Silver and Antibiotic, New Facts to an Old Story
Abstract
1. Introduction
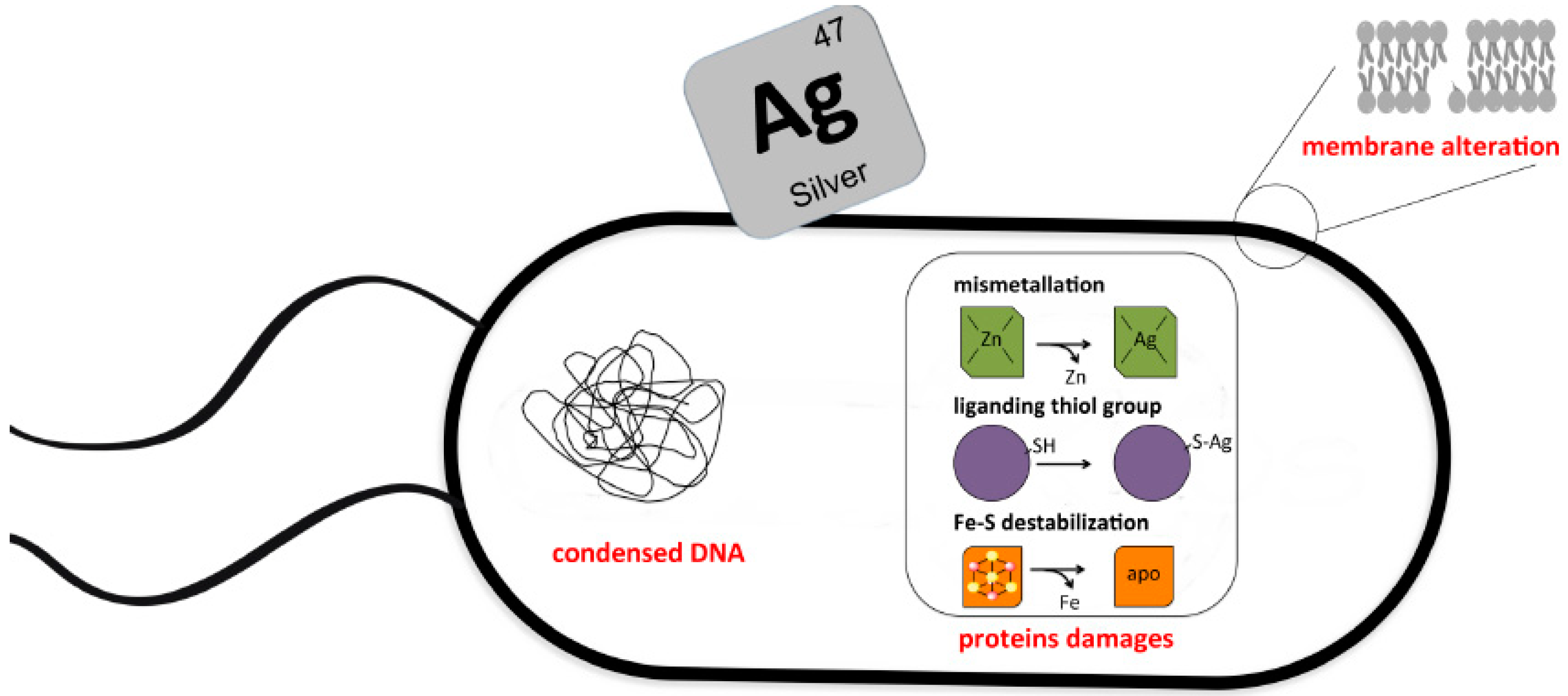
2. Molecular Basis of Silver Toxicity toward Microbes
2.1. Silver Ions Target DNA
2.2. Silver Ions Target Proteins
2.3. Silver Mediated Membrane Alteration
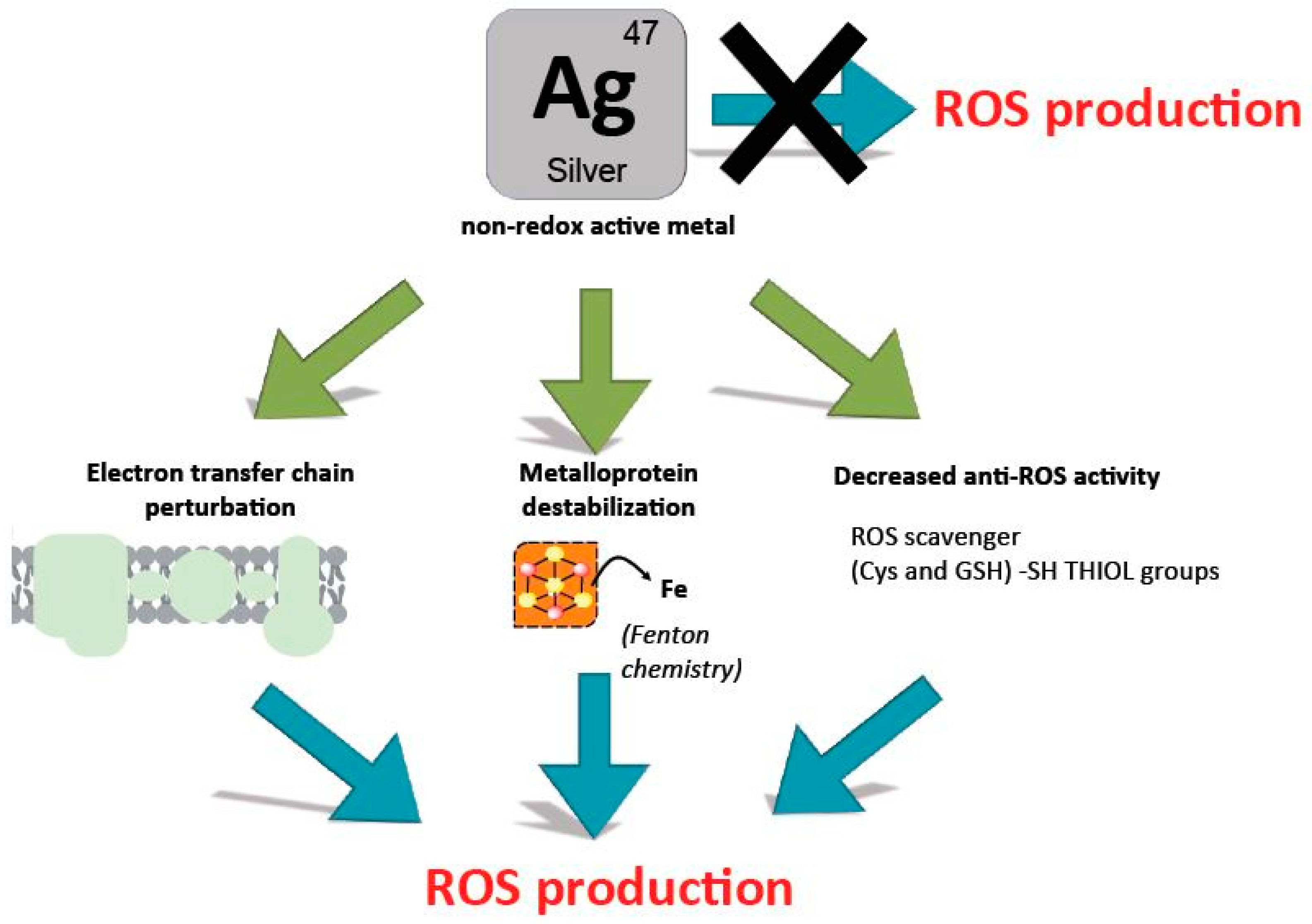
2.4. Are Silver Ions Producing ROS?
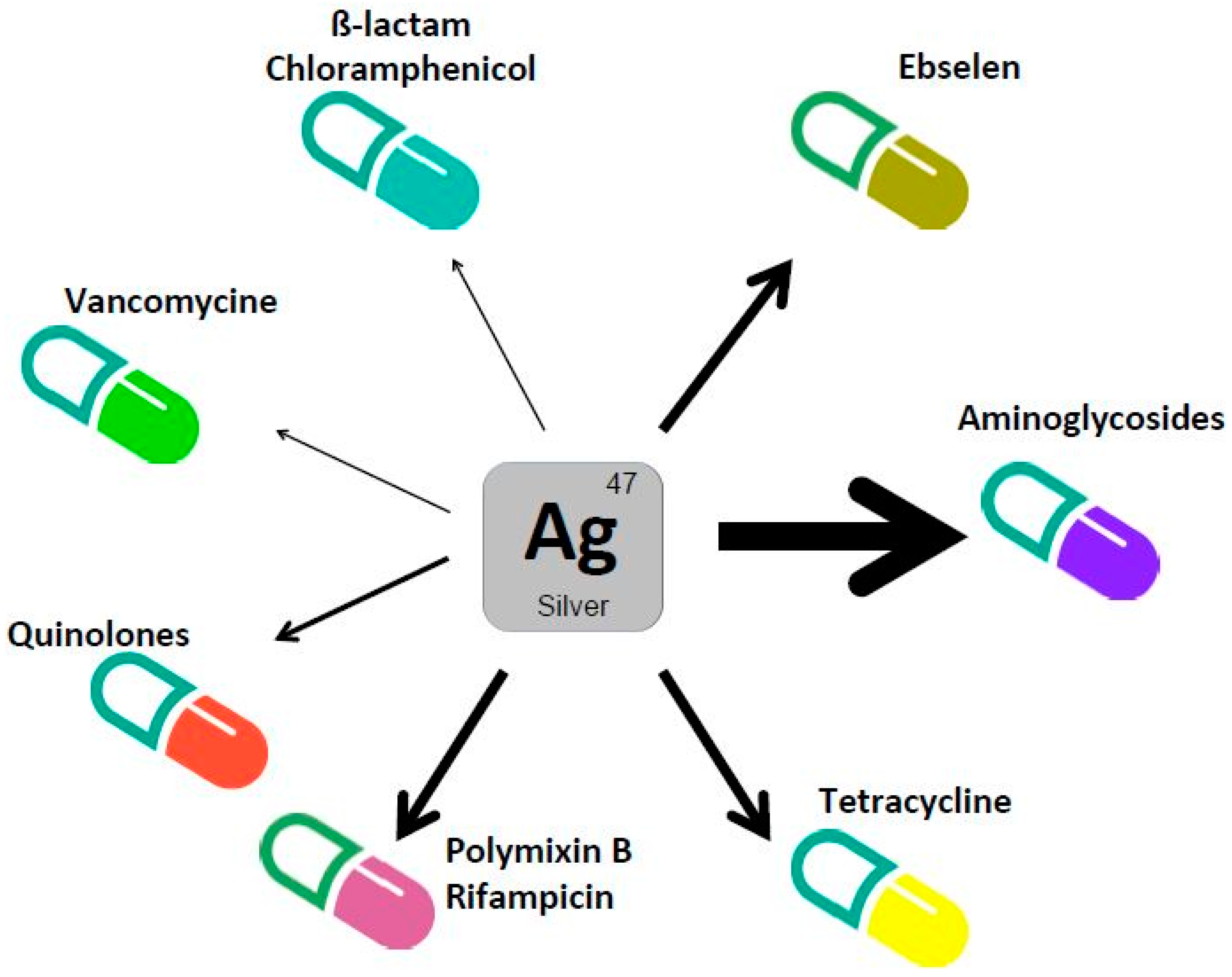
3. Silver Enhances Antibacterial Activity of Antibiotics
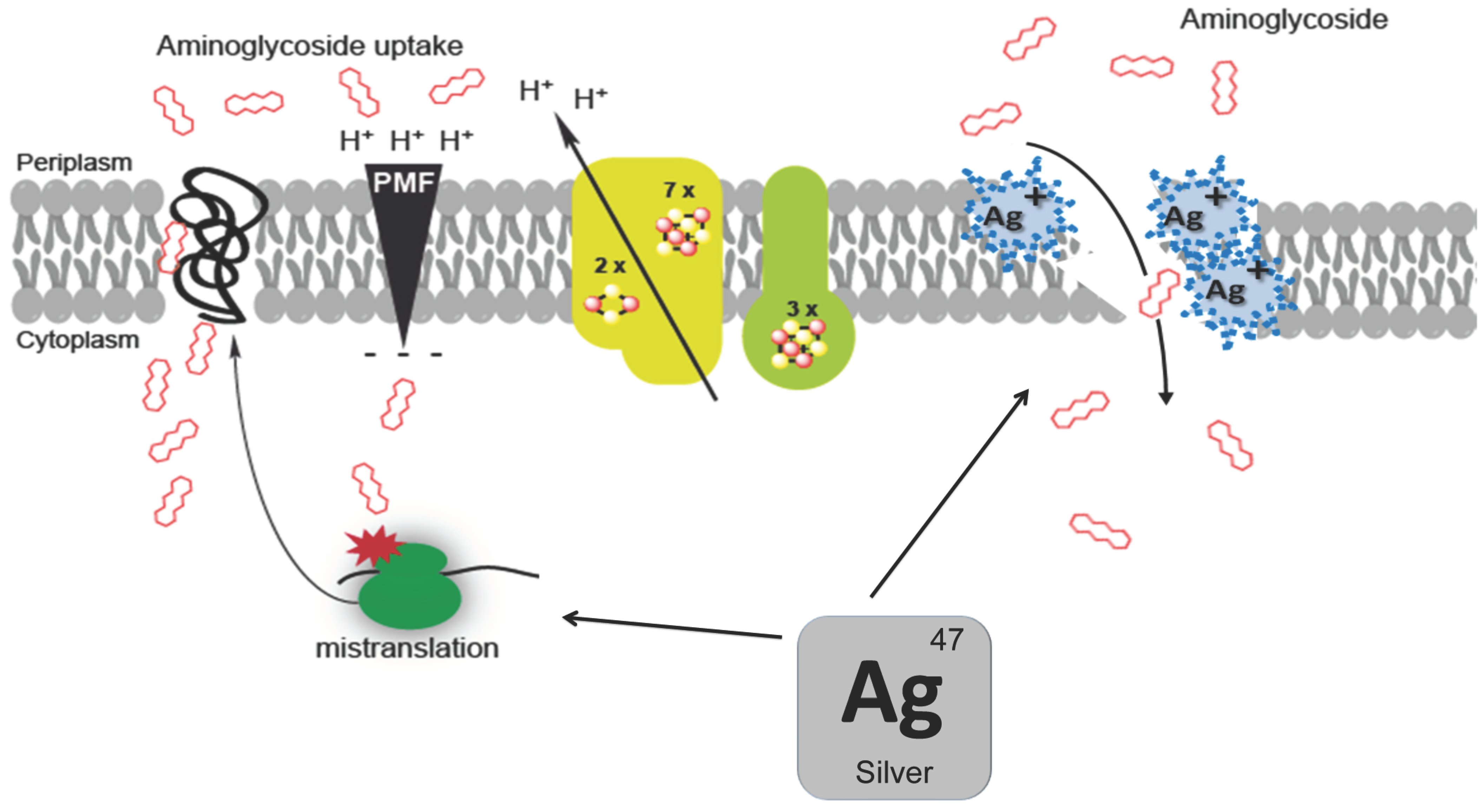
4. Molecular Mechanism in the Aminoglycoside/Silver Synergy
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Mijnendonckx, K.; Leys, N.; Mahillon, J.; Silver, S.; Van Houdt, R. Antimicrobial silver: Uses, toxicity and potential for resistance. BioMetals 2013, 26, 609–621. [Google Scholar] [CrossRef] [PubMed]
- Brochado, A.R.; Telzerow, A.; Bobonis, J.; Banzhaf, M.; Mateus, A.; Selkrig, J.; Huth, E.; Bassler, S.; Beas, J.Z.; Zietek, M.; et al. Species-specific activity of antibacterial drug combinations. Nature 2018, 559, 259–263. [Google Scholar] [CrossRef] [PubMed]
- Morones-Ramirez, J.R.; Winkler, J.A.; Spina, C.S.; Collins, J.J. Silver enhances antibiotic activity against gram-negative bacteria. Sci. Transl. Med. 2013, 5, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Herisse, M.; Duverger, Y.; Martin-Verstraete, I.; Barras, F.; Ezraty, B. Silver potentiates aminoglycoside toxicity by enhancing their uptake. Mol. Microbiol. 2017, 105, 115–126. [Google Scholar] [CrossRef] [PubMed]
