Neisseria gonorrhoeae Aggregation Reduces Its Ceftriaxone Susceptibility
Abstract
1. Introduction
2. Results
2.1. GC Aggregation Promotes Survival through Limiting Ceftriaxone Penetration
2.1.1. GC Aggregation Promotes Survival under Ceftriaxone Treatment
2.1.2. GC Aggregation Limits Ceftriaxone Penetration
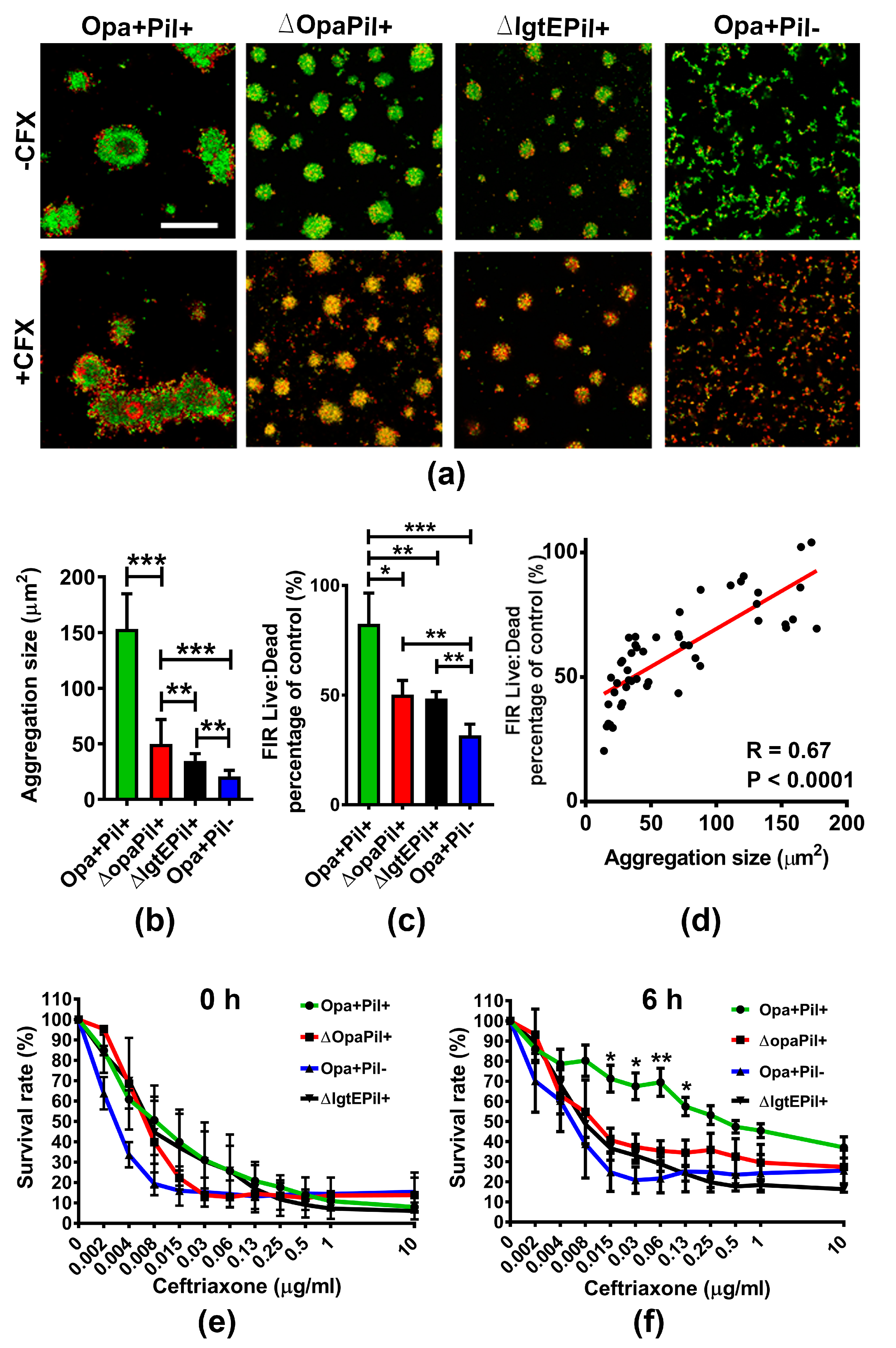
2.2. GC Strains Lacking Opa or Pili or Expressing Truncated LOS Showed a Reduced Survivability against Ceftriaxone Due to Decreased Aggregation
