Occurrence of Ionophores in the Danish Environment
Abstract
:1. Introduction
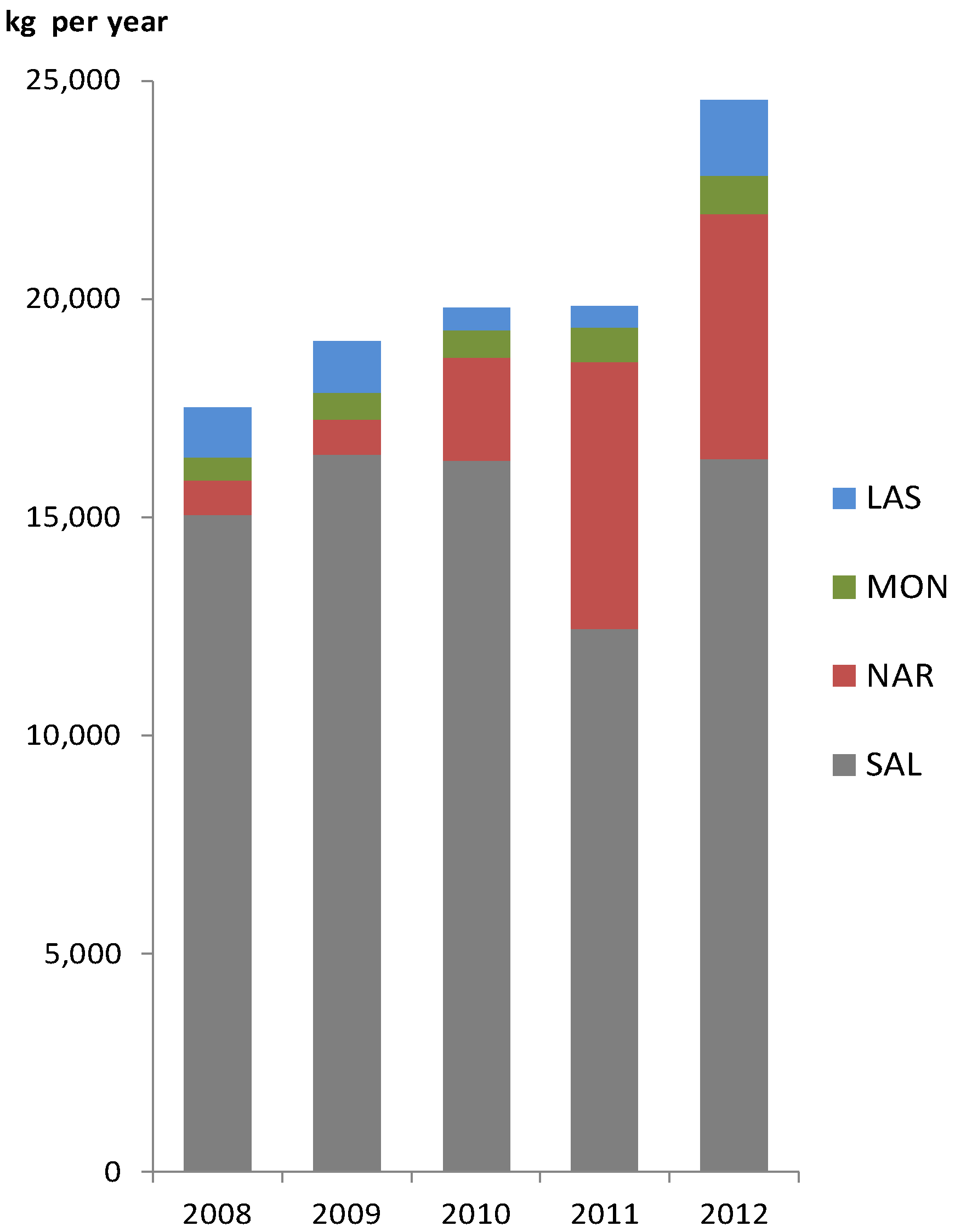
2. Results and Discussion

| July | Matrix | LAS | MON | SAL | NAR | * Unit |
|---|---|---|---|---|---|---|
| Sampling Site 1 | Water | - | - | - | - | ng·L−1 |
| Sediment | - | - | - | - | µg·kg−1 | |
| Sampling Site 2 | Water | - | - | - | - | ng·L−1 |
| Sediment | - | - | <LOQ | 1 | µg·kg−1 | |
| Sampling Site 3 | Water | - | - | - | - | ng·L−1 |
| Sediment | - | - | - | - | µg·kg−1 | |
| Sampling Site 4 | Water | - | - | - | - | ng·L−1 |
| Sediment | - | - | - | - | µg·kg−1 |
| October | Matrix | LAS | MON | SAL | NAR | * Unit |
|---|---|---|---|---|---|---|
| Sampling Site 1 | Water | - | 20 | - | - | ng·L−1 |
| Sediment | - | - | - | - | µg·kg−1 | |
| Soil | - | - | - | 1 | µg·kg−1 | |
| Sampling Site 2 | Water | - | - | - | - | ng·L−1 |
| Sediment | - | 13 | - | - | µg·kg−1 | |
| Soil 2A | - | <LOQ | - | - | µg·kg−1 | |
| Soil 2B | - | 8 | - | - | µg·kg−1 | |
| Soil 2C | - | - | 30 | - | µg·kg−1 | |
| Soil 2D | - | <LOQ | - | - | µg·kg−1 | |
| Soil 2E | - | <LOQ | - | 18 | µg·kg−1 | |
| Sampling Site 3 | Water | - | - | - | - | ng·L−1 |
| Sediment | - | - | - | 2 | µg·kg−1 | |
| Sampling Site 4 | Water | - | - | - | - | ng·L−1 |
| Sediment | - | - | - | - | µg·kg−1 |
3. Experimental Section
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Bak, S.A. Ionophores in the Environment—Analytical Method Development, Occurrence & Degradation Studies. Ph.D. Thesis, University of Copenhagen (UCPH), Copenhagen, Denmark, 2014. [Google Scholar]
- VetStat. 2013. Available online: http://www.foedevarestyrelsen.dk/ (accessed on 22 November 2013).