- Bartłomiejczyk, T.; Lankoff, A.; Kruszewski, M.; Szumiel, I. Silver nanoparticles—Allies or adversaries? Ann. Agric. Environ. Med. 2013, 20, 48–54. [Google Scholar] [PubMed]
- Jakobsen, V.; Viganor, L.; Blanco-Fernández, A.; Howe, O.; Devereux, M.; McKenzie, C.J.; McKee, V. Tetrameric and polymeric silver complexes of the omeprazole scaffold; synthesis, structure, in vitro and in vivo antimicrobial activities and DNA interaction. J. Inorg. Biochem. 2018, 186, 317–328. [Google Scholar] [CrossRef] [PubMed]
- Leonhard, V.; Alasino, R.V.; Muñoz, A.; Beltramo, D.M. Silver nanoparticles with high loading capacity of amphotericin B: Characterization, bactericidal and antifungal effects. Curr. Drug Deliv. 2018. [Google Scholar] [CrossRef] [PubMed]
- De Matteis, V.; Cascione, M.; Toma, C.; Leporatti, S. Silver nanoparticles: Synthetic routes, in vitro toxicity and theranostic applications for cancer disease. Nanomaterials 2018, 8, 319. [Google Scholar] [CrossRef] [PubMed]
- Yudkin, J. The effect of silver ions on some enzymes of Bacterium coli. Enzymologia 1937, 2, 161–170. [Google Scholar]
- Arakawa, H.; Neault, J.F.; Tajmir-Riahi, H.A. Silver(I) complexes with DNA and RNA studied by fourier transform infrared spectroscopy and capillary electrophoresis. Biophys. J. 2001, 81, 1580–1587. [Google Scholar] [CrossRef]
