Determination of the Presence of Three Antimicrobials in Surface Water Collected from Urban and Rural Areas
Abstract
:1. Introduction
2. Results and Discussion
2.1. Concentrations of Pharmaceutical Compounds in Surface Water Samples
| Analyte | Sulfamethazine | Sulfamethoxypyridazine | Trimethoprim |
|---|---|---|---|
| MW | 278.3 | 280.3 | 290.3 |
| Formula | C12H14N4O2S | C11H12N4O3S | C14H18N4O3 |
| Chemical structure |  |  |  |
| LOD (ng·L−1) | 0.2 | 0.1 | 0.2 |
| LOQ (ng·L−1) | 0.5 | 0.5 | 0.5 |
| Maximum concentration (ng·L−1) | 63.6 | 11.2 | 85.4 |
| Minimum concentration (ng·L−1) | 0.5 | 0.5 | 1.1 |
| Mean concentration (ng·L−1) | 5.5 | 1.6 | 13.8 |
| Number of detections | 62 | 37 | 43 |
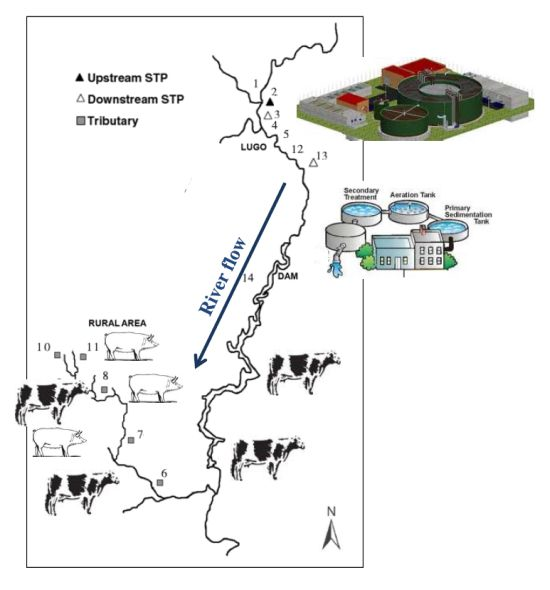
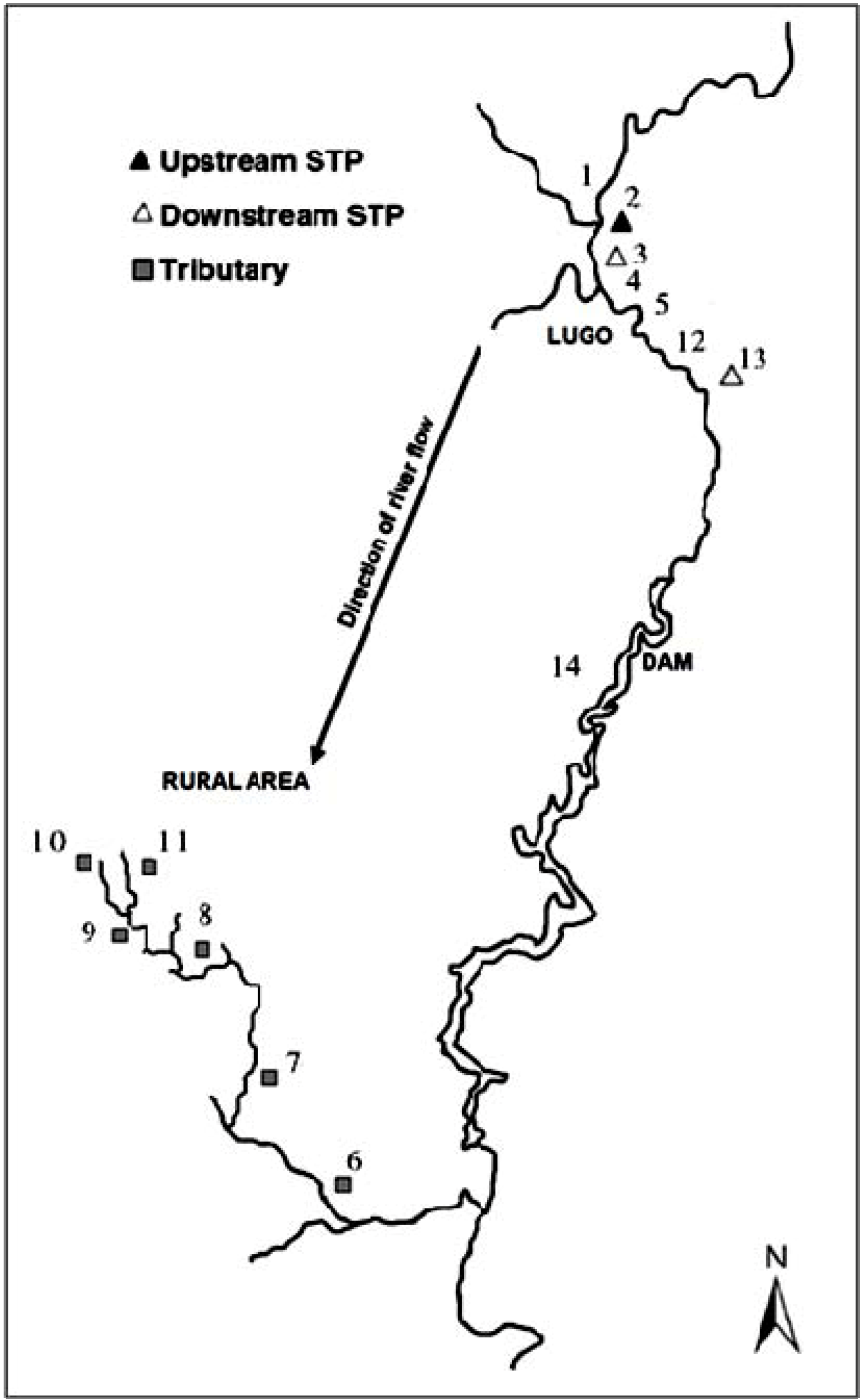
| Site number | Area type | Total number of samples analyzed | Number of positive samples |
|---|---|---|---|
| 1 | Rural without much farming activity | 24 | 8 |
| 2 | Rural without much farming activity | 24 | 9 |
| 3 | Urban | 24 | 17 |
| 4 | Rural without much farming activity | 24 | 5 |
| 5 | Rural without much farming activity | 24 | 7 |
| 6 | Rural with farming activity | 24 | 4 |
| 7 | Rural with farming activity | 24 | 6 |
| 8 | Rural with farming activity | 24 | 9 |
| 9 | Rural with farming activity | 24 | 6 |
| 10 | Rural with farming activity | 24 | 8 |
| 11 | Rural with farming activity | 24 | 6 |
| 12 | Urban | 24 | 6 |
| 13 | Urban | 24 | 22 |
| 14 | Urban | 2 | 2 |
2.2. Statistical Results
3. Experimental
3.1. Study Area
3.2. Water Samples
3.3. Sample Preparation and Analysis
3.4. Chemicals, Reagents and Stock Solutions
3.5. Equipment
3.6. HPLC-MS/MS Method
3.7. Statistical Analysis
4. Conclusions
Acknowledgments
Conflict of Interest
References and Notes
- Moulin, G.; Cavalie, P.; Pellanne, I.; Chevance, A.; Laval, A.; Millemann, Y.; Colin, P.; Chauvin, C. Antimicrobial Resistance ad hoc Group of the French Food Safety Agency. A comparison of antimicrobial usage in human and veterinary medicine in France from 1999 to 2005. J. Antimicrob. Chemother. 2008, 62, 617–625. [Google Scholar] [CrossRef]
- Gracia-Lor, E.; Sancho, J.V.; Serrano, R.; Hernández, F. Occurrence and removal of pharmaceuticals in wastewater treatment plants at the Spanish Mediterranean area of Valencia. Chemosphere 2012, 87, 453–462. [Google Scholar] [CrossRef]
- Díaz-Cruz, M.S.; Barceló, D. Determination of antimicrobial residues and metabolites in the aquatic environment by liquid chromatography tandem mass spectrometry. Anal. Bioanal. Chem. 2006, 386, 973–998. [Google Scholar] [CrossRef]
- Kemper, N. Veterinary antibiotics in the aquatic and terrestrial environment. Ecol. Indic. 2008, 8, 1–13. [Google Scholar] [CrossRef]
