Phenoxymethylpenicillin Versus Amoxicillin for Infections in Ambulatory Care: A Systematic Review
Abstract
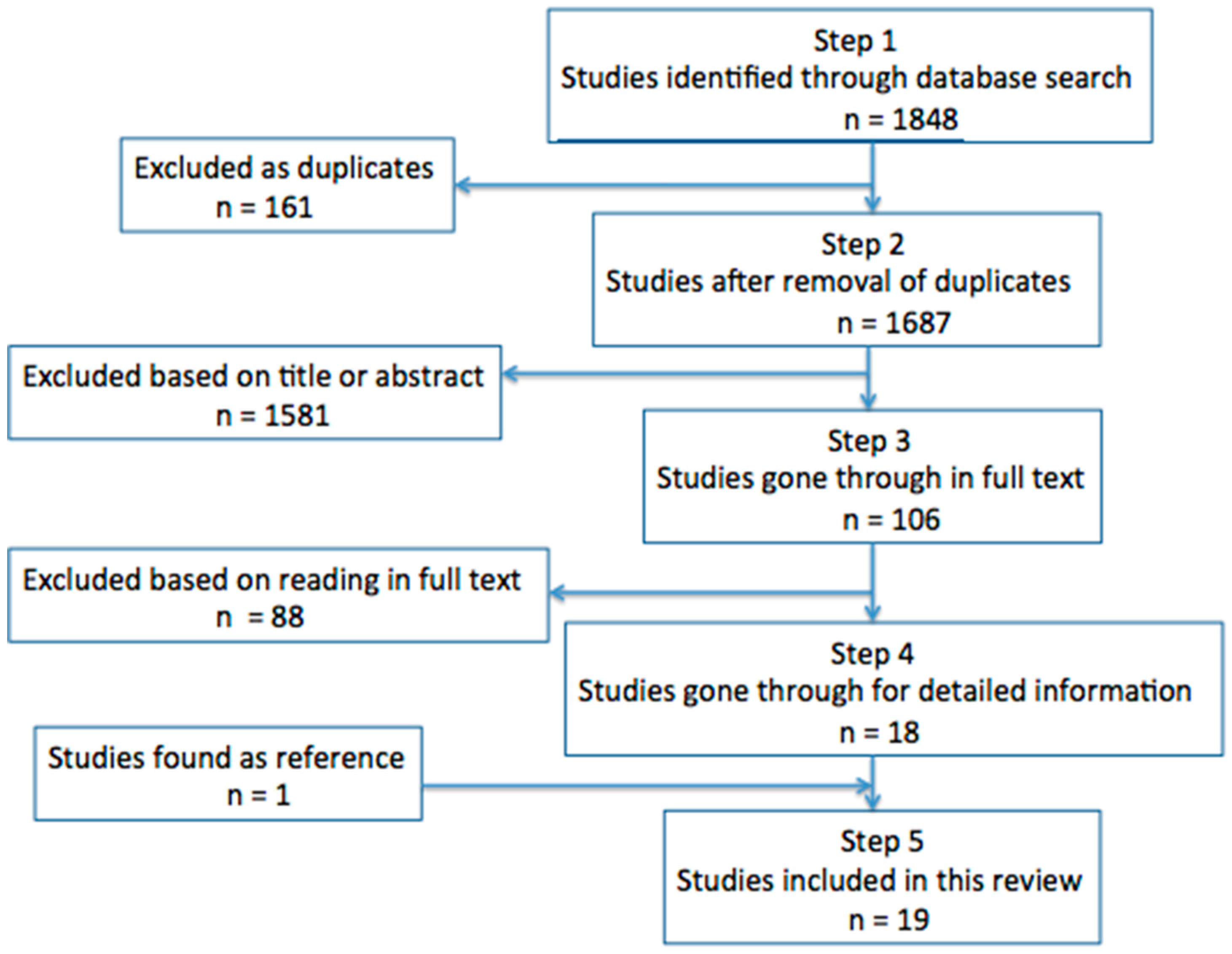
:1. Introduction
2. Results
2.1. Group A Streptococci (GAS) Tonsillitis
2.2. Acute Sinusitis
2.3. Acute Otitis Media
2.4. Lyme Borreliosis
2.5. Pneumonia
3. Discussion
3.1. Summary of Main Results
3.2. Strengths and Limitations
3.3. Comparison with Other Literature
4. Materials and Methods
4.1. Basis of Knowledge
4.2. Inclusion Criteria
4.3. Exclusion Criteria
4.4. Quality Assessment
4.5. Seach Strings
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Straand, J.; Rokstad, K.S.; Sandvik, H. Prescribing systemic antibiotics in general practice: A report from the More & Romsdal Prescription Study. Scand. J. Prim. Health Care 1998, 16, 121–127. [Google Scholar] [PubMed]
- National Guidelines on Antibiotics Use in Primary Care [Nasjonale Faglige Retningslinjer for Antibiotikabruk i Primærhelsetjenesten]. 2012, Directorate of Health: Oslo. Antibiotikabruk i primærhelsetjenesten. Available online: www.antibiotikaiallmennpraksis.no (accessed on 4 September 2018).