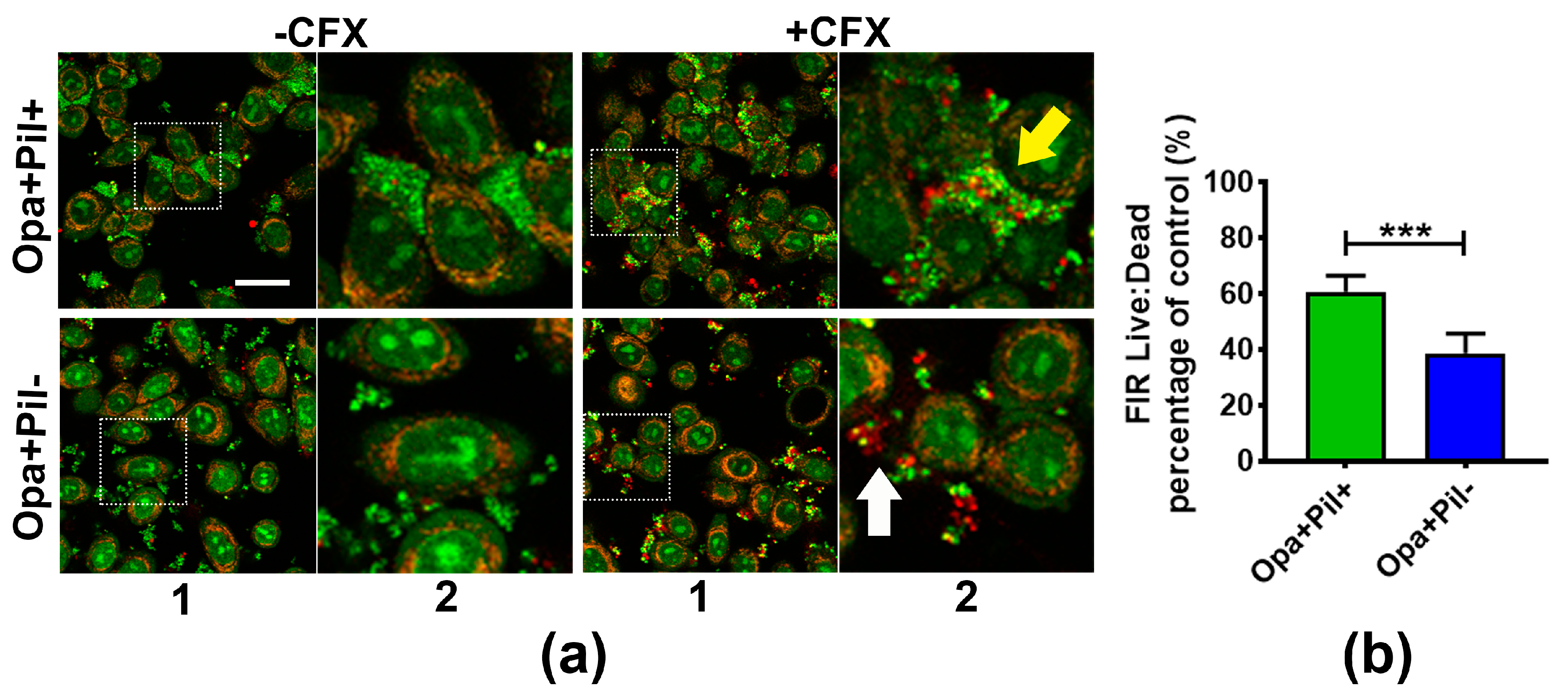
2.3. GC Aggregation on Human Epithelial Cells also Increases Ceftriaxone Survivability
3. Discussion
4. Materials and Methods
4.1. Bacteria Strains
4.2. BacTiter Assay
4.3. Fluorescence Microscopic Analysis of Live and Dead Bacteria in Aggregates
4.4. Statistical Analysis
5. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- CDC. STD Facts. Available online: http://www.cdc.gov/std/gonorrhea/STDFact-gonorrhea-detailed.htm (accessed on 5 October 2017).