- Russell, A.D.; Hugo, W.B. Antimicrobial activity and action of silver. Prog. Med. Chem. 1994, 31, 351–370. [Google Scholar] [PubMed]
- Rainnie, D.J.; Bragg, P.; Bragg, P.D.; Rainnie, D.J. The effect of silver ions on the respiratory chain of Escherichia coli. Can. J. Microbiol. 1974, 20, 883–889. [Google Scholar]
- Dibrov, P.; Dzioba, J.; Gosink, K.K.; Häse, C.C.; Ha, C.C. Chemiosmotic mechanism of antimicrobial activity of Ag+ in vibrio cholerae. Antimicrob. Agents Chemother. 2002, 46, 2668–2670. [Google Scholar] [CrossRef] [PubMed]
- Schreurs, W.J.A.; Rosenberg, H. Effect of silver ions on transport and retention of phosphate by Escherichia coli. J. Bacteriol. 1982, 152, 7–13. [Google Scholar] [PubMed]
- Xu, F.F.; Imlay, J.A. Silver(I), mercury(II), cadmium(II), and zinc(II) target exposed enzymic iron-sulfur clusters when they toxify Escherichia coli. Appl. Environ. Microbiol. 2012, 78, 3614–3621. [Google Scholar] [CrossRef] [PubMed]
- Feng, Q.L.; Wu, J.; Chen, G.Q.; Cui, F.Z.; Kim, T.N.; Kim, J.O. A mechanistic study of the antibacterial effect of silver ions on Escherichia coli and Staphylococcus aureus. J. Biomed. Mater. Res. 2000, 52, 662–668. [Google Scholar] [CrossRef]
- Gu, M.; Imlay, J.A. The SoxRS response of Escherichia coli is directly activated by redox-cycling drugs rather than by superoxide. Mol. Microbiol. 2011, 79, 1136–1150. [Google Scholar] [CrossRef] [PubMed]