- Forrest, F.; Lorenz, K.; Thompson, T.S.; Keenliside, J.; Kendall, J.; Charest, J. A scoping study of livestock antimicrobials in agricultural streams of Alberta. Can. Water Res. 2011, 36, 1–16. [Google Scholar] [CrossRef]
- Kumar, K.; Gupta, C.; Chander, Y.; Singh, A.K. Antibiotic use in agriculture and its impact on the terrestrial environment. In Advances in Agronomy; Donald, L.S., Ed.; Academic Press: Salt Lake City, UT, USA, 2005. [Google Scholar]
- Furtula, V.; Huang, L.; Chambers, P.A. Determination of veterinary pharmaceuticals in poultry litter and soil by methanol extraction and liquid chromatography-tandem mass spectrometry. J. Environ. Sci. Heal. B 2009, 44, 717–723. [Google Scholar] [CrossRef]
- Schlüsener, M.P.; Spiteller, M.; Bester, K. Determination of antibiotics from soil by pressurized liquid extraction and liquid chromatography tandem mass spectrometry. J. Chromatogr. A 2003, 1003, 21–28. [Google Scholar] [CrossRef] [PubMed]
- Eggen, T.; Asp, T.N.; Grave, K.; Hormazabal, V. Uptake and translocation of metformin, ciprofloxacin and narasin in forage- and crop plants. Chemosphere 2011, 85, 26–33. [Google Scholar] [CrossRef] [PubMed]
- Dolliver, H.; Gupta, S. Antibiotic losses in leaching and surface runoff from manure-amended agricultural land. J. Environ. Qual. 2008, 37, 1227–1237. [Google Scholar] [CrossRef] [PubMed]
- Hao, C.; Lissemore, L.; Nguyen, B.; Kleywegt, S.; Yang, P.; Solomon, K. Determination of Pharmaceuticals in environmental waters by liquid chromatography/electrospray ionization/tandem mass spectrometry. Anal. Bioanal. Chem. 2006, 384, 505–513. [Google Scholar] [CrossRef] [PubMed]
- Lissemore, L.; Hao, C.; Yang, P.; Sibley, P.K.; Mabury, S.; Solomon, K.R. An exposure assessment for selected pharmaceuticals within a watershed in Southern Ontario. Chemosphere 2006, 64, 717–729. [Google Scholar] [CrossRef] [PubMed]
- Song, W.; Huang, M.; Rumbeiha, W.; Li, H. Determination of amprolium, carbadox, monensin, and tylosin in surface water by liquid chromatography/tandem mass spectrometry. Rapid Commun. Mass Spectrom. 2007, 21, 1944–1950. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.L.; Zhou, J.L. Simultaneous determination of various pharmaceutical compounds in water by solid-phase extraction—Liquid chromatography—Tandem mass Spectrometry. J. Chromatogr. A 2007, 1154, 205–213. [Google Scholar] [CrossRef] [PubMed]
- Cha, J.M.; Yang, S.; Carlson, K.H. Rapid analysis of trace levels of antibiotic polyether ionophores in surface water by solid-phase extraction and liquid chromatography with ion trap tandem mass spectrometric detection. J. Chromatogr. A 2005, 1065, 187–198. [Google Scholar] [CrossRef] [PubMed]
- Thompson, T.S.; Noot, D.K.; Forrest, F.; van den Heever, J.P.; Kendall, J.; Keenliside, J. Large volume injection for the direct analysis of ionophores and avermectins in surface water by liquid chromatography—Electrospray ionization tandem mass spectrometry. Anal. Chim. Acta 2009, 633, 127–135. [Google Scholar] [CrossRef] [PubMed]
- Bak, S.A.; Hansen, M.; Krogh, K.A.; Brandt, A.; Halling-Sorensen, B.; Björklund, E. Development and validation of an spe methodology combined with LC-MS/MS for the determination of four ionophores in aqueous environmental matrices. Int. J. Environ. Anal. Chem. 2013, 93, 1–13. [Google Scholar] [CrossRef]
