Sperm Quality during Storage Is Not Affected by the Presence of Antibiotics in EquiPlus Semen Extender but Is Improved by Single Layer Centrifugation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Semen
2.2.1. Semen Collection
2.2.2. Semen Preparation with Modified Single Layer Centrifugation
2.3. Evaluation
2.3.1. Sperm Concentration
2.3.2. Computer-Assisted Sperm Analysis (CASA)
2.3.3. Membrane Integrity
2.3.4. Assessment of Mitochondrial Membrane Potential (MMP)
2.3.5. Sperm Chromatin Structure Assay (SCSA)
2.3.6. Bacteriology
2.3.7. Statistics
3. Results
3.1. Sperm Motility
3.2. Membrane Integrity
3.3. Mitochondrial Membrane Potential
3.4. SCSA
3.5. Bacteriology
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Baumber, J. Evaluation of Semen. In Equine Reproduction, 2nd ed.; John Wiley & Sons: Hoboken, NJ, USA, 2011; pp. 1278–1291. [Google Scholar]
- Lindeberg, H.; Karjalainen, H.; Koskinen, E.; Katila, T. Quality of stallion semen obtained by a new semen collection phantom (Equidame) versus a Missouri artificial vagaina. Theriogenology 1999, 51, 1157–1173. [Google Scholar] [CrossRef]
- Bennett, D.G. Therapy of Endometritis in Mares. J. Am. Vet. Med. Assoc. 1986, 188, 1390–1392. [Google Scholar] [PubMed]
- Johansson, A.; Greko, C.; Engstrom, B.E.; Karlsson, M. Antimicrobial susceptibility of Swedish, Norwegian and Danish isolates of Clostridium perfringens from poultry, and distribution of tetracycline resistance genes. Vet. Microbiol. 2004, 99, 251–257. [Google Scholar] [CrossRef] [PubMed]
- Jasko, D.J.; Bedford, S.J.; Cook, N.L.; Mumford, E.L.; Squires, E.L.; Pickett, B.W. Effect of antibiotics on motion characteristics of cooled stallion spermatozoa. Theriogenology 1993, 40, 885–893. [Google Scholar] [CrossRef]