- Park, H.J.; Kim, J.Y.; Kim, J.; Lee, J.H.; Hahn, J.S.; Gu, M.B.; Yoon, J. Silver-ion-mediated reactive oxygen species generation affecting bactericidal activity. Water Res. 2009, 43, 1027–1032. [Google Scholar] [CrossRef] [PubMed]
- Saulou-Bérion, C.; Gonzalez, I.; Enjalbert, B.; Audinot, J.-N.; Fourquaux, I.; Jamme, F.; Cocaign-Bousquet, M.; Mercier-Bonin, M.; Girbal, L. Escherichia coli under ionic silver stress: An integrative approach to explore transcriptional, physiological and biochemical responses. PLoS ONE 2015, 10, e0145748. [Google Scholar] [CrossRef] [PubMed]
- Zou, L.; Lu, J.; Wang, J.; Ren, X.; Zhang, L.; Gao, Y.; Rottenberg, M.E.; Holmgren, A. Synergistic antibacterial effect of silver and ebselen against multidrug-resistant Gram-negative bacterial infections. EMBO Mol. Med. 2017, 9, 1165–1178. [Google Scholar] [CrossRef] [PubMed]
- Wan, G.; Ruan, L.; Yin, Y.; Yang, T.; Ge, M.; Cheng, X. Effects of silver nanoparticles in combination with antibiotics on the resistant bacteria Acinetobacter baumannii. Int. J. Nanomed. 2016, 11, 3789–3800. [Google Scholar] [CrossRef] [PubMed]
- Ruden, S.; Hilpert, K.; Berditsch, M.; Wadhwani, P.; Ulrich, A.S. Synergistic interaction between silver nanoparticles and membrane-permeabilizing antimicrobial peptides. Antimicrob. Agents Chemother. 2009, 53, 3538–3540. