Biogenic Nanosilver against Multidrug-Resistant Bacteria (MDRB)
Abstract
1. Introduction
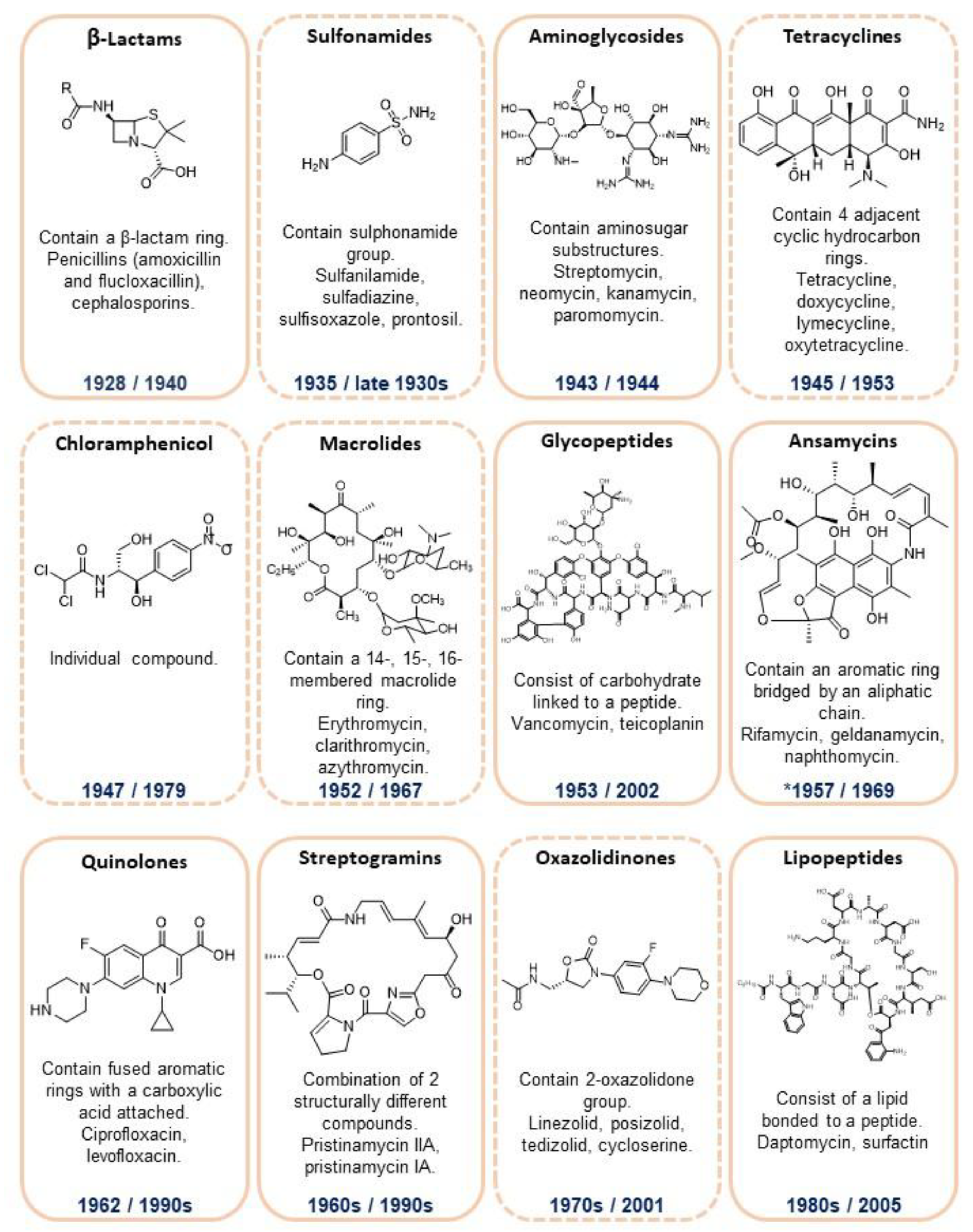
2. Antibiotics
3. The Emerging of Antimicrobial Resistance
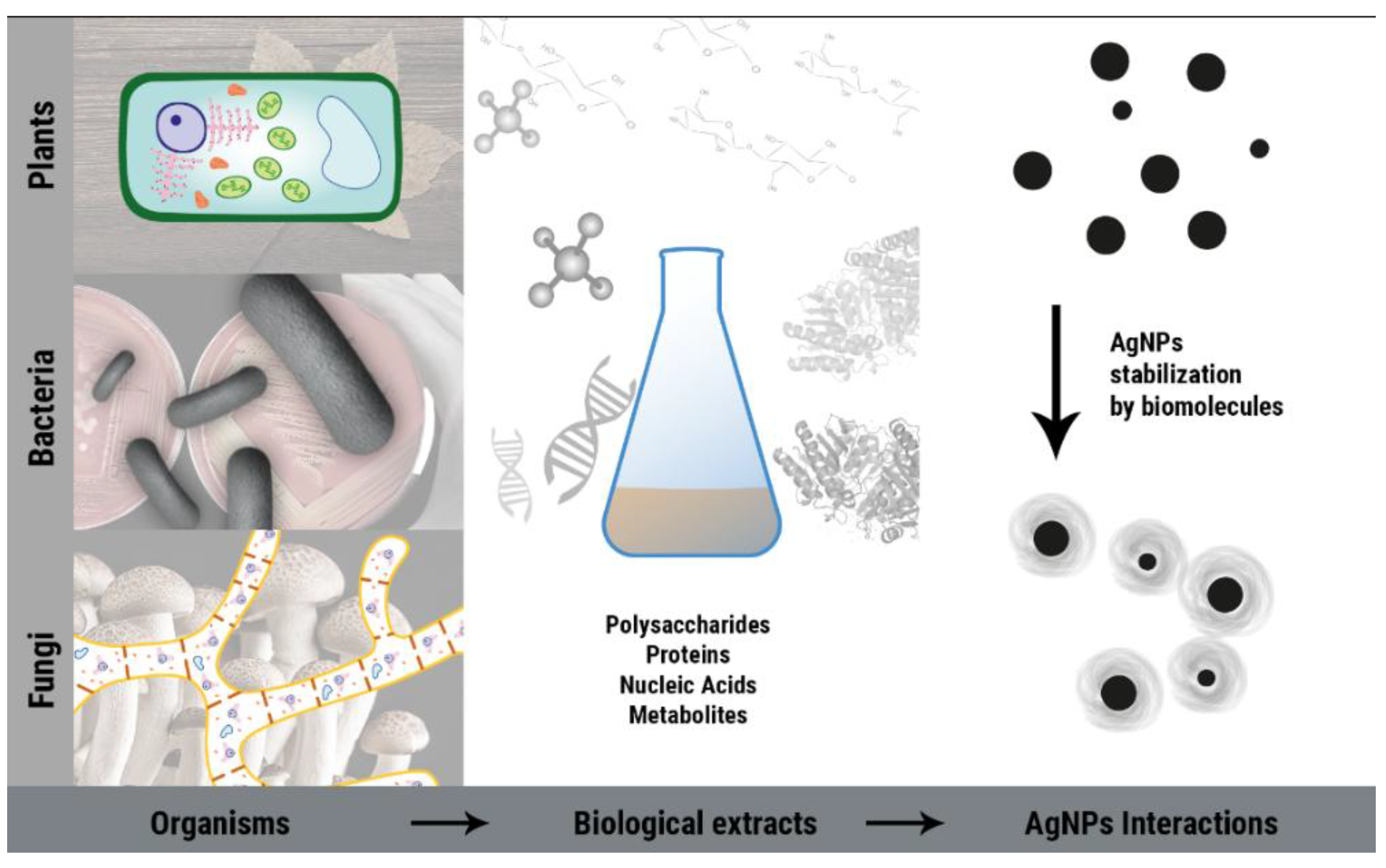
4. Biogenic AgNPs as a Weapon against Multidrug-Resistant Bacteria (MDRB)
4.1. Fungal AgNPs against MDRB
4.2. Bacterial AgNPs against MDRB
4.3. AgNPs from Plants against MDRB
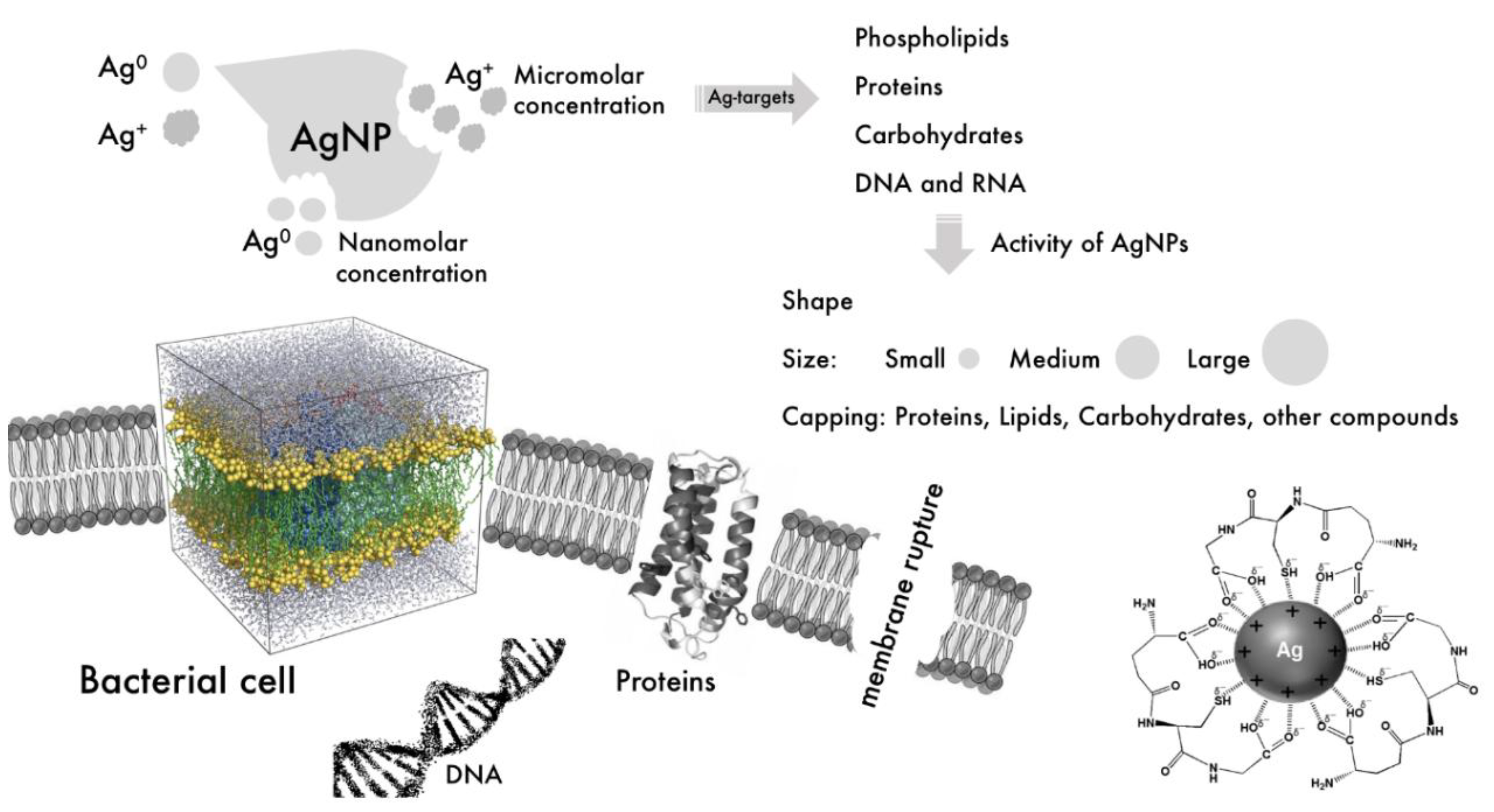
4.4. Modes of Action of AgNPs against Bacteria
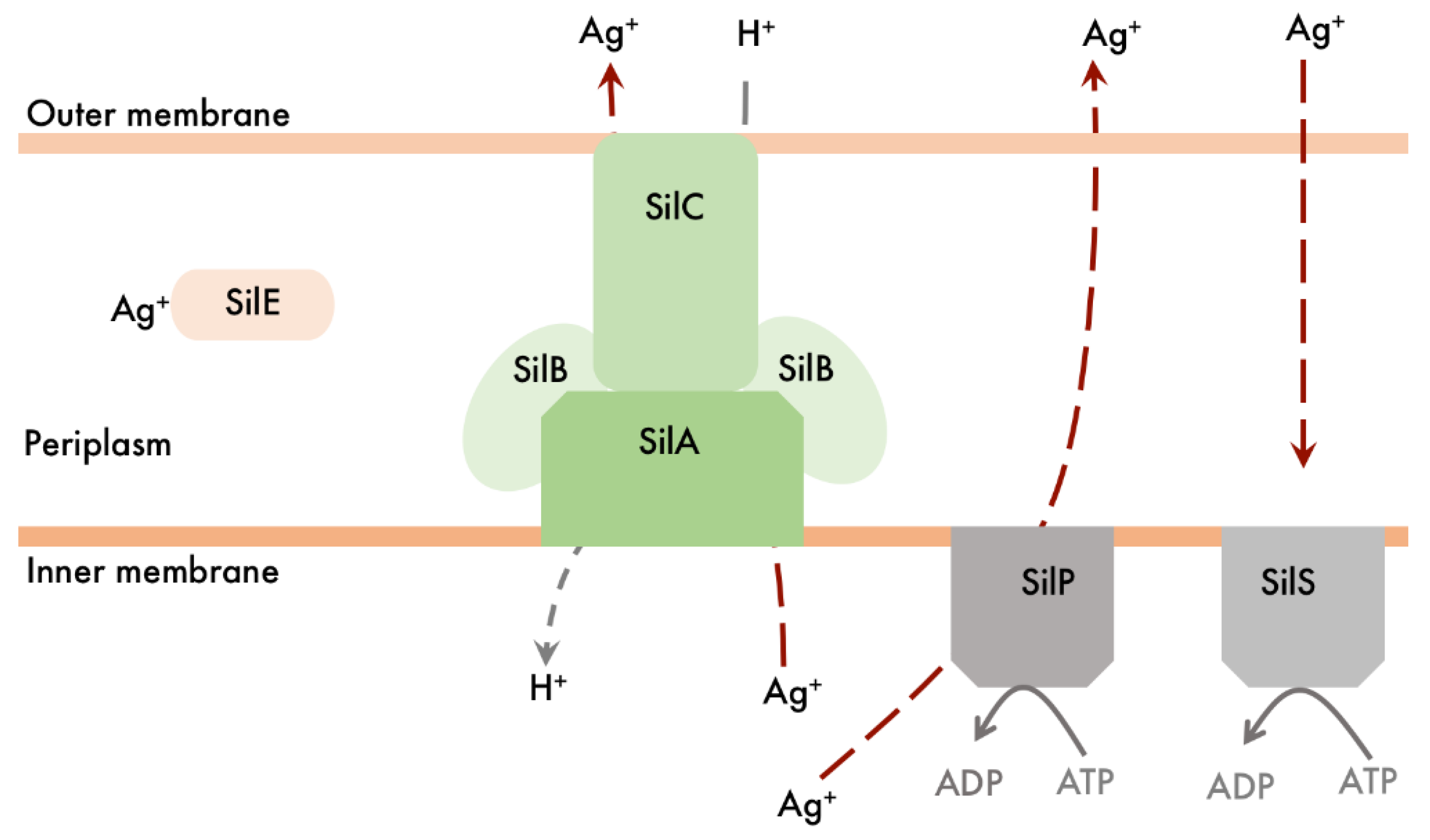
4.5. Bacterial Resistance to Silver
5. Nanosilver Applications in Antimicrobial Products
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- World Health Organization. 10 Facts on Antimicrobial Resistance. Available online: http://www.who.int/features/factfiles/antimicrobial_resistance/en/ (accessed on 10 June 2018).
- Littier, H.M.; Chambers, L.R.; Knowton, K.F. Animal agriculture as a contributor to the global challenge of antibiotic resistance. CAB Rev. 2017, 8, 1–9. [Google Scholar] [CrossRef]
- Roca, I.; Akova, M.; Baquero, F.; Carlet, J.; Cavaleri, M.; Coenen, S.; Cohen, J.; Findlay, D.; Gyssens, I.; Heure, O.E.; et al. The global threat of antimicrobial resistance: Science for intervention. New Microbe New Infect. 2015, 6, 22–29. [Google Scholar] [CrossRef] [PubMed]
- Huebner, C.; Rogellin, M.; Flessa, S. Economic burden of multidrug-resistant bacteria in nursing homes in Germany: A cost analysis based on empirical data. BJM Open 2016, 6, e008458. [Google Scholar] [CrossRef] [PubMed]
- Centers for Disease Control and Prevention. Drug-Resistant TB. Available online: http://www.cdc.gov/tb/topic/drtb/ (accessed on 10 June 2018).
- World Health Organization. WHO Publishes List of Bacteria for Which New Antibiotics Are Urgently Needed. Available online: http://www.who.int/en/news-room/detail/27-02-2017-who-publishes-list-of-bacteria-for-which-new-antibiotics-are-urgently-needed (accessed on 10 June 2018).
- Worthington, R.J.; Melander, C. Combination approaches to combat multidrug-resistant bacteria. Trends Biotechnol. 2013, 31, 177–184. [Google Scholar] [CrossRef] [PubMed]
- Lane, D. Designer combination therapy for cancer. Nat. Biotechnol. 2006, 24, 163–164. [Google Scholar] [CrossRef] [PubMed]
- Richman, D.D. HIV chemotherapy. Nature 2001, 410, 995–1001. [Google Scholar] [CrossRef] [PubMed]
- Nosten, F.; White, M.J. Artemisinin-based combination treatment of falciparum malaria. Am. J. Trop. Med. Hyg. 2007, 77, 191–192. [Google Scholar]
- Xiao, Y.; Wang, D.; Heise, A.; Lang, M. Chemo-enzymatic synthesis of poly (4-piperidine lactone-b-ω-pentadecalactone) block copolymers as biomaterials with antibacterial properties. Biomacromolecules 2018, 19, 2673–2681. [Google Scholar] [CrossRef] [PubMed]
- Zoriasatein, M.; Bidhendi, S.M.; Madani, R. Evaluation of antimicrobial properties of derivative peptide of Naja naja snake’s venom. World Fam. Med. J. 2018, 16, 44–62. [Google Scholar]
- Al-Gbouri, N.M.; Hamzah, A.M. Evaluation of Phyllanthus emblica extract as antibacterial and antibiofilm against biofilm formation. TIJAS 2018, 49, 142–151. [Google Scholar]
- Naqvi, S.Z.H.; Kiran, U.; Ali, M.I.; Jamal, A.; Hameed, A.; Ahmed, S.; Ali, N. Combined efficacy of biologically synthesized silver nanoparticles and different antibiotics against multidrug-resistant bacteria. Int. J. Nanomed. 2013, 8, 3187–3195. [Google Scholar] [CrossRef] [PubMed]
- Sirelkhatim, A.; Mahmud, S.; Seeni, A.; Kaus, N.H.M.; Ann, L.C.; Bakhori, S.K.M.; Hasan, H.; Mohamad, D. Review on zinc oxide nanoparticles: Antibacterial activity. Nano-Micro Lett. 2015, 7, 219–242. [Google Scholar] [CrossRef]
- Ingle, A.P.; Duran, N.; Rai, M. Bioactivity, mechanism of action, and cytotoxicity of copper-based nanoparticles: A review. Appl. Microbiol. Biotechnol. 2014, 98, 1001–1009. [Google Scholar] [CrossRef] [PubMed]
- Dinali, R.; Ebrahiminezhad, A.; Manley-Harris, M.; Ghasemi, Y.; Berenjian, A. Iron oxide nanoparticles in modern microbiology and biotechnology. Crit. Rev. Microb. 2017, 43, 493–507. [Google Scholar] [CrossRef] [PubMed]
- Dastjerdi, R.; Montazer, M. A review on the application of inorganic nano-structured materials in the modification of textiles: Focus on anti-microbial properties. Colloids Surf. B Biointerfaces 2010, 79, 5–18. [Google Scholar] [CrossRef] [PubMed]
- Ballottin, D.; Fulaz, S.; Cabrini, F.; Tsukamoto, J.; Durán, N.; Alves, O.L.; Tasic, L. Antimicrobial textiles: Biogenic silver nanoparticles against Candida and Xanthomonas. Mater. Sci. Eng. C 2017, 75, 582–589. [Google Scholar] [CrossRef] [PubMed]
- Ertem, E.; Guut, B.; Zuber, F.; Allegri, S.; Le Ouay, B.; Mefti, S.; Formentin, K.; Stellacci, F.; Ren, Q. Core-shell silver nanoparticles in endodontic disinfection solutions enable long-term antimicrobial effect on oral biofilms. ACS Appl. Mater. Interfaces 2017, 9, 34762–34772. [Google Scholar] [CrossRef] [PubMed]
- Nakazato, G.; Kobayashi, R.; Seabra, A.B.; Duran, N. Use of nanoparticles as a potential antimicrobial for food packaging. In Food Preservation, 1st ed.; Grumezescu, A., Ed.; Academic Press: Cambridge, MA, USA, 2016. [Google Scholar]
- Holtz, R.D.; Lima, B.A.; Filho, A.G.S.; Brocchi, M.; Alves, O.L. Nanostructured silver vanadate as a promising antibacterial additive to water-based paints. Nanomed. NBM 2012, 8, 935–940. [Google Scholar] [CrossRef] [PubMed]
- LaMer, V.K.; Dinegar, R.H. Theory, production and mechanism of formation of monodispersed hydrosols. J. Am. Chem. Soc. 1950, 72, 4847–4854. [Google Scholar] [CrossRef]
- Rai, M.K.; Deshmukh, S.D.; Ingle, A.P.; Gade, A.K. Silver nanoparticles: The powerful nanoweapon against multidrug-resistant bacteria. J. Appl. Microb. 2012, 112, 841–852. [Google Scholar] [CrossRef] [PubMed]
- Radetic, M. Functionalization of textile materials with silver nanoparticles. J. Mater. Sci. 2013, 48, 95–107. [Google Scholar] [CrossRef]
- Maneerung, T.; Tokura, S.; Rujiravanit, R. Impregnation of silver nanoparticles into bacterial cellulose for antimicrobial wound dressing. Carbohydr. Polym. 2008, 72, 43–51. [Google Scholar] [CrossRef]
- Chen, J.; Han, C.M.; Lin, X.W.; Tang, Z.J.; Su, S.J. Effect of silver nanoparticles dressing on second degree burn wound. Zhonghua Wai Ke Za Zhi 2006, 44, 50–52. [Google Scholar] [PubMed]
- Muangman, P.; Chuntrasakul, C.; Silthram, S.; Suvanchote, S.; Benhathanung, R.; Kttidacha, S.; Rueksomtawin, S. Comparison of efficacy of 1% silver sulfadiazine and Acticoat for treatment of partial-thickness burn wounds. J. Med. Assoc. Thail. 2006, 89, 953–958. [Google Scholar]
- Cohen, M.S.; Stern, J.M.; Vanni, A.J.; Kelley, R.S.; Baumgart, E.; Field, D.; Libertino, J.A.; Summerhayes, I.C. In vitro analysis of a nanocrystalline silver-coated surgical mesh. Surg. Infect. 2007, 8, 397–403. [Google Scholar] [CrossRef] [PubMed]
- Lansdown, A.B. Silver in health care: Antimicrobial effects and safety in use. Curr. Probl. Dermatol. 2006, 33, 17–34. [Google Scholar] [PubMed]
- Zhang, Z.; Yang, M.; Huang, M.; Hu, Y.; Xie, J. Study on germicidal efficacy and toxicity of compound disinfectant gel of nanometer silver and chlorhexidine acetate. Chin. J. Health Lab. Technol. 2007, 17, 1403–1406. [Google Scholar]
- Zhang, Y.; Sun, J. A study on the bio-safety for nano-silver as anti-bacterial materials. Chin. J. Med. Instrum. 2007, 31, 35–38. [Google Scholar]
