Antibiotic Resistance in Pacific Island Countries and Territories: A Systematic Scoping Review
Abstract
1. Introduction
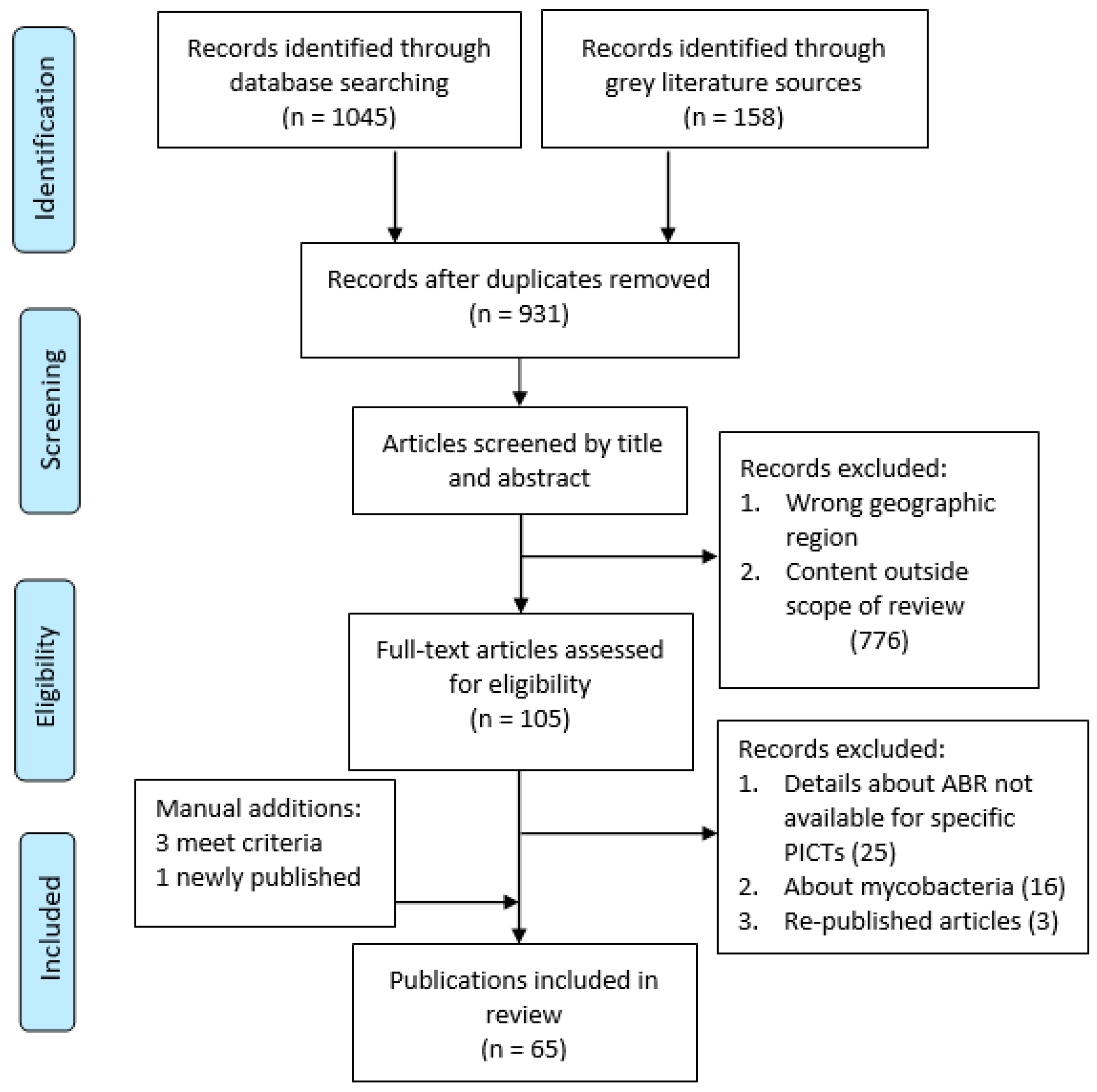
2. Methods
2.1. Geographic Setting
2.2. Search Strategy
- Reports on ABR in humans in PICTs;
- Published in English or French between 1950 and 2018;
- Available in full text.
- Reports about tuberculosis; (in view of solid literature base already known about drug resistant tuberculosis, and globally funded TB program);
- Literature which did not provide details about antibiotic susceptibility in PICTs;
- Conference abstracts and posters; and newspaper articles.
2.3. Selection and Screening
2.4. Data Extraction
2.5. Synthesis of Results
3. Results
3.1. Study Characteristics
3.2. Antibiotic Susceptibility Test Methods
3.3. ABR Reported in PICTs in This Scoping Study
3.3.1. ABR in Gram-Negative Bacteria: Healthcare-Associated Infections (HCAIs)
Klebsiella spp.
Acinetobacter Baumannii
Pseudomonas aeruginosa
Enterobacter spp.
