Surveillance for Antimicrobial Resistance in Gonorrhea: The Alberta Model, 2012–2016
Abstract
1. Introduction
2. Materials and Methods
3. Results
3.1. Culture Representativeness
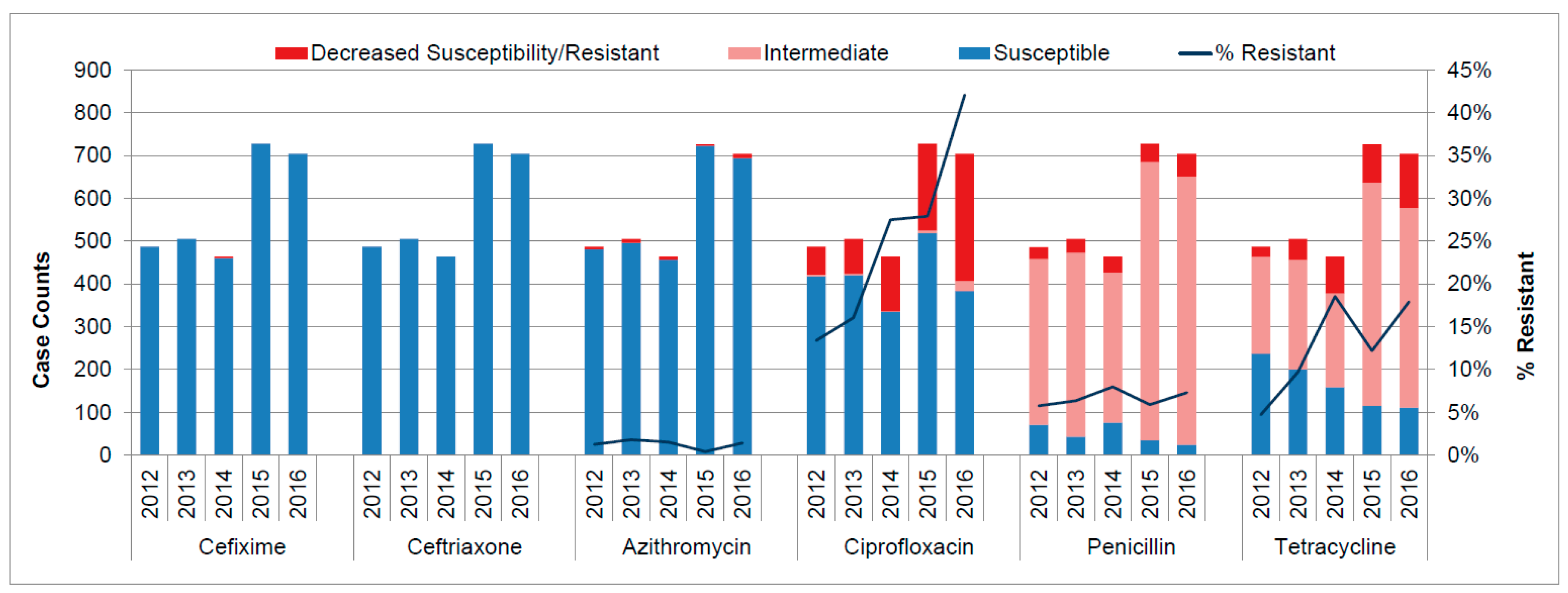
3.2. Antimicrobial Resistance Patterns
3.3. Sequence Types
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Public Health Agency of Canada. Report on sexually transmitted infections in Canada: 2013–2014. Available online: https://www.canada.ca/en/public-health/services/publications/diseases-conditions/report-sexually-transmitted-infections-canada-2013-14.html#a31 (accessed on 14 May 2018).
