Advances in Antarctic Research for Antimicrobial Discovery: A Comprehensive Narrative Review of Bacteria from Antarctic Environments as Potential Sources of Novel Antibiotic Compounds Against Human Pathogens and Microorganisms of Industrial Importance
Abstract
1. Introduction
2. The Antarctic Environment Supports Varied Microbial Life
3. Bacterial Adaptation to Antarctic Extreme Conditions Might Confer the Ability to Produce Diverse and Novel Antimicrobial Compounds
4. Antarctic Exhibits Significant Diversity of Antibiotic-Producing Bacteria with Pharmaceutical Applications
4.1. Proteobacteria Phylum Members
4.2. Actinobacteria Phylum Members
4.3. Other Phylum Members
5. Antarctic Bacterial Strains Exhibit Activity against Microorganisms of Industrial Importance
6. Recent Advances Identified New Antibiotic Molecules from Antarctic Bacteria
7. Concluding Remarks
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Livermore, D.M. Has the era of untreatable infections arrived? J. Antimicrob. Chemother. 2009, 64. [Google Scholar] [CrossRef] [PubMed]
- Roca, I.; Akova, M.; Baquero, F.; Carlet, J.; Cavaleri, M.; Coenen, S.; Cohen, J.; Findlay, D.; Gyssens, I.; Heure, O.E.; et al. The global threat of antimicrobial resistance: Science for intervention. New Microbes New Infect. 2015, 6, 22–29. [Google Scholar] [CrossRef] [PubMed]
- Hughes, D.; Andersson, D.I. Environmental and genetic modulation of the phenotypic expression of antibiotic resistance. FEMS Microbiol. Rev. 2017, 41, 374–391. [Google Scholar] [CrossRef] [PubMed]
- Genilloud, O. The re-emerging role of microbial natural products in antibiotic discovery. Antonie van Leeuwenhoek 2014, 106, 173–188. [Google Scholar] [CrossRef] [PubMed]
- Bax, R.; Mullan, N.; Verhoef, J. The millennium bugs—The need for and development of new antibacterials. Int. J. Antimicrob. Agents 2000, 16, 51–59. [Google Scholar] [CrossRef]
- Axenov-Gribanov, D.V.; Voytsekhovskaya, I.V.; Tokovenko, B.T.; Protasov, E.S.; Gamaiunov, S.V.; Rebets, Y.V.; Luzhetskyy, A.N.; Timofeyev, M.A. Actinobacteria isolated from an underground lake and moonmilk speleothem from the biggest conglomeratic karstic cave in Siberia as sources of novel biologically active compounds. PLoS ONE 2016, 11. [Google Scholar] [CrossRef] [PubMed]
- Newman, D.J.; Cragg, G.M.; Snader, K.M. Natural products as sources of new drugs over the period 1981-2002. J. Nat. Prod. 2003, 66, 1022–1037. [Google Scholar] [CrossRef] [PubMed]
- Katz, L.; Baltz, R.H. Natural product discovery: Past, present, and future. J. Ind. Microbiol. Biotechnol. 2016, 43, 155–176. [Google Scholar] [CrossRef] [PubMed]
- Onaka, H. Novel antibiotic screening methods to awaken silent or cryptic secondary metabolic pathways in actinomycetes. J. Antibiot. 2017, 70, 865–870. [Google Scholar] [CrossRef] [PubMed]
- Bredholt, H.; Fjærvik, E.; Johnsen, G.; Zotchev, S.B. Actinomycetes from sediments in the Trondheim fjord, Norway: Diversity and biological activity. Mar. Drugs 2008, 6, 12–24. [Google Scholar] [CrossRef] [PubMed]
- Lee, L.H.; Cheah, Y.K.; Nurul Syakima, A.M.; Shiran, M.S.; Tang, Y.L.; Lin, H.P.; Hong, K. Analysis of Antarctic protobacteria by PCR fingerprinting and screening for antimicrobial secondary metabolites. Genet. Mol. Res. 2012, 11, 1627–1641. [Google Scholar] [CrossRef] [PubMed]
- Lam, K.S. New aspects of natural products in drug discovery. Trends Microbiol. 2007, 15, 279–289. [Google Scholar] [CrossRef] [PubMed]
- Donadio, S.; Maffioli, S.; Monciardini, P.; Sosio, M.; Jabes, D. Antibiotic discovery in the twenty-first century: Current trends and future perspectives. J. Antibiot. 2010, 63, 423–430. [Google Scholar] [CrossRef] [PubMed]
- Lo Giudice, A.; Bruni, V.; Michaud, L. Characterization of Antarctic psychrotrophic bacteria with antibacterial activities against terrestrial microorganisms. J. Basic Microbiol. 2007, 47, 496–505. [Google Scholar] [CrossRef] [PubMed]
- Gulder, T.A.; Moore, B.S. Chasing the treasures of the sea—Bacterial marine natural products. Curr. Opin. Microbiol. 2009, 12, 252–260. [Google Scholar] [CrossRef] [PubMed]
- Land, M.; Hauser, L.; Jun, S.-R.; Nookaew, I.; Leuze, M.R.; Ahn, T.-H.; Karpinets, T.; Lund, O.; Kora, G.; Wassenaar, T.; et al. Insights from 20 years of bacterial genome sequencing. Funct. Integr. Genom. 2015, 15, 141–161. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, A.; Sharp, R.; Russell, N.J.; Roller, S. Antarctic bacteria inhibit growth of food-borne microorganisms at low temperatures. FEMS Microbiol. Ecol. 2004, 48, 157–167. [Google Scholar] [CrossRef] [PubMed]
- Undabarrena, A.; Beltrametti, F.; Claverías, F.P.; González, M.; Moore, E.R.B.; Seeger, M.; Cámara, B. Exploring the diversity and antimicrobial potential of marine actinobacteria from the comau fjord in Northern Patagonia, Chile. Front. Microbiol. 2016, 7. [Google Scholar] [CrossRef] [PubMed]
- Mehetre, G.; Shah, M.; Dastager, S.G.; Dharne, M.S. Untapped bacterial diversity and metabolic potential within Unkeshwar hot springs, India. Arch. Microbiol. 2018, 200, 753–770. [Google Scholar] [CrossRef] [PubMed]