- Aurich, C.; Spergser, J. Influence of bacteria and gentamicin on cooled-stored stallion spermatozoa. Theriogenology 2007, 67, 912–918. [Google Scholar] [CrossRef] [PubMed]
- Barratt, C.L. Semen analysis is the cornerstone of investigation for male infertility. Practitioner 2007, 251, 8–17. [Google Scholar] [PubMed]
- Rodriguez-Martinez, H. Semen evaluation techniques and their relationship with fertility. Anim. Reprod. Sci. 2013, 10, 148–159. [Google Scholar]
- Quintero-Moreno, A.; Miro, J.; Rigau, A.T.; Rodriguez-Gil, J.E. Identification of sperm subpopulations with specific motility characteristics in stallion ejaculates. Theriogenology 2003, 59, 1973–1990. [Google Scholar] [CrossRef]
- Hossain, M.S.; Johannisson, A.; Wallgren, M.; Nagy, S.; Siqueira, A.P.; Rodriguez-Martinez, H. Flow cytometry for the assessment of animal sperm integrity and functionally: State of the art. Asian J. Androl. 2011, 13, 406–419. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morrell, J.; Klein, C.; Lundeheim, N.; Erol, E.; Troedsson, M. Removal of bacteria from stallion semen by colloid centrifugation. Anim. Reprod. Sci. 2014, 145, 47–53. [Google Scholar] [CrossRef] [PubMed]
- Morrell, J.M.; Wallgren, M. Removal of bacteria from boar ejaculates by Single Layer Centrifugation can reduce the use of antibiotics in semen extenders. Anim. Reprod. Sci. 2011, 123, 64–69. [Google Scholar] [CrossRef] [PubMed]
- Morrell, J.M.; Wallgren, M. Alternatives to antibiotics in semen extenders: A review. Pathogens 2014, 3, 934–946. [Google Scholar] [CrossRef] [PubMed]
- Johannisson, A.; Morrell, J.M.; Thoren, J.; Jönsson, M.; Dalin, A.M.; Rodriguez-Martinez, H. Colloidal centrifugation with Androcoll-ETM prolongs stallion sperm motility, viability and chromatin integrity. Anim. Reprod. Sci. 2009, 116, 119–128. [Google Scholar] [CrossRef] [PubMed]