- Watkinson, A.J.; Murby, E.J.; Kolpin, D.W.; Costanzo, S.D. The occurrence of antibiotics in an urban watershed: From wastewater to drinking water. Sci. Total Environ. 2009, 407, 2711–2723. [Google Scholar] [CrossRef] [PubMed]
- Herrero, P.; Borrull, F.; Pocurull, E.; Marce, R.M. Novel amide polar-embedded reversed-phase column for the fast liquid chromatography-tandem mass spectrometry method to determine polyether ionophores in environmental waters. J. Chromatogr. A 2012, 1263, 7–13. [Google Scholar] [CrossRef]
- Hansen, M. Anticoccidials in the environment: Occurence, fate, effects and risk assessment of ionphores. Ph.D. Thesis, University of Copenhagen (UCPH), Copenhagen, Denmark, 2009. [Google Scholar]
- Watanabe, N.; Harter, T.H.; Bergamaschi, B.A. Environmental occurrence and shallow ground water detection of the antibiotic monensin from dairy farms. J. Environ. Qual. 2008, 37, S78–S85. [Google Scholar] [CrossRef] [PubMed]
- Bartelt-Hunt, S.; Snow, D.D.; Damon-Powell, T.; Miesbach, D. Occurrence of steroid hormones and antibiotics in shallow groundwater impacted by livestock waste control facilities. J. Contam. Hydrol. 2011, 123, 94–103. [Google Scholar] [CrossRef] [PubMed]
- Song, W.; Ding, Y.; Chiou, C.T.; Li, H. Selected Veterinary pharmaceuticals in agricultural water and soil from land application of animal manure. J. Environ. Qual. 2010, 39, 1211–1217. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.C.; Carlson, K. Occurrence of ionophore antibiotics in water and sediments of a mixed-landscape watershed. Water Res. 2006, 40, 2549–2560. [Google Scholar] [CrossRef] [PubMed]
- Bohn, P.; Bak, S.A.; Björklund, E.; Krogh, K.A.; Hansen, M. Abiotic degradation of antibiotic ionophores. Environ. Pollut. 2013, 182, 177–183. [Google Scholar] [CrossRef] [PubMed]
- Bak, S.A.; Hansen, M.; Pedersen, K.M.; Halling-Sørensen, B.; Björklund, E. Quantification of four ionophores in soil, sediment and manure using pressurised liquid extraction. J. Chromatogr. A 2013, 1307, 27–33. [Google Scholar] [CrossRef] [PubMed]
- Hansen, M.; Björklund, E.; Krogh, K.A.; Brandt, A.; Halling-Sorensen, B. Biotic transformation of anticoccidials in soil using a lab-scale bio-reactor as a precursor-tool. Chemosphere 2011, 86, 212–215. [Google Scholar] [CrossRef] [PubMed]
- Hansen, M.; Krogh, K.A.; Björklund, E.; Brandt, A.; Halling-Sorensen, B. Environmental risk assessment of ionophores. Trends Anal. Chem. 2009, 28, 534–542. [Google Scholar] [CrossRef]
- Bak, S.A.; Hansen, M.; Krogh, K.A.; Randt, A.; Halling-Sørensen, B.; jörklund, E. Detection and fate of ionophores in the environment. In Proceedings ot the 6th SETAC World Meeting, Berlin, Germany, 20–24 May 2012.
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bak, S.A.; Björklund, E. Occurrence of Ionophores in the Danish Environment. Antibiotics 2014, 3, 564-571. https://doi.org/10.3390/antibiotics3040564
Bak SA, Björklund E. Occurrence of Ionophores in the Danish Environment. Antibiotics. 2014; 3(4):564-571. https://doi.org/10.3390/antibiotics3040564
Chicago/Turabian StyleBak, Søren Alex, and Erland Björklund. 2014. "Occurrence of Ionophores in the Danish Environment" Antibiotics 3, no. 4: 564-571. https://doi.org/10.3390/antibiotics3040564
APA StyleBak, S. A., & Björklund, E. (2014). Occurrence of Ionophores in the Danish Environment. Antibiotics, 3(4), 564-571. https://doi.org/10.3390/antibiotics3040564