- Jiang, W.; Zhong, Y.; Shen, L.; Wu, X.; Ye, Y.; Chen, C.-T.; Wu, B. Stress-driven discovery of natural products from extreme marine environment- kueishantao hydrothermal vent, a case study of metal switch valve. Curr. Org. Chem. 2014, 18. [Google Scholar] [CrossRef]
- Yogabaanu, U.; Weber, J.F.F.; Convey, P.; Rizman-Idid, M.; Alias, S.A. Antimicrobial properties and the influence of temperature on secondary metabolite production in cold environment soil fungi. Polar Sci. 2017, 14, 60–67. [Google Scholar] [CrossRef]
- Lewis Smith, R.I. Exotic sporomorpha as indicators of potential immigrant colonists in antarctica. Grana 1991, 30, 313–324. [Google Scholar] [CrossRef]
- Bratchkova, A.; Ivanova, V. Bioactive metabolites produced by microorganisms collected in Antarctica and the Arctic. Biotechnol. Biotechnol. Equip. 2011, 25, 1–7. [Google Scholar] [CrossRef]
- Wynn-Williams, D.D. Ecological aspects of Antarctic microbiology. Adv. Microb. Ecol. 1990, 11, 71–146. [Google Scholar]
- Convey, P.; Gibson, J.A.E.; Hillenbrand, C.D.; Hodgson, D.A.; Pugh, P.J.A.; Smellie, J.L.; Stevens, M.I. Antarctic terrestrial life—Challenging the history of the frozen continent? Biol. Rev. 2008, 83, 103–117. [Google Scholar] [CrossRef] [PubMed]
- Corsolini, S. Industrial contaminants in Antarctic biota. J. Chromatogr. A 2009, 1216, 598–612. [Google Scholar] [CrossRef] [PubMed]
- Lo Giudice, A.; Casella, P.; Bruni, V.; Michaud, L. Response of bacterial isolates from Antarctic shallow sediments towards heavy metals, antibiotics and polychlorinated biphenyls. Ecotoxicology 2013, 22, 240–250. [Google Scholar] [CrossRef] [PubMed]
- Cowan, D.A.; Chown, S.L.; Convey, P.; Tuffin, M.; Hughes, K.; Pointing, S.; Vincent, W.F. Non-indigenous microorganisms in the Antarctic: Assessing the risks. Trends Microbiol. 2011, 19, 540–548. [Google Scholar] [CrossRef] [PubMed]
- Martins, C.C.; Aguiar, S.N.; Wisnieski, E.; Ceschim, L.M.M.; Figueira, R.C.L.; Montone, R.C. Baseline concentrations of faecal sterols and assessment of sewage input into different inlets of Admiralty Bay, King George Island, Antarctica. Mar. Pollut. Bull. 2014, 78, 218–223. [Google Scholar] [CrossRef] [PubMed]
- Zreda-Gostynska, G.; Kyle, P.R.; Finnegan, D.; Prestbo, K.M. Volcanic gas emissions from Mount Erebus and their impact on the Antarctic environment. J. Geophys. Res. Solid Earth 1997, 102, 15039–15055. [Google Scholar] [CrossRef]
- Bargagli, R. Environmental contamination in Antarctic ecosystems. Sci. Total Environ. 2008, 400, 212–226. [Google Scholar] [CrossRef] [PubMed]
- Cipro, C.V.Z.Z.; Yogui, G.T.; Bustamante, P.; Taniguchi, S.; Sericano, J.L.; Montone, R.C. Organic pollutants and their correlation with stable isotopes in vegetation from King George Island, Antarctica. Chemosphere 2011, 85, 393–398. [Google Scholar] [CrossRef] [PubMed]
- Nichols, D.; Bowman, J.; Sanderson, K.; Nichols, C.M.; Lewis, T.; McMeekin, T.; Nichols, P.D. Developments with Antarctic microorganisms: Culture collections, bioactivity screening, taxonomy, PUFA production and cold-adapted enzymes. Curr. Opin. Biotechnol. 1999, 10, 240–246. [Google Scholar] [CrossRef]
- Aislabie, J.M.; Jordan, S.; Barker, G.M. Relation between soil classification and bacterial diversity in soils of the Ross Sea region, Antarctica. Geoderma 2008, 144, 9–20. [Google Scholar] [CrossRef]
- Cary, S.C.; McDonald, I.R.; Barrett, J.E.; Cowan, D.A. On the rocks: The microbiology of Antarctic Dry Valley soils. Nat. Rev. Microbiol. 2010, 8, 129–138. [Google Scholar] [CrossRef] [PubMed]
- Loperena, L.; Soria, V.; Varela, H.; Lupo, S.; Bergalli, A.; Guigou, M.; Pellegrino, A.; Bernardo, A.; Calviño, A.; Rivas, F.; et al. Extracellular enzymes produced by microorganisms isolated from maritime Antarctica. World J. Microbiol. Biotechnol. 2012, 28, 2249–2256. [Google Scholar] [CrossRef] [PubMed]
- Pearce, D.A.; Newsham, K.K.; Thorne, M.A.S.; Calvo-Bado, L.; Krsek, M.; Laskaris, P.; Hodson, A.; Wellington, E.M. Metagenomic Analysis of a Southern Maritime Antarctic Soil. Front. Microbiol. 2012, 3. [Google Scholar] [CrossRef] [PubMed]
- Silva, T.R.; Duarte, A.W.F.; Passarini, M.R.Z.; Ruiz, A.L.T.G.; Franco, C.H.; Moraes, C.B.; de Melo, I.S.; Rodrigues, R.A.; Fantinatti-Garboggini, F.; Oliveira, V.M. Bacteria from Antarctic environments: Diversity and detection of antimicrobial, antiproliferative, and antiparasitic activities. Polar Biol. 2018, 41, 1505–1519. [Google Scholar] [CrossRef]
- Singh, B.K.; Munro, S.; Potts, J.M.; Millard, P. Influence of grass species and soil type on rhizosphere microbial community structure in grassland soils. Appl. Soil Ecol. 2007, 36, 147–155. [Google Scholar] [CrossRef]
- Papale, M.; Conte, A.; Mikkonen, A.; Michaud, L.; La Ferla, R.; Azzaro, M.; Caruso, G.; Paranhos, R.; Cabral Anderson, S.; Maimone, G.; et al. Prokaryotic assemblages within permafrost active layer at Edmonson Point (Northern Victoria Land, Antarctica). Soil Biol. Biochem. 2018, 123, 165–179. [Google Scholar] [CrossRef]
- Shivaji, S.; Reddy, G.S.; Aduri, R.P.; Kutty, R.; Ravenschlag, K. Bacterial diversity of a soil sample from Schirmacher Oasis, Antarctica. Cell. Mol. Biol. 2004, 50, 525–536. [Google Scholar] [PubMed]
- Teixeira, L.C.R.S.; Peixoto, R.S.; Cury, J.C.; Sul, W.J.; Pellizari, V.H.; Tiedje, J.; Rosado, A.S. Bacterial diversity in rhizosphere soil from Antarctic vascular plants of Admiralty Bay, maritime Antarctica. Isme J. 2010, 4, 989. [Google Scholar] [CrossRef] [PubMed]