- Morrell, J.M.; Lagerqvist, A.; Humblot, P.; Johannisson, A. Effect of Single Layer Centrifugation on reactive oxygen species and sperm mitochondrial membrane potential in cooled stallion semen. Reprod. Fertil. Dev. 2017, 29, 1039–1045. [Google Scholar] [CrossRef] [PubMed]
- Johannisson, A.; Lundgren, A.; Humblot, P.; Morrell, J.M. Natural and stimulated levels of reactive oxygen species in cooled stallion semen destined for artificial insemination. Animal 2014, 8, 1706–1714. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Goonewardene, L.A. The use of MIXED models in the analysis of animal experiments with repeated measures data. Can. J. Anim. Sci. 2004, 84, 1–11. [Google Scholar] [CrossRef]
- Zampieri, D.; Santos, V.G.; Braga, P.A.; Ferreira, C.R.; Ballottin, D.; Tasic, L.; Basso, A.C.; Sanches, B.V.; Pontes, J.H.F.; Da Silva, B.P.; et al. Microorganisms in cryopreserved semen and culture media used in the in vitro production (IVP) of bovine embryos identified by matrix-assisted laser desorption ionization mass spectrometry (MALDI-MS). Theriogenology 2013, 80, 337–345. [Google Scholar] [CrossRef] [PubMed]
- Althouse, G.C.; Skaife, J.; Loomis, P. Prevalence and types of contamination in extended, chilled equine semen. Anim. Reprod. Sci. 2010, 121S, 224–225. [Google Scholar]
- Yaniz, J.L.; Marco-Aguado, M.A.; Mateos, J.A.; Santolaria, P. Bacterial contamination of ram semen, antibiotic sensitivities, and effects on sperm quality during storage at 15 °C. Anim. Reprod. Sci. 2010, 122, 142–149. [Google Scholar] [CrossRef] [PubMed]