- Nowack, B.; Krug, H.F.; Height, M. 120 Years of nanosilver history: Implications for policy makers. Environ. Sci. Technol. 2011, 45, 1177–1183. [Google Scholar] [CrossRef] [PubMed]
- Waksman, S. History of the word ‘antibiotic’. J. Hist. Med. Allied Sci. 1973, 28, 284–286. [Google Scholar] [CrossRef] [PubMed]
- Davies, J.; Davies, D. Origins and evolution of antibiotic resistance. Microbiol. Mol. Biol. Rev. 2010, 74, 417–433. [Google Scholar] [CrossRef] [PubMed]
- Williams, K. The introduction of ‘chemotherapy’ using arsphenamine—The first magic bullet. J. R. Soc. Med. 2009, 102, 343–348. [Google Scholar] [CrossRef] [PubMed]
- Izumi, Y.; Isozumi, K. Modern Japanese medical history and the European influence. Keio J. Med. 2001, 50, 91–99. [Google Scholar] [CrossRef] [PubMed]
- Amivov, R. A brief history of the antibiotic era: Lessons learned and challenges for the future. Front. Microbiol. 2010, 1, 134. [Google Scholar]
- Bbosa, G.; Mwebaza, N.; Odda, J.; Kyegombe, D.; Ntale, M. Antibiotics/antibacterial drug use, their marketing and promotion during the post-antibiotic golden age and their role in emergence of bacterial resistance. Health 2014, 6, 410–425. [Google Scholar] [CrossRef]
- Thal, L.; Zervos, M. Occurrence and epidemiology of resistance to virginiamycin and streptogramins. J. Antimicrob. Chemother. 1999, 43, 171–176. [Google Scholar] [CrossRef] [PubMed]
- Manten, A.; Van Wijngaarden, L. Development of drug resistance to rifampicin. Chemotherapy 1969, 14, 93–100. [Google Scholar] [CrossRef] [PubMed]
- Chopra, I.; Roberts, M. Tetracycline antibiotics: Mode of action, applications, molecular biology, and epidemiology of bacterial resistance. Microbiol. Mol. Biol. Rev. 2001, 65, 232–260. [Google Scholar] [CrossRef] [PubMed]
- Kirst, H. Introduction to the macrolide antibiotics. In Macrolide Antibiotics Milestones in Drug Therapy MDT; Schönfeld, W., Kirst, H., Eds.; Birkhäuser: Basel, Switzerland, 2002; pp. 1–13. [Google Scholar]
- Jacoby, G. Mechanisms of resistance to quinolones. Clin. Infect. Dis. 2005, 41, S120–S126. [Google Scholar] [CrossRef] [PubMed]
- Madhavan, H.; Bagyalakshmi, R. Farewell, chloramphenicol? Is this true?: A review. J. Microbiol. Biotechnol. 2013, 3, 13–26. [Google Scholar]
- Mutnick, A.; Enne, V.; Jones, R. Linezolid resistance since 2001: SENTRY Antimicrobial Surveillance Program. Ann. Pharmacother. 2003, 37, 769–774. [Google Scholar] [CrossRef] [PubMed]
- Abraham, E.; Chain, E. An enzyme from bacteria able to destroy penicillin. Rev. Infect. Dis. 1940, 10, 677–678. [Google Scholar] [CrossRef]
- D’Costa, V.; McGrann, M.; Hughes, D.; Wright, G. Sampling the antibiotic resistome. Science 2006, 311, 374–377. [Google Scholar] [CrossRef] [PubMed]
- Davies, J. Vicious circles: Looking back on resistance plasmids. Genetics 1995, 139, 1465–1468. [Google Scholar] [PubMed]
- Helinski, D. Introduction to plasmids: A selective view of their history. In Plasmid Biology; Funnell, B., Philips, G., Eds.; ASM Press: Washington, DC, USA, 2004; pp. 1–21. [Google Scholar]
- Hacker, J.; Kaper, J. Pathogenicity islands and the evolution of microbes. Annu. Rev. Microbiol. 2000, 54, 641–679. [Google Scholar] [CrossRef] [PubMed]
- Munita, J.M.; Arias, C.A. Mechanisms of Antibiotic Resistance. Microbiol. Spectr. 2016, 4, 1–37. [Google Scholar]
- Steward, P.; Costerton, J. Antibiotic resistance of bacteria in biofilms. Lancet 2001, 358, 135–138. [Google Scholar] [CrossRef]
- Andersson, D. Persistence of antibiotic resistant bacteria. Curr. Opin. Microbiol. 2003, 6, 452–456. [Google Scholar] [CrossRef] [PubMed]
- Nikaido, H. Multidrug Resistance in Bacteria. Annu. Rev. Biochem. 2009, 78, 119–146. [Google Scholar] [CrossRef] [PubMed]
- Kirbis, A.; Krizman, M. Spread of antibiotic resistant bacteria from food of animal origin to humans and vice versa. Procedia Food Sci. 2015, 5, 148–151. [Google Scholar] [CrossRef]
- Lee Ventola, C. The Antibiotic Resistance Crisis—Part 1: Causes and Threats. Pharm. Ther. 2015, 40, 277–293. [Google Scholar]
- Van Duin, D.; Paterson, D. Multidrug resistant bacteria in the community: Trends and lessons learned. Infect. Dis. Clin. N. Am. 2016, 30, 377–390. [Google Scholar] [CrossRef] [PubMed]
- The World Is Running out of Antibiotics, WHO Report Confirms. Available online: http://www.who.int/news-room/detail/20-09-2017-the-world-is-running-out-of-antibiotics-who-report-confirms (accessed on 10 June 2018).
- Davies, J. Where have all the antibiotics gone? Can. J. Infect. Dis. Med. Microbiol. 2006, 17, 287–290. [Google Scholar] [CrossRef] [PubMed]
- Why Are There So Few Antibiotics in the Research and Development Pipeline? Available online: https://www.pharmaceutical-journal.com/news-and-analysis/features/why-are-there-so-few-antibiotics-in-the-research-and-development-pipeline/11130209.article (accessed on 10 June 2018).
- Thakkar, K.N.; Mhatre, S.S.; Parikh, R.Y. Biological synthesis of metallic nanoparticles. Nanomed. NBM 2010, 6, 257–262. [Google Scholar] [CrossRef] [PubMed]
- Durán, N.; Silveira, C.P.; Durán, M.; Martinez, D.S.T. Silver nanoparticle protein corona and toxicity: A mini-review. J. Nanobiotechnol. 2015, 13, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Ballottin, D.; Fulaz, S.; Souza, M.L.; Corio, P.; Rodrigues, A.G.; Souza, A.O.; Marcato, P.G.; Gomes, A.F.; Gozzo, F.; Tasic, L. Elucidating protein involvement in the stabilization of the biogenic silver nanoparticles. Nanoscale Res. Lett. 2016, 11, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Shannahan, J.H.; Podila, R.; Aldossari, A.A.; Emerson, H.; Powell, B.A.; Ke, P.C.; Rao, A.M.; Brown, J.M. Formation of a protein corona on silver nanoparticles mediates cellular toxicity via scavenger receptors. Toxicol. Sci. 2014, 143, 136–146. [Google Scholar] [CrossRef] [PubMed]
- Rai, M.; Yadav, A.; Gade, A.K. Myconanotechnology: A new and emerging science. In Applied Mycology; Rai, M., Bridge, P.D., Eds.; CABI: Wallingford, UK, 2009; pp. 258–267. [Google Scholar]
- Zhao, X.; Zhou, L.; Rajoka, M.S.R.; Yan, L.; Jiang, C.; Shao, D.; Zhu, J.; Shi, J.; Huang, Q.; Yang, H.; et al. Fungal silver nanoparticles: Synthesis, application and challenges. Crit. Rev. Biotechnol. 2017, 38, 817–835. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, A.; Mukherjee, P.; Senapati, S.; Mandal, D.; Khan, M.I.; Kumar, R.; Sastry, M. Extracellular biosynthesis of silver nanoparticles using the fungus Fusarium oxysporum. Colloids Surf. B 2003, 28, 313–318. [Google Scholar] [CrossRef]
- Durán, N.; Marcato, P.D.; Alves, O.L.; De Souza, G.I.H.; Esposito, E. Mechanistic aspects of biosynthesis of silver nanoparticles by several Fusarium oxysporum strains. J. Nanobiotechnol. 2005, 3, 8. [Google Scholar] [CrossRef] [PubMed]
- Scandorieiro, S.; de Camargo, L.C.; Lancheros, C.A.C.; Yamada-Ogatta, S.F.; Nakamura, C.V.; de Oliveira, A.G.; Andrade, C.G.T.J.; Durán, N.; Nakazato, G.; Kobayashi, R.K.T. Synergistic and additive effect of oregano essential oil and biological silver nanoparticles against multidrug-resistant bacterial strains. Front. Microbiol. 2016, 7, 760. [Google Scholar] [CrossRef] [PubMed]
- Graves, J.L., Jr.; Tajkarimi, M.; Cunningham, Q.; Campbell, A.; Nonga, H.; Harrison, S.H.; Barrick, J.E. Rapid evolution of silver nanoparticle resistance in Escherichia coli. Front. Genet. 2015, 6, 42. [Google Scholar] [CrossRef] [PubMed]