- NORM/NORM-VET 2016. Usage of Antimicrobial Agents and Occurrence of Antimicrobial Resistance in Norway. Available online: https://unn.no/Documents/Kompetansetjenester,%20-sentre%20og%20fagr%C3%A5d/NORM%20-%20Norsk%20overv%C3%A5kingssystem%20for%20antibiotikaresistens%20hos%20mikrober/Rapporter/NORM%20NORM-VET%202016.pdf (accessed on 4 September 2018).
- Versporten, A.; Coenen, S.; Adriaenssens, N.; Muller, A.; Minalu, G.; Faes, C.; Vankerckhoven, V.; Aerts, M.; Hens, N.; Molenberghs, G.; et al. European Surveillance of Antimicrobial Consumption (ESAC): Outpatient penicillin use in Europe (1997–2009). J. Antimicrob. Chemother. 2011, 66, S13–S23. [Google Scholar] [CrossRef] [PubMed]
- Adriaenssens, N.; Coenen, S.; Adriaenssens, N.; Muller, A.; Minalu, G.; Faes, C.; Vankerckhoven, V.; Aerts, M.; Hens, N.; Molenberghs, G.; et al. European surveillance of antimicrobial consumption (ESAC): Outpatient antibiotic use in Europe (1997–2009). J. Antimicrob. Chemother. 2011, 66, S3–S12. [Google Scholar] [CrossRef] [PubMed]
- Pulcini, C.; Bush, K.; Craig, W.A.; Frimodt-Møller, N.; Grayson, M.L.; Mouton, J.W.; Turnidge, J.; Harbarth, S.; Gyssens, I.C.; ESCMID Study Group for Antibiotic Policies. Forgotten antibiotics: An inventory in Europe, the United States, Canada, and Australia. Clin. Infect. Dis. 2012, 54, 268–274. [Google Scholar] [CrossRef] [PubMed]
- Breese, B.B.; Disney, F.A.; Talpey, W.B.; Green, J.L. Treatment of streptococcal pharyngitis with amoxicillin. J. Infect. Dis. 1974, 129, S178–S180. [Google Scholar] [CrossRef]
- Stillerman, M.; Isenberg, H.D.; Facklam, R.R. Treatment of pharyngitis associated with group A Streptococcus: Comparison of amoxicillin and potassium phenoxymethyl penicillin. J. Infect. Dis. 1974, 129, S169–S177. [Google Scholar] [CrossRef]
- Breese, B.B.; Disney, F.A.; Green, J.L.; Talpey, W.B. The treatment of beta hemolytic streptococcal pharyngitis. Comparison of amoxicillin, erythromycin estolate, and penicillin V. Clin. Pediatr. (Phila) 1977, 16, 460–463. [Google Scholar] [CrossRef] [PubMed]
- Pankey, G.A.; Gleason, C.; File, T.M., Jr.; Brown, W. A comparative study of bacampicillin hydrochloride, penicillin V, and amoxicillin in the treatment of acute tonsillitis and/or pharyngitis due to beta-hemolytic streptococci. Rev. Infect. Dis. 1981, 3, 154–158. [Google Scholar] [CrossRef] [PubMed]
- Shvartzman, P.; Tabenkin, H.; Rosentzwaig, A.; Dolginov, F. Treatment of streptococcal pharyngitis with amoxycillin once a day. BMJ 1993, 306, 1170–1172. [Google Scholar] [CrossRef] [PubMed]
- Peyramond, D.; Portier, H.; Geslin, P.; Cohen, R. 6-day amoxicillin versus 10-day penicillin V for group A beta-haemolytic streptococcal acute tonsillitis in adults: A French multicentre, open-label, randomized study. Scand. J. Infect. Dis. 1996, 28, 497–501. [Google Scholar] [CrossRef] [PubMed]