- Bussalleu, E.; Yeste, M.; Sepulveda, L.; Torner, E.; Pinart, E.; Bonet, S. Effects of different concentrations of enterotoxigenic and verotoxi-genic E. coli on boar sperm quality. Anim. Reprod. Sci. 2011, 127, 176–182. [Google Scholar] [CrossRef] [PubMed]
- Arriola, J.; Foote, R.H. Effects of amikacin sulfate on the motility of stallion and bull spermatozoa at different temperatures and intervals of storage. J. Anim. Sci. 1982, 54, 1105–1110. [Google Scholar] [CrossRef] [PubMed]
- Akhter, S.; Ansari, M.S.; Andrabi, S.M.; Ullah, N.; Oayyum, M. Effect of antibiotics in extender on bacterial and spermatozoal quality of cooled buffalo (bubalus bubalis) bull semen. Reprod. Domest. Anim. 2008, 43, 272–278. [Google Scholar] [CrossRef] [PubMed]
- Nicholson, C.M.; Abramsson, L.; Holm, S.E.; Bjurulf, E. Bacterial contamination and sperm recovery after semen preparation by density gradient centrifugation using silane-coated silica particles at different g forces. Hum. Reprod. 2000, 15, 662–666. [Google Scholar] [CrossRef] [PubMed]
- Guimaraes, T.; Lopes, G.; Pinto, M.; Silva, E.; Miranda, C.; Correia, M.J.; Damasio, L.; Thompson, G.; Rocha, A. Colloid centrifugation of fresh stallion semen before cryopreservation decreased microorganism load of frozen- thawed semen without affecting seminal kinetics. Theriogenology 2015, 83, 186–191. [Google Scholar] [CrossRef] [PubMed]
- Morrell, J.M.; Dalin, A.M.; Rodriguez-Martinez, H. Prolongation of stallion sperm survival by centrifugation through coated silica colloids: A preliminary study. Anim. Reprod. 2008, 5, 121–126. [Google Scholar]
- Morrell, J.M.; Rodriguez-Martinez, H.; Johannisson, A. Single layer centrifugation of stallion spermatozoa consistently selects the most robust spermatozoa from the rest of the ejaculate in a large sample size. Equine Vet. J. 2010, 42, 579–585. [Google Scholar] [CrossRef] [PubMed]
