010, 35, 322–332. [Google Scholar] [CrossRef] [PubMed]
- D’Angelo, I.; Conte, C.; La Rotonda, M.I.; Miro, A.; Quaglia, F.; Ungaro, F. Improving the efficacy of inhaled drugs in cystic fibrosis: Challenges and emerging drug delivery strategies. Adv. Drug Deliver. Rev. 2014, 75, 92–111. [Google Scholar] [CrossRef]
- Mah, T.F.C.; O’Toole, G.A. Mechanisms of biofilm resistance to antimicrobial agents. Trends Microbiol. 2001, 9, 34–39. [Google Scholar] [CrossRef]
- Walters, M.C.; Roe, F.; Bugnicourt, A.; Franklin, M.J.; Stewart, P.S. Contributions of antibiotic penetration, oxygen limitation, and low metabolic activity to tolerance of Pseudomonas aeruginosa biofilms to ciprofloxacin and tobramycin. Antimicrob. Agents Chemother. 2003, 47, 317–323. [Google Scholar] [CrossRef]
- Macia, M.D.; Rojo-Molinero, E.; Oliver, A. Antimicrobial susceptibility testing in biofilm-growing bacteria. Clin. Microbiol. Infec. 2014, 20, 981–990. [Google Scholar] [CrossRef] [PubMed]
- Rybtke, M.; Hultqvist, L.D.; Givskov, M.; Tolker-Nielsen, T. Pseudomonas aeruginosa Biofilm Infections: Community Structure, Antimicrobial Tolerance and Immune Response. J. Mol. Biol. 2015, 427, 3628–3645. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Solano, L.; Macia, M.D.; Fajardo, A.; Oliver, A.; Martinez, J.L. Chronic Pseudomonas aeruginosa Infection in Chronic Obstructive Pulmonary Disease. Clin. Infect. Dis. 2008, 47, 1526–1533. [Google Scholar] [CrossRef]
- Oliver, A.; Canton, R.; Campo, P.; Baquero, F.; Blazquez, J. High frequency of hypermutable Pseudomonas aeruginosa in cystic fibrosis lung infection. Science 2000, 288, 1251–1253. [Google Scholar] [CrossRef]
- Shukla, A.; Fleming, K.E.; Chuang, H.F.; Chau, T.M.; Loose, C.R.; Stephanopoulos, G.N.; Hammond, P.T. Controlling the release of peptide antimicrobial agents from surfaces. Biomaterials 2010, 31, 2348–2357. [Google Scholar] [CrossRef]
- Kumar, R.; Munstedt, H. Silver ion release from antimicrobial polyamide/silver composites. Biomaterials 2005, 26, 2081–2088. [Google Scholar] [CrossRef]
- Hetrick, E.M.; Schoenfisch, M.H. Reducing implant-related infections: active release strategies. Chem. Soc. Rev. 2006, 35, 780–789. [Google Scholar] [CrossRef] [PubMed]
- Mi, L.; Jiang, S.Y. Integrated Antimicrobial and Nonfouling Zwitterionic Polymers. Angew. Chem. Int. Edit. 2014, 53, 1746–1754. [Google Scholar] [CrossRef] [PubMed]
- Goda, T.; Ishihara, K.; Miyahara, Y. Critical update on 2-methacryloyloxyethyl phosphorylcholine (MPC) polymer science. J. Appl. Polym. Sci. 2015, 132. [Google Scholar] [CrossRef]
- Nejadnik, M.R.; van der Mei, H.C.; Norde, W.; Busscher, H.J. Bacterial adhesion and growth on a polymer brush-coating. Biomaterials 2008, 29, 4117–4121. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.V.; Vyas, V.; Patil, R.; Sharma, V.; Scopelliti, P.E.; Bongiorno, G.; Podesta, A.; Lenardi, C.; Gade, W.N.; Milani, P. Quantitative Characterization of the Influence of the Nanoscale Morphology of Nanostructured Surfaces on Bacterial Adhesion and Biofilm Formation. PLoS ONE 2011, 6. [Google Scholar] [CrossRef]
- Carman, M.L.; Estes, T.G.; Feinberg, A.W.; Schumacher, J.F.; Wilkerson, W.; Wilson, L.H.; Callow, M.E.; Callow, J.A.; Brennan, A.B. Engineered antifouling microtopographies—correlating wettability with cell attachment. Biofouling 2006, 22, 11–21. [Google Scholar] [CrossRef] [PubMed]