- Cohen, R.; Levy, C.; Doit, C.; De La Rocque, F.; Boucherat, M.; Fitoussi, F.; Langue, J.; Bingen, E. Six-day amoxicillin vs. ten-day penicillin V therapy for group A streptococcal tonsillopharyngitis. Pediatr. Infect. Dis. J. 1996, 15, 78–682. [Google Scholar] [CrossRef]
- Feder, H.M., Jr.; Gerber, M.A.; Randolph, M.F.; Stelmach, P.S.; Kaplan, E.L. Once-daily therapy for streptococcal pharyngitis with amoxicillin. Pediatrics 1999, 103, 47–51. [Google Scholar] [CrossRef] [PubMed]
- Curtin-Wirt, C.; Casey, J.R.; Murray, P.C.; Cleary, C.T.; Hoeger, W.J.; Marsocci, S.M.; Murphy, M.L.; Francis, A.B.; Pichichero, M.E. Efficacy of penicillin vs. amoxicillin in children with group A beta hemolytic streptococcal tonsillopharyngitis. Clin. Pediatr. (Phila) 2003, 42, 219–225. [Google Scholar] [CrossRef] [PubMed]
- Lennon, D.R.; Farrell, E.; Martin, D.R.; Stewart, J.M. Once-daily amoxicillin versus twice-daily penicillin V in group A beta-haemolytic streptococcal pharyngitis. Arch. Dis. Child 2008, 93, 474–478. [Google Scholar] [CrossRef] [PubMed]
- Pichichero, M.E.; Casey, J.R.; Block, S.L.; Guttendorf, R.; Flanner, H.; Markowitz, D.; Clausen, S. Pharmacodynamic analysis and clinical trial of amoxicillin sprinkle administered once daily for 7 days compared to penicillin V potassium administered four times daily for 10 days in the treatment of tonsillopharyngitis due to Streptococcus pyogenes in children. Antimicrob. Agents Chemother. 2008, 52, 2512–2520. [Google Scholar] [PubMed]
- Lindbaek, M.; Hjortdahl, P.; Johnsen, U.L. Randomised, double blind, placebo controlled trial of penicillin V and amoxycillin in treatment of acute sinus infections in adults. BMJ 1996, 313, 325–329. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lindbaek, M.; Kaastad, E.; Dølvik, S.; Johnsen, U.; Laerum, E.; Hjortdahl, P. Antibiotic treatment of patients with mucosal thickening in the paranasal sinuses, and validation of cut-off points in sinus CT. Rhinology 1998, 36, 7–11. [Google Scholar] [PubMed]
- Varonen, H.; Kunnamo, I.; Savolainen, S.; Mäkelä, M.; Revonta, M.; Ruotsalainen, J.; Malmberg, H. Treatment of acute rhinosinusitis diagnosed by clinical criteria or ultrasound in primary care: A placebo-controlled randomised trial. Scand. J. Prim. Health Care 2003, 21, 121–126. [Google Scholar] [CrossRef] [PubMed]
- Howard, J.E.; Nelson, J.D.; Clahsen, J.; Jackson, L.H. Otitis media of infancy and early childhood. A double-blind study of four treatment regimens. Am. J. Dis. Child. 1976, 130, 965–970. [Google Scholar] [CrossRef] [PubMed]
- Puhakka, H.; Virolainen, E.; Aantaa, E. Treatment of acute otitis media in children: Penicillin-V or amoxicilin? Acta Otolaryngol. 1982, 94, S108–S111. [Google Scholar] [CrossRef]