- Chowdhury, S.; Basu, A.; Kundu, S. Green synthesis of protein capped silver nanoparticles from phytopathogenic fungus Macrophomina phaseolina (Tassi) Goid with antimicrobial properties against multidrug-resistant bacteria. Nanoscale Res. Lett. 2014, 9, 365. [Google Scholar] [CrossRef] [PubMed]
- Neethu, S.; Midhun, S.J.; Radhakrishnan, E.K.; Jyothis, M. Green synthesized silver nanoparticles by marine endophytic fungus Penicillium polonicum and its antibacterial efficacy against biofilm forming, multidrug-resistant Acinetobacter baumanii. Microb. Pathog. 2018, 116, 263–272. [Google Scholar] [CrossRef] [PubMed]
- Gopinath, P.M.; Narchonai, G.; Dhanasekaran, D.; Ranjani, A.; Thajuddin, N. Mycosynthesis, characterization and antibacterial properties of AgNPs against multidrug resistant (MDR) bacterial pathogens of female infertility cases. Asian J. Pharm. Sci. 2015, 10, 138–145. [Google Scholar] [CrossRef]
- Fayaz, A.M.; Balaji, K.; Girilal, M.; Yadav, R.; Kalaichelvan, P.T.; Venketesan, R. Biogenic synthesis of silver nanoparticles and their synergistic effect with antibiotics: A study against gram-positive and gram-negative bacteria. Nanomed. NBM 2010, 6, 103–109. [Google Scholar] [CrossRef] [PubMed]
- Bhat, M.A.; Nayak, B.K.; Nanda, A. Exploitation of filamentous fungi for biosynthesis of silver nanoparticle and its enhanced antibacterial activity. Int. J. Pharm. Biol. Sci. 2015, 6, 506–515. [Google Scholar]
- Ray, S.; Sarkar, S.; Kundu, S. Extracellular biosynthesis of silver nanoparticles using the mycorrhizal mushroom Tricholoma crassum (Berk.) SACC: Its antimicrobial activity against pathogenic bacteria and fungus, including multidrug resistant plant and human bacteria. Dig. J. Nanomater. Biostruct. 2011, 6, 1289–1299. [Google Scholar]
- Dhanasekaran, D.; Latha, S.; Saha, S.; Thajuddin, N.; Panneerselvam, A. Extracellular biosynthesis, characterisation and in-vitro antibacterial potential of silver nanoparticles using Agaricus bisporus. J. Exp. Nanosci. 2013, 8, 579–588. [Google Scholar] [CrossRef]
- Saravanan, M.; Nanda, A. Extracellular synthesis of silver bionanoparticles from Aspergillus clavatus and its antimicrobial activity against MRSA and MRSE. Colloids Surf. B Biointerfaces 2010, 77, 214–218. [Google Scholar] [CrossRef] [PubMed]
- Dar, M.A.; Ingle, A.; Rai, M. Enhanced antimicrobial activity of silver nanoparticles synthesized by Cryphonectria sp. evaluated singly and in combination with antibiotics. Nanomed. NBM 2013, 9, 105–110. [Google Scholar] [CrossRef] [PubMed]
- Hiremath, J.; Rathod, V.; Ninganagouda, S.; Singh, D.; Prema, K. Antibacterial activity of silver nanoparticles from Rhizopus spp against Gram negative E. coli-MDR strains. J. Pure Appl. Microbiol. 2014, 8, 555–562. [Google Scholar]
- Singh, R.; Shedbalkar, U.U.; Wadhwani, S.A.; Chopade, B.A. Bacteriagenic silver nanoparticles: Synthesis, mechanism, and applications. Appl. Microbiol. Biotechnol. 2015, 99, 4579–4593. [Google Scholar] [CrossRef] [PubMed]
- Saifuddin, N.; Wong, C.W.; Yasumira, A.A.N. Rapid biosynthesis of silver nanoparticles using culture supernatant of bacteria with microwave irradiation. E-J. Chem. 2009, 6, 61–70. [Google Scholar] [CrossRef]
- Zhang, X.; Yang, C.; Yu, H.; Sheng, G. Light-induced reduction of silver ions to silver nanoparticles in aquatic environments by microbial extracellular polymeric substances (EPS). Water Res. 2016, 106, 242–248. [Google Scholar] [CrossRef] [PubMed]
- Klaus, T.; Joerger, R.; Olsson, E.; Granqvist, C. Silver-based crystalline nanoparticles, microbially fabricated. Proc. Natl. Acad. Sci. USA 1999, 96, 13611–13614. [Google Scholar] [CrossRef] [PubMed]
- Klaus-Joerger, T.; Joerger, R.; Olsson, E.; Granqvist, C. Bacteria as workers in the living factory: Metal-accumulating bacteria and their potential for materials science. Trends Biotechnol. 2001, 19, 15–20. [Google Scholar] [CrossRef]
- Kalishwaralal, K.; Deepak, V.; Pandian, S.R.K.; Kottaisamy, M.; BarathManiKanth, S.; Kartukeyan, B.; Gurunathan, S. Biosynthesis of silver and gold nanoparticles using Brevibacterium casei. Colloids Surf. B 2010, 77, 257–262. [Google Scholar] [CrossRef] [PubMed]
- Singh, H.; Du, J.; Yi, T. Biosynthesis of silver nanoparticles using Aeromonas sp. THG-FG1.2 and its antibacterial activity against pathogenic microbes. Artif. Cells Nanomed. Biotechnol. 2017, 45, 584–590. [Google Scholar] [CrossRef] [PubMed]
- Desai, P.P.; Prabhurajeshwar, C.; Chandrakanth, K.R. Hydrothermal assisted biosynthesis of silver nanoparticles from Streptomyces sp. GUT 21 (KU500633) and its therapeutic antimicrobial activity. J. Nanostruct. Chem. 2016, 6, 235–246. [Google Scholar] [CrossRef]
- Manikprabhu, D.; Cheng, J.; Chen, W.; Sunkara, A.K.; Mane, S.B.; Kumar, R.; Das, M.; Hozzein, W.N.; Duan, Y.; Li, W. Sunlight mediated synthesis of silver nanoparticles by a novel actinobacterium (Sinomonas mesophila MPKL 26) and its antimicrobial activity against multi drug resistant Staphylococcus aureus. J. Photochem. Photobiol. 2016, 158, 202–205. [Google Scholar] [CrossRef] [PubMed]
- Santos, K.S.; Barbosa, A.M.; Costa, L.P.; Pinheiro, M.S.; Oliveira, M.B.P.P.; Padilha, F.F. Silver nanocomposite biosynthesis: Antibacterial activity against multidrug-resistant strains of Pseudomonas aeruginosa and Acinetobacter baumannii. Molecules 2016, 21, 1255. [Google Scholar] [CrossRef] [PubMed]
- Subashini, J.; Khanna, V.G.; Kannabiran, K. Anti-ESBL activity of silver nanoparticles biosynthesized using soil Streptomyces species. Bioprocess Biosyst. Eng. 2014, 37, 999–1006. [Google Scholar] [CrossRef] [PubMed]
- Thomas, R.; Nair, A.P.; Mathew, J.; Ek, R. Antibacterial activity and synergistic effect of biosynthesized AgNPs with antibiotics against multidrug-resistant biofilm-forming coagulase-negative Staphylococci isolated from clinical samples. Appl. Biochem. Biotechnol. 2014, 173, 449–460. [Google Scholar] [CrossRef] [PubMed]
- Arul, D.; Balasubramani, G.; Balasubramanian, V.; Natarajan, T.; Perumal, P. Antibacterial efficacy of silver nanoparticles and ethyl acetate’s metabolites of the potent halophilic (marine) bacterium, Bacillus cereus A30 on multidrug resistant bacteria. Pathog. Glob. Health 2017, 111, 367–382. [Google Scholar] [CrossRef] [PubMed]
- Lateef, A.; Ojo, S.A.; Akinwale, A.S.; Azeez, L.; Gueguim-Kana, E.B.; Beukes, L.S. Biogenic synthesis of silver nanoparticles using cell-free extract of Bacillus safensis LAU 13: Antimicrobial, free radical scavenging and larvicidal activities. Biologia 2015, 70, 1295–1306. [Google Scholar] [CrossRef]
- Jain, D.; Kachhwaha, S.; Jain, R.; Srivastava, G.; Kothari, S.L. Novel microbial route to synthesize silver nanoparticles using spore crystal mixture of Bacillus thuringiensis. Indian J. Exp. Biol. 2010, 48, 1152–1156. [Google Scholar] [PubMed]
- Singh, G.; Babele, P.K.; Shahi, S.K.; Sinha, R.P.; Tyagi, M.B.; Kumar, A. Green synthesis of silver nanoparticles using cell extracts of Anabaena doliolum and screening of its antibacterial and antitumor activity. J. Microbiol. Biotechnol. 2014, 24, 1354–1367. [Google Scholar] [CrossRef] [PubMed]
- Saravanan, M.; Vemu, A.K.; Barik, S.K. Rapid biosynthesis of silver nanoparticles from Bacillus megaterium (NCIM 2326) and their antibacterial activity on multi drug resistant clinical pathogens. Colloids Surf. B Biointerfaces 2011, 88, 325–331. [Google Scholar] [CrossRef] [PubMed]