- Skerlev, M.; Culav-Koscak, I. Gonorrhea: New challenges. Clin. Dermatol. 2014, 32, 275–281. [Google Scholar] [CrossRef] [PubMed]
- Fleming, D.T.; Wasserheit, J.N. From epidemiological synergy to public health policy and practice: The contribution of other sexually transmitted diseases to sexual transmission of HIV infection. Sex. Transm. Infect. 1999, 75, 3–17. [Google Scholar] [CrossRef] [PubMed]
- Weston, E.J.; Wi, T.; Papp, J. Strengthening global surveillance for antimicrobial drug-resistant Neisseria gonorrhoeae through the Enhanced Gonococcal Antimicrobial Surveillance Program. Emerg. Infect. Dis. 2017, 23, S47. [Google Scholar] [CrossRef] [PubMed]
- Lefebvre, B.; Martin, I.; Demczuk, W.; Deshaies, L.; Michaud, S.; Labbé, A.-C.; Beaudoin, M.-C.; Longtin, J. Ceftriaxone-Resistant Neisseria gonorrhoeae, Canada, 2017. Emerg. Infect. Dis. 2018, 24, 381–383. [Google Scholar] [CrossRef] [PubMed]
- Plitt, S.; Boyington, C.; Sutherland, K.; Lovgren, M.; Tilley, P.A.G.; Read, R.; Singh, A.E. Antimicrobial resistance in gonorrhea: The influence of epidemiologic and laboratory surveillance data on treatment guidelines: Alberta, Canada 2001–2007. Sex. Transm. Infect. 2009, 36, 665–669. [Google Scholar] [CrossRef] [PubMed]
- Unemo, M.; Dillon, J.A. Review and international recommendation of methods for typing Neisseria gonorrhoeae isolates and their implications for improved knowledge of gonococcal epidemiology, treatment, and biology. Clin. Microbiol. Rev. 2011, 24, 447–458. [Google Scholar] [CrossRef] [PubMed]
- Clinical and Laboratory Standards Institute. Performance Standards for Antimicrobial Susceptibility Testing: Twenty-Fifth Informational Supplement; M100-S25; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2015. [Google Scholar]
- Kirkcaldy, R.D.; Harvey, A.; Papp, J.R.; Del Rio, C.; Soge, O.O.; Holmes, K.K.; Hook, E.W., 3rd; Kubin, G.; Riedel, S.; Zenilman, J.; Pettus, K.; et al. Neisseria gonorrhoeae Antimicrobial Susceptibility Surveillance—The Gonococcal Isolate Surveillance Project, 27 Sites, United States, 2014. MMWR. Surveill. Summ. 2016, 65, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Martin, I.M.; Ison, C.A.; Aanensen, D.M.; Fenton, K.A.; Spratt, B.G. Rapid sequence-based identification of gonococcal transmission clusters in a large metropolitan area. J. Infect. Dis. 2004, 189, 1497–1505. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.E.; Gratrix, J.; Read, R.; Lovgren, M.; Drews, S.J.; Romanowski, B.; Sutherland, K.; Talbot, J.; Martin, I. Neisseria gonorrhoeae Multi-antigen Sequence Typing (NG-MAST) is Beneficial in Further Characterizing Gonococcal Populations in Alberta, Canada. Sex Transm. Dis. 2013, 40, 744–750. [Google Scholar] [CrossRef] [PubMed]
- Ison, C.A.; Town, K.; Obi, C.; Chisholm, S.; Hughes, G.; Livermore, D.M.; Lowndes, C.M.; GRASP collaborative group. Decreased susceptibility to cephalosporins among gonococci: Data from the Gonococcal Resistance to Antimicrobials Surveillance Programme (GRASP) in England and Wales, 2007–2011. Lancet Infect. Dis. 2013, 13, 762–768. [Google Scholar] [CrossRef]
- Public Health England. Surveillance of antimicrobial resistance in Neisseria gonorrhoeae in England and Wales. Key findings from the Gonococcal Resistance to Antimicrobials Surveillance Programme (GRASP); 2017. Available online: https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/651636/GRASP_Report_2017.pdf (accessed on 13 June 2018).
