Parallel Colorimetric Quantification of Choline and Phosphocholine as a Method for Studying Choline Kinase Activity in Complex Mixtures
Abstract
1. Introduction
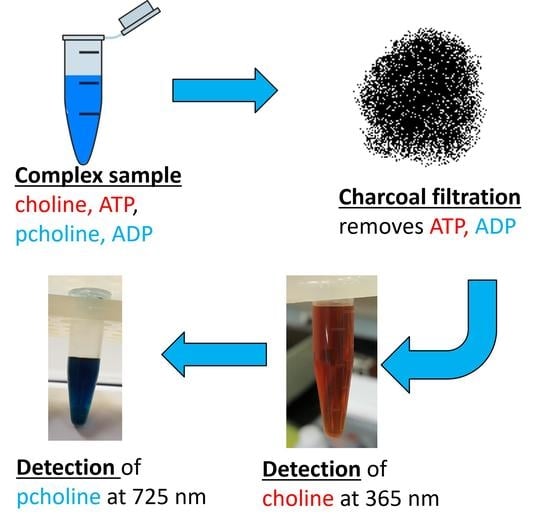
2. Results
Development of the Detection Method
3. Discussion
4. Materials and Methods
4.1. Preparation of Enzyme Extracts
4.2. sChok Enzymatic Reaction
4.3. Activated Charcoal Filtration of ATP
4.4. Triiodide Quantification/Removal of Cho
4.5. MBD Quantification of PCho
4.6. Mass Spectrometry
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Wittenberg, J.; Kornberg, A. Choline phosphokinase. J. Biol. Chem. 1953, 202, 431–444. [Google Scholar] [PubMed]
- Lacal Sanjuan, J.C. Choline kinase as a precision medicine target for therapy in cancer, autoimmune diseases and malaria. Precis. Med. 2015, 1, 2. [Google Scholar]
- Lacal, J.C. Choline kinase is a novel prognostic marker and a therapeutic target in human cancer. EJC Suppl. 2008, 6, 121. [Google Scholar] [CrossRef]
- Zimmerman, T.; Moneriz, C.; Diez, A.; Bautista, J.M.; Gomez Del Pulgar, T.; Cebrian, A.; Lacal, J.C. Antiplasmodial activity and mechanism of action of RSM-932A, a promising synergistic inhibitor of Plasmodium falciparum choline kinase. Antimicrob. Agents Chemother. 2013, 57, 5878–5888. [Google Scholar] [CrossRef] [PubMed]
- Whiting, G.C.; Gillespie, S.H. Incorporation of choline into Streptococcus pneumoniae cell wall antigens: Evidence for choline kinase activity. FEMS Microbiol. Lett. 1996, 138, 141–145. [Google Scholar] [CrossRef] [PubMed]
- Grundling, A.; Schneewind, O. Synthesis of glycerol phosphate lipoteichoic acid in Staphylococcus aureus. Proc. Natl. Acad. Sci. USA 2007, 104, 8478–8483. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Jiang, Y.L.; Zhang, J.R.; Zhou, C.Z.; Chen, Y.X. Structural and Enzymatic Characterization of the Choline Kinase LicA from Streptococcus pneumoniae. PLoS ONE 2015, 10, e0120467. [Google Scholar] [CrossRef] [PubMed]
- Zimmerman, T.; Ibrahim, S. Choline Kinase, A Novel Drug Target for the Inhibition of Streptococcus pneumoniae. Antibiotics 2017, 6, 20. [Google Scholar] [CrossRef] [PubMed]
- Appleton, H.D.; La Du, B.N., Jr.; Levy, B.; Steele, J.M.; Brodie, B.B. A chemical method for the determination of free choline in plasma. J. Biol. Chem. 1953, 205, 803–813. [Google Scholar] [PubMed]
- Wu, W.L.; Adams, C.E.; Stevens, K.E.; Chow, K.H.; Freedman, R.; Patterson, P.H. The interaction between maternal immune activation and alpha 7 nicotinic acetylcholine receptor in regulating behaviors in the offspring. Brain Behav. Immun. 2015, 46, 192–202. [Google Scholar] [CrossRef] [PubMed]
- Murray, J.J.; Dinh, T.T.; Truett, A.P.; Kennerly, D.A. Isolation and Enzymatic Assay of Choline and Phosphocholine Present in Cell-Extracts with Picomole Sensitivity. Biochem. J. 1990, 270, 63–68. [Google Scholar] [CrossRef] [PubMed]
- Elmer, P. Guide to the Safe Handling of Radioactive Materials in Research. 2007. Available online: https://ehsucsfedu/sites/ehsucsfedu/files/documents/2011Guide%20Safe%20HandlingRAD2pdf (accessed on 13 March 2018).