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Pitts, B.; Stewart, P.S.; Camper, A.; Yoon, J. Comparison of the antimicrobial effects of chlorine, silver ion, and tobramycin on biofilm. Antimicrob. Agents Chemother. 2008, 52, 1446–1453. [Google Scholar] [CrossRef] [PubMed]
- Davis, B.D. Mechanism of bactericidal action of aminoglycosides. Microbiol. Rev. 1987, 51, 341–350. [Google Scholar] [PubMed]
- Taber, H.W.; Mueller, J.P.; Miller, P.F.; Arrow, A.M.Y.S. Bacterial uptake of aminoglycoside antibiotics. Microbiol. Rev. 1987, 51, 439–457. [Google Scholar] [PubMed]
- Hurwitz, C.; Braun, C.B.; Rosano, C.L. Role of ribosome recycling in uptake of dihydrostreptomycin by sensitive and resistant Escherichia coli. BBA Sect. Nucleic Acids Protein Synth. 1981, 652, 168–176. [Google Scholar] [CrossRef]
- Lansdown, A.B.G. A Pharmacological and toxicological profile of silver as an antimicrobial agent in medical devices. Adv. Pharmacol. Sci. 2010, 2010, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Bachler, G.; von Goetz, N.; Hungerbühler, K. A physiologically based pharmacokinetic model for ionic silver and silver nanoparticles. Int. J. Nanomed. 2013, 8, 3365–3382. [Google Scholar]
- Ezraty, B.; Barras, F. The ‘liaisons dangereuses’ between iron and antibiotics. FEMS Microbiol. Rev. 2016, 40, 418–435. [Google Scholar] [CrossRef] [PubMed]