3.3.2. ABR in Gram-Negative Bacteria: Community-Acquired Infections
Neisseria gonorrhoeae
Escherichia coli
Salmonella spp.
Shigella spp.
Vibrio cholerae
Campylobacter spp.
3.4. ABR in Gram-Positive Bacteria
3.4.1. Staphylococcus aureus
3.4.2. Streptococcus pneumoniae and Haemophilus influenzae (Respiratory Tract Infections)
3.4.3. Streptococcus pyogenes (Group A Streptococcus)
Enterococcus spp.
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Holmes, A.H.; Moore, L.S.; Sundsfjord, A.; Steinbakk, M.; Regmi, S.; Karkey, A.; Guerin, P.J.; Piddock, L.J.V. Understanding the mechanisms and drivers of antimicrobial resistance. Lancet 2016, 387, 176–187. [Google Scholar] [CrossRef]
- Cecchini, M.; Langer, J. Antimicrobial Resistance in G7 Countries and beyond: Economic Issues, Policies and Options for Action; OECD: Paris, France, 2015; Available online: https://www.oecd.org/els/health-systems/Antimicrobial-Resistance-in-G7-Countries-and-Beyond.pdf (accessed on 1 July 2018).
- The Review on Antimicrobial Resistance. Available online: https://amr-review.org/sites/default/files/160525_Final%20paper_with%20cover.pdf (accessed on 7 July 2018).
- A Primer for Media: Antimicrobial Resistance in the Western Pacific. Available online: http://iris.wpro.who.int/handle/10665.1/13087 (accessed on 8 July 2018).
- National Action Plan for Global Change on Antimicrobial Resistance. Available online: http://iris.wpro.who.int/handle/10665.1/13066 (accessed on 1 May 2018).
- Action Agenda for Antimicrobial Resistance in the Western Pacific Region. Available online: http://www.wpro.who.int/entity/drug_resistance/documents/action_agenda.pdf (accessed on 15 may 2018).
- Antimicrobial Resistance in the Asia Pacific Region: A Development Agenda. Available online: http://iris.wpro.who.int/handle/10665.1/13570 (accessed on 15 May 2018).
- World Bank Country and Lending Groups. Available online: https://datahelpdesk.worldbank.org/knowledgebase/articles/906519 (accessed on 4 July 2018).
- Okeke, I.N.; Laxminarayan, R.; Bhutta, Z.A.; Duse, A.G.; Jenkins, P.; O’Brien, T.F.; Pablo-Mendez, A.; Klugman, K.P. Antimicrobial resistance in developing countries. Part I: Recent trends and current status. Lancet Infect Dis. 2005, 5, 481–493. [Google Scholar] [CrossRef]
- Ekeroma, A.J.; Sharon, B.; Herman, J.; Andrew, H.; Tim, K. Health research systems in six Pacific Island countries and Territories. J. Res. Dev. 2016, 4, 1–9. [Google Scholar]
- Tricco, A.C.; Lillie, E.; Zarin, W.; O’Brien, K.K.; Colquhoun, H.; Levac, D. PRISMA Extension for scoping reviews (PRISMA-ScR): Checklist and explanation. Ann Intern Med. 2018, 169, 467–473. [Google Scholar] [CrossRef]
- As, H.; Laman, M.; Greenhill, A.; Siba, P.; Davis, T.; Laurens, J. Bloodstream infections caused by resistant bacteria in surgical patients admitted to Modilon Hospital, Madang. P. N. G. Med. J. 2012, 55, 5–11. [Google Scholar]
- Duke, T. Antibiotic-resistant bacterial sepsis in Papua New Guinea. P. N. G. Med. J. 2000, 82–90. [Google Scholar]
- Duke, T.; Michael, A. Increase in sepsis due to multi-resistant enteric gram-negative bacilli in Papua New Guinea. Lancet 1999, 353, 2210–2211. [Google Scholar] [CrossRef]
- Kumar, S.; Graham, S.M.; Varman, S.; Kado, J.; Viney, K. Resistance of bacterial Isolates from neonates with suspected Sepsis to recommended first-line antibiotics in Fiji. Pediatr. Infect. Dis. J. 2015, 34, 915–916. [Google Scholar] [CrossRef] [PubMed]
- Lithgow, A.; Kilalang, C. Outbreak of nosocomial sepsis in the Special Care Nursery at Port Moresby General Hospital due to multiresistant Klebsiella pneumoniae: High impact on mortality. P. N. G. Med. J. 2009, 52, 28–34. [Google Scholar] [PubMed]
- Melot, B.; Colot, J.; Guerrier, G. Bacteremic community-acquired infections due to Klebsiella pneumoniae: Clinical and microbiological presentation in New Caledonia, 2008–2013. Int. J. Infect. Dis. 2015, 41, 29–31. [Google Scholar] [CrossRef]
- Montgomery, J.; West, B.; Michael, A.; Kadivaion, B. Bacterial resistance in the Eastern Highlands. P. N. G. Med. J. 1987, 30, 11–19. [Google Scholar] [PubMed]
- Naidu, K.; Nabose, I.; Ram, S.; Viney, K.; Graham, S.M.; Bissell, K. A descriptive study of nosocomial infections in an adult intensive care unit in Fiji: 2011–12. J. Trop. Med. 2014. [Google Scholar] [CrossRef] [PubMed]
- Trevett, A.J.; SenGupta, S.K. Gentamicin resistance in fatal Klebsiella septicaemia. P. N. G. Med. J. 1992, 35, 202–204. [Google Scholar] [PubMed]
- Antimicrobial Resistance: Global Report on Surveillance. Available online: https://www.who.int/drugresistance/documents/surveillancereport/en/ (accessed on 17 May 2018).