- Strle, F.; Maraspin, V.; Pleterski-Rigler, D.; Lotrič-Furlan, S.; Jurca, T.; Cimperman, J.; Ružić-Sabljic, E. Treatment of borrelial lymphocytoma. Infection 1996, 24, 80–84. [Google Scholar] [CrossRef] [PubMed]
- Eliassen, K.E.; Reiso, H.; Berild, D.; Lindbæk, M. Comparison of phenoxymethylpenicillin, amoxicillin, and doxycycline for erythema migrans in general practice. A randomized controlled trial with a 1-year follow-up. Clin. Microbiol. Infect. 2018. [Google Scholar] [CrossRef] [PubMed]
- Llor, C.; Pérez, A.; Carandell, E.; García-Sangenís, A.; Rezola, J.; Llorente, M.; Gestoso, S.; Bobé, F.; Román-Rodríguez, M.; Cots, J.M.; et al. Efficacy of high doses of penicillin versus amoxicillin in the treatment of uncomplicated community acquired pneumonia in adults. A non-inferiority controlled clinical trial. Aten. Primaria 2017. [Google Scholar] [CrossRef] [PubMed]
- Bodey, G.P.; Nance, J. Amoxicillin: In vitro and pharmacological studies. Antimicrob. Agents Chemother. 1972, 1, 358–362. [Google Scholar] [CrossRef] [PubMed]
- Cattoir, V. Mechanisms of Antibiotic Resistance. In Streptococcus pyogenes: Basic Biology to Clinical Manifestations; Ferretti, J.J., Stevens, D.L., Fischetti, V.A., Eds.; University of Oklahoma Health Sciences Center: Oklahoma City, OK, USA, 2016. [Google Scholar]
- Diagnosis, Treatment and Follow-Up of Acute Mediaotitis (AOM)—New Recommendation [Diagnostik, Behandling och Uppföljning av akut Mediaotit (AOM)—Ny Rekommendation]. Information från Läkemedelsverket 2010(5). pp. 13–24. Available online: https://lakemedelsverket.se/upload/halso-och-sjukvard/behandlingsrekommendationer/Akut%20mediaotit_rek_webb.pdf (accessed on 4 September 2018).
- Acute Otitis Media in Children: Treatment. Available online: https://www.uptodate.com/contents/acute-otitis-media-in-children-treatment (accessed on 4 September 2018).
- Otitis Media (Acute): Antimicrobial Prescribing. Available online: https://www.nice.org.uk/guidance/ng91 (accessed on 4 September 2018).
- Uncomplicated Acute Sinusitis and Rhinosinusitis in Adults: Treatment. Available online: https://www.uptodate.com/contents/uncomplicated-acute-sinusitis-and-rhinosinusitis-in-adults-treatment (accessed on 4 September 2018).
- Sinusitis (Acute): Antimicrobial Prescribing. Available online: https://www.nice.org.uk/Guidance/NG79 (accessed on 4 September 2018).
- Treatment of Lyme Disease. Available online: https://www.uptodate.com/contents/treatment-of-lyme-disease (accessed on 4 September 2018).
- Lyme Disease. Available online: https://www.nice.org.uk/guidance/ng95 (accessed on 4 September 2018).
- Drug Treatment of Borrelia Infection—New Recommendation [Läkemedelsbehandling av Borreliainfektion—Ny Rekommendation]. Information från Läkemedelsverket, 2009(4). Available online: https://lakemedelsverket.se/upload/halso-och-sjukvard/behandlingsrekommendationer/Borrelia-rek_webb_bokm%C3%A4rken.pdf (accessed on 4 September 2018).