- Lahra, M.M. Australian Gonococcal Surveillance Programme. Australian Gonococcal Surveillance Programme Annual Report; 2015. Available online: http://www.health.gov.au/internet/main/publishing.nsf/content/cda-cdi4101-pdf-cnt.htm/$FILE/cdi4101i.pdf (accessed on 13 June 2018).
- World Health Organization (WHO). Global Action Plan to Control the Spread and Impact of Antimicrobial Resistance in Neisseria gonorrhoeae. 2012. Available online: http://www.who.int/reproductivehealth/publications/rtis/9789241503501/en/ (accessed on 13 June 2018).
- Public Health Agency of Canada. National Surveillance of Antimicrobial Susceptibilities in Neisseria gonorrhoeae Annual Summary 2016; Public Health Agency of Canada: Ottawa, Canada, 2017.
- Thakur, S.D.; Levett, P.N.; Horsman, G.B.; Dillon, J.R. High levels of susceptibility to new and older antibiotics in Neisseria gonorrhoeae isolates from Saskatchewan (2003–15): Time to consider point-of-care or molecular testing for precision treatment? J. Antimicrob. Chemother. 2018, 73, 118–125. [Google Scholar] [CrossRef] [PubMed]
| Characteristics | Categories | Test Type | Total (N = 13,132) | |
|---|---|---|---|---|
| Culture (n = 2891) | NAAT-Only (n = 10,241) | |||
| Year (p < 0.001) | 2012 | 487 (16.8%) | 1594 (15.6%) | 2081 (15.8%) |
| 2013 | 506 (17.5%) | 1491 (14.6%) | 1997 (15.2%) | |
| 2014 | 465 (16.1%) | 1426 (13.9%) | 1891 (14.4%) | |
| 2015 | 729 (25.2%) | 2682 (26.2%) | 3411 (26.0%) | |
| 2016 | 704 (24.4%) | 3048 (29.7%) | 3752 (28.6%) | |
| Gender (p < 0.0001) | Male | 2285 (79.0%) | 5017 (49.0%) | 7302 (55.6%) |
| Female | 606 (21.0%) | 5224 (51.0%) | 5830 (44.4%) | |
| Age (in year) (p < 0.001) | 0–14 | 10 (0.3%) | 75 (0.7%) | 85 (0.7%) |
| 15–19 | 256 (8.9%) | 1723 (16.8%) | 1979 (15.1%) | |
| 20–24 | 648 (22.4%) | 2818 (27.5%) | 3466 (26.4%) | |
| 25–29 | 780 (27.0%) | 2226 (21.7%) | 3006 (22.9%) | |
| 30–34 | 520 (18.0%) | 1532 (15.0%) | 2052 (15.6%) | |
| 35–39 | 242 (8.4%) | 839 (8.2%) | 1081 (8.2%) | |
| 40 and older | 435 (15.0%) | 1028 (10.1%) | 1463 (11.1%) | |
| Ethnicity (p < 0.001) | First Nation | 373 (12.9%) | 3439 (33.6%) | 3812 (29.0%) |
| Inuit | 2 (0.1%) | 13 (0.1%) | 15 (0.1%) | |
| Métis | 148 (5.1%) | 546 (5.3%) | 694 (5.3%) | |
| Asian | 206 (7.1%) | 332 (3.3%) | 538 (4.1%) | |
| Black | 215 (7.4%) | 448 (4.4%) | 663 (5.0%) | |
| Caucasian | 1761 (60.9%) | 3699 (36.1%) | 5460 (41.6%) | |
| Other | 95 (3.3%) | 155 (1.5%) | 250 (1.9%) | |
| Unknown | 91 (3.2%) | 1609 (15.7%) | 1700 (13.0%) | |