- Le Hello, S.; Falcot, V.; Lacassin, F.; Baumann, F.; Nordmann, P.; Naas, T. Molecular epidemiology of carbapenem-resistant Acinetobacter baumannii in New Caledonia. Clin. Microbiol. Infect. 2008, 14, 977–981. [Google Scholar] [CrossRef] [PubMed]
- Le Hello, S.; Falcot, V.; Lacassin, F.; Mikulski, M.; Baumann, F. Risk factors for carbapenem-resistant acinetobacter baumannii infections at a tertiary care hospital in New Caledonia, South Pacific. Scand. J. Infect. Dis. 2010, 42, 821–826. [Google Scholar] [CrossRef] [PubMed]
- Naas, T.; Levy, M.; Hirschauer, C.; Marchandin, H.; Nordmann, P. Outbreak of carbapenem-resistant Acinetobacter baumannii producing the carbapenemase OXA-23 in a tertiary care hospital of Papeete, French Polynesia. J. Clin. Microbiol. 2005, 43, 4826–4829. [Google Scholar] [CrossRef]
- Cook Islands Ministry of Health; Everts, R. Antibiotic Guidelines Cook Islands 2018: Guidelines for Empiric and Targeted Antibiotic Treatment, Prophylaxis, Dosing and Allergies; Ministry of Health: Rarotonga, Cook Islands, 2018.
- Samoa Ministry of Health; Everts, R. Antibiotic Guidelines Samoa 2016: Guidelines for Empiric and Targeted Antibiotic Treatment, Prophylaxis, Dosing and Allergies; Ministry of Health: Apia, Samoa, 2016.
- Cuzon, G.; Levy, M.; Jacob, E.; Dortet, L.; Naas, T. IMI-1-producing Enterobacter cloacae clinical isolate from Tahiti, French Polynesia. J. Glob. Antimicrob. Resist. 2016, 5, 1–2. [Google Scholar] [CrossRef] [PubMed]
- Narayan, S.A.; Kool, J.L.; Vakololoma, M.; Steer, A.C.; Mejia, A.; Drake, A.; Jenney, A.; Turton, J.F.; Kado, J.; Tikoduadua, L. Investigation and control of an outbreak of enterobacter aerogenes bloodstream infection in a neonatal intensive care unit in Fiji. Infect. Control Hosp. Epidemiol. 2009, 30, 797–800. [Google Scholar] [CrossRef]
- Lahra, M.; Lo, Y.; Whiley, D. Gonococcal antimicrobial resistance in the Western Pacific Region. Sex Transm. Infect. 2013, 89 (Suppl. 4), iv19–iv23. [Google Scholar] [CrossRef]
- WHO Western Pacific and South East Asian Gonococcal Antimicrobial Surveillance Programmes. Surveillance of Antibiotic Resistance in Neisseria Gonorrhoeae in the WHO Western Pacific and South East Asian Regions, 2009. Commun. Dis. Intell. Q. Rep. 2011, 35, 2–7. [Google Scholar]
- Lahra, M.; WHO Western Pacific and South East Asian Gonococcal Antimicrobial Surveillance Programmes. Surveillance of Antibiotic Resistance in Neisseria Gonorrhoeae in the WHO Western Pacific and South East Asian Regions, 2010. Commun. Dis. Intell. Q. Rep. 2012, 36, 95–100. [Google Scholar] [PubMed]
- WHO Western Pacific Programme; South East Asian Gonococcal Antimicrobial Surveillance Programme; Tapsall, J.W.; Limnios, E.A.; Abu Bakar, H.M.; Darussalam, B.; Ping, Y.Y.; Buadromo, E.M.; Kumar, P.; Singh, S. Surveillance of Antibiotic Resistance in Neisseria Gonorrhoeae in the WHO Western Pacific and South East Asian Regions 2007–2008. Commun. Dis. Intell. Q. Rep. 2010, 34, 1–7. [Google Scholar] [PubMed]
- Tapsall, J.W. Implications of current recommendations for third generation cephalosporin use in the WHO Western Pacific Region following the emergence of multiresistant gonococci. Sex Transm. Infect. 2009, 85, 256–258. [Google Scholar] [CrossRef] [PubMed]
- Toliman, P.; Lupiwa, T.; Law, G.; Reeder, J.; Siba, P. Neisseria gonorrhoeae isolates from four centres in Papua New Guinea remain susceptible to amoxycillin-clavulanate therapy. P. N. G. Med. J. 2010, 53, 15–20. [Google Scholar] [PubMed]
- Vernel-Pauillac, F.F.; Hogan, T.R.; Tapsall, J.W.; Goarant, C. Quinolone resistance in Neisseria gonorrhoeae: Rapid genotyping of quinolone resistance-determining regions in gyrA and parC genes by melting curve analysis predicts susceptibility. Antimicrob. Agents Chemother. 2009, 53, 1264–1267. [Google Scholar] [CrossRef] [PubMed]
- Vernel-Pauillac, F.F.; Nandi, S.; Nicholas, R.; Goarant, C. Genotyping as a tool for antibiotic resistance surveillance of Neisseria gonorrhoeae in New Caledonia: Evidence of a novel genotype associated with reduced penicillin susceptibility. Antimicrob. Agents Chemother. 2008, 52, 3293–3300. [Google Scholar] [CrossRef] [PubMed]