- Pneumonia in Adults: Diagnosis and Management. Available online: https://www.nice.org.uk/guidance/cg191 (accessed on 4 September 2018).
- Butler, C.C.; Hood, K.; Verheij, T.; Little, P.; Melbye, H.; Nuttall, J.; Kelly, M.J.; Mölstad, S.; Godycki-Cwirko, M.; Almirall, J.; et al. Variation in antibiotic prescribing and its impact on recovery in patients with acute cough in primary care: Prospective study in 13 countries. BMJ 2009, 338. [Google Scholar] [CrossRef] [PubMed]
- Gjelstad, S.; Straand, J.; Dalen, I.; Fetveit, A.; Strøm, H.; Lindbæk, M. Do general practitioners’ consultation rates influence their prescribing patterns of antibiotics for acute respiratory tract infections? J. Antimicrob. Chemother. 2011, 66, 2425–2433. [Google Scholar] [CrossRef] [PubMed]
- Rafii, F.; Sutherland, J.B.; Cerniglia, C.E. Effects of treatment with antimicrobial agents on the human colonic microflora. Ther. Clin. Risk Manag. 2008, 4, 1343–1358. [Google Scholar] [CrossRef] [PubMed]
- European Centre for Disease Prevention and Control. Surveillance of Antimicrobial Resistance in Europe 2016. Annual Report of the European Antimicrobial Resistance Surveillance Network (EARS-Net). Stockholm: ECDC. 2017. Available online: https://ecdc.europa.eu/sites/portal/files/documents/AMR-surveillance-Europe-2016.pdf (accessed on 4 September 2018).
- Blandhol, M.; Tysland, T.; Blix, H.S.; Høye, S. Antibiotic switch during treatment with antibiotics against respiratory tract infections in ambulatory care in Norway. Infect. Dis. 2017, 49, 854–858. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Checklist for Assessing a Randomized Controlled Study (RCT) [Sjekkliste for Vurdering av en Randomisert Kontrollert Studie (RCT)]. Available online: https://www.fhi.no/globalassets/dokumenterfiler/tema/brukererfaring/sjekkliste-rct-2014.pdf (accessed on 4 September 2017).
| Lead Author (Reference) | Year, Country | Design, Population | Age in Years | Daily Dose (days) PcV-Amoxicillin | Clinical Outcome PcV-Amoxicillin | Signific-ance | Quality |
|---|---|---|---|---|---|---|---|
| GAS | |||||||
| Breese, B.B. [7] | 1974, USA | RCT, 159 | “Children” | 375 mg (10)–375 mg (10) | Cure rate after five weeks: 80.8–76.6% | NSD | Medium |
| Stillerman, M. [8] | 1974, USA | RCT, 112 | “Children” | 375 mg (10)–375 mg (10) | Cure rate within 72 h: 91–89% | * | Medium |
| Breese, B.B. [9] | 1977, USA | RCT, 172 1 | 1–17 | 375–750 mg (10)–375–750 mg (10) | Cure rate after six weeks: 87.9–90.6% | NSD | Medium |
| Pankey, G. A. [10] | 1981, USA | RCT, 83 2 | 6–40 | 1000 mg (10)–750 mg (10) | Cure rate at day 13–14: 100–100% | NSD | Low |
| Shvartzman, P. [11] | 1993, Israel | RCT, 157 | >3 | 750–1000 mg (10)–750 mg (10) | Days off school/work: 139 days for 64 patients–100 days for 57 patients | NSD | Medium |
| Peyramond, D. [12] | 1996, France | RCT, 342 | >15 | 1980 mg (10)–2000 mg (6) | Cure rate at end of treatment: 96.5–96.4% | NSD | High |