| Sexual Partner (p < 0.001) | Opposite sex | 1434 (49.6%) | 7144 (69.8%) | 8578 (65.3%) |
| Same sex | 1196 (41.4%) | 801 (7.8%) | 1997 (15.2%) | |
| Bisexual | 187 (6.5%) | 290 (2.8%) | 477 (3.6%) | |
| Case < 12 years | 7 (0.2%) | 6 (0.1%) | 13 (0.1%) | |
| Unknown | 67 (2.3%) | 2000 (19.5%) | 2067 (15.8%) | |
| Geographic Area (p < 0.001) | South | 33 (1.1%) | 246 (2.4%) | 279 (2.1%) |
| Calgary | 1150 (39.8%) | 1924 (18.8%) | 3074 (23.4%) | |
| Central | 15 (0.5%) | 902 (8.8%) | 917 (6.9%) | |
| Edmonton | 1624 (56.2%) | 4627 (45.2%) | 6251 (47.6%) | |
| North | 69 (2.4%) | 2542 (24.8%) | 2611 (20.0%) | |
| Testing Agency (p < 0.001) | Calgary STI Clinic | 1114 (38.5%) | 281 (2.7%) | 1395 (10.6%) |
| Edmonton STI Clinic | 1468 (50.8%) | 878 (8.6%) | 2346 (17.9%) | |
| Fort McMurray STI Clinic | 46 (1.6%) | 118 (1.2%) | 164 (1.2%) | |
| Other Providers | 263 (9.1%) | 8964 (87.5%) | 9227 (70.3%) | |
| Sequence Group | Cases/Isolates (n) | Predominant ST (n) | Differ ≤1% for porB ST (n) | Differ ≤1% for tbpB ST (n) |
|---|---|---|---|---|
| SG-7638 | 367 | ST-7638 (328) | ST-10815 (12), ST-12095 (1), ST-12863 (17), ST-13828 (1), ST-14537 (1), ST-14878 (1), ST-14984 (1) | ST-13826 (5) |
| SG-5985 | 145 | ST-5985 (133) | ST-6968 (3), ST-11348 (1), ST-11471 (1), ST-11544 (1), ST-11841 (1), ST-14865 (3) | ST-10131 (2) |
| SG-11299 | 127 | ST-11299 (116) | ST-8695 (4), ST-11837 (1), ST-12389 (5), ST-15200 (1) | -- |
| Characteristics | Categories | NG-MAST Group n (%) | ||
|---|---|---|---|---|
| SG-7638 (n = 367) | SG-5985 (n = 145) | SG-11299 (n = 127) | ||
| Gender | Female | 147 (40.1%) | 17 (11.7%) | 15 (11.8%) |
| Male | 220 (59.9%) | 128 (88.3%) | 112 (88.2%) | |
| Age (in years) | 0–14 | 1 (0.3%) | 1 (0.7%) | 0 |
| 15–19 | 35 (9.5%) | 9 (6.2%) | 6 (4.7%) | |
| 20–24 | 93 (25.3%) | 43 (29.7%) | 25 (19.7%) | |
| 25–29 | 103 (28.1%) | 45 (31.0%) | 31 (24.4%) | |
| 30–34 | 59 (16.1%) | 23 (15.9%) | 22 (17.3%) | |
| 35–39 | 38 (10.4%) | 5 (3.4%) | 20 (15.8%) | |
| 40+ | 38 (10.4%) | 19 (13.1%) | 23 (18.1%) | |
| Ethnicity | Asian | 13 (3.5%) | 8 (5.5%) | 14 (11.0%) |
| Black | 19 (5.2% | 6 (4.1%) | 4 (3.2%) | |
| Caucasian | 118 (32.2%) | 109 (75.2%) | 90 (70.9%) | |
| First Nation | 134 (36.5%) | 7 (4.8%) | 4 (3.2%) | |
| Metis | 44 (12.0%) | 3 (2.1%) | 5 (3.9%) | |
| Other | 6 (1.6%) | 5 (3.5%) | 4 (3.2%) | |
| Unknown | 33 (9.0%) | 7 (4.8%) | 6 (4.7%) | |
| Sexual Partners | Opposite Sex | 302 (82.3%) | 42 (29.0%) | 35 (27.6%) |
| Same Sex | 16 (4.3%) | 93 (64.1%) | 69 (54.3%) | |