- Marizcurrena, J.J.; Morel, M.A.; Braña, V.; Morales, D.; Martinez-López, W.; Castro-Sowinski, S. Searching for novel photolyases in UVC-resistant Antarctic bacteria. Extremophiles 2017, 21, 409–418. [Google Scholar] [CrossRef] [PubMed]
- Lamilla, C.; Pavez, M.; Santos, A.; Hermosilla, A.; Llanquinao, V.; Barrientos, L. Bioprospecting for extracellular enzymes from culturable Actinobacteria from the South Shetland Islands, Antarctica. Polar Biol. 2017, 40, 719–726. [Google Scholar] [CrossRef]
- Subramani, R.; Aalbersberg, W. Culturable rare Actinomycetes: Diversity, isolation and marine natural product discovery. Appl. Microbiol. Biotechnol. 2013, 97, 9291–9321. [Google Scholar] [CrossRef] [PubMed]
- Tribelli, P.M.; Rossi, L.; Ricardi, M.M.; Gomez-Lozano, M.; Molin, S.; Raiger Iustman, L.J.; Lopez, N.I. Microaerophilic alkane degradation in Pseudomonas extremaustralis: A transcriptomic and physiological approach. J. Ind. Microbiol. Biotechnol. 2018, 45, 15–23. [Google Scholar] [CrossRef] [PubMed]
- Dube, S.; Singh, L.; Alam, S.I. Proteolytic anaerobic bacteria from lake sediments of Antarctica. Enzyme Microb. Technol. 2001, 28, 114–121. [Google Scholar] [CrossRef]
- Mountfort, D.O.; Rainey, F.A.; Burghardt, J.; Kaspar, H.F.; Stackebrandt, E. Clostridium vincentii sp. nov., a new obligately anaerobic, saccharolytic, psychrophilic bacterium isolated from low-salinity pond sediment of the McMurdo Ice Shelf, Antarctica. Arch. Microbiol. 1997, 167, 54–60. [Google Scholar] [CrossRef] [PubMed]
- Bowman, J.P.; Cavanagh, J.; Austin, J.J.; Sanderson, K. Novel Psychrobacter species from Antarctic ornithogenic soils. Int. J. Syst. Bacteriol. 1996, 46, 841–848. [Google Scholar] [CrossRef] [PubMed]
- Vishniac, H. Soil microgeology. In Antarctic Microbiology; Friedmann, Ed.; Wiley–Liss: New York, NY, USA, 1993; pp. 297–341. [Google Scholar]
- Long, R.A.; Azam, F. Antagonistic Interactions among Marine Pelagic Bacteria. Appl. Environ. Microbiol. 2001, 67, 4975–4983. [Google Scholar] [CrossRef] [PubMed]
- Nair, S.; Simidu, U. Distribution and significance of heterotrophic marine bacteria with antibacterial activity. Appl. Environ. Microbiol. 1987, 53, 2957–2962. [Google Scholar] [PubMed]
- Gram, L.; Grossart, H.P.; Schlingloff, A.; Kiørboe, T. Possible quorum sensing in marine snow bacteria: Production of acylated homoserine lactones by Roseobacter strains isolated from marine snow. Appl. Environ. Microbiol. 2002, 68, 4111–4116. [Google Scholar] [CrossRef] [PubMed]
- Tedesco, P.; Maida, I.; Esposito, F.P.; Tortorella, E.; Subko, K.; Ezeofor, C.C.; Zhang, Y.; Tabudravu, J.; Jaspars, M.; Fani, R.; et al. Antimicrobial activity of monoramnholipids produced by bacterial strains isolated from the Ross Sea (Antarctica). Mar. Drugs 2016, 14, 83. [Google Scholar] [CrossRef] [PubMed]
- Rojas, J.L.; Martín, J.; Tormo, J.R.; Vicente, F.; Brunati, M.; Ciciliato, I.; Losi, D.; Van Trappen, S.; Mergaert, J.; Swings, J.; et al. Bacterial diversity from benthic mats of Antarctic lakes as a source of new bioactive metabolites. Mar. Genom. 2009, 2, 33–41. [Google Scholar] [CrossRef] [PubMed]
- Tomova, I.; Stoilova-Disheva, M.; Lazarkevich, I.; Vasileva-Tonkova, E. Antimicrobial activity and resistance to heavy metals and antibiotics of heterotrophic bacteria isolated from sediment and soil samples collected from two Antarctic islands. Front. Life Sci. 2015, 8, 348–357. [Google Scholar] [CrossRef]
- Lee, L.H.; Cheah, Y.K.; Sidik, S.M.; Mutalib, N.S.A.; Tang, Y.L.; Lin, H.P.; Hong, K. Molecular characterization of Antarctic actinobacteria and screening for antimicrobial metabolite production. World J. Microbiol. Biotechnol. 2012, 28, 2125–2137. [Google Scholar] [CrossRef] [PubMed]
- Cheah, Y.K.; Lee, L.H.; Chieng, C.Y.C.; Wong, V.L.C.M. Isolation, identification and screening of actinobacteria in volcanic soil of deception island (the Antarctic) for antimicrobial metabolites. Polish Polar Res. 2015, 36, 67–78. [Google Scholar] [CrossRef]
- Gesheva, V.; Vasileva-Tonkova, E. Production of enzymes and antimicrobial compounds by halophilic Antarctic Nocardioides sp. grown on different carbon sources. World J. Microbiol. Biotechnol. 2012, 28, 2069–2076. [Google Scholar] [CrossRef] [PubMed]
- Lavin, P.L.; Yong, S.T.; Wong, C.M.V.L.; De Stefano, M. Isolation and characterization of Antarctic psychrotroph Streptomyces sp. strain INACH3013. Antarct. Sci. 2016, 28, 433–442. [Google Scholar] [CrossRef]
- Asencio, G.; Lavin, P.; Alegría, K.; Domínguez, M.; Bello, H.; González-Rocha, G.; González-Aravena, M. Antibacterial activity of the Antarctic bacterium Janthinobacterium sp. SMN 33.6 against multi-resistant Gram-negative bacteria. Electron. J. Biotechnol. 2014, 17, 1–5. [Google Scholar] [CrossRef]
- Mojib, N.; Philpott, R.; Huang, J.P.; Niederweis, M.; Bej, A.K. Antimycobacterial activity in vitro of pigments isolated from Antarctic bacteria. Antonie van Leeuwenhoek 2010, 98, 531–540. [Google Scholar] [CrossRef] [PubMed]