- Vernel-Pauillac, F.F.; Whiley, D.; Merien, F. Rapid detection of a chromosomally mediated penicillin resistance-associated ponA mutation in Neisseria gonorrhoeae using a real-time PCR assay. FEMS Microbiol. Lett. 2006, 255, 66–74. [Google Scholar] [CrossRef]
- Vernel-Pauillac, F.F.; Guillard, E.H.; Goursaud, B.; Lethezer, R.; Hem, C.; Merien, F.; Goarant, C. Correlation between antibiotic susceptibilities and genotypes in Neisseria gonorrhoeae from different geographical origins: Determinants monitoring by realtime PCR as a complementary tool for surveillance. Sex Transm. Infect. 2010, 86, 106–111. [Google Scholar] [CrossRef]
- Wandi, F.; Kiagi, G.; Duke, T. Long-term outcome for children with bacterial meningitis in rural Papua New Guinea. J. Trop. Pediatr. 2005, 51, 51–53. [Google Scholar] [CrossRef]
- Surveillance of Antimicrobial Resistance: Western Pacific Region, 10 Years’ Experience and Future Directions. Available online: http://who.int/medicinedocs/documents/s16877e/s16877e.pdf (accessed on 4 June 2018).
- Greenhill, A.; Guwada, C.; Siba, V.; Michael, A.; Yoannes, M.; Wawarie, Y.; Ford, R.; Siba, P.M.; Horwood, P. Antibiotic resistant Shigella is a major cause of diarrhoea in the Highlands of Papua New Guinea. J. Infect. Dev. Ctries 2014, 8, 1391–1397. [Google Scholar] [CrossRef]
- Lane, R.J.; Holland, D.; McBride, S.; Perera, S.; Zeng, I.; Wilson, M.; Read, K.; Jelleyman, T.; Ingram, R. Enteric fever in the Pacific: A regional retrospective study from Auckland, New Zealand. Intern. Med. J. 2015, 45, 148–155. [Google Scholar] [CrossRef] [PubMed]
- Le Hello, S.; Maillard, F.; Mallet, H.P.; Daudens, E.; Levy, M.; Roy, V.; Branaa, P.; Bertrand, S.; Fabre, L.; Weill, F.X. Salmonella enterica serotype enteritidis in French Polynesia, South Pacific, 2008–2013. Emerg. Infect. Dis. 2015, 21, 1045–1048. [Google Scholar] [CrossRef] [PubMed]
- Thompson, C.; Kama, M.; Acharya, S.; Bera, U.; Clemens, J.; Crump, J.; Dawainavesi, A.; Dougan, G.; Edmunds, W.J.; Fox, K.; et al. Typhoid fever in Fiji: A reversible plague? Trop. Med. Int. Health. 2014, 19, 1284–1292. [Google Scholar] [CrossRef] [PubMed]
- Poka, H.; Duke, T. Clinical management of diarrhoea in children. P. N. G. Med. J. 2013, 56, 156–161. [Google Scholar] [PubMed]
- Rosewell, A.; Ropa, B.; Posanai, E.; Dutta, S.R.; Mola, G.; Zwi, A.; MacIntyre, C. Shigella spp. antimicrobial drug resistance, Papua New Guinea, 2000–2009. Emerg. Infect. Dis. 2010, 16, 1797–1799. [Google Scholar] [CrossRef] [PubMed]
- Watson, M. Death from multi resistant shigelloses: A case study from Fiji. Pac. Health Dialog 2006, 13, 111–114. [Google Scholar]
- Murhekar, M.; Dutta, S.; Ropa, B.; Dagina, R.; Posanai, E.; Rosewell, A. Vibrio cholerae antimicrobial drug resistance, Papua New Guinea, 2009–2011. West. Pac. Surveill. Response J. 2013, 4, 60–62. [Google Scholar] [CrossRef] [PubMed]
- Aglua, I.; Jaworski, J.; Drekore, J.; Urakoko, B.; Poka, H.; Michael, A. Methicillin-Resistant Staphylococcus Aureus in Melanesian Children with Haematogenous Osteomyelitis from the Central Highlands of Papua New Guinea. Int. J. Pediatr. 2018, 6. [Google Scholar] [CrossRef]
- Alesana-Slater, J.; Ritchie, S.; Heffernan, H.; Camp, T.; Richardson, A.; Herbison, P.; Norris, P. Methicillin-resistant staphylococcus aureus: Samoa, 2007–2008. Emerg. Infect. Dis. 2011, 17, 1023–1029. [Google Scholar] [CrossRef]
- Brian, M.J.; Michael, A. Community-acquired infection with methicillin-resistant Staphylococcus aureus in Papua New Guinea. Pediatr. Infect. Dis. J. 1989, 8, 807–808. [Google Scholar] [CrossRef]
- Gosbell, I.B.; Mercer, J.L.; Neville, S.A.; Crone, S.A.; Chant, K.G.; Jalaludin, B.B.; Munro, R. Non-multiresistant and multiresistant methicillin-resistant Staphylococcus aureus in community-acquired infections. Med. J. Aust. 2001, 174, 627–630. [Google Scholar] [PubMed]
- The Clinical and Molecular Epidemiology of Staphylococcus aureus Infections in Fiji. Available online: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3998116/pdf/1471-2334-14-160.pdf (accessed on 11 November 2018).