- Morrell, J.M.; Johannisson, A.; Dalin, A.M.; Rodriguez-Martinez, H. Single Layer Centrifugation with AndrocollTM—E can be scaled-up to allow large volumes of stallion ejaculate to be processed easily. Theriogenology 2009, 72, 879–884. [Google Scholar] [CrossRef] [PubMed]
- Ortiz, I.; Dorado, J.; Ramirez, L.; Morrell, J.M.; Acha, D.; Urbano, M.; Galvez, M.J.; Carrasco, J.J.; Gomez-Arrones, V.; Calero-Carretero, R.; et al. Effect of single layer centrifugation using Androcoll-E-Large on the sperm quality parameters of cooled-stored donkey semen doses. Animal 2014, 8, 308–315. [Google Scholar] [CrossRef] [PubMed]
- Morrell, J.M.; Alsina, M.S.; Abraham, M.C.; Sjunnesson, Y. Practical applications of sperm selection techniques for improving reproduction efficiency. Anim. Reprod. 2016, 13, 340–345. [Google Scholar] [CrossRef]
- Lindahl, J.; Dalin, A.M.; Stuhtmann, G.; Morrell, J.M. Stallion spermatozoa selected by single layer centrifugation are capable of fertilization after storage for up to 96 h at 6 °C prior to artificial insemination. Acta Vet. Scand. 2012, 54. [Google Scholar] [CrossRef] [PubMed]
- Morrell, J.M.; Rodriguez-Martinez, H. Colloid Centrifugation of Semen: Applications in Assisted Reproduction. J. Anal. Chem. 2016, 7, 597–610. [Google Scholar] [CrossRef]

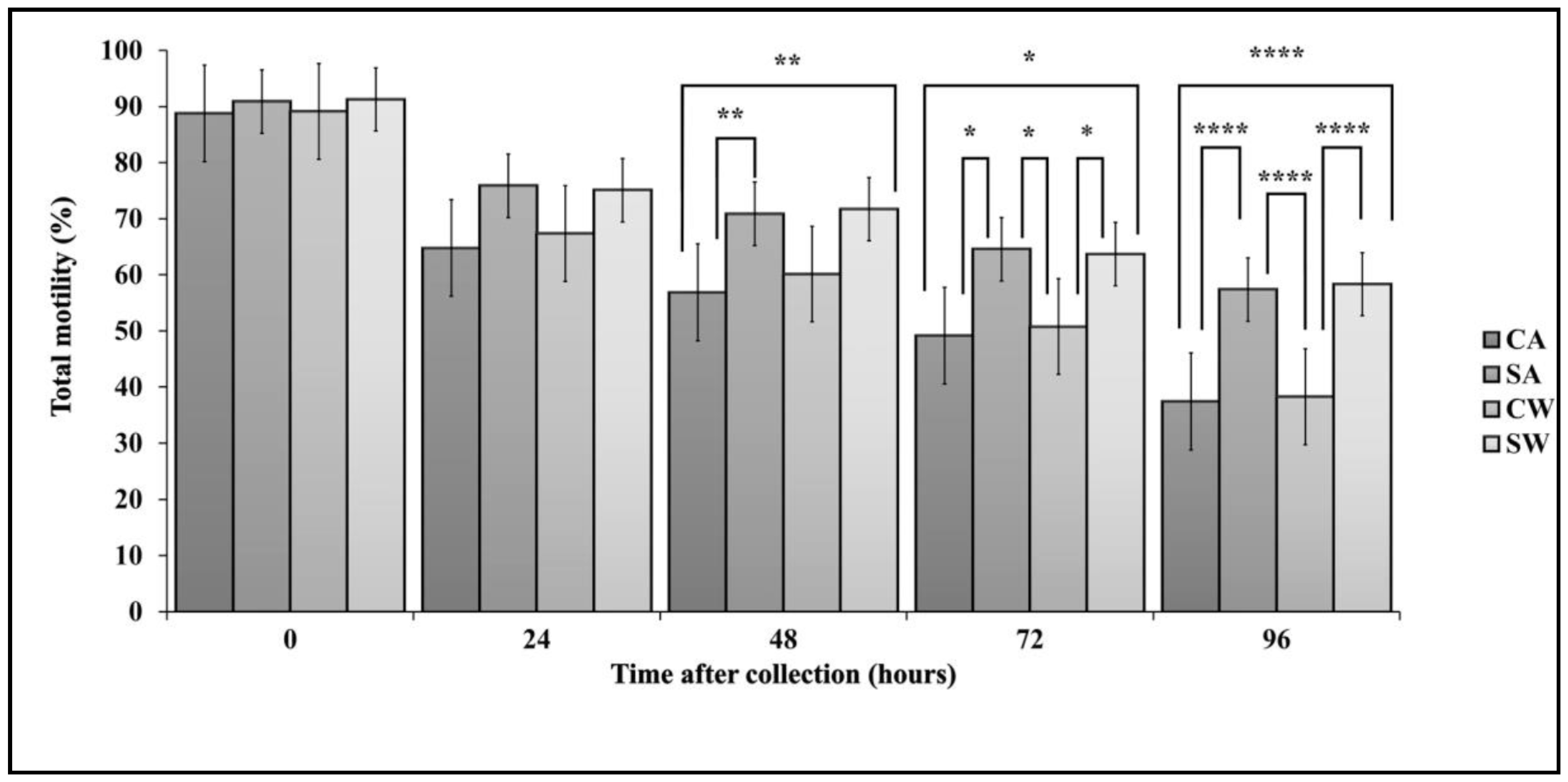
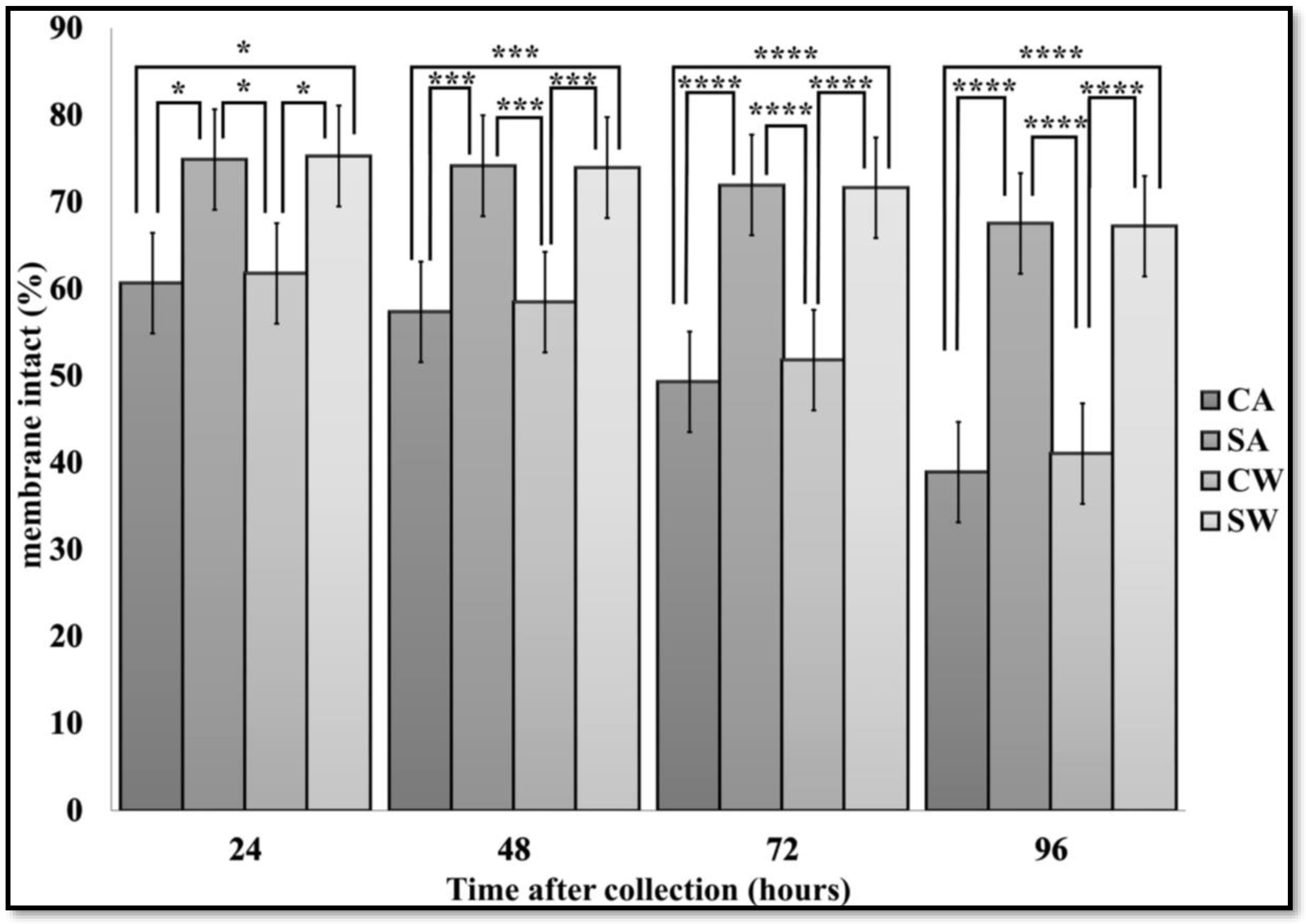
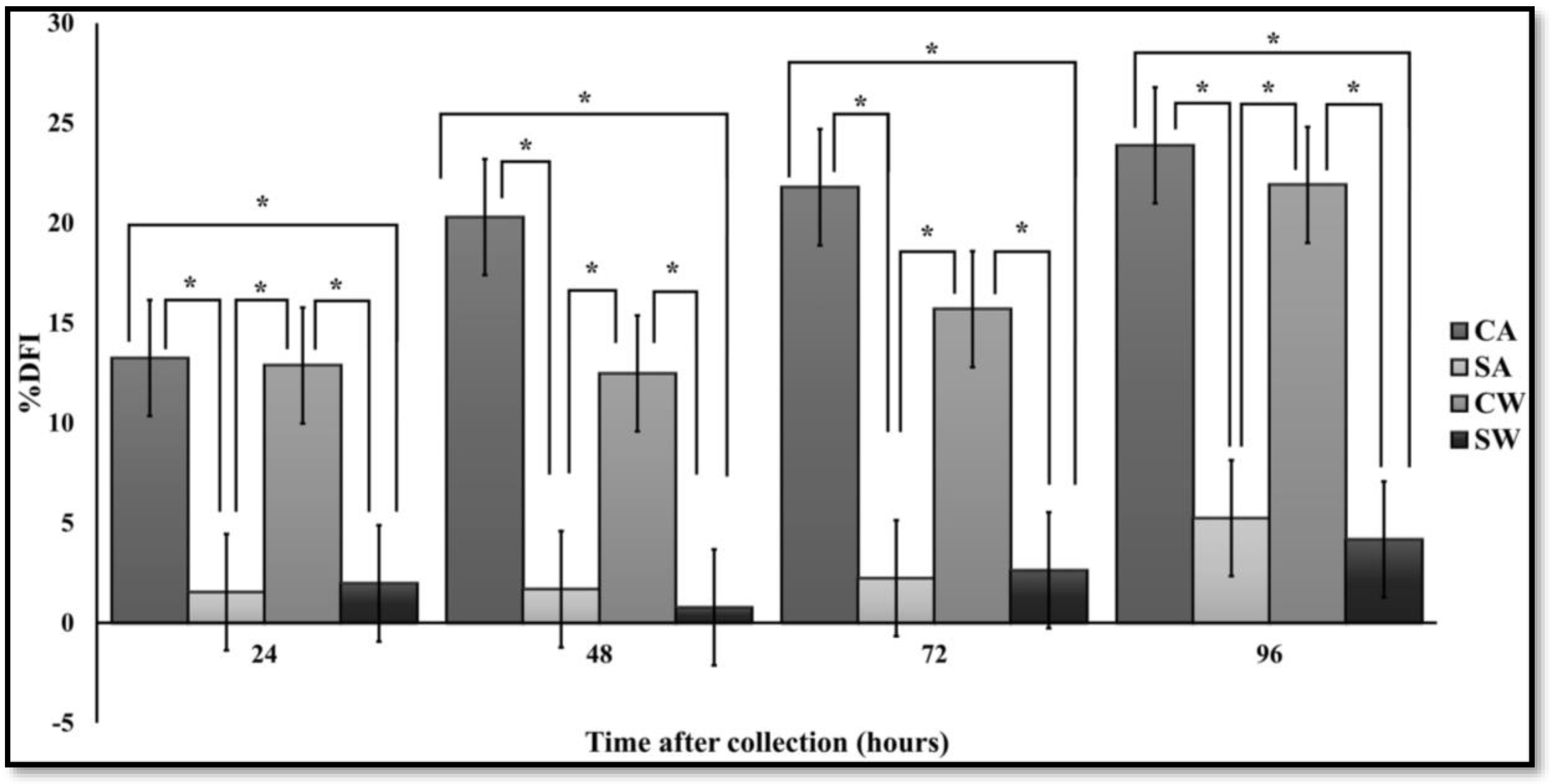
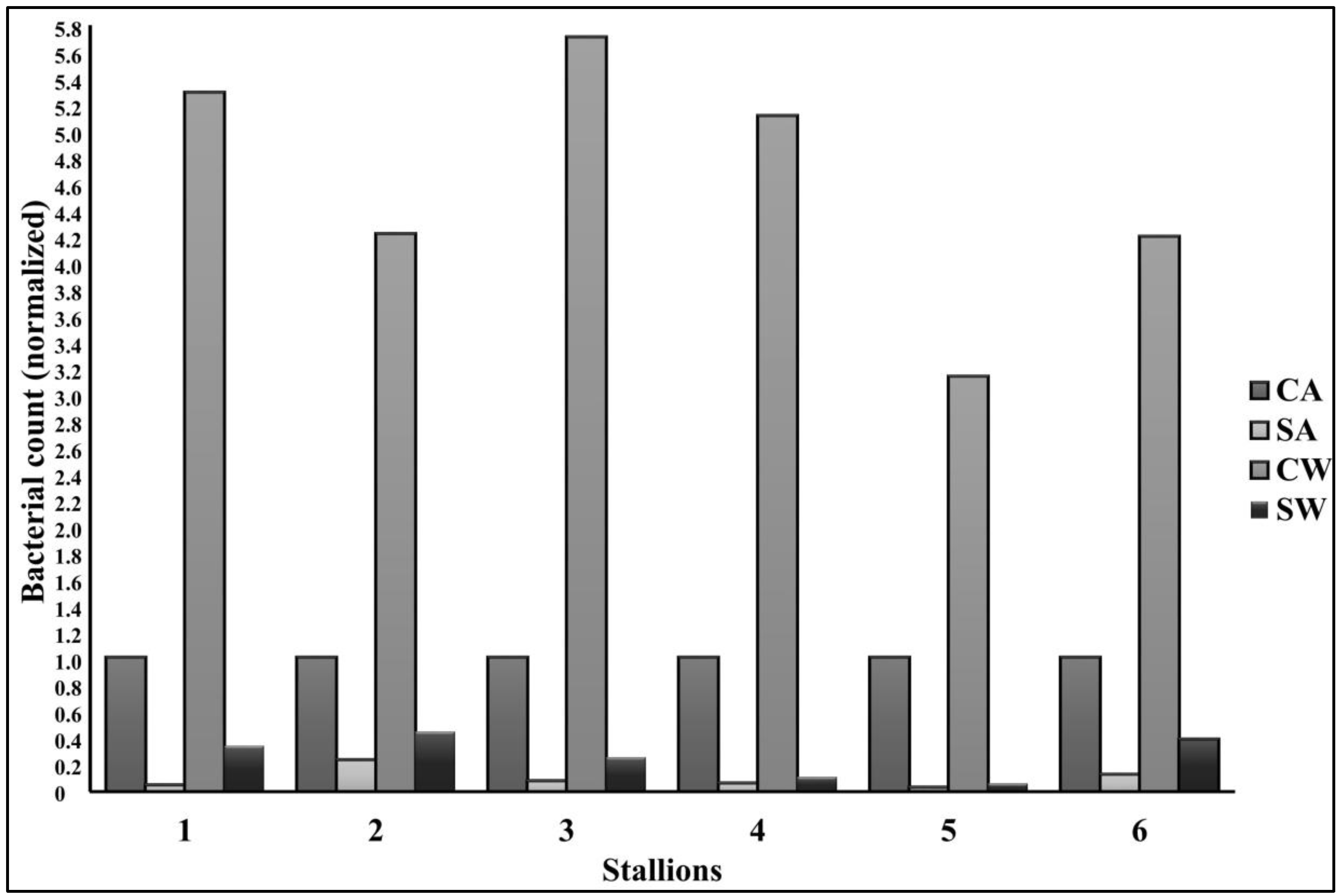

| Time | CA | SA | CW | SW | |
|---|---|---|---|---|---|
| 0 h | PM% | 80.83 ± 4.62 | 83.60 ± 4.62 | 81.27 ± 4.62 | 84.29 ± 4.62 |
| VAP (µm/s) | 93.97 ± 3.20 a | 79.02 ± 3.20 a,c | 95.30 ± 3.20 c | 82.13 ± 3.20 | |
