Blue Light Disinfection in Hospital Infection Control: Advantages, Drawbacks, and Pitfalls
Abstract
1. Introduction
2. Methods
3. Hospital Acquired-Infections
3.1. Reservoirs and Transmission
3.2. Challenges and Costs
4. Prevention and Control of Hospital-Acquired Infections
4.1. Surveillance Methods
4.2. Infection Control Programs
4.3. Limitations of Current Methods and New Perspectives
5. Photodynamic Therapy as an Alternative Approach in the Control of Colonization/Infection in Hospital Settings and Facilities
5.1. Antimicrobial Photodynamic Therapy (aPDT)
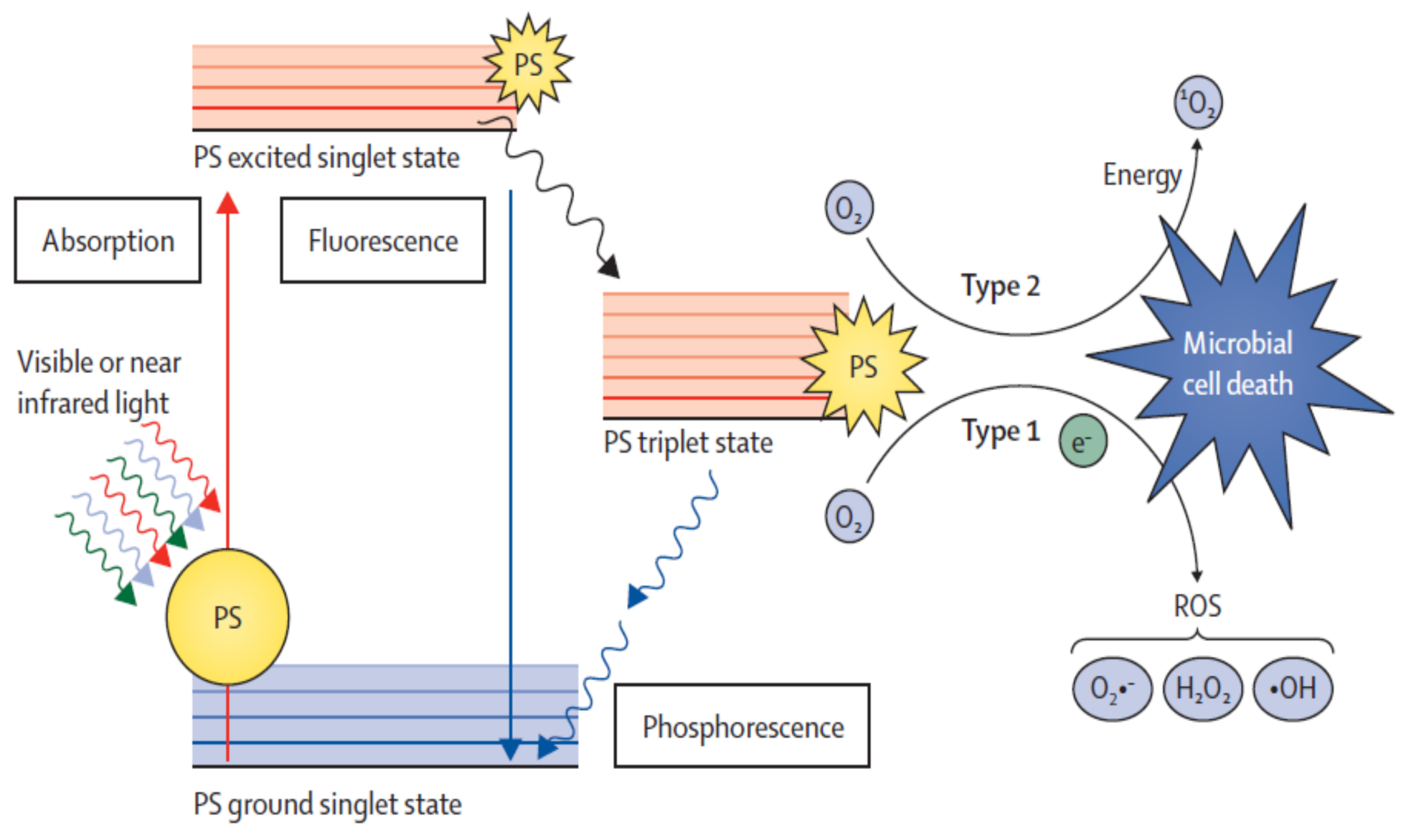
5.1.1. Mechanisms of Action (Type I and Type II)
5.1.2. Photosensitizers
5.1.3. Light Conditions
5.1.4. Microbial Targets
5.2. In Vitro and Clinical Effectiveness of Photodynamic Therapy
5.3. Effectiveness of Photodynamic Therapy in Healthcare Settings/Facilities
5.4. Disinfection/Sterilization of Polymeric Materials
5.5. Advantages and Drawbacks of Photodynamic Therapy Relatively to Conventional Antimicrobials
6. Blue Light Microbial Photoinactivation
6.1. Mechanism of Action of Blue Light
6.2. Endogenous Photosensitizers of Microorganisms
6.3. Effectiveness of Blue Light in the Inactivation of Microorganisms
6.4. Advantages and Drawbacks of Antimicrobial Blue Light Relatively to aPDT
6.5. High Intensity Narrow Spectrum Light
7. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kasper, D.L.; Fauci, A.S.; Hauser, S.L.; Longo, D.L.; Jameson, J.L.; Loscalzo, J. Harrison’s Principles of Internal Medicine, 19th ed.; McGraw Hill Education: New York, NY, USA, 2015. [Google Scholar]
- Cardoso, T.; Almeida, M.; Carratala, J.; Aragao, I.; Costa-Pereira, A.; Sarmento, A.E.; Azevedo, L. Microbiology of healthcare-associated infections and the definition accuracy to predict infection by potentially drug resistant pathogens: A systematic review. BMC Infect. Dis. 2015, 15, 565. [Google Scholar] [CrossRef] [PubMed]
- O’Neill, J. Tackling Drug-Resistant Infection Globally: Final Report and Reccommendations; AMR Review: London, UK, 2016. [Google Scholar]
- WHO. Antimicrobial Resistance; WHO: Geneva, Switzerland, 2018. [Google Scholar]
- Cookson, B. Clinical significance of emergence of bacterial antimicrobial resistance in the hospital environment. J. Appl. Microbiol. 2005, 99, 989–996. [Google Scholar] [CrossRef] [PubMed]
- Dai, T.; Gupta, A.; Huang, Y.Y.; Yin, R.; Murray, C.K.; Vrahas, M.S.; Sherwood, M.E.; Tegos, G.P.; Hamblin, M.R. Blue light rescues mice from potentially fatal Pseudomonas aeruginosa burn infection: Efficacy, safety, and mechanism of action. Antimicrob. Agents Chemother. 2013, 57, 1238–1245. [Google Scholar] [CrossRef]
- Maclean, M.; McKenzie, K.; Anderson, J.G.; Gettinby, G.; MacGregor, S.J. 405 nm light technology for the inactivation of pathogens and its potential role for environmental disinfection and infection control. J. Hosp. Infect. 2014, 88, 1–11. [Google Scholar] [CrossRef]
- Boyce, J.M. Modern technologies for improving cleaning and disinfection of environmental surfaces in hospitals. Antimicrob. Resist. Infect. Control 2016, 5, 10–11. [Google Scholar] [CrossRef]
- Messina, G.; Fattorini, M.; Nante, N.; Rosadini, D.; Serafini, A.; Tani, M.; Cevenini, G. Time Effectiveness of Ultraviolet C Light (UVC) Emitted by Light Emitting Diodes (LEDs) in Reducing Stethoscope Contamination. Int. J. Environ. Res. Public Health 2016, 13. [Google Scholar] [CrossRef]