- Services UDS. Safe Handling of Radioisotopes. 2015. Available online: http://safetyservicesucdavisedu/article/safe-handling-radioisotopes (accessed on 15 March 2108).
- Shah, T.; Wildes, F.; Penet, M.F.; Winnard, P.T., Jr.; Glunde, K.; Artemov, D.; Ackerstaff, E.; Gimi, B.; Kakkad, S.; Raman, V.; et al. Choline kinase overexpression increases invasiveness and drug resistance of human breast cancer cells. NMR Biomed. 2010, 23, 633–642. [Google Scholar] [CrossRef] [PubMed]
- Makoto Hayashi, T.U.; Komei, Miyaki. Colorimetric Method for the Determination of Phosphorylcholine. Yakugaku Zasshi 1961, 81, 1039. [Google Scholar] [CrossRef][Green Version]
- Mo, J.; Duncan, J.A. Assessing ATP binding and hydrolysis by NLR proteins. Methods Mol. Biol. 2013, 1040, 153–168. [Google Scholar] [PubMed]
- Bligh, E.G.; Dyer, W.J. A Rapid Method of Total Lipid Extraction and Purification. Can. J. Biochem. Physiol. 1959, 37, 911–917. [Google Scholar] [CrossRef] [PubMed]


| Control Reaction (μM) | HC-3 Reaction (μM) | |||||||
|---|---|---|---|---|---|---|---|---|
| Minutes | [PCho] | Inverse [Cho] | [Cho] | [Cho] MS | [PCho] MS | [Pcholine] | [Cho] MS | [Pcho] MS |
| 0 | * | 72.6 | 927.4 | - | - | * | - | - |
| 20 | 284.3 | 270.4 | 729.6 | - | - | * | - | - |
| 40 | 374.8 | 367.7 | 632.3 | - | - | 258.5 | - | - |
| 60 | 451.7 | 445.2 | 554.8 | 521.1 | 422.2 | 284.9 | 741.1 | 295.2 |
| 80 | 514.4 | 502.5 | 497.5 | 478.7 | 540.9 | 325.5 | 693.1 | 326.2 |
| 100 | 551.7 | 579.2 | 420.8 | 462.6 | 549.5 | 354.3 | 668.9 | 375.3 |
| Name | Precursor m/z | Product m/z |
|---|---|---|
| Choline | 104 | 45 |
| Phosphocholine | 184 | 86 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zimmerman, T.; Ibrahim, S.A. Parallel Colorimetric Quantification of Choline and Phosphocholine as a Method for Studying Choline Kinase Activity in Complex Mixtures. Antibiotics 2018, 7, 24. https://doi.org/10.3390/antibiotics7010024
Zimmerman T, Ibrahim SA. Parallel Colorimetric Quantification of Choline and Phosphocholine as a Method for Studying Choline Kinase Activity in Complex Mixtures. Antibiotics. 2018; 7(1):24. https://doi.org/10.3390/antibiotics7010024
Chicago/Turabian StyleZimmerman, Tahl, and Salam A. Ibrahim. 2018. "Parallel Colorimetric Quantification of Choline and Phosphocholine as a Method for Studying Choline Kinase Activity in Complex Mixtures" Antibiotics 7, no. 1: 24. https://doi.org/10.3390/antibiotics7010024
APA StyleZimmerman, T., & Ibrahim, S. A. (2018). Parallel Colorimetric Quantification of Choline and Phosphocholine as a Method for Studying Choline Kinase Activity in Complex Mixtures. Antibiotics, 7(1), 24. https://doi.org/10.3390/antibiotics7010024