- Danilovich, M.E.; Sánchez, L.A.; Acosta, F.; Delgado, O.D. Antarctic bioprospecting: In pursuit of microorganisms producing new antimicrobials and enzymes. Polar Biol. 2018, 47, 1417–1433. [Google Scholar] [CrossRef]
- Fukuda, W.; Kimura, T.; Araki, S.; Miyoshi, Y.; Atomi, H.; Imanaka, T. Lysobacter oligotrophicus sp. nov., isolated from an Antarctic freshwater lake in Antarctica. Int. J. Syst. Evol. Microbiol. 2013, 63, 3313–3318. [Google Scholar] [CrossRef] [PubMed]
- Maida, I.; Bosi, E.; Fondi, M.; Perrin, E.; Orlandini, V.; Papaleo, M.C.; Mengoni, A.; de Pascale, D.; Tutino, M.L.; Michaud, L.; et al. Antimicrobial activity of Pseudoalteromonas strains isolated from the Ross Sea (Antarctica) versus Cystic Fibrosis opportunistic pathogens. Hydrobiologia 2015, 761, 443–457. [Google Scholar] [CrossRef]
- Papa, R.; Parrilli, E.; Sannino, F.; Barbato, G.; Tutino, M.L.; Artini, M.; Selan, L. Anti-biofilm activity of the Antarctic marine bacterium Pseudoalteromonas haloplanktis TAC125. Res. Microbiol. 2013, 164, 450–456. [Google Scholar] [CrossRef] [PubMed]
- Médigue, C.; Krin, E.; Pascal, G.; Barbe, V.; Bernsel, A.; Bertin, P.N.; Cheung, F.; Cruveiller, S.; D’Amico, S.; Duilio, A.; et al. Coping with cold: The genome of the versatile marine Antarctica bacterium Pseudoalteromonas haloplanktis TAC125. Genome Res. 2005, 15, 1325–1335. [Google Scholar] [CrossRef] [PubMed]
- Sannino, F.; Parrilli, E.; Apuzzo, G.A.; de Pascale, D.; Tedesco, P.; Maida, I.; Perrin, E.; Fondi, M.; Fani, R.; Marino, G.; et al. Pseudoalteromonas haloplanktis produces methylamine, a volatile compound active against Burkholderia cepacia complex strains. New Biotechnol. 2017, 35, 13–18. [Google Scholar] [CrossRef] [PubMed]
- Wong, C.M.V.L.; Tam, H.K.; Alias, S.A.; González, M.; González-Rocha, G.; Domínguez-Yévenes, M. Pseudomonas and pedobacter isolates from King George Island inhibited the growth of foodborne pathogens. Polish Polar Res. 2011, 32, 3–14. [Google Scholar] [CrossRef]
- Leyton, A.; Urrutia, H.; Vidal, J.M.; de la Fuente, M.; Alarcón, M.; Aroca, G.; González-Rocha, G.; Sossa, K. Actividad inhibitoria del sobrenadante de la bacteria Antártica Pseudomonas sp. M19B en la formación de biopelículas de Flavobacterium psychrophilum 19749. Rev. Biol. Mar. Oceanogr. 2015, 50, 375–381. [Google Scholar] [CrossRef]
- Taton, A.; Grubisic, S.; Ertz, D.; Hodgson, D.A.; Piccardi, R.; Biondi, N.; Tredici, M.R.; Mainini, M.; Losi, D.; Marinelli, F.; et al. Polyphasic study of antarctic cyanobacterial strains. J. Phycol. 2006, 42, 1257–1270. [Google Scholar] [CrossRef]
- Asthana, R.K.; Deepali; Tripathi, M.K.; Srivastava, A.; Singh, A.P.; Singh, S.P.; Nath, G.; Srivastava, R.; Srivastava, B.S. Isolation and identification of a new antibacterial entity from the Antarctic cyanobacterium Nostoc CCC 537. J. Appl. Phycol. 2009, 21, 81–88. [Google Scholar] [CrossRef]
- Guo, W.; Cui, P.; Chen, X. Complete genome of Bacillus sp. Pc3 isolated from the Antarctic seawater with antimicrobial activity. Mar. Genom. 2015, 20, 1–2. [Google Scholar] [CrossRef] [PubMed]
- Vollú, R.E.; Jurelevicius, D.; Ramos, L.R.; Peixoto, R.S.; Rosado, A.S.; Seldin, L. Aerobic endospore-forming bacteria isolated from Antarctic soils as producers of bioactive compounds of industrial interest. Polar Biol. 2014, 37, 1121–1131. [Google Scholar] [CrossRef]
- Shekh, R.M.; Singh, P.; Singh, S.M.; Roy, U. Antifungal activity of Arctic and Antarctic bacteria isolates. Polar Biol. 2011, 34, 139–143. [Google Scholar] [CrossRef]
- Van den Burg, B. Extremophiles as a source for novel enzymes. Curr. Opin. Microbiol. 2003, 6, 213–218. [Google Scholar] [CrossRef]
- Casanueva, A.; Tuffin, M.; Cary, C.; Cowan, D.A. Molecular adaptations to psychrophily: The impact of “omic” technologies. Trends Microbiol. 2010, 18, 374–381. [Google Scholar] [CrossRef] [PubMed]
- D’Amico, S.; Collins, T.; Marx, J.C.; Feller, G.; Gerday, C. Psychrophilic microorganisms: Challenges for life. EMBO Rep. 2006, 7, 385–389. [Google Scholar] [CrossRef] [PubMed]
- Lo Giudice, A.; Brilli, M.; Bruni, V.; De Domenico, M.; Fani, R.; Michaud, L. Bacterium-bacterium inhibitory interactions among psychrotrophic bacteria isolated from Antarctic seawater (Terra Nova Bay, Ross Sea). FEMS Microbiol. Ecol. 2007, 60, 383–396. [Google Scholar] [CrossRef] [PubMed]
- Leiva, S.; Alvarado, P.; Huang, Y.; Wang, J.; Garrido, I. Diversity of pigmented Gram-positive bacteria associated with marine macroalgae from Antarctica. FEMS Microbiol. Lett. 2015, 362. [Google Scholar] [CrossRef] [PubMed]
- Cristóbal, H.A.; López, M.A.; Kothe, E.; Abate, C.M. Diversity of protease-producing marine bacteria from sub-antarctic environments. J. Basic Microbiol. 2011, 51, 590–600. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.H.; Cho, K.H.; Lee, Y.M.; Yim, J.H.; Lee, H.K.; Cho, J.C.; Hong, S.G. Diversity of cold-active protease-producing bacteria from arctic terrestrial and marine environments revealed by enrichment culture. J. Microbiol. 2010, 48, 426–432. [Google Scholar] [CrossRef] [PubMed]
- Lo Giudice, A.; Caruso, C.; Mangano, S.; Bruni, V.; De Domenico, M.; Michaud, L. Marine Bacterioplankton Diversity and Community Composition in an Antarctic Coastal Environment. Microb. Ecol. 2012, 63, 210–223. [Google Scholar] [CrossRef] [PubMed]