- Adlhart, C.; Verran, J.; Azevedo, N.F.; Olmez, H.; Keinanen-Toivola, M.M.; Gouveia, I.; Melo, L.F.; Crijns, F. Surface modifications for antimicrobial effects in the healthcare setting: A critical overview. J. Hosp. Infect. 2018, 99, 239–249. [Google Scholar] [CrossRef] [PubMed]
- Cobrado, L.; Silva-Dias, A.; Azevedo, M.M.; Rodrigues, A.G. High-touch surfaces: Microbial neighbours at hand. Eur. J. Clin. Microbiol. Infect. Dis 2017, 36, 2053–2062. [Google Scholar] [CrossRef] [PubMed]
- Weber, D.J.; Anderson, D.; Rutala, W.A. The role of the surface environment in healthcare-associated infections. Curr. Opin. Infect. Dis. 2013, 26, 338–344. [Google Scholar] [CrossRef]
- Kramer, A.; Schwebke, I.; Kampf, G. How long do nosocomial pathogens persist on inanimate surfaces? A systematic review. BMC Infect. Dis. 2006, 6, 130–138. [Google Scholar] [CrossRef] [PubMed]
- Smibert, O.C.; Aung, A.K.; Woolnough, E.; Carter, G.P.; Schultz, M.B.; Howden, B.P.; Seemann, T.; Spelman, D.; McGloughlin, S.; Peleg, A.Y. Mobile phones and computer keyboards: Unlikely reservoirs of multidrug-resistant organisms in the tertiary intensive care unit. J. Hosp. Infect. 2018, 99, 295–298. [Google Scholar] [CrossRef] [PubMed]
- Clesham, K.; Ryan, P.R.; Murphy, C.G. Assessment of theatre shoe contamination in an orthopaedic theatre. J. Hosp. Infect. 2018, 99, 299–302. [Google Scholar] [CrossRef] [PubMed]
- Chander, Y.; Rai, R. Hospital Acquired Infection. Med. J. Armed Forces India 1998, 54, 179–181. [Google Scholar] [CrossRef]
- WHO. Hospital Hygiene and Infection Control; Health, W.S., Ed.; WHO: Geneva, Switzerland, 2015. [Google Scholar]
- Qian, H.; Zheng, X. Ventilation control for airborne transmission of human exhaled bio-aerosols in buildings. J. Thorac. Dis. 2018, 10, S2295–S2304. [Google Scholar] [CrossRef]
- Decker, B.K.; Palmore, T.N. The role of water in healthcare-associated infections. Curr. Opin. Infect. Dis. 2013, 26, 345–351. [Google Scholar] [CrossRef] [PubMed]
- Calfee, D.P. Crisis in hospital-acquired, healthcare-associated infections. Annu. Rev. Med. 2012, 63, 359–371. [Google Scholar] [CrossRef]
- Sydnor, E.R.; Perl, T.M. Hospital epidemiology and infection control in acute-care settings. Clin. Microbiol. Rev. 2011, 24, 141–173. [Google Scholar] [CrossRef]
- WHO. Health Care-Associated Infections FACT SHEET; WHO: Geneva, Switzerland, 2017. [Google Scholar]
- Klevens, R.M.; Edwards, J.R.; Richards, C.L., Jr.; Horan, T.C.; Gaynes, R.P.; Pollock, D.A.; Cardo, D.M. Estimating health care-associated infections and deaths in U.S. hospitals, 2002. Public Health Rep. 2007, 122, 160–166. [Google Scholar] [CrossRef]
- ECDC. Point Prevalence Survey of Healthcare Associated Infections and Antimicrobial Use in European Acute Care Hospitals 2011–2012. ECDC: Solna Sweden, 2013. [Google Scholar]
- Douglas, S.R. The Direct Medical Costs of Healthcare-Associated Infections in U.S. Hospitals and the Benefits of Prevention. CDC: Atlanta, GA, USA, 2009. [Google Scholar]
- Surveillance of nosocomial infections in Europe: Which methodologies? Clin. Microbiol. Infect. 1999, 5. [CrossRef]
- Implementation of a Region Wide Computerized Surveillance Program for Nosocomial Postoperative Wound Infections in Surgical Units of Styria. Available online: http://www.pasq.eu/DesktopModules/BlinkQuestionnaires/QFiles/448_WP4_KAGes_NISS_Poster.pdf (accessed on 24 November 2018).
- Nosocomial Infection Surveillance System—Intensive Care Units (NISS-ITS). Lessons Learned after One Year. Available online: http://www.pasq.eu/DesktopModules/BlinkQuestionnaires/QFiles/448_WP4_KAGes_NISS_Poster.pdf (accessed on 24 November 2018).