| Bisexual | 15 (4.1%) | 5 (3.4%) | 17 (13.4%) | |
| Case < 12 years | 1 (0.3%) | 1 (0.7%) | 0 | |
| Unknown | 33 (9.0%) | 4 (2.8%) | 6 (4.7%) | |
| Year | 2012 | 0 | 3 (2.1%) | 0 |
| 2013 | 1 (0.3%) | 22 (15.2%) | 4 (3.2%) | |
| 2014 | 8 (2.2%) | 47 (32.4%) | 22 (17.3%) | |
| 2015 | 229 (62.4%) | 45 (31.0%) | 52 (40.9%) | |
| 2016 | 129 (35.1%) | 28 (19.3%) | 49 (38.6%) | |
| Geographic Area | South | 2 (0.5%) | 1 (0.7%) | 1 (0.8%) |
| Calgary | 29 (8.0%) | 82 (56.5%) | 48 (37.8%) | |
| Central | 2 (0.5%) | 1 (0.7%) | 0 | |
| Edmonton | 153 (41.7%) | 42 (29.0%) | 64 (50.4%) | |
| North | 181 (49.3%) | 19 (13.1%) | 14 (11.0%) | |
| Testing Agency | Calgary STI Clinic | 28 (7.6%) | 81 (55.9%) | 45 (35.4%) |
| Edmonton STI Clinic | 131 (35.7%) | 36 (24.8%) | 60 (47.2%) | |
| Fort McMurray STI Clinic | 13 (3.5%) | 5 (3.4%) | 1 (0.8%) | |
| Other providers | 195 (53.1%) | 23 (15.9%) | 21 (16.5%) | |
| Antibiotic Resistance or Decreased Susceptibility (MIC Value; µg/mL) 1 | Culture-positive Isolates | 192 (52.3%) | 131 (90.3%) | 114 (89.8%) |
| Cefixime (>0.25) | 0 | 0 | 0 | |
| Ceftriaxone (>0.25) | 0 | 0 | 0 | |
| Azithromycin (>2) | 1 (0.5%) | 0 | 0 | |
| Ciprofloxacin (≥1) | 6 (3.1%) | 1 (0.8%) | 108 (94.7%) | |
| Penicillin (≥2.0) | 0 | 0 | 21 (18.4%) | |
| Tetracycline (≥2.0) | 3 (1.6%) | 128 (97.7%) | 8 (7.0%) | |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gratrix, J.; Kamruzzaman, A.; Martin, I.; Smyczek, P.; Read, R.; Bertholet, L.; Naidu, P.; Singh, A.E. Surveillance for Antimicrobial Resistance in Gonorrhea: The Alberta Model, 2012–2016. Antibiotics 2018, 7, 63. https://doi.org/10.3390/antibiotics7030063
Gratrix J, Kamruzzaman A, Martin I, Smyczek P, Read R, Bertholet L, Naidu P, Singh AE. Surveillance for Antimicrobial Resistance in Gonorrhea: The Alberta Model, 2012–2016. Antibiotics. 2018; 7(3):63. https://doi.org/10.3390/antibiotics7030063
Chicago/Turabian StyleGratrix, Jennifer, Anmmd Kamruzzaman, Irene Martin, Petra Smyczek, Ron Read, Lindsay Bertholet, Prenilla Naidu, and Ameeta E. Singh. 2018. "Surveillance for Antimicrobial Resistance in Gonorrhea: The Alberta Model, 2012–2016" Antibiotics 7, no. 3: 63. https://doi.org/10.3390/antibiotics7030063
APA StyleGratrix, J., Kamruzzaman, A., Martin, I., Smyczek, P., Read, R., Bertholet, L., Naidu, P., & Singh, A. E. (2018). Surveillance for Antimicrobial Resistance in Gonorrhea: The Alberta Model, 2012–2016. Antibiotics, 7(3), 63. https://doi.org/10.3390/antibiotics7030063