- Bosi, E.; Fondi, M.; Orlandini, V.; Perrin, E.; Maida, I.; de Pascale, D.; Tutino, M.L.; Parrilli, E.; Lo Giudice, A.; Filloux, A.; et al. The pangenome of (Antarctic) Pseudoalteromonas bacteria: Evolutionary and functional insights. BMC Genom. 2017, 18. [Google Scholar] [CrossRef] [PubMed]
- Weintraub, S.J.; Manson, S.R. Asparagine deamidation: A regulatory hourglass. Mech. Ageing Dev. 2004, 125, 255–257. [Google Scholar] [CrossRef] [PubMed]
- Parrilli, E.; Giuliani, M.; Giordano, D.; Russo, R.; Marino, G.; Verde, C.; Tutino, M.L. The role of a 2-on-2 haemoglobin in oxidative and nitrosative stress resistance of Antarctic Pseudoalteromonas haloplanktis TAC125. Biochimie 2010, 92, 1003–1009. [Google Scholar] [CrossRef] [PubMed]
- Wilmes, B.; Hartung, A.; Lalk, M.; Liebeke, M.; Schweder, T.; Neubauer, P. Fed-batch process for the psychrotolerant marine bacterium Pseudoalteromonas haloplanktis. Microb. Cell Fact. 2010, 9. [Google Scholar] [CrossRef] [PubMed]
- Mocali, S.; Chiellini, C.; Fabiani, A.; Decuzzi, S.; Pascale, D.; Parrilli, E.; Tutino, M.L.; Perrin, E.; Bosi, E.; Fondi, M.; et al. Ecology of cold environments: New insights of bacterial metabolic adaptation through an integrated genomic-phenomic approach. Sci. Rep. 2017, 7. [Google Scholar] [CrossRef] [PubMed]
- Dieser, M.; Greenwood, M.; Foreman, C.M. Carotenoid Pigmentation in Antarctic Heterotrophic Bacteria as a Strategy to Withstand Environmental Stresses. Arctic Antarct. Alp. Res. 2010, 42, 396–405. [Google Scholar] [CrossRef]
- Lo Giudice, A.; Fani, R. Antimicrobial Potential of Cold-Adapted Bacteria and Fungi from Polar Regions. In Biotechnology of Extremophiles; Springer International Publishing: New York, NY, USA, 2016; pp. 83–115. ISBN 978-3-319-13520-5. [Google Scholar]
- Fondi, M.; Bosi, E.; Lo Giudice, A.; Fani, R. A Systems Biology View on Bacterial Response to Temperature Shift. In Biotechnology of Extremophiles; Springer International Publishing: New York, NY, USA, 2016; pp. 597–618. ISBN 978-3-319-13520-5. [Google Scholar]
- Holmström, C.; Kjelleberg, S. Marine Pseudoalteromonas species are associated with higher organisms and produce biologically active extracellular agents. FEMS Microbiol. Ecol. 1999, 30, 285–293. [Google Scholar] [CrossRef]
- Isnansetyo, A.; Kamei, Y. MC21-A, a bactericidal antibiotic produced by a new marine bacterium, Pseudoalteromonas phenolica sp. nov. O-BC30T, against methicillin-resistant Staphylococcus aureus. Antimicrob. Agents Chemother. 2003, 47, 480–488. [Google Scholar] [CrossRef] [PubMed]
- Xie, B.; Qin, Y.L.S.; Rong, J.C.; Zhang, X.Y.; Chen, X.L.; Zhou, B.C.; Zhang, Y.Z. Genome Sequence of the Cycloprodigiosin-Producing Bacterial Strain Pseudoalteromonas rubra ATCC 29570 T. J. Bacteriol. 2012, 194, 1637–1638. [Google Scholar] [CrossRef] [PubMed]
- Holmström, C.; Egan, S.; Franks, A.; McCloy, S.; Kjelleberg, S. Antifouling activities expressed by marine surface associated Pseudoalteromonas species. FEMS Microbiol. Ecol. 2002, 41, 47–58. [Google Scholar] [CrossRef]
- Goodfellow, M.; Fiedler, H.P. A guide to successful bioprospecting: Informed by actinobacterial systematics. Antonie van Leeuwenhoek 2010, 98, 119–142. [Google Scholar] [CrossRef] [PubMed]
- Sen, A.; Daubin, V.; Abrouk, D.; Gifford, I.; Berry, A.M.; Normand, P.; Philippe Normand, C. Phylogeny of the class Actinobacteria revisited in the light of complete genomes. Int. J. Syst. Evol. Microbiol. 2014, 64, 3821–3832. [Google Scholar] [CrossRef] [PubMed]
- Doroghazi, J.R.; Metcalf, W.W. Comparative genomics of actinomycetes with a focus on natural product biosynthetic genes. BMC Genom. 2013, 14. [Google Scholar] [CrossRef] [PubMed]
- Okoro, C.K.; Brown, R.; Jones, A.L.; Andrews, B.A.; Asenjo, J.A.; Goodfellow, M.; Bull, A.T. Diversity of culturable actinomycetes in hyper-arid soils of the Atacama Desert, Chile. Antonie van Leeuwenhoek 2009, 95, 121–133. [Google Scholar] [CrossRef] [PubMed]
- Thakur, D.; Yadav, A.; Gogoi, B.K.; Bora, T.C. Isolation and screening of Streptomyces in soil of protected forest areas from the states of Assam and Tripura, India, for antimicribial metabolites. J. Mycol. Med. 2007, 17, 242–249. [Google Scholar] [CrossRef]
- Fischbach, M.A.; Walsh, C.T. Assembly-line enzymology for polyketide and nonribosomal peptide antibiotics: Logic machinery, and mechanisms. Chem. Rev. 2006, 106, 3468–3496. [Google Scholar] [CrossRef] [PubMed]
- Maida, I.; Fondi, M.; Papaleo, M.C.; Perrin, E.; Orlandini, V.; Emiliani, G.; de Pascale, D.; Parrilli, E.; Tutino, M.L.; Michaud, L.; et al. Phenotypic and genomic characterization of the Antarctic bacterium Gillisia sp. CAL575, a producer of antimicrobial compounds. Extremophiles 2014, 18, 35–49. [Google Scholar] [CrossRef] [PubMed]
- Vincent, W.F. Cyanobacterial Dominance in the Polar Regions. In The Ecology of Cyanobacteria; Springer: Dordrecht, The Netherlands, 2000; pp. 321–340. ISBN 978-0-7923-4735-4. [Google Scholar]
- Zakhia, F.; Jungblut, A.D.; Taton, A.; Vincent, W.F.; Wilmotte, A. Cyanobacteria in cold ecosystems. In Psychrophiles: From Biodiversity to Biotechnology; Springer: Berlin/Heidelberg, Germany, 2008; pp. 121–135. ISBN 9783540743347. [Google Scholar]