- ECDC. Healthcare-Associated Infections in European Hospitals; ECDC: Solna, Sweden, 2015. [Google Scholar]
- Harbarth, S.; Sax, H.; Gastmeier, P. The preventable proportion of nosocomial infections: An overview of published reports. J. Hosp. Infect. 2003, 54, 258–266. [Google Scholar] [CrossRef]
- WHO. Guidelines on Hand Hygiene in Health Care: A Summary; WHO: Geneva, Switzerland, 2014. [Google Scholar]
- Landelle, C.; Pagani, L.; Harbarth, S. Is patient isolation the single most important measure to prevent the spread of multidrug-resistant pathogens? Virulence 2013, 4, 163–171. [Google Scholar] [CrossRef]
- Boyce, J.M.; Havill, N.L.; Lipka, A.; Havill, H.; Rizvani, R. Variations in hospital daily cleaning practices. Infect. Control Hosp. Epidemiol. 2010, 31, 99–101. [Google Scholar] [CrossRef] [PubMed]
- Fernando, S.A.; Gray, T.J.; Gottlieb, T. Healthcare-acquired infections: Prevention strategies. Intern. Med. J. 2017, 47, 1341–1351. [Google Scholar] [CrossRef] [PubMed]
- Carling, P.C.; Bartley, J.M. Evaluating hygienic cleaning in health care settings: What you do not know can harm your patients. Am. J. Infect. Control 2010, 38, S41–S50. [Google Scholar] [CrossRef] [PubMed]
- Boyce, J.M.; Sullivan, L.; Booker, A.; Baker, J. Quaternary Ammonium Disinfectant Issues Encountered in an Environmental Services Department. Infect. Control Hosp. Epidemiol. 2016, 37, 340–342. [Google Scholar] [CrossRef][Green Version]
- WHO. Natural Ventilation for Infection Control in Health-Care Settings; WHO: Geneva, Switzerland, 2009. [Google Scholar]
- Decker, B.K.; Palmore, T.N. Hospital water and opportunities for infection prevention. Curr. Infect. Dis. Rep. 2014, 16, 432–445. [Google Scholar] [CrossRef]
- Dancer, S.J. Controlling hospital-acquired infection: Focus on the role of the environment and new technologies for decontamination. Clin. Microbiol. Rev. 2014, 27, 665–690. [Google Scholar] [CrossRef] [PubMed]
- Hamblin, M.R.; Hasan, T. Photodynamic therapy: A new antimicrobial approach to infectious disease? Photochem. Photobiol. Sci. 2004, 3, 436–450. [Google Scholar] [CrossRef]
- Costa, L.; Faustino, M.A.F.; Neves, M.G.P.M.S.; Cunha, Â.; Almeida, A. Photodynamic Inactivation of Mammalian Viruses and Bacteriophages. Viruses 2012, 4, 1034. [Google Scholar] [CrossRef]
- Dalla Via, L.; Marciani Magno, S. Photochemotherapy in the treatment of cancer. Curr. Med. Chem. 2001, 8, 1405–1418. [Google Scholar]
- DeRosa, M.C.; Crutchley, R.J. Photosensitized singlet oxygen and its applications. Coord. Chem. Rev. 2002, 233–234, 351–371. [Google Scholar] [CrossRef]
- Jori, G.; Brown, S.B. Photosensitized inactivation of microorganisms. Photochem. Photobiol. Sci. 2004, 3, 403–405. [Google Scholar] [CrossRef]
- Alves, E.; Faustino, M.A.; Neves, M.G.; Cunha, A.; Tome, J.; Almeida, A. An insight on bacterial cellular targets of photodynamic inactivation. Future Med. Chem. 2014, 6, 141–164. [Google Scholar] [CrossRef] [PubMed]
- Almeida, A.; Faustino, M.A.; Tome, J.P. Photodynamic inactivation of bacteria: Finding the effective targets. Future Med. Chem. 2015, 7, 1221–1224. [Google Scholar] [CrossRef] [PubMed]
- Bonnett, R. Chemical Aspects of Photodynamic Therapy; Gordon and Breach Science Publishers: Amsterdam, The Netherlands, 2000. [Google Scholar]
- Choe, E.; Min, D.B. Chemistry and reactions of reactive oxygen species in foods. Crit. Rev. Food Sci. Nutr. 2006, 46, 1–22. [Google Scholar] [CrossRef] [PubMed]
- Wainwright, M. Photodynamic antimicrobial chemotherapy (PACT). J. Antimicrob. Chemother. 1998, 42, 13–28. [Google Scholar] [CrossRef] [PubMed]
- Ackroyd, R.; Kelty, C.; Brown, N.; Reed, M. The history of photodetection and photodynamic therapy. Photochem. Photobiol. 2001, 74, 656–669. [Google Scholar] [CrossRef]
- Hamblin, M.; Mroz, P. Advances in Photodynamic Therapy: Basic, Translational, and Clinical, 1st ed.; Artech House: London, UK, 2008; p. 559. [Google Scholar]
- Castano, A.P.; Demidova, T.N.; Hamblin, M.R. Mechanisms in photodynamic therapy: Part one-photosensitizers, photochemistry and cellular localization. Photodiagnosis Photodyn. Ther. 2004, 1, 279–293. [Google Scholar] [CrossRef]
- Almeida, A.; Cunha, A.; Faustino, M.A.F.; Tomé, A.C.; Neves, M.G.P.M.S. Neves MGPMS Porphyrins as antimibrobial photosensitizing agents. In Photodynamic Inactivation of Microbial Pathogens: Medical and Environmental Applications; Royal Society of Chemistry: Cambridge, UK, 2011; pp. 83–160. [Google Scholar]
- Wainwright, M.; Maisch, T.; Nonell, S.; Plaetzer, K.; Almeida, A.; Tegos, G.P.; Hamblin, M.R. Photoantimicrobials—are we afraid of the light? Lancet Infect. Dis. 2017, 17, e49–e55. [Google Scholar] [CrossRef]
- Costa, L.; Alves, E.; Carvalho, C.M.; Tome, J.P.; Faustino, M.A.; Neves, M.G.; Tome, A.C.; Cavaleiro, J.A.; Cunha, A.; Almeida, A. Sewage bacteriophage photoinactivation by cationic porphyrins: A study of charge effect. Photochem. Photobiol. Sci. 2008, 7, 415–422. [Google Scholar] [CrossRef]