- Keogan, D.M.; Griffith, D.M. Current and potential applications of bismuth-based drugs. Molecules 2014, 19, 15258–15297. [Google Scholar] [CrossRef] [PubMed]
- Barras, F.; Fontecave, M. Cobalt stress in Escherichia coli and Salmonella enterica: Molecular bases for toxicity and resistance. Metallomics 2011, 3, 1130–1134. [Google Scholar] [CrossRef] [PubMed]
- Chandrangsu, P.; Rensing, C.; Helmann, J.D. Metal homeostasis and resistance in bacteria. Nat. Rev. Microbiol. 2017, 15, 338–350. [Google Scholar] [CrossRef] [PubMed]
| Antibiotics | Organism | Culture Condition | Effects | References | |
|---|---|---|---|---|---|
| ß-lactams | Ampicillin | E. coli | Laboratory medium | 10-fold increase in antimicrobial activity | [3] |
| Quinolones | Ofloxacine, Nalidixic Acid, Norfloxacin | E. coli | Laboratory medium | 10-fold increase in antimicrobial activity. MIC value decreased 10–25% | [3,4] |
| Aminoglycosides | Gentamicin | E. coli | Laboratory medium. Animal models | 100-fold increase in antimicrobial activity. MIC value decreased more than 10-fold | [3,4] |
| C. difficile | Laboratory medium | MIC value decreased 4-fold | [4] | ||
| Tobramycin | E. coli., P. aeruginosa | Laboratory medium | MIC value decreased 10-fold (E. coli). 3-fold increase in antimicrobial activity (P. aeruginosa) | [4,23] | |
| Kanamycin Streptomycin | E. coli | Laboratory medium | MIC value decreased more than 10-fold | [4] | |
| Spectinomycin | E. coli | Laboratory medium | MIC value decreased 2-fold | [4] | |