- Priyadarshini, S.; Gopinath, V.; Priyadharsshini, N.M.; MubarakAli, D.; Velusamy, P. Synthesis of anisotropic silver nanoparticles using novel strain, Bacillus flexus and its biomedical application. Colloids Surf. B Biointerfaces 2013, 102, 232–237. [Google Scholar] [CrossRef] [PubMed]
- Saravanan, M.; Barik, S.K.; MubarakAli, D.; Prakash, P.; Pugazhendhi, A. Synthesis of silver nanoparticles from Bacillus brevis (NCIM 2533) and their antibacterial activity against pathogenic bacteria. Microb. Pathog. 2018, 116, 221–226. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, S.; Ahmad, M.; Swami, B.L.; Ikram, S. A review on plants extract mediated synthesis of silver nanoparticles for antimicrobial applications: A green expertise. J. Adv. Res. 2016, 7, 17–28. [Google Scholar] [CrossRef] [PubMed]
- Prathna, T.C.; Chandrasekaran, N.; Raichur, A.M.; Mukherjee, A. Biomimetic synthesis of silver nanoparticles by Citrus limon (lemon) aqueous extract and theoretical prediction of particle size. Colloids Surf. B Biointerfaces 2011, 82, 152–159. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.C.; Chen, C.Y.; Chen, W.M.; Yu, A.B. Role of citric acid in the formation of silver nanoplates through a synergistic reduction approach. Langmuir 2010, 26, 4400–4408. [Google Scholar] [CrossRef] [PubMed]
- Makarov, V.; Love, A.; Sinitsyna, O.; Yaminsky, S.M.; Taliansky, M.; Kalinina, N. Green nanotechnologies: Synthesis of metal nanoparticles using plants. Acta Naturae 2014, 6, 35–44. [Google Scholar] [PubMed]
- Singh, A.K.; Talat, M.; Singh, D.P.; Srivastava, O.N. Biosynthesis of gold and silver nanoparticles by natural precursor clove and their functionalization with amine group. J. Nanopart. Res. 2010, 12, 1667–1675. [Google Scholar] [CrossRef]
- Barros, C.H.N.; Cruz, G.C.F.; Mayrink, M.; Tasic, L. Bio-based synthesis of silver nanoparticles from orange waste: Effects of distinct biomolecule coatings on size, morphology, and antimicrobial activity. Nanotechnol. Sci. Appl. 2018, 11, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Liu, C.; Qu, D.; Chen, Y.; Huang, M.; Liu, Y. Antibacterial evaluation of silver nanoparticles synthesized by polysaccharides from Astragalus membranaceus roots. Biomed. Pharmacother. 2017, 89, 351–357. [Google Scholar] [CrossRef] [PubMed]
- Anjum, S.; Abbasi, B.H. Biomimetic synthesis of antimicrobial silver nanoparticles using in vitro-propagated plantlets of a medicinally important endangered species: Phlomis bracteosa. Int. J. Nanomed. 2016, 11, 1663–1675. [Google Scholar]
- Jeeva, K.; Thiyagarajan, M.; Elangovan, V.; Geetha, N.; Venkatachalam, P. Caesalpinia coriaria leaf extracts mediated biosynthesis of metallic silver nanoparticles and their antibacterial activity against clinically isolated pathogens. Ind. Crops Prod. 2012, 52, 714–720. [Google Scholar] [CrossRef]
- Jinu, U.; Jayalakshmi, N.; Anbu, A.S.; Mahendran, D.; Sahi, S.; Venkatachalam, P. Biofabrication of cubic phase silver nanoparticles loaded with phytochemicals from Solanum nigrum leaf extracts for potential antibacterial, antibiofilm and antioxidant activities against MDR human pathogens. J. Clust. Sci. 2017, 28, 489–505. [Google Scholar] [CrossRef]
- Prasannaraj, G.; Venkatachalam, P. Enhanced antibacterial, anti-biofilm and antioxidant (ROS) activities of biomolecules engineered silver nanoparticles against clinically isolated Gram positive and Gram negative microbial pathogens. J. Clust. Sci. 2017, 28, 645–664. [Google Scholar] [CrossRef]
- Das, B.; Dash, S.K.; Mandal, D.; Ghosh, T.; Chattopadhyay, S.; Tripathy, S.; Das, S.; Dey, S.K.; Das, D.; Roy, S. Green synthesized silver nanoparticles destroy multidrug resistant bacteria via reactive oxygen species mediated membrane damage. Arab. J. Chem. 2017, 10, 862–876. [Google Scholar] [CrossRef]
- Gopinath, V.; Priyadarshini, S.; Priyadharsshini, N.M.; Pandian, K.; Velusamy, P. Biogenic synthesis of antibacterial silver chloride nanoparticles using leaf extracts of Cissus quadrangularis Linn. Mater. Lett. 2013, 91, 224–227. [Google Scholar] [CrossRef]
- Khalil, M.M.H.; Ismail, E.H.; El-Baghdady, K.Z.; Mohamed, D. Green synthesis of silver nanoparticles using olive leaf extract and its antibacterial activity. Arab. J. Chem. 2014, 7, 1131–1139. [Google Scholar] [CrossRef]
- Singh, K.; Panghal, M.; Kadyan, S.; Chaudhary, U.; Yadav, J.P. Green silver nanoparticles of Phyllanthus amarus: As an antibacterial agent against multi drug resistant clinical isolates of Pseudomonas aeruginosa. J. Nanobiotechnol. 2014, 12, 40. [Google Scholar] [CrossRef] [PubMed]
- Kasithevar, M.; Periakaruppan, P.; Muthupandian, S.; Mohan, M. Antibacterial efficacy of silver nanoparticles against multi-drug resistant clinical isolates from post-surgical wound infections. Microb. Pathog. 2017, 107, 327–334. [Google Scholar] [CrossRef] [PubMed]
- Gopinath, V.; MubarakAli, D.; Priyadarshini, S.; Priyadharsshini, N.M.; Thajuddin, N.; Velusamy, P. Biosynthesis of silver nanoparticles from Tribulus terrestris and its antimicrobial activity: A novel biological approach. Colloids Surf. B Biointerfaces 2012, 96, 69–74. [Google Scholar] [CrossRef] [PubMed]
- Veerasamy, R.; Xin, T.Z.; Gunasagaran, S.; Xiang, T.F.W.; Yang, E.F.C.; Jeyakumar, N.; Dhanaraj, S.A. Biosynthesis of silver nanoparticles using mangosteen leaf extract and evaluation of their antimicrobial activities. J. Saudi Chem. Soc. 2011, 15, 113–120. [Google Scholar] [CrossRef]
- Singh, A.; Mittal, S.; Shrivastav, R.; Dass, S.; Srivastava, J.N. Biosynthesis of silver nanoparticles using Ricinus communis L. leaf extract and its antibacterial activity. Dig. J. Nanomater. Biostruct. 2012, 7, 1157–1163. [Google Scholar]
- Prakash, P.; Gnanaprakasam, P.; Emmanuel, R.; Arokiyaraj, S.; Saravanan, M. Green synthesis of silver nanoparticles from leaf extract of Mimusops elengi, Linn. for enhanced antibacterial activity against multi drug resistant clinical isolates. Colloids Surf. B Biointerfaces 2013, 108, 255–259. [Google Scholar] [CrossRef] [PubMed]
- Garg, M.; Devi, B.; Devi, R. In vitro antibacterial activity of biosynthesized silver nanoparticles from ethyl acetate extract of Hydrocotyle sibthorpioides against multidrug resistant microbes. Asian J. Pharm. Clin. Res. 2017, 10, 263–266. [Google Scholar] [CrossRef]
- Li, K.; Ma, C.; Jian, T.; Sun, H.; Wang, L.; Xu, H.; Li, W.; Su, H.; Cheng, X. Making good use of the byproducts of cultivation: Green synthesis and antibacterial effects of silver nanoparticles using the leaf extract of blueberry. J. Food Sci. Technol. 2017, 54, 3569–3576. [Google Scholar] [CrossRef] [PubMed]
- Miri, A.; Sarani, M.; Bazaz, M.R.; Darroudi, M. Plant-mediated biosynthesis of silver nanoparticles using Prosopis farcta extract and its antibacterial properties. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2015, 141, 287–291. [Google Scholar] [CrossRef] [PubMed]
- Das, J.; Das, M.P.; Velusamy, P. Sesbania grandiflora leaf extract mediated green synthesis of antibacterial silver nanoparticles against selected human pathogens. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2013, 104, 265–270. [Google Scholar] [CrossRef] [PubMed]
- Lateef, A.; Azeez, M.A.; Asafa, T.B.; Yekeen, T.A.; Akinboro, A.; Oladipo, I.C.; Azeez, L.; Ajibade, S.E.; Ojo, S.A.; Gueguim-Kana, E.B.; et al. Biogenic synthesis of silver nanoparticles using a pod extract of Cola nitida: Antibacterial and antioxidant activities and application as a paint additive. J. Taibah Univ. Sci. 2016, 10, 551–562. [Google Scholar] [CrossRef]