- Dinh, Q.T.; Alliot, F.; Moreau-Guigon, E.; Eurin, J.; Chevreuil, M.; Labadie, P. Measurement of trace levels of antibiotics in river water using on-line enrichment and triple-quadrupole LC-MS/MS. Talanta 2011, 85, 1238–1245. [Google Scholar] [CrossRef]
- Sanderson, H.; Johnson, D.J.; Reitsma, T.; Brain, R.A.; Wilson, C.J.; Solomon, K.R. Ranking and prioritization of environmental risks of pharmaceuticals in surface waters. Regul. Toxicol. Pharmacol. 2004, 39, 158–183. [Google Scholar] [CrossRef]
- Madden, J.C.; Enoch, S.J.; Hewitt, M.; Cronin, M.T.D. Pharmaceuticals in the environment: Good practice in predicting acute ecotoxicological effects. Toxicol. Lett. 2009, 185, 85–101. [Google Scholar]
- Halling-Sørensen, B.; Nors Nielsen, S.; Lanzky, P.F.; Ingerslev, F.; Holten Lützhøft, H.C.; Jørgensen, S.E. Occurrence, fate and effects of pharmaceutical substances in the environment—A review. Chemosphere 1998, 36, 357–393. [Google Scholar] [CrossRef]
- Monteiro, S.C.; Boxall, A.B.A. Occurrence and Fate of Human Pharmaceuticals in the Environment. Rev. Environ. Contam. Toxicol. 2010, 202, 53–154. [Google Scholar]
- Kim, S.C.; Carlson, K. Quantification of human and veterinary antibiotics in water and sediment using SPE/LC/MS/MS. Anal. Bioanal. Chem. 2007, 387, 1301–1315. [Google Scholar] [CrossRef]
- Chang, H.; Hu, J.; Asami, M.; Kunikane, S. Simultaneous analysis of 16 sulfonamide and trimethoprim antibiotics in environmental waters by liquid chromatography-electrospray tandem mass spectrometry. J. Chromatogr. A 2008, 1190, 390–393. [Google Scholar] [CrossRef]
- Kasprzyk-Hordern, B.; Dinsdale, R.M.; Guwy, A.J. The occurrence of pharmaceuticals, personal care products, endocrine disruptors and illicit drugs in surface water in South Wales, UK. Water Res. 2008, 42, 3498–3518. [Google Scholar] [CrossRef]
- Rao, R.N.; Venkateswarlu, N.; Narsimha, R. Determination of antibiotics in aquatic environment by solid-phase extraction followed by liquid chromatography-electrospray ionization mass spectrometry. J. Chromatogr. A 2008, 1187, 151–164. [Google Scholar] [CrossRef]
- Senta, I.; Terzic, S.; Ahel, M. Simultaneous determination of sulfonamides, fluoroquinolones, macrolides and trimethoprim in wastewater and river water by LC-tandem-MS. Chromatographia 2008, 68, 747–758. [Google Scholar] [CrossRef]
- Wille, K.; Noppe, H.; Verheyden, K.; Vanden Bussche, J.; de Wulf, E.; van Caeter, P.; Janssen, C.; de Brabander, H.; Vanhaecke, L. Validation and application of an LC-MS/MS method for the simultaneous quantification of 13 pharmaceuticals in seawater. Anal. Bioanal. Chem. 2010, 397, 1797–1808. [Google Scholar]