- Ranjit, J.; Sirus, N.; McDonnell, G. The clinical spectrum of Staphylococcal bacteraemia: A review of 101 Melanesian patients From Papua New Guinea. P. N. G. Med. J. 1990, 33, 229–233. [Google Scholar]
- Unger, H.W.; Aho, C.; Ome-Kaius, M.; Wangnapi, R.A.; Umbers, A.J.; Jack, W.; Lafana, A.; Michael, A.; Hanieh, S.; Siba, P.; et al. Impact of intermittent preventive treatment in pregnancy with azithromycin-containing regimens on maternal nasopharyngeal carriage and antibiotic sensitivity of Streptococcus pneumoniae, Haemophilus influenzae, and Staphylococcus aureus: A cross-sectional survey at delivery. J. Clin. Microbiol. 2015, 53, 1317–1323. [Google Scholar] [PubMed]
- Weber, J.T. Community-associated methicillin-resistant Staphylococcus aureus. Clin. Infect. Dis. 2005, 41 (Suppl. 4), S269–S272. [Google Scholar] [CrossRef] [PubMed]
- Markham, N.; Stenhouse, A. A bacteriological investigation of wound infections in Rarotonga, Cook Islands. Trans. R. Soc. Trop. Med. Hyg. 1959, 53, 404–409. [Google Scholar] [CrossRef]
- Montgomery, J. The aerobic bacteriology of infected skin lesions in children in the Eastern Highlands Province. P. N. G. Med. J. 1985, 28, 93–103. [Google Scholar] [PubMed]
- Miles, F.; Voss, L.; Segedin, E.; Anderson, B.J. Review of Staphylococcus aureus infections requiring admission to a paediatric intensive care unit. Arch. Dis. Child. 2005, 90, 1274–1278. [Google Scholar] [CrossRef]
- Charveriat, M.; Chomarat, M.; Watson, M.; Garin, B. Nasopharyngeal carriage of Streptococcus pneumoniae in healthy children, 2 to 24 months of age, in New-Caledonia. Med. Maladies Infect. 2005, 35, 500–506. [Google Scholar]
- Duke, T.; Michael, A.; Mokela, D.; Wal, T.; Reeder, J. Chloramphenicol or ceftriaxone, or both, as treatment for meningitis in developing countries? Arch. Dis. Child. 2003, 88, 536–539. [Google Scholar] [CrossRef]
- Gratten, M.; Montgomery, J. The bacteriology of acute pneumonia and meningitis in children in Papua New Guinea: Assumptions, facts and technical strategies. P. N. G. Med. J. 1991, 34, 185–198. [Google Scholar]
- Douglas, R.; Sturt, J. Penicillin-resistant pneumococci and pneumonia. Med. J. Aust. 1975, 1, 82. [Google Scholar] [PubMed]
- Gratten, M.; Naraqi, S.; Hansman, D. High prevalence of penicillin-insensitive pneumococci in Port Moresby, Papua New Guinea. Lancet 1980, 2, 192–195. [Google Scholar] [CrossRef]
- Greenhill, A.R.; Phuanukoonnon, S.; Michael, A.; Yoannes, M.; Orami, T.; Smith, H.; Murphy, D.; Blyth, C.; Reeder, J.; Siba, P.; et al. Streptococcus pneumoniae and Haemophilus influenzae in paediatric meningitis patients at Goroka General Hospital, Papua New Guinea: Serotype distribution and antimicrobial susceptibility in the pre-vaccine era. BMC Infect. Dis. 2015, 15, 485. [Google Scholar] [CrossRef] [PubMed]
- Jenkins, C.; Montgomery, J.; Michael, A. Penicillin-resistant streptococcus pneumoniae and other nasal bacteria among children in remote areas of the fringe highlands of Papua New Guinea. P. N. G. Med. J. 1989, 32, 185–188. [Google Scholar]
- Laman, M. Acute bacterial meningitis in Papua New Guinea: New treatment guidelines in response to increasing antibiotic resistance. P. N. G. Med. J. 2011, 54, 1–3. [Google Scholar]
- Le Hello, S.; Watson, M.; Levy, M.; Marcon, S.; Brown, M.; Yvon, J.F.; Missotte, I.; Garin, B. Invasive serotype 1 Streptococcus pneumoniae outbreaks in the South Pacific from 2000 to 2007. J. Clin. Microbiol. 2010, 48, 2968–2971. [Google Scholar] [CrossRef] [PubMed]