- Gudipati, C.S.; Greenlief, C.M.; Johnson, J.A.; Prayongpan, P.; Wooley, K.L. Hyperbranched fluoropolymer and linear poly(ethylene glycol) based Amphiphilic crosslinked networks as efficient antifouling coatings: An insight into the surface compositions, topographies, and morphologies. J. Polym. Sci. Pol. Chem. 2004, 42, 6193–6208. [Google Scholar] [CrossRef]
- Cao, Z.Q.; Mi, L.; Mendiola, J.; Ella-Menye, J.R.; Zhang, L.; Xue, H.; Jiang, S.Y. Reversibly Switching the Function of a Surface between Attacking and Defending against Bacteria. Angew. Chem. Int. Edit. 2012, 51, 2602–2605. [Google Scholar] [CrossRef]
- Dorner, F.; Boschert, D.; Schneider, A.; Hartleb, W.; Al-Ahmad, A.; Lienkamp, K. Toward Self-Regenerating Antimicrobial Polymer Surfaces. Acs. Macro. Lett. 2015, 4, 1337–1340. [Google Scholar] [CrossRef]
- Cook, G.S.; Costerton, J.W.; Lamont, R.J. Biofilm formation by Porphyromonas gingivalis and Streptococcus gordonii. J. Periodontal. Res. 1998, 33, 323–327. [Google Scholar] [CrossRef]
- Bossier, P.; Verstraete, W. Triggers for microbial aggregation in activated sludge? Appl. Microbiol. Biotechnol. 1996, 45, 1–6. [Google Scholar] [CrossRef]
- Edzwald, J.K. Coagulation in Drinking-Water Treatment—Particles, Organics and Coagulants. Water Sci. Technol. 1993, 27, 21–35. [Google Scholar] [CrossRef]
- Wickramasinghe, S.R.; Leong, Y.K.; Mondal, S.; Liow, J.L. Influence of cationic flocculant properties on the flocculation of yeast suspensions. Adv. Powder Technol. 2010, 21, 374–379. [Google Scholar] [CrossRef]
- Schwarz-Linek, J.; Winkler, A.; Wilson, L.G.; Pham, N.T.; Schilling, T.; Poon, W.C.K. Polymer-induced phase separation in Escherichia coli suspensions. Soft Matter 2010, 6, 4540–4549. [Google Scholar] [CrossRef]
- Dorken, G.; Ferguson, G.P.; French, C.E.; Poon, W.C.K. Aggregation by depletion attraction in cultures of bacteria producing exopolysaccharide. J. R. Soc. Interface 2012, 9, 3490–3502. [Google Scholar] [CrossRef]
- Lee, C.S.; Robinson, J.; Chong, M.F. A review on application of flocculants in wastewater treatment. Process Saf. Environ. 2014, 92, 489–508. [Google Scholar] [CrossRef]
- Lui, L.T.; Xue, X.; Sui, C.; Brown, A.; Pritchard, D.I.; Halliday, N.; Winzer, K.; Howdle, S.M.; Fernandez-Trillo, F.; Krasnogor, N.; et al. Bacteria clustering by polymers induces the expression of quorum-sensing-controlled phenotypes. Nat. Chem. 2013, 5, 1058–1065. [Google Scholar] [CrossRef]
- Zhang, P.; Lu, H.; Chen, H.; Zhang, J.; Liu, L.; Lv, F.; Wang, S. Cationic Conjugated Polymers-Induced Quorum Sensing of Bacteria Cells. Anal. Chem. 2016, 88, 2985–2988. [Google Scholar] [CrossRef] [PubMed]
- Kenawy, E.R.; Worley, S.D.; Broughton, R. The chemistry and applications of antimicrobial polymers: A state-of-the-art review. Biomacromolecules 2007, 8, 1359–1384. [Google Scholar] [CrossRef]
- Gregory, J. Rates of Flocculation of Latex Particles by Cationic Polymers. J. Colloid Interface Sci. 1973, 42, 448–456. [Google Scholar] [CrossRef]
- Gregory, J. Flocculation of Polystyrene Particles with Cationic Polyelectrolytes. T. Faraday Soc. 1969, 65, 2260–2268. [Google Scholar] [CrossRef]
- Hancock, R.E.W. Hancock Laboratory Methods. Available online: http://cmdr.ubc.ca/bobh/methods.htm (accessed on 9 May 2019).