- Kagithoju, S.; Godishala, V.; Nanna, R.S. Eco-friendly and green synthesis of silver nanoparticles using leaf extract of Strychnos potatorum Linn.F. and their bactericidal activities. 3 Biotech 2015, 5, 709–714. [Google Scholar] [CrossRef] [PubMed]
- Prabakar, K.; Sivalingam, P.; Rabeek, S.I.M.; Muthuselvam, M.; Devarajan, N.; Arjunan, A.; Karthick, R.; Suresh, M.M.; Wembonyama, J.P. Evaluation of antibacterial efficacy of phyto fabricated silver nanoparticles using Mukia scabrella (Musumusukkai) against drug resistance nosocomial gram negative bacterial pathogens. Colloids Surf. B Biointerfaces 2013, 104, 282–288. [Google Scholar] [CrossRef] [PubMed]
- Meva, F.E.; Ebongue, C.O.; Fannang, S.V.; Segnou, M.L.; Ntoumba, A.A.; Kedi, P.B.E.; Loudang, R.N.; Wanlao, A.Y.; Mang, E.R.; Mpondo, E.A.M. Natural substances for the synthesis of silver nanoparticles against Escherichia coli: The case of Megaphrynium macrostachyum (Marantaceae), Corchorus olitorus (Tiliaceae), Ricinodendron heudelotii (Euphorbiaceae), Gnetum bucholzianum (Gnetaceae), and Ipomoea batatas (Convolvulaceae). J. Nanomater. 2017, 2017, 6834726. [Google Scholar]
- Shruthi, G.; Prasad, K.S.; Vinod, T.P.; Balamurugan, V.; Shivamallu, C. Green synthesis of biologically active silver nanoparticles through a phyto-mediated approach using Areca catechu leaf extract. ChemistrySelect 2017, 2, 10354–10359. [Google Scholar] [CrossRef]
- Azeez, M.A.; Lateef, A.; Asafa, T.B.; Yekeen, T.A.; Akinboro, A.; Oladipo, I.C.; Gueguim-Kana, E.B.; Beukes, L.S. Biomedical applications of cocoa bean extract-mediated silver nanoparticles as antimicrobial, larvicidal and anticoagulant agents. J. Clust. Sci. 2017, 28, 149–164. [Google Scholar] [CrossRef]
- Lateef, A.; Azeez, M.A.; Asafa, T.B.; Yekeen, T.A.; Akinboro, A.; Oladipo, I.C.; Azeez, L.; Ojo, S.A.; Gueguim-Kana, E.B.; Beukes, L.S. Cocoa pod husk extract-mediated biosynthesis of silver nanoparticles: Its antimicrobial, antioxidant and larvicidal activities. J. Nanostruct. Chem. 2016, 6, 159–169. [Google Scholar] [CrossRef]
- Swamy, M.K.; Akhtar, M.S.; Mohanty, S.K.; Sinniah, U.R. Synthesis and characterization of silver nanoparticles using fruit extract of Momordica cymbalaria and assessment of their in vitro antimicrobial, antioxidant and cytotoxicity activities. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2015, 151, 939–944. [Google Scholar] [CrossRef] [PubMed]
- Durán, N.; Durán, M.; Jesus, M.B.; Seabra, A.B.; Fávaro, W.J.; Nakazato, G. Silver nanoparticles: A new view on mechanistic aspects on antimicrobial activity. Nanomed. NBM 2016, 12, 789–799. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Mahendra, S.; Lyon, D.Y.; Brunet, L.; Liga, M.V.; Li, D.; Alvarez, P.J.J. Antimicrobial nanomaterials for water disinfection and microbial control: Potential applications and implications. Water Res. 2008, 42, 4591–4602. [Google Scholar] [CrossRef] [PubMed]
- Manke, A.; Wang, L.; Rojanasakul, Y. Mechanisms of nanoparticle-induced oxidative stress and toxicity. BioMed Res. Int. 2013, 2013, 942916. [Google Scholar] [CrossRef] [PubMed]
- Siddiqi, K.S.; Husen, A.; Rao, R.A.K. A review on biosynthesis of silver nanoparticles and their biocidal properties. J. Nanobiotechnol. 2018, 16, 14. [Google Scholar] [CrossRef] [PubMed]
- Zheng, K.; Setyawati, M.I.; Leong, D.T.; Xie, J. Antimicrobial silver nanomaterials. Coord. Chem. Rev. 2018, 357, 1–17. [Google Scholar] [CrossRef]
- Lok, C.; Ho, C.M.; Chen, R.; He, Q.Y.; Yu, W.Y.; Sun, H.; Tam, P.K.; Chiu, J.F.; Che, C.M. Proteomic analysis of the mode of antibacterial action of silver nanoparticles. J. Proteome Res. 2006, 5, 916–924. [Google Scholar] [CrossRef] [PubMed]
- Xiu, Z.; Zhang, Q.; Puppala, H.L.; Colvin, V.L.; Alvarez, P.J.J. Negligible particle-specific antibacterial activity of silver nanoparticles. Nano Lett. 2012, 12, 4271–4275. [Google Scholar] [CrossRef] [PubMed]
- Dibrov, P.; Dzioba, J.; Gosink, K.K.; Hase, C.C. Chemiosmotic mechanism of antimicrobial activity of Ag+ in Vibrio cholerae. Antimicrob. Agents Chemother. 2002, 46, 2668–2670. [Google Scholar] [CrossRef] [PubMed]
- Liau, S.Y.; Read, D.C.; Pugh, W.J.; Furr, J.R.; Russell, A.D. Interaction of silver nitrate with readily identifiable groups: Relationship to the antibacterial action of silver ions. Lett. Appl. Microbiol. 1997, 25, 279–283. [Google Scholar] [CrossRef] [PubMed]
- Liao, X.; Yang, F.; Li, H.; So, P.K.; Yao, Z.; Wia, W.; Sun, H. Targeting the thioredoxin reductase–thioredoxin system from Staphylococcus aureus by silver ions. Inorg. Chem. 2017, 56, 14823–14830. [Google Scholar] [CrossRef] [PubMed]
- Holt, K.B.; Bard, A.J. Interaction of silver(I) ions with the respiratory chain of Escherichia coli: An electrochemical and scanning electrochemical microscopy study of the antimicrobial mechanism of micromolar Ag+. Biochemistry 2005, 44, 13214–13223. [Google Scholar] [CrossRef] [PubMed]
- Wen, Y.; Geitner, N.K.; Chen, R.; Ding, F.; Chen, P.; Andorfer, R.E.; Govindan, P.N.; Ke, P.C. Binding of cytoskeletal proteins with silver nanoparticles. RSC Adv. 2013, 3, 22002–22007. [Google Scholar] [CrossRef]
- Hong, X.; Wen, J.; Xiong, X.; Hu, Y. Shape effect on the antibacterial activity of silver nanoparticles synthesized via a microwave-assisted method. Environ. Sci. Pollut. Res. 2016, 23, 4489–4497. [Google Scholar] [CrossRef] [PubMed]
- Alshareef, A.; Laird, K.; Cross, R.B.M. Shape-dependent antibacterial activity of silver nanoparticles on Escherichia coli and Enterococcus faecium bacterium. Appl. Surf. Sci. 2017, 424, 310–315. [Google Scholar] [CrossRef]
- Pal, S.; Tak, Y.K.; Song, J.M. Does the antibacterial activity of silver nanoparticles depend on the shape of the nanoparticle? A study of the Gram-negative bacterium Escherichia coli. Appl. Environ. Microb. 2007, 73, 1712–1720. [Google Scholar] [CrossRef] [PubMed]
- Acharya, D.; Singha, K.M.; Pandey, P.; Mohanta, B.; Rajkumari, J.; Singha, L.P. Shape dependent physical mutilation and lethal effects of silver nanoparticles on bacteria. Sci. Rep. 2018, 8, 201. [Google Scholar] [CrossRef] [PubMed]
- Ivask, A.; Kurvet, I.; Kasemets, K.; Blinova, I.; Aruoja, V.; Suppi, S.; Vija, H.; Kakinen, A.; Titma, T.; Heinlaan, M.; et al. Size-dependent toxicity of silver nanoparticles to bacteria, yeast, algae, crustaceans and mammalian cells in vitro. PLoS ONE 2014, 9, e102108. [Google Scholar] [CrossRef] [PubMed]
- Kumari, M.; Pandey, S.; Giri, V.P.; Bhattacharya, A.; Shukla, R.; Mishra, A.; Nautiyal, C.S. Tailoring shape and size of biogenic silver nanoparticles to enhance antimicrobial efficacy against MDR bacteria. Microb. Pathog. 2017, 105, 346–355. [Google Scholar] [CrossRef] [PubMed]
- Lu, Z.; Rong, K.; Li, J.; Yang, H.; Chen, R. Size-dependent antibacterial activities of silver nanoparticles against oral anaerobic pathogenic bacteria. J. Mater. Sci. Mater. Med. 2013, 24, 1465–1471. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Yang, Z.; Wu, H.; Pan, X.; Xie, X.; Wu, C. Antimicrobial activity and the mechanism of silver. Int. J. Nanomed. 2011, 6, 2873–2877. [Google Scholar]
- Kora, A.J.; Sashidhar, R.B. Biogenic silver nanoparticles synthesized with rhamnogalacturonan gum: Antibacterial activity, cytotoxicity and its mode of action. Arab. J. Chem. 2018, 11, 313–323. [Google Scholar] [CrossRef]