- Handsfield, H.H. Gonorrhea and Uncomplicated Gonococcal Infection; McGraw-Hill Book Co.: New York, NY, USA, 1984. [Google Scholar]
- Den Heijer, C.D.J.; Hoebe, C.; van Liere, G.; van Bergen, J.; Cals, J.W.L.; Stals, F.S.; Dukers-Muijrers, N. A comprehensive overview of urogenital, anorectal and oropharyngeal Neisseria gonorrhoeae testing and diagnoses among different STI care providers: A cross-sectional study. BMC Infect. Dis. 2017, 17. [Google Scholar] [CrossRef] [PubMed]
- Hein, K.; Marks, A.; Cohen, M.I. Asymptomatic gonorrhea: Prevalence in a population of urban adolescents. J. Pediatr. 1977, 90, 634–635. [Google Scholar] [CrossRef]
- Hananta, I.P.; van Dam, A.P.; Bruisten, S.M.; Schim van der Loeff, M.F.; Soebono, H.; de Vries, H.J. Gonorrhea in Indonesia: High Prevalence of Asymptomatic Urogenital Gonorrhea but No Circulating Extended Spectrum Cephalosporins-Resistant Neisseria gonorrhoeae Strains in Jakarta, Yogyakarta, and Denpasar, Indonesia. Sex. Transm. Dis. 2016, 43, 608–616. [Google Scholar] [CrossRef] [PubMed]
- WHO. Chlamydia trachomatis, Neisseria gonorrhoeae, Syphilis and Trichomonas vaginalis. Methods and Results Used by WHO to Generate 2005 Estimates; Prevalence and Incidence of Selected Sexually Transmitted Infections; World Health Organisation: Geneva, Switzerland, 2011. [Google Scholar]
- Mayor, M.T.; Roett, M.A.; Uduhiri, K.A. Diagnosis and management of gonococcal infections. Am. Fam. Physician 2012, 86, 931–938. [Google Scholar] [PubMed]
- Silva, J., Jr.; Wilson, K. Disseminated gonococcal infections (DGI). Cutis 1979, 24, 601–606. [Google Scholar] [PubMed]
- Jarvis, G.A.; Chang, T.L. Modulation of HIV transmission by Neisseria gonorrhoeae: molecular and immunological aspects. Curr. HIV Res. 2012, 10, 211–217. [Google Scholar] [CrossRef] [PubMed]
- Alirol, E.; Wi, T.E.; Bala, M.; Bazzo, M.L.; Chen, X.S.; Deal, C.; Dillon, J.R.; Kularatne, R.; Heim, J.; Hooft van Huijsduijnen, R.; et al. Multidrug-resistant gonorrhea: A research and development roadmap to discover new medicines. PLoS Med. 2017, 14, e1002366. [Google Scholar] [CrossRef] [PubMed]
- Workowski, K.A.; Bolan, G.A. Sexually Transmitted Diseases Treatment Guidelines, 2015. Morb. Mortal. Wkly. Rep. 2015, 64, 1–138. [Google Scholar]
- Hananta, I.P.Y.; De Vries, H.J.C.; van Dam, A.P.; van Rooijen, M.S.; Soebono, H.; Schim van der Loeff, M.F. Persistence after treatment of pharyngeal gonococcal infections in patients of the STI clinic, Amsterdam, the Netherlands, 2012–2015: A retrospective cohort study. Sex. Transm. Infect. 2017, 19. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Singh, S.K.; Chowdhury, I.; Singh, R. Understanding the Mechanism of Bacterial Biofilms Resistance to Antimicrobial Agents. Open Microbiol. J. 2017, 11, 53–62. [Google Scholar] [CrossRef] [PubMed]
- Greiner, L.L.; Edwards, J.L.; Shao, J.; Rabinak, C.; Entz, D.; Apicella, M.A. Biofilm Formation by Neisseria gonorrhoeae. Infect. Immun. 2005, 73, 1964–1970. [Google Scholar] [CrossRef] [PubMed]
- Zollner, R.; Oldewurtel, E.R.; Kouzel, N.; Maier, B. Phase and antigenic variation govern competition dynamics through positioning in bacterial colonies. Sci. Rep. 2017, 7, 017–12472. [Google Scholar] [CrossRef] [PubMed]
- Stein, D.C.; LeVan, A.; Wang, L.-C.; Zimmerman, L.; Song, W. Expression of opacity proteins interferes with the transmigration of Neisseria gonorrhoeae across polarized epithelial cells. PLoS ONE 2015, 10, e0134342. [Google Scholar] [CrossRef] [PubMed]
- LeVan, A.; Zimmerman, L.I.; Mahle, A.C.; Swanson, K.V.; DeShong, P.; Park, J.; Edwards, V.L.; Song, W.; Stein, D.C. Construction and characterization of a derivative of Neisseria gonorrhoeae strain MS11 devoid of all opa genes. J. Bacteriol. 2012, 194, 6468–6478. [Google Scholar] [CrossRef] [PubMed]
- Steichen, C.T.; Shao, J.Q.; Ketterer, M.R.; Apicella, M.A. Gonococcal cervicitis: A role for biofilm in pathogenesis. J. Infect. Dis. 2008, 198, 1856–1861. [Google Scholar] [CrossRef] [PubMed]