- Alves, E.; Costa, L.; Carvalho, C.M.; Tome, J.P.; Faustino, M.A.; Neves, M.G.; Tome, A.C.; Cavaleiro, J.A.; Cunha, A.; Almeida, A. Charge effect on the photoinactivation of Gram-negative and Gram-positive bacteria by cationic meso-substituted porphyrins. BMC Microbiol. 2009, 9, 70–83. [Google Scholar] [CrossRef] [PubMed]
- Costa, L.; Carvalho, C.M.; Faustino, M.A.; Neves, M.G.; Tome, J.P.; Tome, A.C.; Cavaleiro, J.A.; Cunha, A.; Almeida, A. Sewage bacteriophage inactivation by cationic porphyrins: Influence of light parameters. Photochem. Photobiol. Sci. 2010, 9, 1126–1133. [Google Scholar] [CrossRef]
- Gabor, F.; Szolnoki, J.; Toth, K.; Fekete, A.; Maillard, P.; Csik, G. Photoinduced inactivation of T7 phage sensitized by symmetrically and asymmetrically substituted tetraphenyl porphyrin: Comparison of efficiency and mechanism of action. Photochem. Photobiol. 2001, 73, 304–311. [Google Scholar] [CrossRef]
- Sperandio, F.F.; Huang, Y.Y.; Hamblin, M.R. Antimicrobial photodynamic therapy to kill Gram-negative bacteria. Recent Pat. Antiinfect. Drug Discov. 2013, 8, 108–120. [Google Scholar] [CrossRef] [PubMed]
- Costa, L.; Tome, J.P.; Neves, M.G.; Tome, A.C.; Cavaleiro, J.A.; Cunha, A.; Faustino, M.A.; Almeida, A. Susceptibility of non-enveloped DNA- and RNA-type viruses to photodynamic inactivation. Photochem. Photobiol. Sci. 2012, 11, 1520–1563. [Google Scholar] [CrossRef]
- Girotti, A.W. Photosensitized oxidation of membrane lipids: Reaction pathways, cytotoxic effects, and cytoprotective mechanisms. J. Photochem. Photobiol. B 2001, 63, 103–113. [Google Scholar] [CrossRef]
- Stark, G. Functional consequences of oxidative membrane damage. J. Membr. Biol. 2005, 205, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Marciel, L.; Teles, L.; Moreira, B.; Pacheco, M.; Lourenco, L.M.; Neves, M.G.; Tome, J.P.; Faustino, M.A.; Almeida, A. An effective and potentially safe blood disinfection protocol using tetrapyrrolic photosensitizers. Future Med. Chem. 2017, 9, 365–379. [Google Scholar] [CrossRef] [PubMed]
- Jori, G.; Fabris, C.; Soncin, M.; Ferro, S.; Coppellotti, O.; Dei, D.; Fantetti, L.; Chiti, G.; Roncucci, G. Photodynamic therapy in the treatment of microbial infections: Basic principles and perspective applications. Lasers Surg. Med. 2006, 38, 468–481. [Google Scholar] [CrossRef] [PubMed]
- Maisch, T.; Szeimies, R.M.; Jori, G.; Abels, C. Antibacterial photodynamic therapy in dermatology. Photochem. Photobiol. Sci. 2004, 3, 907–917. [Google Scholar] [CrossRef]
- Nikaido, H. Prevention of drug access to bacterial targets: Permeability barriers and active efflux. Science 1994, 264, 382–388. [Google Scholar] [CrossRef] [PubMed]
- Hamblin, M.R.; O’Donnell, D.A.; Murthy, N.; Rajagopalan, K.; Michaud, N.; Sherwood, M.E.; Hasan, T. Polycationic photosensitizer conjugates: Effects of chain length and Gram classification on the photodynamic inactivation of bacteria. J. Antimicrob. Chemother. 2002, 49, 941–951. [Google Scholar] [CrossRef] [PubMed]
- Ehrenberg, B.; Gross, E.; Nitzan, Y.; Malik, Z. Electric depolarization of photosensitized cells: Lipid vs. protein alterations. Biochim. Biophys. Acta 1993, 1151, 257–264. [Google Scholar] [CrossRef]
- Grinholc, M.; Szramka, B.; Kurlenda, J.; Graczyk, A.; Bielawski, K.P. Bactericidal effect of photodynamic inactivation against methicillin-resistant and methicillin-susceptible Staphylococcus aureus is strain-dependent. J. Photochem. Photobiol. B 2008, 90, 57–63. [Google Scholar] [CrossRef]
- Nitzan, Y.; Arielly, H.; Maayan, M.C.; Rozenszajn, A. Gram negative bacteria isolated from blood cultures in a general hospital. New Microbiol. 1994, 17, 111–122. [Google Scholar] [PubMed]
- Arrojado, C.; Pereira, C.; Tome, J.P.; Faustino, M.A.; Neves, M.G.; Tome, A.C.; Cavaleiro, J.A.; Cunha, A.; Calado, R.; Gomes, N.C.; et al. Applicability of photodynamic antimicrobial chemotherapy as an alternative to inactivate fish pathogenic bacteria in aquaculture systems. Photochem. Photobiol. Sci. 2011, 10, 1691–1700. [Google Scholar] [CrossRef] [PubMed]
- Moore, C.; Wallis, C.; Melnick, J.L.; Kuns, M.D. Photodynamic treatment of herpes keratitis. Infect. Immun. 1972, 5, 169–171. [Google Scholar]
- Abramson, A.L.; Hirschfield, L.S.; Shikowitz, M.J.; Barrezueta, N.X. The pathologic effects of photodynamic therapy on the larynx. Experimental study. Arch. Otolaryngol. Head Neck Surg. 1988, 114, 33–39. [Google Scholar] [CrossRef]
- Perlin, M.; Mao, J.C.; Otis, E.R.; Shipkowitz, N.L.; Duff, R.G. Photodynamic inactivation of influenza and herpes viruses by hematoporphyrin. Antivir. Res. 1987, 7, 43–51. [Google Scholar] [CrossRef]
- Mohr, H.; Knuver-Hopf, J.; Gravemann, U.; Redecker-Klein, A.; Muller, T.H. West Nile virus in plasma is highly sensitive to methylene blue-light treatment. Transfusion 2004, 44, 886–890. [Google Scholar] [CrossRef]
- Wainwright, M. The emerging chemistry of blood product disinfection. Chem. Soc. Rev. 2002, 31, 128–136. [Google Scholar] [CrossRef] [PubMed]