- Kim, J.S.; Kuk, E.; Yu, N.K.; Kim, J.; Park, S.J.; Lee, H.J.; Kim, S.H.; Park, Y.K.; Park, Y.H.; Hwang, C.; et al. Antimicrobial effects of silver nanoparticles. Nanomed. NBM 2007, 3, 95–101. [Google Scholar] [CrossRef] [PubMed]
- Choi, O.; Hu, Z. Size dependent and reactive oxygen species related nanosilver toxicity to nitrifying bacteria. Environ. Sci. Technol. 2008, 42, 4583–4588. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Xie, X.; Shi, Q.; Zeng, H.; OU-Yang, Y.; Chen, Y. Antibacterial activity and mechanism of silver nanoparticles on Escherichia coli. Appl. Microbiol. Biotechnol. 2010, 85, 1115–1122. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, A.; Wei, Y.; Syed, F.; Rehman, A.U.; Khan, A.; Ullah, S.; Yuan, Q. The effects of bacteria-nanoparticles interface on the antibacterial activity of green synthesized silver nanoparticles. Microb. Pathog. 2017, 102, 133–142. [Google Scholar] [CrossRef] [PubMed]
- Qayyum, S.; Oves, M.; Khan, A.U. Obliteration of bacterial growth and biofilm through ROS generation by facilely synthesized green silver nanoparticles. PLoS ONE 2017, 12, e0181363. [Google Scholar] [CrossRef] [PubMed]
- Pareek, V.; Gupta, R.; Panwar, J. Do physico-chemical properties of silver nanoparticles decide their interaction with biological media and bactericidal action? A review. Mater. Sci. Eng. C 2018, 90, 739–749. [Google Scholar] [CrossRef] [PubMed]
- Mchugh, G.L.; Moellering, R.C.; Hopkins, C.C.; Swartz, M.N. Salmonella typhimurium resistant to silver nitrate, chloramphenicol, and ampicillin. Lancet 1975, 1, 235–240. [Google Scholar] [CrossRef]
- Gupta, A.; Matsui, K.; Lo, J.; Silver, S. Molecular basis for resistance to silver cations in Salmonella. Nat. Med. 1999, 5, 183–185. [Google Scholar] [CrossRef] [PubMed]
- Finley, P.J.; Norton, R.; Austin, C.; Mitchell, A.; Zank, S.; Durham, P. Unprecedented silver resistance in clinically isolated Enterobacteriaceae: Major implications for burn and wound management. Antimicrob. Agents Chemother. 2015, 59, 4734–4741. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Nikaido, H.; Williams, K.E. Silver-resistant mutants of Escherichia coli display active efflux of Ag+ and are deficient in porins. J. Bacteriol. 1997, 179, 6127–6132. [Google Scholar] [CrossRef] [PubMed]
- Randall, C.P.; Gupta, A.; Jackson, N.; Busse, D.; O’Neill, A.J. Silver resistance in Gram-negative bacteria: A dissection of endogenous and exogenous mechanisms. J. Antimicrob. Chemother. 2015, 70, 1037–1046. [Google Scholar] [CrossRef] [PubMed]
- Su, C.; Long, F.; Zimmermann, M.T.; Rajashankar, K.R.; Jernigan, R.L.; Yu, E.W. Crystal structure of the CusBA heavy-metal efflux complex of Escherichia coli. Nature 2011, 470, 558–563. [Google Scholar] [CrossRef] [PubMed]
- Xue, Y.; Davis, A.V.; Balakrishnan, G.; Stasser, J.P.; Staehlin, B.M.; Focia, P.; Spiro, T.G.; Penner-Hahn, J.E.; O’Halloran, T.V. Cu(I) recognition via cation-π and methionine interactions in CusF. Nat. Chem. Biol. 2008, 4, 107–109. [Google Scholar] [CrossRef] [PubMed]
- Gudipaty, S.A.; McEvoy, M.M. The histidine kinase CusS senses silver ions through direct binding by its sensor domain. Biochim. Biophys. Acta 2014, 1844, 1656–1661. [Google Scholar] [CrossRef] [PubMed]
- Franke, S.; Grass, G.; Nies, D.H. The product of the ybdE gene of the Escherichia coli chromosome is involved in detoxification of silver ions. Microbiology 2001, 147, 965–972. [Google Scholar] [CrossRef] [PubMed]
- Panáček, A.; Kvítek, L.; Smékalová, M.; Večeřová, R.; Kolář, M.; Röderová, M.; Dyčka, F.; Šebela, M.; Prucek, R.; Tomanec, O.; et al. Bacterial resistance to silver nanoparticles and how to overcome it. Nat. Nanotechnol. 2018, 13, 65–71. [Google Scholar] [CrossRef] [PubMed]
- Holladay, R.; Moeller, W.; Mehta, D.; Brooks, J.H.J.; Roy, R.; Mortenson, M. Silver/Water, Silver Gels and Silver-Based Compositions; and Methods for Making and Using the Same. World Intellectual Property Organization 2006074117A2, 5 January 2005. [Google Scholar]
- Jinjun, L.; Qiangbai, L.; Jianchao, S. Use of Medicinal Nanomaterial Composition Dg-5 Applied to Anti-Drug Resistant Bacteria. World Intellectual Property Organization 2018010403A1, 13 July 2016. [Google Scholar]
- Jiachong, C.; Jixiong, Y. Manufacturing Methods and Applications of Antimicrobial Plant Fibers Having Silver Particles. U.S. Patent 20100003296, 7 January 2010. [Google Scholar]
- Nano Silver and Perfume Contain an Apron. Korean Patent 200384433Y1, 16 May 2005.
- Method for Preparing Nano-Silver Particle and Detergent Composition by Using Them. Korean Patent 100933736B1, 26 June 2008.
- Composite Nanometer Antibacterial Material Used for Treating Vancomycin Drug Resistant Pathogenic Bacteria. Chinese Patent 105412940A, 2 December 2015.
- Paknikar, K.M. Anti-Microbial Activity of Biologically Stabilized Silver Nano Particles. World Intellectual Property Organization 2005120173A2, 22 December 2005. [Google Scholar]
- Holladay, R.J.; Christensen, H.; Moeller, W.D. Treatment of Humans with Colloidal Silver Composition. U.S. Patent 7135195B2, 14 November 2006. [Google Scholar]
- Liang, D.; Lu, Z.; Yang, H.; Gao, J.; Chen, R. Novel asymmetric wettable AgNPs/chitosan wound dressing: In vitro and in vivo evaluation. ACS Appl. Mater. Interfaces 2016, 8, 3958–3968. [Google Scholar] [CrossRef] [PubMed]
- Yabanoglu, H.; Basaran, O.; Aydogan, C.; Azap, O.K.; Karakayali, F.; Moray, G. Assessment of the effectiveness of silver-coated dressing, chlorhexidine acetate (0.5%), citric acid (3%), and silver sulfadiazine (1%) for topical antibacterial effects against the multi-drug resistant Pseudomonas aeruginosa infecting full-skin thickness burn wounds on rats. Int. Surg. 2013, 98, 416–423. [Google Scholar] [PubMed]
- Huang, Y.; Li, X.; Liao, Z.; Zhang, G.; Liu, Q.; Tang, J.; Peng, Y.; Liu, X.; Luo, Q. A randomized comparative trial between Acticoat and SD-Ag in the treatment of residual burn wounds, including safety analysis. Burns 2007, 33, 161–166. [Google Scholar] [CrossRef] [PubMed]
| Fungus | AgNPs Size (nm) | Target MDR Microorganism | Test Type a | Test Result b | Reference |
|---|---|---|---|---|---|
| Aspergillus flavus | 5–30 | E. coli | ZI | 15 ± 1.5 mm | [14] |
| S. aureus | ZI | 16 ± 2 mm | |||
| M. luteus | ZI | 14 ± 1 mm | |||
| P. aeruginosa | ZI | 14 ± 1.5 mm | |||
| E. faecalis | ZI | 15 ± 1.5 mm | |||
| A. baumanii | ZI | 15 ± 1 mm | |||
| K. pneumoniae | ZI | 14 ± 0.6 mm | |||
| Bacillus spp. | ZI | 15 ± 1.5 mm | |||
| Fusarium oxysporum NGD | 16.3–70 | Enterobacter sp. | ZI | 31 mm | [74] |
| P. aeruginosa | ZI | 20 mm | |||
| K. pneumoniae | ZI | 19 mm | |||
| E. coli | ZI | 2 mm | |||
| Trichoderma viride | 5–40 | E. coli | ZI | 16–28 mm (*) | [75] |
| S. typhi | ZI | 19–36 mm (*) | |||
| S. aureus | ZI | 10–19 mm (*) | |||
| M. luteus | ZI | 9–17 mm (*) | |||
| Aspergillus niger | 30–40 | S. aureus | ZI | 15 ± 0.23 mm | [76] |
| B. cereus | ZI | 16 ± 0.32 mm | |||
| P. vulgaris | ZI | 14 ± 0.26 mm | |||
| E. coli | ZI | 14 ± 0.44 mm | |||
| V. cholerae | ZI | 13 ± 0.51 mm | |||
| Tricholoma crassum | 5–50 | E. coli (DH5 α) | ZI | 17.5 ± 0.5 (**) | [77] |
| A. tumifaciens (LBA4404) | ZI | 20.0 ± 0.5 (**) | |||
| Agaricus bisporus | - | E. coli | ZI | 14 mm | [78] |
| Klebsiella sp. | ZI | 15 mm | |||
| Pseudomonas sp. | ZI | - | |||
| Enterobacter sp. | ZI | 18 mm | |||