- Biondi, N.; Tredici, M.R.; Taton, A.; Wilmotte, A.; Hodgson, D.A.; Losi, D.; Marinelli, F. Cyanobacteria from benthic mats of Antarctic lakes as a source of new bioactivities. J. Appl. Microbiol. 2008, 105, 105–115. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.N.; Nambisan, B.; Sundaresan, A.; Mohandas, C.; Anto, R.J. Isolation and identification of antimicrobial secondary metabolites from Bacillus cereus associated with a rhabditid entomopathogenic nematode. Ann. Microbiol. 2014, 64, 209–218. [Google Scholar] [CrossRef]
- Li, B.; Li, Q.; Xu, Z.; Zhang, N.; Shen, Q.; Zhang, R. Responses of beneficial Bacillus amyloliquefaciens SQR9 to different soilborne fungal pathogens through the alteration of antifungal compounds production. Front. Microbiol. 2014, 5. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Wang, Q.; Wang, K.; Brian, K.; Liu, C.; Gu, Y. Study of the antifungal activity of Bacillus vallismortis ZZ185 in vitro and identification of its antifungal components. Bioresour. Technol. 2010, 101, 292–297. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Jansen, R.; Nimtz, M.; Johri, B.N.; Wray, V. Rhamnolipids from the rhizosphere bacterium Pseudomonas sp. GRP3that reduces damping-off disease in chilli and tomato nurseries. J. Nat. Prod. 2007, 70, 941–947. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Mawgoud, A.M.; Lépine, F.; Déziel, E. Rhamnolipids: Diversity of structures, microbial origins and roles. Appl. Microbiol. Biotechnol. 2010, 86, 1323–1336. [Google Scholar] [CrossRef] [PubMed]
- Sotirova, A.V.; Spasova, D.I.; Galabova, D.N.; Karpenko, E.; Shulga, A. Rhamnolipid-biosurfactant permeabilizing effects on gram-positive and gram-negative bacterial strains. Curr. Microbiol. 2008, 56, 639–644. [Google Scholar] [CrossRef] [PubMed]
- Papaleo, M.C.; Fondi, M.; Maida, I.; Perrin, E.; Lo Giudice, A.; Michaud, L.; Mangano, S.; Bartolucci, G.; Romoli, R.; Fani, R. Sponge-associated microbial Antarctic communities exhibiting antimicrobial activity against Burkholderia cepacia complex bacteria. Biotechnol. Adv. 2012, 30, 272–293. [Google Scholar] [CrossRef] [PubMed]
- Papaleo, M.C.; Romoli, R.; Bartolucci, G.; Maida, I.; Perrin, E.; Fondi, M.; Orlandini, V.; Mengoni, A.; Emiliani, G.; Tutino, M.L.; et al. Bioactive volatile organic compounds from Antarctic (sponges) bacteria. New Biotechnol. 2013, 30, 824–838. [Google Scholar] [CrossRef] [PubMed]
- Thomas, T.R.A.; Kavlekar, D.P.; LokaBharathi, P.A. Marine drugs from sponge-microbe association—A review. Mar. Drugs 2010, 8, 1417–1468. [Google Scholar] [CrossRef] [PubMed]
- Alvarado, P.; Huang, Y.; Wang, J.; Garrido, I.; Leiva, S. Phylogeny and bioactivity of epiphytic Gram-positive bacteria isolated from three co-occurring antarctic macroalgae. Antonie Van Leeuwenhoek 2018, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Makhalanyane, T.P.; Van Goethem, M.W.; Cowan, D.A. Microbial diversity and functional capacity in polar soils. Curr. Opin. Biotechnol. 2016, 38, 159–166. [Google Scholar] [CrossRef] [PubMed]
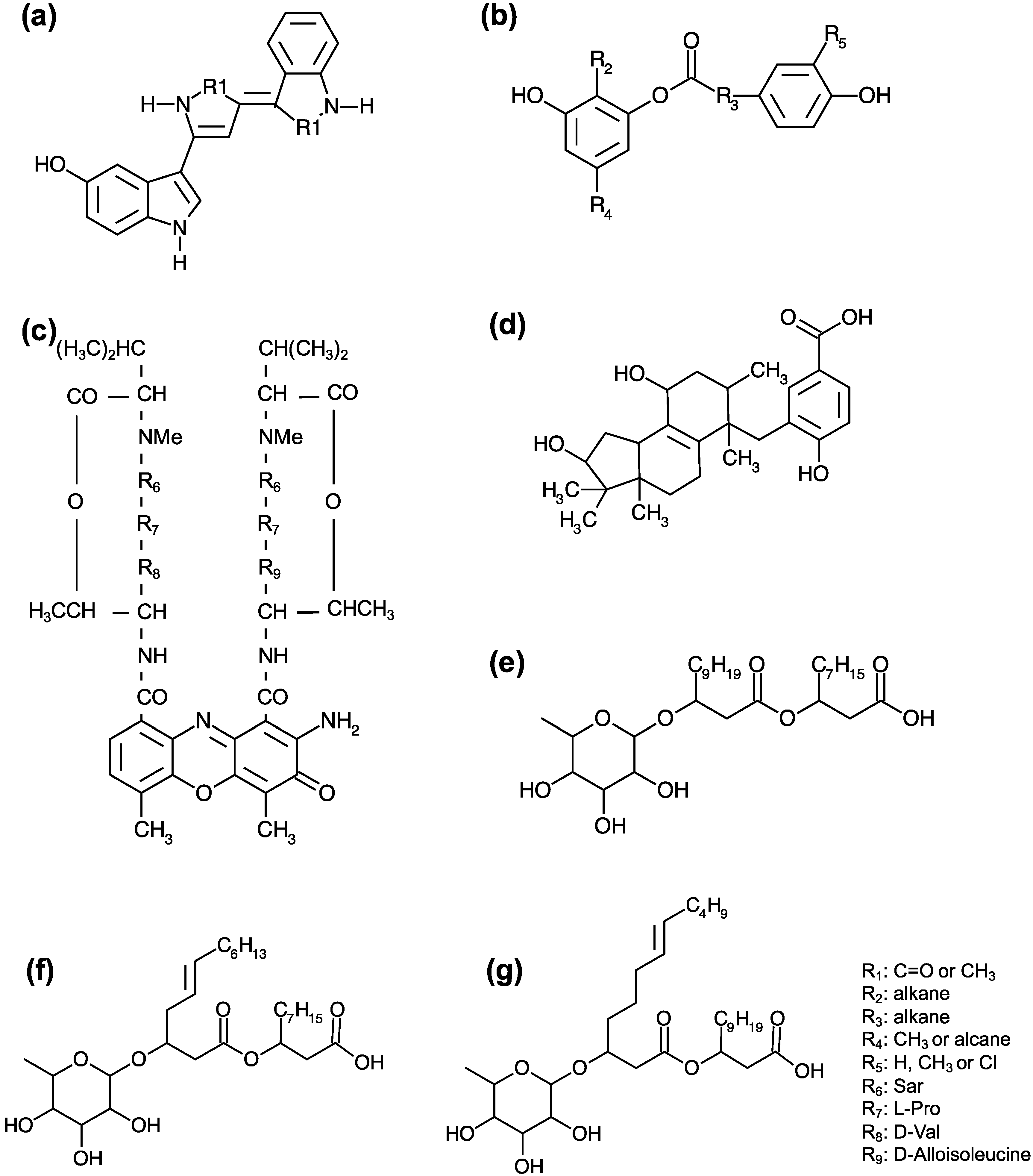
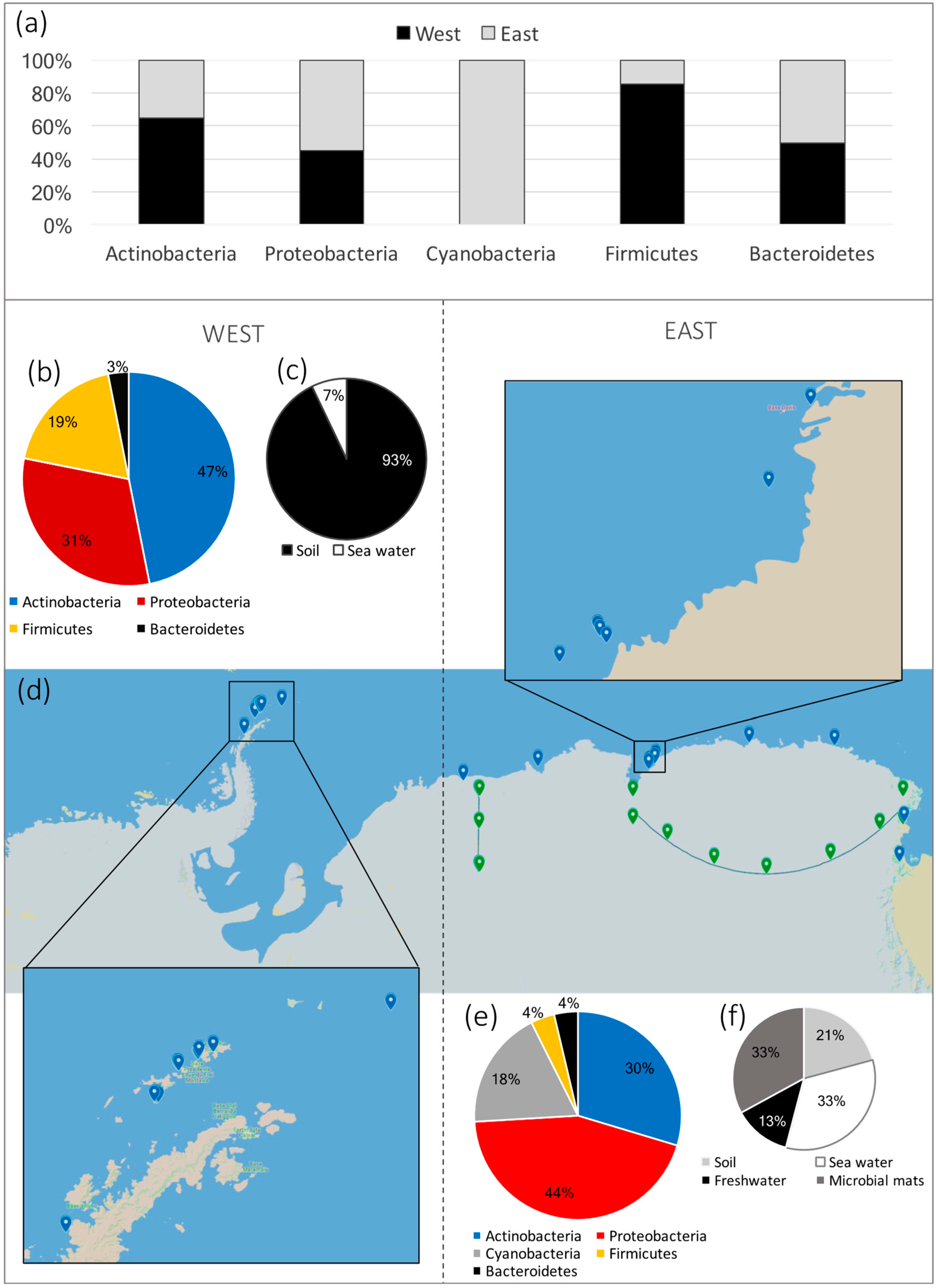
| Isolate Identification | Type of Sample | Place of Sampling | Antagonist Activity | Reference |
|---|---|---|---|---|
| Actinobacteria | ||||
| Arthrobacter sp. | Sea water | 72°19′ S to 74°53′ S–163°48′ E to 70°16′ E: Stations Mergellina Santa Maria Novella, Tiburtina, Road Bay, Gerlache Inlet, Evans Cove, Inexpressible Island, Cape Hallet, and Tethys Bay | Escherichia coli, Micrococcus luteus, Bacillus subtilis, Proteus mirabilis | [14] |
| Soil | 72°19′ S to 77°83′ S–16° 55′ E to 17° 16′ E: Cape Hallett, Edmonson Point, Kay Island, Cape Russell, Lake Hoare, Harrow Peaks, Crater Circe, Battleship Promontory, Mount, McGee, Mount Rittmann, Mount Melbourne | Listeria spp., Brochothrix thermosphacta | [17] | |
| Marine sediment | 74°41′36.96″ S, 164°6′42.12″ E: Terranova Bay, Ross Sea. | Burkholderia cepacia complex pathogens | [54] | |
| Benthic microbial mat | Larsemann Hills, Vestfold Hills and McMurdo Dry Valleys | Staphylococcus aureus, Enterococcus faecium, E. coli | [55] | |
| Ornithogenic soil | 62°59′ S, 60°34′ W: Whalers Bay on Deception Island, South Shetland Islands and 65°14′44.6″ S, 64°15′26″ W: Galindez Island, Argentine Islands | B. subtilis, Bacillus cereus, M. luteus, Pseudomonas aeruginosa, Acinetobacter johnsonii, Xanthomonas oryzae, Candida sp., Cryptococcus sp. | [56] | |
| Brevibacterium sp. | Soil | 62°24′ S, 59°47′ W: Barrientos Island | S. aureus, Candida albicans | [57] |
| Demetria sp. | Soil | 62°24′ S, 59°47′ W: Barrientos Island | S. aureus | [57] |
| Gordonia sp. | Soil | 62°24′ S, 59°47′ W: Barrientos Island | S. aureus, C. albicans | [57] |
| Gordonia terrae | Soil | 62°58′42.2″ S, 60°42′71.5″ W: Deception Island | Salmonella enterica serotype Paratyphi, S. enterica serotype Enteritidis | [58] |
| Janibacter sp. | Soil | 62°24′ S, 59°47′ W: Barrientos Island | S. aureus | [57] |
| Janibacter thuringensis | Sea water | 72°19′ S to 74°53′ S–163°48′ E to 70°16′ E: Stations Mergellina Santa Maria Novella, Tiburtina, Road Bay, Gerlache Inlet, Evans Cove, Inexpressible Island, Cape Hallet, and Tethys Bay | E. coli, M. luteus, P. mirabilis | [14] |