| VCL (µm/s) | 166.14 ± 5.44 c | 137.84 ± 5.44 c,d | 163.80 ± 5.44 d | 143.15 ± 5.44 | |
| VSL (µm/s) | 83.75 ± 2.86 a | 70.76 ± 2.86 a,c | 85.46 ± 2.86 b,c | 73.29 ± 2.86 b | |
| STR% | 0.89 ± 0.01 | 0.89 ± 0.01 | 0.89 ± 0.01 | 0.89 ± 0.01 | |
| LIN% | 0.50 ± 0.01 | 0.50 ± 0.01 | 0.51 ± 0.01 | 0.50 ± 0.01 | |
| WOB% | 0.56 ± 0.01 | 0.56 ± 0.01 | 0.57 ± 0.01 | 0.57 ± 0.01 | |
| ALH (µm) | 3.68 ± 0.14 | 3.18 ± 0.14 | 3.74 ± 0.14 | 3.19 ± 0.14 | |
| BCF(Hz) | 33.90 ± 0.63 | 33.14 ± 0.63 | 34.40 ± 0.63 | 32.68 ± 0.63 | |
| 24 h | PM% | 49.74 ± 4.62 | 59.41 ± 4.62 | 52.61 ± 4.62 | 57.97 ± 4.62 |
| VAP (µm/s) | 78.60 ± 3.20 | 68.40 ± 3.20 | 78.71 ± 3.20 | 65.59 ± 3.20 | |
| VCL (µm/s) | 144.83 ± 5.44 | 129.60 ± 5.44 | 145.28 ± 5.44 | 124.82 ± 5.44 | |
| VSL (µm/s) | 61.38 ± 2.86 a | 52.31 ± 2.86 | 61.91 ± 2.86 b | 49.32 ± 2.86 a,b | |
| STR% | 0.78 ± 0.01 | 0.76 ± 0.01 | 0.78 ± 0.01 | 0.75 ± 0.01 | |
| LIN% | 0.42 ± 0.01 | 0.40 ± 0.01 | 0.42 ± 0.01 | 0.39 ± 0.01 | |
| WOB% | 0.54 ± 0.01 | 0.52 ± 0.01 | 0.54 ± 0.01 | 0.52 ± 0.01 | |
| ALH (µm) | 3.91 ± 0.14 | 3.62 ± 0.14 | 3.84 ± 0.14 | 3.45 ± 0.14 | |
| BCF (Hz) | 31.30 ± 0.63 | 29.79 ± 0.63 | 31.27 ± 0.63 | 29.41 ± 0.63 | |
| 48 h | PM% | 44.20 ± 4.62 | 52.28 ± 4.62 | 44.92 ± 4.62 | 50.94 ± 4.64 |
| VAP (µm/s) | 73.58 ± 3.20 a,c | 59.66 ± 3.20 a | 72.58 ± 3.20 b | 57.39 ± 3.26 c,b | |
| VCL (µm/s) | 139.63 ± 5.44 a,c | 114.81 ± 5.44 a,b | 138.72 ± 5.44 d,b | 111.84 ± 5.43 c,d | |
| VSL (µm/s) | 56.01 ± 2.86 c | 44.19 ± 2.86 | 55.07 ± 2.86 a | 41.71 ± 2.91 c,a | |
| STR% | 0.75 ± 0.01 | 0.73 ± 0.01 | 0.75 ± 0.01 | 0.72 ± 0.01 | |
| LIN% | 0.39 ± 0.01 | 0.38 ± 0.01 | 0.39 ± 0.01 | 0.37 ± 0.01 | |
| WOB% | 0.52 ± 0.01 | 0.52 ± 0.01 | 0.52 ± 0.01 | 0.51 ± 0.01 | |