- St Denis, T.G.; Dai, T.; Izikson, L.; Astrakas, C.; Anderson, R.R.; Hamblin, M.R.; Tegos, G.P. All you need is light: Antimicrobial photoinactivation as an evolving and emerging discovery strategy against infectious disease. Virulence 2011, 2, 509–520. [Google Scholar] [CrossRef] [PubMed]
- Demidova, T.N.; Hamblin, M.R. Effect of cell-photosensitizer binding and cell density on microbial photoinactivation. Antimicrob. Agents Chemother. 2005, 49, 2329–2335. [Google Scholar] [CrossRef] [PubMed]
- Calzavara-Pinton, P.; Rossi, M.T.; Sala, R.; Venturini, M. Photodynamic antifungal chemotherapy. Photochem. Photobiol. 2012, 88, 512–522. [Google Scholar] [CrossRef] [PubMed]
- Smijs, T.G.; Pavel, S. The susceptibility of dermatophytes to photodynamic treatment with special focus on Trichophyton rubrum. Photochem. Photobiol. 2011, 87, 2–13. [Google Scholar] [CrossRef] [PubMed]
- Donnelly, R.F.; McCarron, P.A.; Tunney, M.M.; David Woolfson, A. Potential of photodynamic therapy in treatment of fungal infections of the mouth. Design and characterisation of a mucoadhesive patch containing toluidine blue O. J. Photochem. Photobiol. B 2007, 86, 59–69. [Google Scholar] [CrossRef] [PubMed]
- Pereira, C.A.; Romeiro, R.L.; Costa, A.C.; Machado, A.K.; Junqueira, J.C.; Jorge, A.O. Susceptibility of Candida albicans, Staphylococcus aureus, and Streptococcus mutans biofilms to photodynamic inactivation: An in vitro study. Lasers Med. Sci. 2011, 26, 341–348. [Google Scholar] [CrossRef] [PubMed]
- Morgenthaler, J.B.; Peters, S.J.; Cedeno, D.L.; Constantino, M.H.; Edwards, K.A.; Kamowski, E.M.; Passini, J.C.; Butkus, B.E.; Young, A.M.; Lash, T.D.; et al. Carbaporphyrin ketals as potential agents for a new photodynamic therapy treatment of leishmaniasis. Bioorg. Med. Chem. 2008, 16, 7033–7038. [Google Scholar] [CrossRef] [PubMed]
- Van der Snoek, E.M.; Robinson, D.J.; van Hellemond, J.J.; Neumann, H.A. A review of photodynamic therapy in cutaneous leishmaniasis. J. Eur. Acad. Dermatol. Venereol. 2008, 22, 918–922. [Google Scholar] [CrossRef]
- Grellier, P.; Santus, R.; Mouray, E.; Agmon, V.; Maziere, J.C.; Rigomier, D.; Dagan, A.; Gatt, S.; Schrevel, J. Photosensitized inactivation of Plasmodium falciparum- and Babesia divergens-infected erythrocytes in whole blood by lipophilic pheophorbide derivatives. Vox Sang. 1997, 72, 211–220. [Google Scholar] [CrossRef]
- Alouini, Z.; Jemli, M. Destruction of helminth eggs by photosensitized porphyrin. J. Environ. Monit. 2001, 3, 548–551. [Google Scholar] [CrossRef] [PubMed]
- Alves, E.; Esteves, A.C.; Correia, A.; Cunha, A.; Faustino, M.A.; Neves, M.G.; Almeida, A. Protein profiles of Escherichia coli and Staphylococcus warneri are altered by photosensitization with cationic porphyrins. Photochem. Photobiol. Sci. 2015, 14, 1169–1178. [Google Scholar] [CrossRef] [PubMed]
- Mesquita, Q.M.; Cristina, J.D.; Maria, G.P.M.S.N.; Almeida, A.; Faustino, M.A.F. Revisiting Current Photoactive Materials for Antimicrobial Photodynamic Therapy. Molecules 2018, 23, 2424–2471. [Google Scholar] [CrossRef] [PubMed]
- Alvarez, M.G.; Gomez, M.L.; Mora, S.J.; Milanesio, M.E.; Durantini, E.N. Photodynamic inactivation of Candida albicans using bridged polysilsesquioxane films doped with porphyrin. Bioorg. Med. Chem. 2012, 20, 4032–4039. [Google Scholar] [CrossRef]
- Bozja, J.; Sherrill, J.; Michielsen, S.; Stojiljkovic, I. Porphyrin-based, light-activated antimicrobial materials. J. Appl. Polym. Sci. 2003, 41, 2297–2303. [Google Scholar] [CrossRef]
- Krouit, M.; Granet, R.; Krausz, P. Photobactericidal films from porphyrins grafted to alkylated cellulose—Synthesis and bactericidal properties. Eur. Polymer J. 2009, 45, 1250–1259. [Google Scholar] [CrossRef]
- Wilson, M. Light-activated antimicrobial coating for the continuous disinfection of surfaces. Infect. Control. Hosp. Epidemiol. 2003, 24, 782–784. [Google Scholar] [CrossRef]
- Perni, S.; Piccirillo, C.; Pratten, J.; Prokopovich, P.; Chrzanowski, W.; Parkin, I.P.; Wilson, M. The antimicrobial properties of light-activated polymers containing methylene blue and gold nanoparticles. Biomaterials 2009, 30, 89–93. [Google Scholar] [CrossRef]
- Perni, S.; Prokopovich, P.; Parkin, I.P.; Wilson, M.; Pratten, J. Prevention of biofilm accumulation on a light-activated antimicrobial catheter material. J. Mater. Chem. 2010, 20, 8668–8673. [Google Scholar] [CrossRef]
- Bovis, M.J.; Noimark, S.; Woodhams, J.H.; Kay, C.W.M.; Weiner, J.; Peveler, W.J.; Correia, A.; Wilson, M.; Allan, E.; Parkin, I.P.; et al. Photosensitisation studies of silicone polymer doped with methylene blue and nanogold for antimicrobial applications. RSC Adv. 2015, 5, 54830–54842. [Google Scholar] [CrossRef]
- Macdonald, T.J.; Wu, K.; Sehmi, S.K.; Noimark, S.; Peveler, W.J.; du Toit, H.; Voelcker, N.H.; Allan, E.; MacRobert, A.J.; Gavriilidis, A.; et al. Thiol-Capped Gold Nanoparticles Swell-Encapsulated into Polyurethane as Powerful Antibacterial Surfaces Under Dark and Light Conditions. Sci. Rep. 2016, 6, 39272–39283. [Google Scholar] [CrossRef]