| Kocuria sp. | Soil | 62°24′ S, 59°47′ W: Barrientos Island | S. aureus | [57] |
| Lapillicoccus sp. | Soil | 62°24′ S, 59°47′ W: Barrientos Island | C. albicans | [57] |
| Leifsonia soli | Soil | 62°58′42.2″ S, 60°42′71.5″ W: Deception Island | S. enterica serotype Paratyphi, S. enterica serotype Enteritidis | [58] |
| Micromonospora sp. | Soil | 62°24′ S, 59°47′ W: Barrientos Island | S. aureus, P. aeruginosa | [57] |
| Nesterenkonia sp. | Sea water | 72°19′ S to 74°53′ S–163°48′ E to 70°16′ E: Stations Mergellina Santa Maria Novella, Tiburtina, Road Bay, Gerlache Inlet, Evans Cove, Inexpressible Island, Cape Hallet, and Tethys Bay | E. coli | [14] |
| Nocardioides sp. | Soil | Casey Station, Wilkes Land | S. aureus, B. subtilis, X. oryzae | [59] |
| 62°24′ S, 59°47′ W: Barrientos Island | C. albicans | [57] | ||
| Rhodococcus sp. | Soil | 62°24′ S, 59°47′ W: Barrientos Island | S. aureus | [57] |
| Ornithogenic soil | 62°59′ S, 60°34′ W: Whalers Bay on Deception Island, South Shetland Islands and 65°14′44.6″ S, 64°15′26″ W: Galindez Island, Argentine Islands | B. subtilis, B. cereus, P. aeruginosa, Candida sp., Cryptococcus sp. | [56] | |
| Rhodococcus fascians | Sea water | 72°19′ S to 74°53′ S–163°48′ E to 70°16′ E: Stations Mergellina Santa Maria Novella, Tiburtina, Road Bay, Gerlache Inlet, Evans Cove, Inexpressible Island, Cape Hallet, and Tethys Bay | E. coli, M. luteus, B. subtilis, P. mirabilis | [14] |
| Streptomyces sp. | Soil | 62°12′26.4″ S, 58°58′28.7″ W: Fildes Peninsula, King George Island. | Vibrio parahaemolyticus, S. enterica serovar Typhimurium, Enterobacter cloacae, Klebsiella pneumoniae, B. cereus, Listeria monocytogenes (serotypes I, IV and aa), Enterococcus. faecalis, Staphylococcus haemolyticus, Staphylococcus equorum, S. aureus | [60] |
| Terrabacter lapilli | Soil | 62°58′42.2″ S, 60°42′71.5″ W: Deception Island | S. enterica serotype Paratyphi, S. enterica serotype Enteritidis | [58] |
| Proteobacteria | ||||
| Bradyrhizobium sp. | Soil | 62°24′ S, 59°47′ W: Barrientos Island | C. albicans | [57] |
| Burkholderia sp. | Ornithogenic soil | 62°59′ S, 60°34′ W: Whalers Bay on Deception Island, South Shetland Islands and 65°14′44.6″ S, 64°15′26″ W: Galindez Island, Argentine Islands | B. subtilis, B. cereus, A. johnsonii, X. oryzae. | [56] |
| Janthinobacterium sp. | Soil | 62°12′ S, 58°57′ W: Fildes Peninsula, King George Island | Acinetobacter baumannii, P. aeruginosa, E. coli, K. pneumoniae, Serratia marcescens | [61] |
| Freshwater | 70°45′52.3″ S, 11°37′10.7″ E: Lake Podprudnoye, Schirmacher Oasis, Dronning Maud Land | Mycobacterium smegmatis, Mycobacterium tuberculosis | [62] | |
| Benthic microbial mat | Larsemann Hills, Vestfold Hills and McMurdo Dry Valleys | S. aureus, E. faecium, E. coli | [55] | |
| Ornithogenic soil | 62°59′ S, 60°34′ W: Whalers Bay on Deception Island, South Shetland Islands and 65°14′44.6″ S, 64°15′26″ W: Galindez Island, Argentine Islands | B. subtilis, B. cereus, P. aeruginosa, E. coli, Candida sp., Cryptococcus sp. | [56] | |
| Halomonas sp. | Sea water | 57°59′422” to 64°33′779″ S–45°27′440” to 63°16′554”: Antarctic Peninsula and South Shetland Islands area | E. coli, S. enterica, Enterobacter aerogenes, Citrobacter freundii, S. marcescens, Shigella sp., S. aureus, Staphylococcus epidermidis, B. subtilis, Xanthomonas sp., Erwinia sp. | [63] |
| Lysobacter oligotrophicus | Bottom of freshwater lake | Skarvsnes region | E. coli, Lysobacter enzymogenes, Rhodoligotrophos appendicifer, Saccharomyces cerevisiae. | [64] |
| Methylobacterium sp. | Soil | 62°24′ S, 59°47′ W: Barrientos Island | S. aureus, C. albicans | [57] |
| Paracoccus sp. | Soil | 62°24′ S, 59°47′ W: Barrientos Island | C. albicans | [57] |
| Pseudoalteromonas sp. | Sea water | 72°19′ S to 74°53′ S–163°48′ E to 70°16′ E: Stations Mergellina Santa Maria Novella, Tiburtina, Road Bay, Gerlache Inlet, Evans Cove, Inexpressible Island, Cape Hallet, and Tethys Bay | E. coli, M. luteus, P. mirabilis. | [14] |
| B. cepacia complex pathogens | [65] | |||
| Pseudoalteromonas haloplanktis | Sea water | 66°40′ S; 140° 01′ E: French Antarctic station Dumont d’ Urville, Terre Adélie | Biofilms formation of S. epidermidis | [66] |
| B. cepacia complex pathogens | [67,68] | |||
| Pseudomonas sp. | Soil | 72°19′ S to 77°83′ S, 16° 55′ E to 17° 16′ E: Cape Hallett, Edmonson Point, Kay Island, Cape Russell, Lake Hoare, Harrow Peaks, Crater Circe, Battleship Promontory, Mount, McGee, Mount Rittmann, Mount Melbourne | Listeria spp., B. thermosphacta | [17] |
| Sub-sea sediment | 74°41′36.96′ S, 164°6′42.12′ E: Terranova Bay, Ross sea | B. cepacia complex pathogens | [54] | |
| Marine sediment | 62°58′788” to 62°05′948″ S–60°33′464” to 58°23′622″ W: Deception Island, Martel Bay, King George Island and Punta Hannah sediment | M. luteus, S. aureus | [38] | |
| Benthic microbial mat | Larsemann Hills, Vestfold Hills and McMurdo Dry Valleys | S. aureus, E. faecium, E. coli | [55] | |
| Ornithogenic soil | 62°59′ S, 60°34′ W: Whalers Bay on Deception Island, South Shetland Islands and 65°14′44.6″ S, 64°15′26″ W: Galindez Island, Argentine Islands | B. subtilis, B. cereus, Sarcina lutea, M. luteus P. aeruginosa, E. coli, A. johnsonii, X. oryzae, Candida sp., Cryptococcus sp. | [56] | |