- Giacometti, A.; Cirioni, O.; Barchiesi, F.; Del Prete, M.S.; Fortuna, M.; Caselli, F.; Scalise, G. In vitro susceptibility tests for cationic peptides: Comparison of broth microdilution methods for bacteria that grow aerobically. Antimicrob. Agents Chemother. 2000, 44, 1694–1696. [Google Scholar] [CrossRef] [PubMed]
- Schleheck, D.; Barraud, N.; Klebensberger, J.; Webb, J.S.; McDougald, D.; Rice, S.A.; Kjelleberg, S. Pseudomonas aeruginosa PAO1 Preferentially Grows as Aggregates in Liquid Batch Cultures and Disperses upon Starvation. PloS ONE 2009, 4. [Google Scholar] [CrossRef] [PubMed]
- Christensen, G.D.; Simpson, W.A.; Younger, J.J.; Baddour, L.M.; Barrett, F.F.; Melton, D.M.; Beachey, E.H. Adherence of Coagulase-Negative Staphylococci to Plastic Tissue-Culture Plates—A Quantitative Model for the Adherence of Staphylococci to Medical Devices. J. Clin. Microbiol. 1985, 22, 996–1006. [Google Scholar]
- O’Toole, G.A. Microtiter Dish Biofilm Formation Assay. Jove-J. Vis. Exp. 2011. [Google Scholar] [CrossRef] [PubMed]
- Rivas, B.L.; Pereira, E.D.; Mondaca, M.A. Biostatic behavior of side chain charged-polycations and polymer-Ag complexes. Polym. Bull. 2003, 50, 327–333. [Google Scholar] [CrossRef]
- Morgan, H.C.; Meier, J.F.; Merker, R.L. Method of creating a biostatic agent using interpenetrating network polymers. U.S. patent No. US6146688A 2000, 2000. [Google Scholar]
- Melrose, G.J.H.; Kleppe, C.M.; Langley, J.W.; Stewart, J.M.; Van Dyk, J. Biostatic and biocidal compositions. patent No. WO1988004671A1, 1988. [Google Scholar]
- Yang, R.; Li, H.J.; Huang, M.; Yang, H.; Li, A.M. A review on chitosan-based flocculants and their applications in water treatment. Water Res. 2016, 95, 59–89. [Google Scholar] [CrossRef]
- ImQuest BioSciences Biofilm Protocol Optimization for Pseudomonas aeruginosa: Culture Media, Incubation Time, and Biofilm Measurement. 2016. Available online: http://imquestbio.com/resources/technical-documents/ (accessed on 9 May 2019).
- Harrison-Balestra, C.; Cazzaniga, A.L.; Davis, S.C.; Mertz, P.M. A wound-isolated Pseudomonas aeruginosa grows a biofilm in vitro within 10 hours and is visualized by light microscopy. Dermatol. Surg. 2003, 29, 631–635. [Google Scholar] [PubMed]

| Polymer | DP | Mn (g/mol) (1H NMR) | Mn (g/mol) (GPC) | Mw (g/mol) (GPC) | Đ |
|---|---|---|---|---|---|
| P-1 | 116 | 25,900 | 19,600 | 20,580 | 1.05 |
| P-2 | 99 | 20,589 | 15,333 | 20,392 | 1.33 |
| Polymers | MIC (µg/mL) | ||
|---|---|---|---|
| P. aeruginos a) | E. coli b) | S. aureus b) | |
| P-1 | >1000 | >1000 | >1000 |
| P-2 | >1000 | >1000 | >1000 |
| PEG | >1000 | >1000 | >1000 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Foster, L.L.; Yusa, S.-i.; Kuroda, K. Solution-Mediated Modulation of Pseudomonas aeruginosa Biofilm Formation by a Cationic Synthetic Polymer. Antibiotics 2019, 8, 61. https://doi.org/10.3390/antibiotics8020061
Foster LL, Yusa S-i, Kuroda K. Solution-Mediated Modulation of Pseudomonas aeruginosa Biofilm Formation by a Cationic Synthetic Polymer. Antibiotics. 2019; 8(2):61. https://doi.org/10.3390/antibiotics8020061
Chicago/Turabian StyleFoster, Leanna L., Shin-ichi Yusa, and Kenichi Kuroda. 2019. "Solution-Mediated Modulation of Pseudomonas aeruginosa Biofilm Formation by a Cationic Synthetic Polymer" Antibiotics 8, no. 2: 61. https://doi.org/10.3390/antibiotics8020061
APA StyleFoster, L. L., Yusa, S.-i., & Kuroda, K. (2019). Solution-Mediated Modulation of Pseudomonas aeruginosa Biofilm Formation by a Cationic Synthetic Polymer. Antibiotics, 8(2), 61. https://doi.org/10.3390/antibiotics8020061