- Lehmann, D.; Gratten, M.; Montgomery, J. Susceptibility of pneumococcal carriage isolates to penicillin provides a conservative estimate of susceptibility of invasive pneumococci. Pediatr. Infect. Dis. J. 1997, 16, 297–305. [Google Scholar] [CrossRef]
- Michel, N.; Watson, M.; Baumann, F.; Perolat, P.; Garin, B. Distribution of Streptococcus pneumoniae serotypes responsible for penicillin resistance and the potential role of new conjugate vaccines in New Caledonia. J. Clin. Microbiol. 2005, 43, 6060–6063. [Google Scholar] [CrossRef]
- Russell, F.; Carapetis, J.; Ketaiwai, S.; Kunabuli, V.; Taoi, M.; Biribo, S.; Seduadua, A.; Mulholland, E.K. Pneumococcal nasopharyngeal carriage and patterns of penicillin resistance in young children in Fiji. Ann. Trop. Paediatr. 2006, 26, 187–197. [Google Scholar] [CrossRef]
- Shann, F.; Gratten, M.; Germer, S.; Linnemann, V.; Hazlett, D.; Payne, R. Aetiology of pneumonia in children in Goroka Hospital, Papua New Guinea. Lancet 1984, 2, 537–541. [Google Scholar] [CrossRef]
- Hansman, D.; Devitt, L.; Riley, I. Pneumococci with increased resistance to penicillin. Br. Med. J. (Clin. Res. Ed.) 1973, 405. [Google Scholar] [CrossRef]
- Hansman, D.; Glasgow, H.; Sturt, J.; Devitt, L. Pneumococci insensitive to penicillin. Nature 1971, 230, 407–408. [Google Scholar] [CrossRef] [PubMed]
- Daimen, M. Acute bacterial meningitis in adult patients at the Port Moresby General Hospital 1998–2008. In Public Private Partnership in Health Care: 45th Annual Medical Symposium, 30 August–4 September 2009; Medical Society Papua New Guinea: Port Moresby, Papua New Guinea, 2009. [Google Scholar]
- Manning, L.; Laman, M.; Greenhill, A.; Michael, A.; Siba, P.; Mueller, I.; Davis, T. Increasing Chloramphenicol Resistance in Streptococcus pneumoniae Isolates from Papua New Guinean Children with Acute Bacterial Meningitis. Antimicrob. Agents Chemother. 2011, 55, 4454–4456. [Google Scholar] [CrossRef] [PubMed]
- Tikhomirova, A.; Kidd, S. Haemophilus influenzae and Streptococcus pneumoniae: Living together in a biofilm. Pathog. Dis. 2013, 62, 114–126. [Google Scholar] [CrossRef] [PubMed]
- Le Hello, S.; Doloy, A.; Baumann, F.; Roques, N.; Coudene, P.; Bouchon, B.; Lacassin, F.; Bouvert, A. Clinical and microbial characteristics of invasive streptococcus pyogenes disease in New Caledonia, a region in Oceania with a high incidence of acute rheumatic fever. J. Clin. Microbiol. 2010, 48, 256–530. [Google Scholar] [CrossRef]
- Grayson, M.L.; Eliopoulos, G.M.; Wennersten, C.B.; Ruoff, K.L.; De Girolami, P.C.; Ferraro, M.J.; Mollering, R.C. Increasing resistance to beta-lactam antibiotics among clinical isolates of Enterococcus faecium: A 22-year review at one institution. Antimicrob. Agents Chemother. 1991, 35, 2180–2184. [Google Scholar] [CrossRef]
- Garin, B. First case of infection due to vancomycin resistant Enterococcus faecalis in New Caledonia. Med. Maladies Infect. 2006, 36, 297–298. [Google Scholar] [CrossRef]
- Lee, Y.; Wakabayashi, M. Key informant interview on antimicrobial resistance (AMR) in some countries in the Western Pacific region. Glob. Health 2013, 9. [Google Scholar] [CrossRef]