- Novotny, P.; Short, J.A.; Walker, P.D. An electron-microscope study of naturally occurring and cultured cells of Neisseria Gonorrhoeae. J. Med. Microbiol. 1975, 8, 413–427. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.C.; Yu, Q.; Edwards, V.; Lin, B.; Qiu, J.; Turner, J.R.; Stein, D.C.; Song, W. Neisseria gonorrhoeae infects the human endocervix by activating non-muscle myosin II-mediated epithelial exfoliation. PLoS Pathog. 2017, 13, e1006269. [Google Scholar] [CrossRef] [PubMed]
- Bhoopalan, S.V.; Piekarowicz, A.; Lenz, J.D.; Dillard, J.P.; Stein, D.C. nagZ Triggers Gonococcal Biofilm Disassembly. Sci. Rep. 2016, 6, 22372. [Google Scholar] [CrossRef] [PubMed]
- Edwards, J.L.; Butler, E.K. The Pathobiology of Neisseria gonorrhoeae Lower Female Genital Tract Infection. Front. Microbiol. 2011, 2, 102. [Google Scholar] [CrossRef] [PubMed]
- Wachter, J.; Hill, S. Positive Selection Pressure Drives Variation on the Surface-Exposed Variable Proteins of the Pathogenic Neisseria. PLoS ONE 2016, 11, e0161348. [Google Scholar] [CrossRef] [PubMed]
- Eisenstein, B.I.; Lee, T.J.; Sparling, P.F. Penicillin sensitivity and serum resistance are independent attributes of strains of Neisseria gonorrhoeae causing disseminated gonococcal infection. Infect. Immun. 1977, 15, 834–841. [Google Scholar] [PubMed]
- Wiesner, P.J.; Handsfield, H.H.; Holmes, K.K. Low antibiotic resistance of gonococci causing disseminated infection. N. Engl. J. Med. 1973, 288, 1221–1222. [Google Scholar] [CrossRef] [PubMed]
- Harvey, H.A.; Jennings, M.P.; Campbell, C.A.; Williams, R.; Apicella, M.A. Receptor-mediated endocytosis of Neisseria gonorrhoeae into primary human urethral epithelial cells: The role of the asialoglycoprotein receptor. Mol. Microbiol. 2001, 42, 659–672. [Google Scholar] [CrossRef] [PubMed]
- Edwards, J.L.; Brown, E.J.; Uk-Nham, S.; Cannon, J.G.; Blake, M.S.; Apicella, M.A. A co-operative interaction between Neisseria gonorrhoeae and complement receptor 3 mediates infection of primary cervical epithelial cells. Cell. Microbiol. 2002, 4, 571–584. [Google Scholar] [CrossRef] [PubMed]
- Johnson, M.B.; Criss, A.K. Resistance of Neisseria gonorrhoeae to neutrophils. Front. Microbiol. 2011, 2, 77. [Google Scholar] [CrossRef] [PubMed]
- Ball, L.M.; Criss, A.K. Constitutively Opa-expressing and Opa-deficient Neisseria gonorrhoeae strains differentially stimulate and survive exposure to human neutrophils. J. Bacteriol. 2013, 195, 2982–2990. [Google Scholar] [CrossRef] [PubMed]
- Grassme, H.; Gulbins, E.; Brenner, B.; Ferlinz, K.; Sandhoff, K.; Harzer, K.; Lang, F.; Meyer, T.F. Acidic sphingomyelinase mediates entry of N. gonorrhoeae into nonphagocytic cells. Cell 1997, 91, 605–615. [Google Scholar] [CrossRef]
- Minor, S.Y.; Banerjee, A.; Gotschlich, E.C. Effect of alpha-oligosaccharide phenotype of Neisseria gonorrhoeae strain MS11 on invasion of Chang conjunctival, HEC-1-B endometrial, and ME-180 cervical cells. Infect. Immun. 2000, 68, 6526–6534. [Google Scholar] [CrossRef] [PubMed]
- White, L.A.; Kellogg, D.S. An improved fermentation medium for Neisseria gonorrhoeae and other Neisseria. Health Lab. Sci. 1965, 2, 238–241. [Google Scholar] [PubMed]

© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, L.-C.; Litwin, M.; Sahiholnasab, Z.; Song, W.; Stein, D.C. Neisseria gonorrhoeae Aggregation Reduces Its Ceftriaxone Susceptibility. Antibiotics 2018, 7, 48. https://doi.org/10.3390/antibiotics7020048
Wang L-C, Litwin M, Sahiholnasab Z, Song W, Stein DC. Neisseria gonorrhoeae Aggregation Reduces Its Ceftriaxone Susceptibility. Antibiotics. 2018; 7(2):48. https://doi.org/10.3390/antibiotics7020048
Chicago/Turabian StyleWang, Liang-Chun, Madeline Litwin, Zahraossadat Sahiholnasab, Wenxia Song, and Daniel C. Stein. 2018. "Neisseria gonorrhoeae Aggregation Reduces Its Ceftriaxone Susceptibility" Antibiotics 7, no. 2: 48. https://doi.org/10.3390/antibiotics7020048
APA StyleWang, L.-C., Litwin, M., Sahiholnasab, Z., Song, W., & Stein, D. C. (2018). Neisseria gonorrhoeae Aggregation Reduces Its Ceftriaxone Susceptibility. Antibiotics, 7(2), 48. https://doi.org/10.3390/antibiotics7020048