| ALH (µm) | 3.79 ± 0.14 a,c | 3.20 ± 0.14 a,b | 3.82 ± 0.14 b,d | 3.10 ± 0.14 c,d | |
| BCF (Hz) | 29.11 ± 0.63 | 29.27 ± 0.63 | 29.53 ± 0.63 | 28.61 ± 0.63 | |
| 72 h | PM% | 35.13 ± 4.62 | 42.97 ± 4.62 | 37.44 ± 4.62 | 43.46 ± 4.62 |
| VAP (µm/s) | 67.02 ± 3.20 a | 55.49 ± 3.20 | 65.79 ± 3.20 | 53.26 ± 3.20 a | |
| VCL (µm/s) | 129.66 ± 5.44 | 107.80 ± 5.44 | 128.44 ± 5.44 a | 104.56 ± 5.44 a | |
| VSL (µm/s) | 49.33 ± 2.86 | 39.17 ± 2.86 | 48.40 ± 2.86 | 37.87 ± 2.86 | |
| STR% | 0.73 ± 0.01 | 0.70 ± 0.01 | 0.73 ± 0.01 | 0.71 ± 0.01 | |
| LIN% | 0.38 ± 0.01 | 0.36 ± 0.01 | 0.37 ± 0.01 | 0.36 ± 0.01 | |
| WOB% | 0.52 ± 0.01 | 0.51 ± 0.01 | 0.51 ± 0.01 | 0.51 ± 0.01 | |
| ALH (µm) | 3.70 ± 0.14 c,d | 3.04 ± 0.14 c | 3.56 ± 0.14 | 3.00 ± 0.14 d | |
| BCF (Hz) | 27.54 ± 0.63 | 28.21 ± 0.63 | 27.86 ± 0.63 | 28.30 ± 0.63 | |
| 96 h | PM% | 25.77 ± 4.62 | 34.97 ± 4.62 | 28.52 ± 4.62 | 35.11 ± 4.62 |
| VAP (µm/s) | 58.22 ± 3.20 | 53.72 ± 3.20 | 60.50 ± 3.20 | 51.10 ± 3.20 | |
| VCL (µm/s) | 110.36 ± 5.44 | 104.95 ± 5.44 | 121.38 ± 5.44 | 98.54 ± 5.44 | |
| VSL (µm/s) | 42.47 ± 2.86 | 37.33 ± 2.86 | 44.71 ± 2.86 | 35.84 ± 2.86 | |
| STR% | 0.73 ± 0.01 a | 0.69 ± 0.01 a,c | 0.73 ± 0.01 b,c | 0.69 ± 0.01 b | |
| LIN% | 0.39 ± 0.01 a | 0.35 ± 0.01 a | 0.36 ± 0.01 | 0.36 ± 0.01 | |
| WOB% | 0.54 ± 0.01 | 0.51 ± 0.01 | 0.49 ± 0.01 | 0.52 ± 0.01 | |
| ALH (µm) | 3.56 ± 0.14 | 3.12 ± 0.14 | 3.42 ± 0.14 | 3.00 ± 0.14 | |
| BCF (Hz) | 25.55 ± 0.63 | 27.89 ± 0.63 | 27.38 ± 0.63 | 27.41 ± 0.63 |
| JC-1 Low % | CA | SA | CW | SW |
| 24 h | 48.97 ± 6.63 a,b | 34.60 ± 6.63 a | 46.83 ± 6.63 | 33.61 ± 6.66 b |
| 48 h | 55.96 ± 6.63 | 51.70 ± 6.63 | 56.03 ± 6.63 | 48.74 ± 6.63 |
| 72 h | 69.64 ± 6.63 a | 59.11 ± 6.63 | 64.93 ± 6.63 | 55.37 ± 6.63 a |