- Noimark, S.; Allan, E.; Parkin, I.P. Light-activated antimicrobial surfaces with enhanced efficacy induced by a dark-activated mechanism. Chem. Sci. 2014, 5, 2216–2223. [Google Scholar] [CrossRef]
- Noimark, S.; Bovis, M.; MacRobert, A.J.; Correia, A.; Allan, E.; Wilson, M.; Parkin, I.P. Photobactericidal polymers; the incorporation of crystal violet and nanogold into medical grade silicone. RSC Adv. 2013, 3, 18383–18394. [Google Scholar] [CrossRef]
- Noimark, S.; Dunnill, C.W.; Kay, C.W.M.; Perni, S.; Prokopovich, P.; Ismail, S.; Wilson, M.; Parkin, I.P. Incorporation of methylene blue and nanogold into polyvinyl chloride catheters; a new approach for light-activated disinfection of surfaces. J. Mater. Chem. 2012, 22, 15388–15396. [Google Scholar] [CrossRef]
- Walker, T.; Canales, M.; Noimark, S.; Page, K.; Parkin, I.; Faull, J.; Bhatti, M.; Ciric, L. A Light-Activated Antimicrobial Surface Is Active Against Bacterial, Viral and Fungal Organisms. Sci. Rep. 2017, 7, 15298–15308. [Google Scholar] [CrossRef]
- Page, K.; Correia, A.; Wilson, M.; Allan, E.; Parkin, I.P. Light-activated antibacterial screen protectors for mobile telephones and tablet computers. J. Photochem. Photobiol. 2015, 296, 19–24. [Google Scholar] [CrossRef]
- Chen, J.; Wang, W.; Hu, P.; Wang, D.; Lin, F.; Xue, J.; Chen, Z.; Iqbal, Z.; Huang, M. Dual antimicrobial actions on modified fabric leads to inactivation of drug-resistant bacteria. Dyes Pigment. 2017, 140, 236–243. [Google Scholar] [CrossRef]
- Almeida, A.; Cunha, A.; Gomes, N.C.; Alves, E.; Costa, L.; Faustino, M.A. Phage therapy and photodynamic therapy: Low environmental impact approaches to inactivate microorganisms in fish farming plants. Mar. Drugs 2009, 7, 268–313. [Google Scholar] [CrossRef]
- Tavares, A.; Carvalho, C.M.; Faustino, M.A.; Neves, M.G.; Tome, J.P.; Tome, A.C.; Cavaleiro, J.A.; Cunha, A.; Gomes, N.C.; Alves, E.; et al. Antimicrobial photodynamic therapy: Study of bacterial recovery viability and potential development of resistance after treatment. Mar. Drugs 2010, 8, 91–105. [Google Scholar] [CrossRef]
- Costa, L.; Tome, J.P.; Neves, M.G.; Tome, A.C.; Cavaleiro, J.A.; Faustino, M.A.; Cunha, A.; Gomes, N.C.; Almeida, A. Evaluation of resistance development and viability recovery by a non-enveloped virus after repeated cycles of aPDT. Antivir. Res. 2011, 91, 278–282. [Google Scholar] [CrossRef]
- Bartolomeu, M.; Rocha, S.; Cunha, A.; Neves, M.G.; Faustino, M.A.; Almeida, A. Effect of Photodynamic Therapy on the Virulence Factors of Staphylococcus aureus. Front. Microbiol. 2016, 7, 267. [Google Scholar] [CrossRef]
- Bourre, L.; Giuntini, F.; Eggleston, I.M.; Mosse, C.A.; Macrobert, A.J.; Wilson, M. Effective photoinactivation of Gram-positive and Gram-negative bacterial strains using an HIV-1 Tat peptide-porphyrin conjugate. Photochem. Photobiol. Sci. 2010, 9, 1613–1620. [Google Scholar] [CrossRef]
- Yin, R.; Agrawal, T.; Khan, U.; Gupta, G.K.; Rai, V.; Huang, Y.Y.; Hamblin, M.R. Antimicrobial photodynamic inactivation in nanomedicine: Small light strides against bad bugs. Nanomedicine 2015, 10, 2379–2404. [Google Scholar] [CrossRef]
- Alves, E.; Rodrigues, J.M.M.; Faustino, M.A.F.; Neves, M.G.P.M.S.; Cavaleiro, J.A.S.; Lin, Z.; Cunha, Â.; Nadais, M.H.; Tomé, J.P.C.; Almeida, A. A new insight on nanomagnet–porphyrin hybrids for photodynamic inactivation of microorganisms. Dyes Pigment. 2014, 110, 80–88. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, Y.; Wang, Y.; Murray, C.K.; Hamblin, M.R.; Hooper, D.C.; Dai, T. Antimicrobial blue light inactivation of pathogenic microbes: State of the art. Drug Resist. Updat. 2017, 33–35, 1–22. [Google Scholar] [CrossRef]
- Hamblin, M.R.; Viveiros, J.; Yang, C.; Ahmadi, A.; Ganz, R.A.; Tolkoff, M.J. Helicobacter pylori accumulates photoactive porphyrins and is killed by visible light. Antimicrob. Agents Chemother. 2005, 49, 2822–2827. [Google Scholar] [CrossRef]
- Kim, M.J.; Yuk, H.G. Antibacterial Mechanism of 405-Nanometer Light-Emitting Diode against Salmonella at Refrigeration Temperature. Appl. Environ. Microbiol. 2017, 83, e02582-16. [Google Scholar] [CrossRef]
- Maclean, M.; Macgregor, S.J.; Anderson, J.G.; Woolsey, G.A. The role of oxygen in the visible-light inactivation of Staphylococcus aureus. J. Photochem. Photobiol. B 2008, 92, 180–184. [Google Scholar] [CrossRef]
- Wang, Y.; Wu, X.; Chen, J.; Amin, R.; Lu, M.; Bhayana, B.; Zhao, J.; Murray, C.K.; Hamblin, M.R.; Hooper, D.C.; et al. Antimicrobial Blue Light Inactivation of Gram-Negative Pathogens in Biofilms: In Vitro and In Vivo Studies. J. Infect. Dis. 2016, 213, 1380–1387. [Google Scholar] [CrossRef]
- Tielker, D.; Eichhof, I.; Jaeger, K.E.; Ernst, J.F. Flavin mononucleotide-based fluorescent protein as an oxygen-independent reporter in Candida albicans and Saccharomyces cerevisiae. Eukaryot. Cell 2009, 8, 913–915. [Google Scholar] [CrossRef]
- Kwon, S.J.; de Boer, A.L.; Petri, R.; Schmidt-Dannert, C. High-level production of porphyrins in metabolically engineered Escherichia coli: Systematic extension of a pathway assembled from overexpressed genes involved in heme biosynthesis. Appl. Environ. Microbiol. 2003, 69, 4875–4883. [Google Scholar] [CrossRef]