- Martín, J.; Camacho-Muñoz, D.; Santos, J.L.; Aparicio, I.; Alonso, E. Monitoring of pharmaceutically active compounds on the Guadalquivir River basin (Spain): Occurrence and risk assessment. J. Environ. Monit. 2011, 13, 2042–2049. [Google Scholar] [CrossRef]
- Seifrtová, M.; Pena, A.; Lino, C.M.; Solich, P. Determination of fluoroquinolone antibiotics in hospital and municipal wastewaters in Coimbra by liquid chromatography with a monolithic column and fluorescence detection. Anal. Bioanal. Chem. 2008, 391, 799–805. [Google Scholar] [CrossRef]
- Pena, A.; Pina, J.; Silva, L.J.G.; Meisel, L.; Lino, C.M. Fluoroquinolone antibiotics determination in piggeries environmental waters. J. Environ. Monit. 2010, 12, 642–646. [Google Scholar] [CrossRef]
- Conley, J.M.; Symes, S.J.; Schorr, M.S.; Richards, S.M. Spatial and temporal analysis of pharmaceutical concentrations in the upper Tennessee River basin. Chemosphere 2008, 73, 1178–1187. [Google Scholar] [CrossRef]
- Kümmerer, K. Antibiotics in the aquatic environment—A review. Part II. Chemosphere 2009, 75, 435–441. [Google Scholar] [CrossRef]
- Forbes, B.; Sahm, D.F.; Weissfeld, A.S. Principios de la acción y de la resistencia a los antimicrobianos. In Bailey & Scott’s Diagnóstico microbiológico, 12th ed.; Médica Panamericana: Buenos Aires, Argentina, 2009; pp. 172–186. [Google Scholar]
- Díaz-Cruz, M.S.; García-Galán, M.J.; Barceló, D. Highly sensitive simultaneous determination of sulfonamide antibiotics and one metabolite in environmental waters by liquid chromatography-quadrupole linear ion trap-mass spectrometry. J. Chromatogr. A 2008, 1193, 50–59. [Google Scholar] [CrossRef]
- Watkinson, A.J.; Murby, E.J.; Kolpin, D.W.; Costanzo, S.D. The occurrence of antibiotics in an urban watershed: From wastewater to drinking water. Sci. Total Environ. 2009, 407, 2711–2723. [Google Scholar] [CrossRef]
- Ferrer, I.; Zweigenbaum, J.A.; Thurman, E.M. Analysis of 70 Environmental Protection Agency priority pharmaceuticals in water by EPA Method 1694. J. Chromatogr. A 2010, 1217, 5674–5686. [Google Scholar] [CrossRef]
- García-Galán, M.J.; Díaz-Cruz, M.S.; Barceló, D. Determination of 19 sulfonamides in environmental water samples by automated on-line solid-phase extraction-liquid chromatography-tandem mass spectrometry (SPE-LC-MS/MS). Talanta 2010, 81, 355–366. [Google Scholar]
- Kools, S.A.E.; Moltmann, J.F.; Knacker, T. Estimating the use of veterinary medicines in the European Union. Regul. Toxicol. Pharmacol. 2008, 50, 59–65. [Google Scholar] [CrossRef]
- Hilton, M.; Thomas, K.V. Determination of selected human pharmaceutical compounds in effluent and surface water samples by high-performance liquid chromatography-electrospray tandem mass spectrometry. J. Chromatogr. A 2003, 1015, 129–141. [Google Scholar] [CrossRef]