| Proteus sp. | ZI | 20 mm | |||
| S. aureus | ZI | 17 mm | |||
| S. typhi | ZI | 22 mm | |||
| S. paratyphi | ZI | 17 mm | |||
| Aspergillus clavatus | 550–650 (AFM) | S. aureus | ZI | 20.5 mm | [79] |
| S. epidermidis | ZI | 19 mm | |||
| Penicilium polonicum | 10–15 | A. baumanii | MIC, MBC, ZI | 15.62 μg mL−1 (MIC), 31.24 μg mL−1 (MBC), 21.2 ± 0.4 mm (ZI) | [73] |
| Cryphonectria sp. | 30–70 | S. aureus (ATCC-25923) | ZI | 16 ± 0.69 mm | [80] |
| S. typhi (ATCC-51812) | ZI | 12 ± 0.29 mm | |||
| E. coli (ATCC-39403) | ZI | 13 ± 1.54 mm | |||
| Rhizoppus spp. | 27–50 | E. coli | ZI | 15–22 mm (***) | [81] |
| Fusarium oxysporum | 77.68 | S. aureus (MRSA 101) | MIC, MBC | 250 μM (MIC), 500 μM (MBC) | [70] |
| S. aureus (MRSA 107) | MIC, MBC | 250 μM (MIC), 500 μM (MBC) | |||
| E. coli (ESBL 167) | MIC, MBC | 125 μM (MIC), 125 μM (MBC) | |||
| E. coli (ESBL 169) | MIC, MBC | 125 μM (MIC), 125 μM (MBC) | |||
| E. coli (ESBL 176) | MIC, MBC | 125 μM (MIC), 125 μM (MBC) | |||
| E. coli (ESBL 192) | MIC, MBC | 125 μM (MIC), 125 μM (MBC) | |||
| E. coli (KPC 131) | MIC, MBC | 125 μM (MIC), 125 μM (MBC) | |||
| E. coli (KPC 133) | MIC, MBC | 125 μM (MIC), 125 μM (MBC) | |||
| A. baumannii (CR 01) | MIC, MBC | 125 μM (MIC), 125 μM (MBC) | |||
| Aspergillus flavus | 5–30 | E. coli | ZI | 15 ± 1.5 mm | [14] |
| S. aureus | ZI | 16 ± 2 mm | |||
| M. luteus | ZI | 14 ± 1 mm | |||
| P. aeruginosa | ZI | 14 ± 1.5 mm | |||
| E. faecalis | ZI | 15 ± 1.5 mm | |||
| A. baumannii | ZI | 15 ± 1 mm | |||
| K. pneumoniae | ZI | 14 ± 0.6 mm | |||
| Bacillus spp. | ZI | 15 ± 1.5 mm | |||
| Macrophomina phaseolina | 5–40 | E. coli (DH5α-MDR) | ZI | 3.0 ± 0.2 mm (**) | [72] |
| A. tumefaciens (LBA4404-MDR) | ZI | 3.3 ± 0.2 mm (**) |
| Bacteria | AgNPs Size (nm) | Target MDR Microorganism | Test Type a | Test Result b | Reference |
|---|---|---|---|---|---|
| Streptomyces | 20–70 | K. pneumoniae (ATCC 100603) | MIC | 4 μg mL−1 | [92] |
| K. pneumoniae | MIC | 1.4 μg mL−1 | |||
| E. coli | MIC | 2 μg mL−1 | |||
| Citrobacter | MIC | 2 μg mL−1 | |||
| Bacillus sp. | 14–42 | S. epidermidis strain 73 (pus) | ZI | 15 mm | [93] |
| S. epidermidis strain 145 (catheter tips) | ZI | 19 mm | |||
| S. epidermidis strain 152 (blood) | ZI | 19 mm | |||
| S. aureus (MTCC 87) | ZI | 18 mm | |||
| S. typhi | ZI | 13 mm | |||
| S. paratyphi | ZI | 15 mm | |||
| V. cholerae (MTCC 3906) | ZI | 18 mm | |||
| Bacillus cereus | 24–46 | E. coli | MIC, ZI | 6.25 μg mL−1 (MIC), 16 ± 1 mm (ZI) | [94] |
| S. aureus | MIC, ZI | 12.5 μg mL−1 (MIC), 14 ± 1 (ZI) | |||
| K. pneumoniae | MIC, ZI | >3.12 μg mL−1 (MIC), 17 ± 1 mm (ZI) | |||
| P. aeruginosa | MIC, ZI | 3.12 μg mL−1 (MIC), 23 ± 1 mm (ZI) | |||
| Bacillus safensis (LAU 13) | 5–95 | E. coli | ZI | 11–19 mm | [95] |
| K. granulomatis | ZI | 11–19 mm | |||
| P. vulgaris | ZI | 11–19 mm | |||
| P. aeruginosa | ZI | 11–19 mm | |||
| S. aureus | ZI | 11–19 mm | |||
| Aeromonas sp. THG-FG1.2 | 8–16 | B. cereus (ATCC 14579) | ZI | 13.5 ± 0.5 mm | [88] |
| B. subtilis (KACC 14741) | ZI | 13 ± 1.0 mm | |||
| S. aureus (ATCC 6538) | ZI | 15.5 ± 0.5 mm | |||
| E. coli (ATCC 10798) | ZI | 13 ± 0.2 mm | |||
| P. aeruginosa (ATCC 6538) | ZI | 16 ± 0.1 mm | |||
| V. parahaemolyticus (ATCC 33844) | ZI | 16 ± 0.1 mm | |||
| S. enterica (ATCC 13076) | ZI | 11 ± 0.2 mm | |||
| C. albicans (KACC 30062) | ZI | 20 ± 0.1 mm | |||
| C. tropicalis (KCTC 7909) | ZI | 15 ± 0.5 mm | |||
| Bacillus thuringiensis | 15 | E. coli | ZI | 12 ± 1 mm (*) | [96] |
| P. aeruginosa | ZI | 16 ± 1 mm (*) | |||
| S. aureus | ZI | 9 ± 1 mm (*) | |||
| Anabaena diololum | 10–50 | K. pneumoniae DF12SA (HQ114261) | ZI | 36 ± 0.82 mm | [97] |
| 10–50 | E. coli DF39TA (HQ163793) | ZI | 33 ± 1.63 mm | ||
| 10–50 | S. aureus DF8TA (JN642261) | ZI | 34 ± 0.81 mm | ||
| Streptomyces sp. GUT 21 | 23–48 | E. coli (MTCC 9537) | MIC, ZI | 14 μg mL−1 (MIC), 27.05 ± 3.20 mm (ZI) | [89] |
| K. pneumoniae (MTCC 109) | MIC, ZI | 12 μg mL−1 (MIC), 28.50 ± 2.60 mm (ZI) | |||
| S. aureus (MTCC 96) | MIC, ZI | 15 μg mL−1 (MIC), 24.25 ± 2.09 mm (ZI) | |||
| P. aeruginosa (MTCC 1688) | MIC, ZI | 10 μg mL−1 (MIC), 10.05 ± 3.60 mm (ZI) | |||
| Bacillus megaterium | 80–98.56 (AFM) | S. pneumoniae | ZI | 21 mm | [98] |
| S. typhi | ZI | 18 mm | |||
| Xanthomonas spp. | 5–40 | P. aeruginosa | ZI | 10.0 ± 1.0 mm | [91] |
| baumannii | ZI | 10.6 ± 0.6 mm | |||
| Sinomonas mesophila MPKL 26 | 4–50 | S. aureus | ZI | 12 mm | [90] |
| Bacillus flexus | 12–65 | E. coli | ZI | 11.55 mm | [99] |
| P. aeruginosa | ZI | 11.05 mm | |||
| S. pyogenes | ZI | 11.65 mm | |||
| subtilis | ZI | 11.55 mm | |||
| Bacillus brevis (NCIM 2533) | 41–68 | S. aureus | ZI | 19 mm | [100] |
| S. typhi | ZI | 7.5 mm |
| Plant | Part | AgNPs Size (nm) | Target MDR Microorganism | Test Type a | Test Result b | Reference |
|---|---|---|---|---|---|---|
| Olive | leaf | 20–25 | S. aureus | ZI | 2.4 ± 0.2 cm (*) | [114] |
| P. aeruginosa | ZI | 2.4 ± 0.2 cm (*) | ||||
| E. coli | ZI | 1.8 ± 0.2 cm (*) | ||||
| Phyllanthus amarus | Whole plant | 24 ± 8 | P. aeruginosa | MIC, ZI | 6.25–12.5 μg mL−1 (MIC), 10 ± 0.53 to 21 ± 0.11 mm (ZI) | [115] |
| Corchorus capsularis | leaf | 5–45 | P. aeruginosa | ZI | 17 mm | [116] |
| S. aureus | ZI | 21 mm | ||||
| Coagulase negative staphylococci | ZI | 20 mm | ||||
| Tribulus terrestris | fruit | 16–28 | S. pyogens | ZI | 10 mm | [117] |
| E. coli | ZI | 10.75 mm | ||||
| P. aeruginosa | ZI | 9.25 mm | ||||
| B. subtilis | ZI | 9.25 mm | ||||
| S. aureus | ZI | 9.75 mm | ||||
| Garcinia mangostana | leaf | 35 | E. coli | ZI | 15 mm | [118] |
| S. aureus | ZI | 20 mm | ||||
| Ricinus communis | leaf | 29.18 (X-ray diffraction) | B. fusiformis | ZI | 2.90 cm | [119] |
| E.coli | ZI | 2.89 cm | ||||
| Caesalpinia coriaria | leaf | 40–52 | E. coli | ZI | 12.0 ± 0.50 mm | [109] |
| P. aeruginosa | ZI | 18.3 ± 0.80 mm | ||||
| K. pneumonia | ZI | 14.6 ± 1.20 mm | ||||
| S. aureus | ZI | 10.3 ± 1.20 mm | ||||
| 78–98 | E. coli | ZI | 9.6 ± 0.80 mm | [109] | ||
| P. aeruginosa | ZI | 18.3 ± 1.20 mm | ||||
| K. pneumonia | ZI | 13.3 ± 0.30 mm | ||||
| S. aureus | ZI | 11.0 ± 0.00 mm | ||||
| Mimusops elengi | leaf | 55–83 | K. pneumoniae | ZI | 18 mm | [120] |
| S. aureus | ZI | 10 mm | ||||
| M. luteus | ZI | 11 mm | ||||
| Ocimum gratissimum | leaf | 16 ± 2 | E. coli (MC-2) | MIC, MBC, ZI | 4 μg mL−1 (MIC), 8 μg mL−1 (MBC), 12 ± 0.6 mm (ZI) | [112] |
| S. aureus (MMC-20) | MIC, MBC, ZI | 8 μg mL−1 (MIC), 16 μg mL−1 (MBC), 16 ± 1.0 mm (ZI) | ||||
| Hydrocotyle sibthorpioides | Whole plant | 13.37 ± 10 | K. pneumonia | ZI | 3.0 ± 0.17 mm | [121] |
| P. aeruginosa | ZI | 2.7 ± 0.32 mm | ||||
| S. aureus | ZI | 3.6 ± 0.57 mm | ||||
| Vaccinium corymbosum | leaf | 10–30 | E. coli (ATCC 25922) | MIC, MBC, ZI | 11.22 ± 0.29 mm | [122] |
| S. aureus (ATCC 25923) | MIC, MBC, ZI | 13.1 ± 1.1 mm | ||||
| P. aeruginosa (ATCC 27853) | MIC, MBC, ZI | 11.6 ± 0.32 mm | ||||
| B. subtilis (ATCC 21332) | MIC, MBC, ZI | 12.4 ± 0.40 mm | ||||
| Prosopis farcta | leaf | 10.8 ± 3.54 | S. aureus (PTCC 1431) | ZI | 9.5 mm | [123] |
| B. subtilis (PTCC 1420) | ZI | 9 mm | ||||
| E. coli (PTCC 1399) | ZI | 9.5 mm | ||||
| P. aeruginosa (PTCC 1074) | ZI | 9.5 mm | ||||
| Sesbania gradiflora | leaf | 10–25 | S. enterica | ZI | 15.67 ± 0.09 mm | [124] |
| S. aureus | ZI | 10.54 ± 0.23 mm | ||||
| Solanum nigrum | leaf | 20 | K. pneumoniae | ZI | 21.5 mm | [110] |