| Cohen, R. M.D. [13] | 1996, France | RCT, 321 | 3–15 | 45 mg/kg (10)–50 mg/kg (6) | Cure rate: 89.0–90.8% | NSD | Medium |
| Feder, H.M., Jr. [14] | 1999, USA | RCT, 152 | 4–18 | 750 mg (10)–750 mg (10) | Cure rate after 18–24 h: 90–90% | NSD | Medium |
| Curtin-Wirt, C. [15] | 2003, USA | CT, 389 | 0–20 | 35 mg/kg–1000 mg (10)–35 mg/kg–1000 mg (10) | Cure rate at day 10 +/− 4: 73–84% | Amoxicillin superior, p = 0.03 | High |
| Lennon, D. R. [16] | 2008, NZ | RCT, 353 | 5–12 | 500–1000 (10)–750–1500 (10) | “No difference in clinical symptom resolution” | * | High |
| Pichichero, M. E. [17] | 2008, USA | RCT, 579 | 0–12 | 40 mg/kg (10)–475–775 mg (7) | Cure rate: 91.9–86.1% | NSD | High |
| Acute Sinusitis | |||||||
| Lindbaek, M. [18] | 1996, Norway | RCT, 130 3 | 16–74 | 3960 mg (10)–1500 mg (10) | Cure rate at day 10: 82.1–88.6% | NSD | High |
| Lindbaek, M. [19] | 1998, Norway | RCT, 70 3 | 16–83 | 3960 mg (10)–1500 mg (10) | Cure rates at day 10: 75–77% | NSD | Medium |
| Varonen, H. [20] | 2003, Finland | RCT, 150 3,4 | 18–75 | 3000 mg (7)–1500 mg (7) | Cure rate at day 14–16: 81–78% | NSD | High |
| Acute Otitis | |||||||
| Howard, J. E. [21] | 1976, USA | RCT, 383 5 | 0–5 | 50 mg/kg (10)–30 mg/kg (10) | Cure rate at day 10: 75–92% | Amoxicillin superior, p < 0.05 | High |
| Puhakka, H. [22] | 1982, Finland | RCT, 65 | 0–9 | 75–80 mg/kg (10)–40 mg/kg (10) | Cure rate at day 10 and day 24: 44–32%, 88–87% | NSD | Medium |
| Lyme Borreliosis | |||||||
| Strle, F. [23] | 1996, Slovenia | CT, 65 4,6 | 1–72 | 3000 mg (14)–3000 mg (14) | Median duration of lymphocytoma: 2 weeks–1.5 weeks. | * | Medium |
| Eliassen K.E. [24] | 2018, Norway | RCT, 188 4 | 18–85 | 3900 mg (14)–1500 mg (14) | Median duration of erythema migrans: 14 days–13 days | NSD | High |
| Pneumonia | |||||||
| Llor, C. [25] | 2017, Spain | RCT, 36 | 18–75 | 3200 mg (10)–3000 mg (10) | Cure rate at day 14: 71.4–100% (ITT) 90.9–100% (PP) | ITT: Amoxicillin superio, p = 0.009. PP: NSD | High |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Skarpeid, P.L.; Høye, S. Phenoxymethylpenicillin Versus Amoxicillin for Infections in Ambulatory Care: A Systematic Review. Antibiotics 2018, 7, 81. https://doi.org/10.3390/antibiotics7030081
Skarpeid PL, Høye S. Phenoxymethylpenicillin Versus Amoxicillin for Infections in Ambulatory Care: A Systematic Review. Antibiotics. 2018; 7(3):81. https://doi.org/10.3390/antibiotics7030081
Chicago/Turabian StyleSkarpeid, Philip Lawrence, and Sigurd Høye. 2018. "Phenoxymethylpenicillin Versus Amoxicillin for Infections in Ambulatory Care: A Systematic Review" Antibiotics 7, no. 3: 81. https://doi.org/10.3390/antibiotics7030081
APA StyleSkarpeid, P. L., & Høye, S. (2018). Phenoxymethylpenicillin Versus Amoxicillin for Infections in Ambulatory Care: A Systematic Review. Antibiotics, 7(3), 81. https://doi.org/10.3390/antibiotics7030081