| Vancomycin | E. coli | Laboratory medium. Animal models | 10-fold increase in antimicrobial activity | [3] | |
| Chloramphenicol | E. coli | Laboratory medium | MIC value decreased 1.5-fold | [4] | |
| Ebselen | K. pneumoniae, A. baumanni, P. aeruginosa, E. cloacae, E. coli | Laboratory medium. Animal models | 10-fold increase in MIC value | [20] | |
| Polymixin B | E. coli | Laboratory medium | MIC value decreased 5- to 10-fold | [21,22] | |
| Rifampicin | A. baumannii | Laboratory medium | MIC value decreased 5-10 fold | [21] | |
| Tetracycline | E. coli (TetR) | Laboratory medium | MIC value decreased 2-fold | [3] | |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barras, F.; Aussel, L.; Ezraty, B. Silver and Antibiotic, New Facts to an Old Story. Antibiotics 2018, 7, 79. https://doi.org/10.3390/antibiotics7030079
Barras F, Aussel L, Ezraty B. Silver and Antibiotic, New Facts to an Old Story. Antibiotics. 2018; 7(3):79. https://doi.org/10.3390/antibiotics7030079
Chicago/Turabian StyleBarras, Frédéric, Laurent Aussel, and Benjamin Ezraty. 2018. "Silver and Antibiotic, New Facts to an Old Story" Antibiotics 7, no. 3: 79. https://doi.org/10.3390/antibiotics7030079
APA StyleBarras, F., Aussel, L., & Ezraty, B. (2018). Silver and Antibiotic, New Facts to an Old Story. Antibiotics, 7(3), 79. https://doi.org/10.3390/antibiotics7030079