- Hobbs, C.; Reid, J.D.; Shepherd, M. The coproporphyrin ferrochelatase of Staphylococcus aureus: Mechanistic insights into a regulatory iron-binding site. Biochem. J. 2017, 474, 3513–3522. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhu, Y.; Chen, J.; Wang, Y.; Sherwood, M.E.; Murray, C.K.; Vrahas, M.S.; Hooper, D.C.; Hamblin, M.R.; Dai, T. Antimicrobial blue light inactivation of Candida albicans: In vitro and in vivo studies. Virulence 2016, 7, 536–545. [Google Scholar] [CrossRef]
- Amin, R.M.; Bhayana, B.; Hamblin, M.R.; Dai, T. Antimicrobial blue light inactivation of Pseudomonas aeruginosa by photo-excitation of endogenous porphyrins: In vitro and in vivo studies. Lasers Surg. Med. 2016, 48, 562–568. [Google Scholar] [CrossRef]
- Fotinos, N.; Convert, M.; Piffaretti, J.-C.; Gurny, R.; Lange, N. Effects on Gram-Negative and Gram-Positive Bacteria Mediated by 5-Aminolevulinic Acid and 5-Aminolevulinic Acid Derivatives. Antimicrob. Agents Chemother. 2008, 52, 1366–1373. [Google Scholar] [CrossRef] [PubMed]
- Ashkenazi, H.; Malik, Z.; Harth, Y.; Nitzan, Y. Eradication of Propionibacterium acnes by its endogenic porphyrins after illumination with high intensity blue light. FEMS Immunol. Med. Microbiol. 2003, 35, 17–24. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhu, Y.; Gupta, A.; Huang, Y.; Murray, C.K.; Vrahas, M.S.; Sherwood, M.E.; Baer, D.G.; Hamblin, M.R.; Dai, T. Antimicrobial blue light therapy for multidrug-resistant Acinetobacter baumannii infection in a mouse burn model: Implications for prophylaxis and treatment of combat-related wound infections. J. Infect. Dis. 2014, 209, 1963–1971. [Google Scholar] [CrossRef]
- Dai, T.; Gupta, A.; Huang, Y.Y.; Sherwood, M.E.; Murray, C.K.; Vrahas, M.S.; Kielian, T.; Hamblin, M.R. Blue light eliminates community-acquired methicillin-resistant Staphylococcus aureus in infected mouse skin abrasions. Photomed. Laser Surg. 2013, 31, 531–538. [Google Scholar] [CrossRef]
- Davies, A.; Pottage, T.; Bennett, A.; Walker, J. Gaseous and air decontamination technologies for Clostridium difficile in the healthcare environment. J. Hosp. Infect. 2011, 77, 199–203. [Google Scholar] [CrossRef]
- Murdoch, L.E.; Maclean, M.; Endarko, E.; MacGregor, S.J.; Anderson, J.G. Bactericidal effects of 405 nm light exposure demonstrated by inactivation of Escherichia, Salmonella, Shigella, Listeria, and Mycobacterium species in liquid suspensions and on exposed surfaces. Sci. World J. 2012, 2012, 137805–137812. [Google Scholar] [CrossRef]
- Maclean, M.; MacGregor, S.J.; Anderson, J.G.; Woolsey, G. Inactivation of bacterial pathogens following exposure to light from a 405-nanometer light-emitting diode array. Appl. Environ. Microbiol. 2009, 75, 1932–1937. [Google Scholar] [CrossRef]
- Enwemeka, C.S.; Williams, D.; Enwemeka, S.K.; Hollosi, S.; Yens, D. Blue 470-nm light kills methicillin-resistant Staphylococcus aureus (MRSA) in vitro. Photomed. Laser Surg. 2009, 27, 221–226. [Google Scholar] [CrossRef]
- Enwemeka, C.S.; Williams, D.; Hollosi, S.; Yens, D.; Enwemeka, S.K. Visible 405 nm SLD light photo-destroys methicillin-resistant Staphylococcus aureus (MRSA) in vitro. Lasers Surg. Med. 2008, 40, 734–737. [Google Scholar] [CrossRef]
- Dai, T. The antimicrobial effect of blue light: What are behind? Virulence 2017, 8, 649–652. [Google Scholar] [CrossRef]
- Guffey, J.S.; Payne, W.; Martin, K.; Dodson, C. Delaying the onset of resistance formation: Effect of manipulating dose, wavelength, and rate of energy delivery of 405-, 464-, and 850-nanometer light for Staphylococcus aureus. Wounds 2014, 26, 95–100. [Google Scholar]
- Tomb, R.M.; Maclean, M.; Coia, J.E.; MacGregor, S.J.; Anderson, J.G. Assessment of the potential for resistance to antimicrobial violet-blue light in Staphylococcus aureus. Antimicrob. Resist. Infect. Control 2017, 6, 100–112. [Google Scholar] [CrossRef]
- Yin, R.; Dai, T.; Avci, P.; Jorge, A.E.; de Melo, W.C.; Vecchio, D.; Huang, Y.Y.; Gupta, A.; Hamblin, M.R. Light based anti-infectives: Ultraviolet C irradiation, photodynamic therapy, blue light, and beyond. Curr. Opin. Pharmacol. 2013, 13, 731–762. [Google Scholar] [CrossRef]
- Huang, S.T.; Wu, C.Y.; Lee, N.Y.; Cheng, C.W.; Yang, M.J.; Hung, Y.A.; Wong, T.W.; Liang, J.Y. Effects of 462 nm Light-Emitting Diode on the Inactivation of Escherichia coli and a Multidrug-Resistant by Tetracycline Photoreaction. J. Clin. Med. 2018, 7, 1–15. [Google Scholar] [CrossRef]
- Fila, G.; Kawiak, A.; Grinholc, M.S. Blue light treatment of Pseudomonas aeruginosa: Strong bactericidal activity, synergism with antibiotics and inactivation of virulence factors. Virulence 2017, 8, 938–958. [Google Scholar] [CrossRef]
- Halstead, F.D.; Thwaite, J.E.; Burt, R.; Laws, T.R.; Raguse, M.; Moeller, R.; Webber, M.A.; Oppenheim, B.A. Antibacterial Activity of Blue Light against Nosocomial Wound Pathogens Growing Planktonically and as Mature Biofilms. Appl. Environ. Microbiol. 2016, 82, 4006–4016. [Google Scholar] [CrossRef]