- Steer, A.C.; Jenny, A.; Kado, J.; Good, M.; Batzloff, M.; Waqatakirewa, L.; Mullholland, E.K.; Carapetis, J.R. Prospective surveillance of group A. streptococcal disease, Fiji 2005–2007. Emerg. Infect. Dis. 2009, 15, 216–222. [Google Scholar] [CrossRef]
| K. pneumoniae | ||||
| Antibiotic Class | Antibiotics | PICTs | 1980–2000 % or % Range (No. Isolates Tested per Study) | 2011–2017 % or % Range [No. Isolates Tested per Study] |
| Cephalosporin | Ceftriaxone Cefotaxime Ceftazidime | Fiji Micronesia PNG | 25 (2900) 71 (87) 63.5 (252) | |
| Ceftriaxone | Samoa | 7.7–19.8 (116, 119) | ||
| Carbapenem | Imipenem Meropenem | Fiji | 0.7 (2175) | |
| Aminoglycoside | Gentamicin | PNG | 100–78.4 (14, 41) | |
| Tetracycline | Tetracycline | PNG | 36 (11) | |
| Chloramphenicol | Chloramphenicol | PNG | 40–100 (14, 22) | |
| Nalidixic acid | New Caledonia | 54 (119) | ||
| Fluoroquinolone | Ciprofloxacin | New Caledonia | 54 (119) | |
| Diaminopyrimidine | Co-trimoxazole | New Caledonia | 54 (119) | |
| PNG | 32 (22) | |||
| E. coli | ||||
| Antibiotic Class | Antibiotics | PICTS | 1980–2000 % or % Range (No. Isolates Tested per Study) | 2011–2017 % or % Range (No. Isolates Tested per Study) |
| Ampicillin | PNG Fiji | 46 (37) | 87 (25) | |
| Cephalosporin | Ceftriaxone Ceftazidime Cefotaxime | Fiji Micronesia PNG Samoa | 12.2 (2895) 77 (158) 24.1 (174) 45 (25) | |
| Aminoglycoside | Gentamicin | PNG | 3–37 (37, 61) | 45 (25) |
| Chloramphenicol | Chloramphenicol | PNG | 32–96 (37, 61) | |
| Fluoroquinolone | Ciprofloxacin | Fiji Kiribati Marshall Islands Micronesia PNG Samoa | 11.9(2566) 3 (72) 13 (202) 16 (158) 13.3 (526) 13.9 (43) | |
| N. gonorrhoeae | ||||
|---|---|---|---|---|
| Antibiotic Class | Antibiotics | PICTs | 2000–2010 % or % Range (No. Isolates Tested per Study) | 2011–2017 % or % Range (No. Isolates Tested per Study) |
| Penicillin | Penicillin | Fiji | 8.3 (336, 541) | 6.7 (252) |
| New Caledonia | 0.5–47 (197, 208 *, 110) | 9 (166) | ||
| PNG | 3.6–63 (52, 82, 54) | |||
| Cephalosporin | Ceftriaxone | Fiji | 0.4 (541 *) | |
| Solomon Islands | 10 (10) | |||
| Fluoroquinolone | Ciprofloxacin | Fiji | 0.2–0.6 (541, 336) | |
| New Caledonia | 1.3–0.5 (79, 197) | 6 (166) | ||
| PNG | 2 (52) | |||
| Tetracycline | Tetracycline | PNG | 19 (52) | |
| Shigella spp. | ||||
| Antibiotics | PICTs | 1980–2000 % (No. Isolates Tested by Study) | 2001–2010 % (No. Isolates Tested by Study) | 2011–2017 % (No. Isolates Tested by Study) |
| Ampicillin | Fiji | 79 (205} | ||
| PNG | 86 (94) | 91.5 (47) | ||
| Amoxicillin | PNG | 96 (98) | ||
| Tetracycline | PNG | 91(54) | 76.6 (47) | |
| Doxycycline | Fiji | 63.3 (205) | 63.3 (205) | |
| Tigecycline | PNG | 76.6 (47) | ||
| Trimethoprim | FIJI | 29 (160) | ||
| Co-trimoxazole | PNG | 86 (76) | 70.2 (47) | |
| Chloramphenicol | Fiji | 69.7 (205) | ||
| Fiji | 60 (114) | |||
| PNG | 83 (94) | 55.3 (47) | ||
| Nalidixic acid | Fiji | 3 (37) | ||
| PNG | 15 (13) | |||
| Salmonella spp. | ||||
| Antibiotics | PICTs | 1980–2000 % (No. Isolates Tested by Study) | 2001–2010 % (No. Isolates Tested by Study) | 2011–2017 % or % Range (No. Isolates Tested by Study) |
| Ampicillin-sulbactam | PNG | 53 (38) | 8.5 (5) | |
| Amoxicillin-clavulanate | Fr. Polynesia | 3.1 (96) | ||
| Ceftriaxone | PNG | 19 (315) | ||
| Doxycycline | Fiji | 6 (33) | 2.3 (215) | |
| Nalidixic acid | PNG | 58 (38) | ||
| Ciprofloxacin | Fiji | 0.3 (383) | ||
| PNG | 20, 33.3 (5, 15) | |||
| S. aureus and MRSA * | |||||