| Soil | 62°09′30.0″ S, 58°56′15.2″ W: King George Island | E. coli, S. enterica serovar Typhimurium, K. pneumoniae, E. cloacae, B. cereus, V. parahaemolyticus | [69] | |
| Pseudomonas fragi | Water column, rock surfaces | 62°12′ S, 58°57′ W: King George Island | Flavobacterium psychrophilum biofilm | [70] |
| Psychrobacter | Sub-sea sediment | 74°41′36.96″ S, 164°6′42.12″ E: Terranova Bay, Ross sea | B. cepacia complex pathogens | [54] |
| Benthic microbial mat | Larsemann Hills, Vestfold Hills and McMurdo Dry Valleys | S. aureus, E. coli | [55] | |
| Shewanella sp. | Lake ponds benthic microbial mats | Larsemann Hills, Vestfold Hills andMcMurdo Dry Valleys | S. aureus, E. coli | [55] |
| Sphingomonas sp. | Soil | 62°24′ S, 59°47′ W: Barrientos Island | C. albicans | [57] |
| Cyanobacteria | ||||
| Leptolyngbya antarctica | Benthic microbial mat | Larsemann Hills, Bølingen Islands, Vestfold Hills, Rauer Islands, and the McMurdo Dry Valleys | S. aureus | [71] |
| Nostoc sp. | Benthic microbial mat | Larsemann Hills, Bølingen Islands, Vestfold Hills, Rauer Islands, and the McMurdo Dry Valleys | M. tuberculosis, S. aureus, S. typhi, P. aeruginosa, E. aerogenes, multidrug-resistant strains of E. coli, C. neoformans | [71,72] |
| Phormidium priestleyi | Benthic microbial mat | Larsemann Hills, Bølingen Islands, Vestfold Hills, Rauer Islands, and the McMurdo Dry Valleys | S. aureus, A. fumigatus, Cryptococcus neoformans | [71] |
| Phormidium murrayi | Benthic microbial mat | Larsemann Hills, Bølingen Islands, Vestfold Hills, Rauer Islands, and the McMurdo Dry Valleys | S. aureus | [71] |
| Pseudophormidium sp. | Benthic microbial mat | Larsemann Hills, Bølingen Islands, Vestfold Hills, Rauer Islands, and the McMurdo Dry Valleys | S. aureus, C. neoformans | [71] |
| Firmicutes | ||||
| Bacillus sp. | Sea water | 50°76′ W, 61°16′ S | Paecilomyces variotii, Colletotrichum gloeosporioides, Fusarium oxysporum, Trichoderma viride, Rhizoctonia solani | [73] |
| Ornithogenic soil | 62°59′ S, 60°34′ W: Whalers Bay on Deception Island, South Shetland Islands and 65°14′44.6′ S, 64°15′26″ W: Galindez Island, Argentine Islands | B. subtilis, B. cereus, P. aeruginosa, E. coli, A. johnsonii, Candida sp. | [56] | |
| Soil | 62°04′ S, 58°21′ W: King George Island | Methicillin resistant S. aureus, C. albicans | [74] | |
| Enterococcus sp. | Soil | 69°21.68′ S, 76°07.76′ E; 69°21.68′ S, 76°07.70′ E and 69º22.433′ S, 76°08.940′ E: Penguin rookeries Larsemann Hills, East Antarctica | Multidrug-resistant strains of C. albicans | [75] |
| Planococcus sp. | Soil | 72°19′ S to 77°83′ S–16° 55′ E to 17° 16′ E: Cape Hallett, Edmonson Point, Kay Island, Cape Russell, Lake Hoare, Harrow Peaks, Crater Circe, Battleship Promontory, Mount, McGee, Mount Rittmann, Mount Melbourne | Listeria spp., B. thermosphacta | [17] |
| Sporosarcina sp. | Ornithogenic soil | 62°59′ S, 60°34′ W: Whalers Bay on Deception Island, South Shetland Islands and 65°14′44.6″ S, 64°15′26″ W: Galindez Island, Argentine Islands | B. subtilis, B. cereus, S. lutea, P. aeruginosa, E. coli, A. johnsonii, X. oryzae, Candida sp., Cryptococcus sp. | [56] |
| 62°04′ S, 58°21′ W: King George Island | Methicillin resistant S. aureus, C. albicans | [74] | ||
| Bacteroidetes | ||||
| Flavobacterium sp. | Freshwater | 70°45′52.3″ S, 11°37′10.7″ E: Lake Podprudnoye, Schirmacher Oasis, Dronning Maud Land | M. smegmatis, M. tuberculosis | [62] |
| Pedobacter sp. | Soil | 62°09′30.0″ S, 58°56′15.2″ W: King George Island | E. coli, S. enterica serovar Typhimurium, S. enterica serovar Typhi, K. pneumoniae, E. cloacae, B. cereus | [69] |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Núñez-Montero, K.; Barrientos, L. Advances in Antarctic Research for Antimicrobial Discovery: A Comprehensive Narrative Review of Bacteria from Antarctic Environments as Potential Sources of Novel Antibiotic Compounds Against Human Pathogens and Microorganisms of Industrial Importance. Antibiotics 2018, 7, 90. https://doi.org/10.3390/antibiotics7040090
Núñez-Montero K, Barrientos L. Advances in Antarctic Research for Antimicrobial Discovery: A Comprehensive Narrative Review of Bacteria from Antarctic Environments as Potential Sources of Novel Antibiotic Compounds Against Human Pathogens and Microorganisms of Industrial Importance. Antibiotics. 2018; 7(4):90. https://doi.org/10.3390/antibiotics7040090
Chicago/Turabian StyleNúñez-Montero, Kattia, and Leticia Barrientos. 2018. "Advances in Antarctic Research for Antimicrobial Discovery: A Comprehensive Narrative Review of Bacteria from Antarctic Environments as Potential Sources of Novel Antibiotic Compounds Against Human Pathogens and Microorganisms of Industrial Importance" Antibiotics 7, no. 4: 90. https://doi.org/10.3390/antibiotics7040090
APA StyleNúñez-Montero, K., & Barrientos, L. (2018). Advances in Antarctic Research for Antimicrobial Discovery: A Comprehensive Narrative Review of Bacteria from Antarctic Environments as Potential Sources of Novel Antibiotic Compounds Against Human Pathogens and Microorganisms of Industrial Importance. Antibiotics, 7(4), 90. https://doi.org/10.3390/antibiotics7040090