- Moorhead, S.; MacLean, M.; Coia, J.; MacGregor, S. Inactivation of C. difficile by 405 nm HINS-light. In Proceedings of the 8th Annual Scottish Environmental and Clean Technology Conference, Glasgow, Scotland, 26 June 2014. [Google Scholar]
- Kuse, Y.; Ogawa, K.; Tsuruma, K.; Shimazawa, M.; Hara, H. Damage of photoreceptor-derived cells in culture induced by light emitting diode-derived blue light. Sci. Rep. 2014, 4, 5223–5235. [Google Scholar] [CrossRef] [PubMed]
- Almeida, J.; Tome, J.P.; Neves, M.G.; Tome, A.C.; Cavaleiro, J.A.; Cunha, A.; Costa, L.; Faustino, M.A.; Almeida, A. Photodynamic inactivation of multidrug-resistant bacteria in hospital wastewaters: Influence of residual antibiotics. Photochem. Photobiol. Sci. 2014, 13, 626–633. [Google Scholar] [CrossRef]
- Costa, D.C.; Gomes, M.C.; Faustino, M.A.; Neves, M.G.; Cunha, A.; Cavaleiro, J.A.; Almeida, A.; Tome, J.P. Comparative photodynamic inactivation of antibiotic resistant bacteria by first and second generation cationic photosensitizers. Photochem. Photobiol. Sci. 2012, 11, 1905–1913. [Google Scholar] [CrossRef] [PubMed]
- Maclean, M.; Macgregor, S.J.; Anderson, J.G.; Woolsey, G.A.; Coia, J.E.; Hamilton, K.; Taggart, I.; Watson, S.B.; Thakker, B.; Gettinby, G. Environmental decontamination of a hospital isolation room using high-intensity narrow-spectrum light. J. Hosp. Infect. 2010, 76, 247–251. [Google Scholar] [CrossRef]
- Bache, S.E.; Maclean, M.; MacGregor, S.J.; Anderson, J.G.; Gettinby, G.; Coia, J.E.; Taggart, I. Clinical studies of the High-Intensity Narrow-Spectrum light Environmental Decontamination System (HINS-light EDS), for continuous disinfection in the burn unit inpatient and outpatient settings. Burns 2012, 38, 69–76. [Google Scholar] [CrossRef]
- Maclean, M.; Booth, M.; Anderson, J.; MacGregor, S.; Woolsey, G.; Coia, J.; Hamilton, K.; Gettinby, G. Continuous decontamination of an intensive care isolation room during patient occupancy using 405 nm light technology. J. Infect. Prev. 2013, 14, 176–181. [Google Scholar] [CrossRef]
- Endarko, E.; Maclean, M.; Timoshkin, I.V.; MacGregor, S.J.; Anderson, J.G. High-intensity 405 nm light inactivation of Listeria monocytogenes. Photochem. Photobiol. 2012, 88, 1280–1286. [Google Scholar] [CrossRef]
- Moorhead, S.; Maclean, M.; Coia, J.E.; MacGregor, S.J.; Anderson, J.G. Synergistic efficacy of 405 nm light and chlorinated disinfectants for the enhanced decontamination of Clostridium difficile spores. Anaerobe 2016, 37, 72–77. [Google Scholar] [CrossRef]
- Maclean, M.; MacGregor, S.J.; Anderson, J.; Woolsey, G. HINS-light Environmental Decontamination System: A new method for pathogen control in the clinical envionment. In Proceedings of the Infection Prevention Society, Glasgow, UK, 27 October 2010. [Google Scholar]
- Ramakrishnan, P.; Maclean, M.; MacGregor, S.J.; Anderson, J.G.; Grant, M.H. Differential sensitivity of osteoblasts and bacterial pathogens to 405-nm light highlighting potential for decontamination applications in orthopedic surgery. J. Biomed. Opt. 2014, 19, 105001–105009. [Google Scholar] [CrossRef]
| Equation | No. |
|---|---|
| SubstractH2 + PS → PSH• + SubstractH• | (1) |
| PS* + Substract → PS•− + Substract•+ ou PS* + Substract → PS•+ + Substract•− | (2) |
| PS•− + 3O2 → PS + O2•− | (3) |
| O2•− + H+ ⇋ HOO• | (4) |
| 2 HOO• → H2O2 + O2 | (5) |
| H2O2 + Fe2+ → HO• + OH− + Fe3+ | (6) |
| BiomoleculeH + HO• → Biomolecule• + H2O | (7) |
| Biomolecule• + 3O2 → Biomolecule-OO• → products | (8) |
| PS* + 3O2 → PS + 1O2 | (9) |
| Biomolecules + 1O2 → oxidative products | (10) |
| aBLT | aPDT | |
|---|---|---|
| Requirement of exogenous photosensitizers | Not required [7] | Required [7] |
| Damage of self-cells | Reduced, risk of eye damage [137] | Negligible [53] |
| Resistance development | Improvable [107,108,109] | Improvable [107,108,109] |
| Effectiveness | High, even against MDRO [138] | High, even against MDRO [138,139] |
| Multitarget capacity | Yes, lipids, proteins, and nucleic acids [104,105,106] | Yes, lipids, proteins, and nucleic acids [104,105,106] |
| Response time | Quick lethal effects [104] | Quick lethal effects [104] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cabral, J.; AG, R. Blue Light Disinfection in Hospital Infection Control: Advantages, Drawbacks, and Pitfalls. Antibiotics 2019, 8, 58. https://doi.org/10.3390/antibiotics8020058
Cabral J, AG R. Blue Light Disinfection in Hospital Infection Control: Advantages, Drawbacks, and Pitfalls. Antibiotics. 2019; 8(2):58. https://doi.org/10.3390/antibiotics8020058
Chicago/Turabian StyleCabral, João, and Rodrigues AG. 2019. "Blue Light Disinfection in Hospital Infection Control: Advantages, Drawbacks, and Pitfalls" Antibiotics 8, no. 2: 58. https://doi.org/10.3390/antibiotics8020058
APA StyleCabral, J., & AG, R. (2019). Blue Light Disinfection in Hospital Infection Control: Advantages, Drawbacks, and Pitfalls. Antibiotics, 8(2), 58. https://doi.org/10.3390/antibiotics8020058