| 96 h | 77.23 ± 6.63 | 66.14 ± 6.63 | 73.11 ± 6.63 | 66.88 ± 6.63 |
| JC-1 high % | ||||
| 24 h | 48.92 ± 6.63 a,b | 62.96 ± 6.63 a | 50.87 ± 6.63 | 64.05 ± 6.63 b |
| 48 h | 41.85 ± 6.63 | 46.65 ± 6.63 | 42.22 ± 6.63 | 49.36 ± 6.66 |
| 72 h | 29.15 ± 6.63 b | 39.83 ± 6.63 | 34.14 ± 6.63 | 43.59 ± 6.63 b |
| 96 h | 21.83 ± 6.63 | 33.16 ± 6.63 | 26.27 ± 6.63 | 32.40 ± 6.63 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Al-Kass, Z.; Spergser, J.; Aurich, C.; Kuhl, J.; Schmidt, K.; Johannisson, A.; Morrell, J.M. Sperm Quality during Storage Is Not Affected by the Presence of Antibiotics in EquiPlus Semen Extender but Is Improved by Single Layer Centrifugation. Antibiotics 2018, 7, 1. https://doi.org/10.3390/antibiotics7010001
Al-Kass Z, Spergser J, Aurich C, Kuhl J, Schmidt K, Johannisson A, Morrell JM. Sperm Quality during Storage Is Not Affected by the Presence of Antibiotics in EquiPlus Semen Extender but Is Improved by Single Layer Centrifugation. Antibiotics. 2018; 7(1):1. https://doi.org/10.3390/antibiotics7010001
Chicago/Turabian StyleAl-Kass, Ziyad, Joachim Spergser, Christine Aurich, Juliane Kuhl, Kathrin Schmidt, Anders Johannisson, and Jane M. Morrell. 2018. "Sperm Quality during Storage Is Not Affected by the Presence of Antibiotics in EquiPlus Semen Extender but Is Improved by Single Layer Centrifugation" Antibiotics 7, no. 1: 1. https://doi.org/10.3390/antibiotics7010001
APA StyleAl-Kass, Z., Spergser, J., Aurich, C., Kuhl, J., Schmidt, K., Johannisson, A., & Morrell, J. M. (2018). Sperm Quality during Storage Is Not Affected by the Presence of Antibiotics in EquiPlus Semen Extender but Is Improved by Single Layer Centrifugation. Antibiotics, 7(1), 1. https://doi.org/10.3390/antibiotics7010001