| P. aeruginosa | ZI | 21.3 mm | ||||
| S. epidermidis | ZI | 19.6 mm | ||||
| E. coli | ZI | 15.3 mm | ||||
| P. vulgaris | ZI | 13.3 mm | ||||
| S. aureus | ZI | 9.6 mm | ||||
| Cissus quadrangularis | leaf | 15–23 (**) | S. pyogens | MIC, ZI | 4 μg mL−1 (MIC), 7.77 ± 0.25 mm (ZI) | [113] |
| S. aureus | MIC, ZI | 3 μg mL−1 (MIC), 8.83 ± 0.26 mm (ZI) | ||||
| E. coli | MIC, ZI | 5 μg mL−1 (MIC), 7.9 ± 0.31 mm (ZI) | ||||
| P. vulgaris | MIC, ZI | 7 μg mL−1 (MIC), 8.4 ± 0.40 mm (ZI) | ||||
| Cola nitida | pod | 12–80 | E. coli | ZI | 19 ± 0.9 mm | [125] |
| K. granulomatis | ZI | 11 ± 0.8 mm | ||||
| P. aeruginosa | ZI | 28 ± 0.1 mm | ||||
| Strychnos potatorum | leaf | 28 | S. aureus | ZI | 8 mm | [126] |
| K. pneumoniae | ZI | 10 mm | ||||
| Alstonia scholaris | leaf | 80 | E. coli | ZI | 10.0 ± 2.8 mm | [111] |
| P. aeruginosa | ZI | 8.0 ± 1.4 mm | ||||
| K. pneumoniae | ZI | 11.0 ± 1.0 mm | ||||
| S. aureus | ZI | 10.0 ± 3.0 mm | ||||
| P. vulgaris | ZI | 8.3 ± 0.6 mm | ||||
| S. epidermidis | ZI | 10.6 ± 1.2 mm | ||||
| Andrographis paniculata | leaf | 70 | E. coli | ZI | 8.0 ± 1.4 mm | [111] |
| P. aeruginosa | ZI | 6.7 ± 0.7 mm | ||||
| K. pneumoniae | ZI | 9.3 ± 0.6 mm | ||||
| S. aureus | ZI | 8.0 ± 1.0 mm | ||||
| P. vulgaris | ZI | 8.3 ± 0.6 mm | ||||
| S. epidermidis | ZI | 9.0 ± 1.0 mm | ||||
| Aegle marmelos | leaf | 70 | E. coli | ZI | 11.0 ± 2.8 mm | [111] |
| P. aeruginosa | ZI | 9.0 ± 1.4 mm | ||||
| K. pneumoniae | ZI | 9.3 ± 1.6 mm | ||||
| S. aureus | ZI | 9.7 ± 1.5 mm | ||||
| P. vulgaris | ZI | 9.7 ± 0.6 mm | ||||
| S. epidermidis | ZI | 8.0 ± 1.0 mm | ||||
| Centella asiatica | leaf | 90 | E. coli | ZI | 12.7 ± 0.7 mm | [111] |
| P. aeruginosa | ZI | 8.0 ± 1.4 mm | ||||
| K. pneumoniae | ZI | 12.0 ± 1.0 mm | ||||
| S. aureus | ZI | 13.0 ± 2.0 mm | ||||
| P. vulgaris | ZI | 9.7 ± 0.6 mm | ||||
| S. epidermidis | ZI | 14.0 ± 1.0 mm | ||||
| Eclipta prostrata | leaf | 70 | E. coli | ZI | 10.0 ± 4.0 mm | [111] |
| P. aeruginosa | ZI | 8.3 ± 2.5 mm | ||||
| K. pneumoniae | ZI | 10.0 ± 5.2 mm | ||||
| S. aureus | ZI | 12.6 ± 4.9 mm | ||||
| P. vulgaris | ZI | 6.6 ± 0.5 mm | ||||
| S. epidermidis | ZI | 8.0 ± 0.0 mm | ||||
| Moringa oleifera | leaf | 50 | E. coli | ZI | 7.7 ± 0.6 mm | [111] |
| P. aeruginosa | ZI | 8.0 ± 1.7 mm | ||||
| K. pneumoniae | ZI | 7.0 ± 1.0 mm | ||||
| S. aureus | ZI | 9.0 ± 2.6 mm | ||||
| P. vulgaris | ZI | 7.0 ± 2.0 mm | ||||
| S. epidermidis | ZI | 7.0 ± 0.0 mm | ||||
| Thespesia populnea | bark | 70 | E. coli | ZI | 9.0 ± 1.7 mm | [111] |
| P. aeruginosa | ZI | 10.3 ± 2.1 mm | ||||
| K. pneumoniae | ZI | 11.3 ± 1.2 mm | ||||
| S. aureus | ZI | 9.3 ± 2.4 mm | ||||
| P. vulgaris | ZI | 8.6 ± 1.2 mm | ||||
| S. epidermidis | ZI | 8.6 ± 0.7 mm | ||||
| Terminalia arjuna | bark | 70 | E. coli | ZI | 8.0 ± 0.7 mm | [111] |
| P. aeruginosa | ZI | 9.0 ± 2.0 mm | ||||
| K. pneumoniae | ZI | 14.0 ± 1.0 mm | ||||
| S. aureus | ZI | 12.7 ± 1.1 mm | ||||
| P. vulgaris | ZI | 8.3 ± 0.6 mm | ||||
| S. epidermidis | ZI | 9.0 ± 2.0 mm | ||||
| Plumbago zeylanica | Root bark | 90 | E. coli | ZI | 8.0 ± 1.4 mm | [111] |
| P. aeruginosa | ZI | 14.7 ± 0.7 mm | ||||
| K. pneumoniae | ZI | 8.3 ± 0.8 mm | ||||
| S. aureus | ZI | 7.7 ± 0.6 mm | ||||
| P. vulgaris | ZI | 8.3 ± 0.6 mm | ||||
| S. epidermidis | ZI | 8.0 ± 1.0 mm | ||||
| Semecarpus anacardium | nuts | 60 | E. coli | ZI | 10.0 ± 2.0 mm | [111] |
| P. aeruginosa | ZI | 9.3 ± 1.5 mm | ||||
| K. pneumoniae | ZI | 10.0 ± 1.0 mm | ||||
| S. aureus | ZI | 7.7 ± 1.1 mm | ||||
| P. vulgaris | ZI | 8.3 ± 0.6 mm | ||||
| S. epidermidis | ZI | 9.3 ± 1.5 mm | ||||
| Mukia scabrella | leaf | 18–21 | Acinetobacter sp. | ZI | 22 mm | [127] |
| K. pneumoniae | ZI | 19 mm | ||||
| P. aeruginosa | ZI | 20 mm | ||||
| Phyllanthus amarus | Whole plant | 24 ± 8 | P. aeruginosa (***) | MIC, ZI | 6.25–12.5 μg mL−1 (MIC), 21 ± 0.11 mm (ZI) | [115] |
| Ricinodendron heudelotti | Seed kernel | 89.0 | E. coli | MIC, MBC | 1.68 μg mL−1 (MIC), 6.75 μg mL−1 (MBC) | [128] |
| Gnetum bucholzianum | leaf | 67.4 | E. coli | MIC, MBC | 1.687 μg mL−1 (MIC), 1.687 μg mL−1 (MBC) | [129] |
| Megaphrynium macrostachyum | leaf | 33.7 (Ag), 44.2 (AgCl) | E. coli | MIC, MBC | 0.515 μg mL−1 (MIC), 4.12 μg mL−1 (MBC) | [129] |
| Corchorus olitorus | leaf | 30.0 (nm), 37.9 (AgCl) | E. coli | MIC, MBC | 8.25 μg mL−1 (MIC), 16.5 μg mL−1 (MBC) | [129] |
| Ipomoea batatas | leaf | 67.3 (Ag), 37.9 (AgCl) | E. coli | MIC, MBC | 5.3 μg mL−1 (MIC), 5.3 μg mL−1 (MBC) | [129] |
| Areca catechu | leaf | 22–40 | E. coli | ZI | 20 mm | [129] |
| P. aeruginosa | ZI | 24 mm | ||||
| S. typhi | ZI | 19 mm | ||||
| P. vulgaris | ZI | 23 mm | ||||
| K. pneumoniae | ZI | 26 mm | ||||
| Cocoa | bean | 8.96–54.22 | S. aureus | ZI | 12 mm (*) | [130] |
| K. pneumoniae (wound) | ZI | 12 mm (*) | ||||
| K. pneumoniae (urine) | ZI | 13 mm (*) | ||||
| E. coli | ZI | 14 mm (*) | ||||
| Cocoa | Pod husk | 4–32 | K. pneumoniae | ZI | 10–14 mm | [131] |
| E. coli | ZI | 10–14 mm | ||||
| Phomis bracteosa | Whole plant | 22.41 | E. coli (ATCC 15224) | ZI | 13.2 ± 0.12 | [108] |
| S. aureus (ATCC 6538) | ZI | 11.1 ± 0.10 | ||||
| K. pneumoniae (ATCC 4619) | ZI | 10.3 ± 0.11 | ||||
| Momordica cymbalaria | fruit | 15.5 | E. coli | ZI | 24.0 ± 1.0 | [132] |
| M. luteus | ZI | 20.0 ± 1.4 | ||||
| B. cereus | ZI | 22.0 ± 1.0 | ||||
| K. pneumoniae | ZI | 26.0 ± 1.4 | ||||
| S. pneumoniae | ZI | 26.0 ± 1.7 | ||||
| Astragalus membranaceus | root | 65.08 | S. aureus (MRSA) | MIC, ZI | 0.063 mg mL−1 (MIC), 12.83 ± 1.04 mm (ZI) | [107] |
| S. epidermidis (MRSE) | MIC, ZI | 0.063 mg mL−1 (MIC), 12.33 ± 0.29 mm (ZI) | ||||
| P. aeruginosa | MIC, ZI | 0.032 mg mL−1 (MIC), 15.17 ± 0.76 mm (ZI) | ||||
| E. coli | MIC, ZI | 0.032 mg mL−1 (MIC), 14.67 ± 0.76 mm (ZI) |
| Patent Number | Application | Resistant Bacteria | Reference |
|---|---|---|---|
| WO2006074117A2 | Hydrogel | E. cloacae, K. pneumoniae, E. coli, P. aeruginosa, A. Acinetobacter | [170] |
| WO2018010403A1 | Pharmaceuticals | E. cloacae, K. pneumoniae, E. coli, P. aeruginosa, A. Acinetobacter | [171] |
| US20100003296A1 | Textiles | Methicillin-resistant S. aureus (MRSA) | [172] |
| KR200384433Y1 | Apron, perfume | Methicillin-resistant S. aureus (MRSA) | [173] |
| KR100933736B1 | Detergent additive | E. coli | [174] |
| CN105412940A | General | Vancomycin-resistant Enterococcus faecalis | [175] |
| WO2005120173A2 | General | P. aeruginosa | [176] |
| US7135195B2 | General | Methicillin-resistant S. aureus (MRSA) | [177] |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barros, C.H.N.; Fulaz, S.; Stanisic, D.; Tasic, L. Biogenic Nanosilver against Multidrug-Resistant Bacteria (MDRB). Antibiotics 2018, 7, 69. https://doi.org/10.3390/antibiotics7030069
Barros CHN, Fulaz S, Stanisic D, Tasic L. Biogenic Nanosilver against Multidrug-Resistant Bacteria (MDRB). Antibiotics. 2018; 7(3):69. https://doi.org/10.3390/antibiotics7030069
Chicago/Turabian StyleBarros, Caio H. N., Stephanie Fulaz, Danijela Stanisic, and Ljubica Tasic. 2018. "Biogenic Nanosilver against Multidrug-Resistant Bacteria (MDRB)" Antibiotics 7, no. 3: 69. https://doi.org/10.3390/antibiotics7030069
APA StyleBarros, C. H. N., Fulaz, S., Stanisic, D., & Tasic, L. (2018). Biogenic Nanosilver against Multidrug-Resistant Bacteria (MDRB). Antibiotics, 7(3), 69. https://doi.org/10.3390/antibiotics7030069