|---|---|---|---|---|---|
| Antibiotic Classes | Antibiotics | PICTs | 1978–2000 % (No. Isolates Tested per Study) | 2001–2010 % or % Range (No. Isolates Tested per Study) | 2011–2017 % or % Range (No. Isolates Tested per Study) |
| Penicillin | Oxacillin | PNG | 44 * (9) | 6.4–89.4 * (202, 164, 47) | |
| Samoa | 64.7 * (34) | ||||
| Fiji | 6.7 * (323) | 0.2–2.4 * (437, 2502) | |||
| Kiribati | 31 * (36) | ||||
| FSM | 4 * (113) | ||||
| Tonga | 17.2 * (430) | ||||
| Methicillin | New Caledonia | 33.5 *–21 * (202, 544) | |||
| Samoa | 51 * (246) | ||||
| PNG | 3 (73) | 27.2 * (11) | 43.9–81.5 * (164, 47) | ||
| Samoa | 24 (389) | ||||
| Flucloxacillin | Cook Is | 22 (588) | |||
| PNG | 36.3 * (11) | ||||
| Samoa | 100 * (34) | 42 (428) | |||
| Ampicillin | Cook Is | 90 (550) | |||
| PNG | 93–95.5 * (202, 47) | ||||
| Tetracycline | Tetracycline | PNG | 3–14.8 (66,101) | 0.99 * (202) | |
| Lincomycin | Clindamycin | Cook Is | 10 (327) | ||
| Samoa | 19 (278) | ||||
| Macrolide | Azithromycin | PNG | 46 * (202) | ||
| Erythromycin | Cook Is | 17 (557) | |||
| Samoa | 29.4 * (34) | 33 (312) | |||
| Chloramphenicol | Chloramphenicol | Cook Is | 5 (259) | ||
| PNG | 39.6 (101) | 36.3 * (11) | 7.4 * (202) | ||
| Samoa | 47 (216) | ||||
| Diaminopyrimidine | Co-trimoxazole | PNG | 37.4 * (202) | ||
| S. pneumoniae | |||||
|---|---|---|---|---|---|
| Antibiotic Classes | Antibiotics | PICTs | 1970–2000 % or % Range (No. Isolates Tested per Study) | 2001–2010 % or % Range (No. Isolates Tested per Study) | 2011–2017 % or % Range (No. Isolates Tested per Study) |
| Penicillins | Penicillin | Fiji | 11.4 (195) | ||
| New Caledonia | 21 (544) | ||||
| PICTs | 14.4 (298) | ||||
| PNG | 12–60.3 (350, 58, 5, 50, 140, 177) | 11.7–21.5 (17, 40, 177) | 47.9 (121) | ||
| Amoxicillin | New Caledonia | 2.7 (544) | |||
| PICTs | 3.7 (298) | ||||
| Amoxicillin/ Clavulanic acid | PICTs | 1. 7 (298) | |||
| Cephalosporins | Ceftriaxone | Fiji | 0.8 (195) | ||
| New Caledonia | 2.2 (544) | ||||
| PICTs | 1.7 (298) | ||||
| Tetracyclines | Tetracycline | PNG | 4.2–5.8 (176, 17) | ||
| Macrolides | Azithromycin | PNG | 4.9 (121) | ||
| Erythromycin | Fiji | 1.6 (195) | |||
| Chloramphenicol | Chloramphenicol | PNG | 1.5 (121) | 2.3–5.8 (176, 17) | 0.82 (121) |
| Diaminopyrimidines | Co-trimoxazole | Fiji | 20.3 (195) | ||
| New Caledonia | 2.2 (544) | ||||
| PNG | 4–17.6 (176,6,17) | 38 (121) | |||
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Foxlee, N.D.; Townell, N.; McIver, L.; Lau, C.L. Antibiotic Resistance in Pacific Island Countries and Territories: A Systematic Scoping Review. Antibiotics 2019, 8, 29. https://doi.org/10.3390/antibiotics8010029
Foxlee ND, Townell N, McIver L, Lau CL. Antibiotic Resistance in Pacific Island Countries and Territories: A Systematic Scoping Review. Antibiotics. 2019; 8(1):29. https://doi.org/10.3390/antibiotics8010029
Chicago/Turabian StyleFoxlee, Nicola D., Nicola Townell, Lachlan McIver, and Colleen L. Lau. 2019. "Antibiotic Resistance in Pacific Island Countries and Territories: A Systematic Scoping Review" Antibiotics 8, no. 1: 29. https://doi.org/10.3390/antibiotics8010029
APA StyleFoxlee, N. D., Townell, N., McIver, L., & Lau, C. L. (2019). Antibiotic Resistance in Pacific Island Countries and Territories: A Systematic Scoping Review. Antibiotics, 8(1), 29. https://doi.org/10.3390/antibiotics8010029