- Metcalfe, C.D.; Miao, X.; Koenig, B.G.; Struger, J. Distribution of acidic and neutral drugs in surface waters near sewage treatment plants in the lower Great Lakes, Canada. Environ. Toxicol. Chem. 2003, 22, 2881–2889. [Google Scholar] [CrossRef]
- Vieno, N.M.; Tuhkanen, T.; Kronberg, L. Seasonal variation in the occurrence of pharmaceuticals in effluents from a sewage treatment plant and in the recipient water. Environ. Sci. Technol. 2005, 39, 8220–8226. [Google Scholar] [CrossRef]
- Hua, W.Y.; Bennett, E.R.; Maio, X.-S.; Metcalfe, C.D.; Letcher, R.J. Seasonality effects on pharmaceuticals and s-triazine herbicides in wastewater effluent and surface water from the Canadian side of the upper Detroit River. Environ. Toxicol. Chem. 2006, 25, 2356–2365. [Google Scholar] [CrossRef]
- Zuccato, E.; Castiglioni, S.; Bagnati, R.; Melis, M.; Fanelli, R. Source, occurrence and fate of antibiotics in the Italian aquatic environment. J. Hazard. Mater. 2010, 179, 1042–1048. [Google Scholar] [CrossRef]
- Kasprzyk-Hordern, B.; Dinsdale, R.M.; Guwy, A.J. Multi-residue method for the determination of basic/neutral pharmaceuticals and illicit drugs in surface water by solid-phase extraction and ultra performance liquid chromatography-positive electrospray ionization tandem mass spectrometry. J. Chromatogr. A 2007, 1161, 132–145. [Google Scholar] [CrossRef]
- Muñóz, I.; López-Doval, J.C.; Ricart, M.; Villagrasa, M.; Brix, R.; Geiszinger, A.; Ginebreda, A.; Guasch, H.; López de Alda, M.J.; Romaní, A.M.; et al. Bridging levels of pharmaceuticals in river water with biological community structure in the Llobregat river basin (northeast Spain). Environ. Toxicol. Chem. 2009, 28, 2706–2714. [Google Scholar] [CrossRef]
- Postigo, C.; López de Alda, M.J.; Barceló, D. Drugs of abuse and their metabolites in the Ebro River basin: Occurrence in sewage and surface water, sewage treatment plants removal efficiency, and collective drug usage estimation. Environ. Int. 2010, 36, 75–84. [Google Scholar] [CrossRef]
- Jung, J.; Kim, Y.; Kim, J.; Jeong, D.-H.; Choi, K. Environmental levels of ultraviolet light potentiate the toxicity of sulfonamide antibiotics in Daphnia magna. Ecotoxicology 2008, 17, 37–45. [Google Scholar] [CrossRef]
- Cancel-Barrio, J.J.; Fandiño, M. Management of irrigation water in Terra Chá: Indicators. (in spanish). IBADER: Instituto de Biodiversidade Agraria e Desenvolvemento Rural Recursos Rurais. 2009, 5, 49–57. [Google Scholar]
- INE. Instituto Nacional de Estadística. Anuario Estadístico de España 2011. Demografía. (in Spanish). 2011, pp. 30–71. Available online: http://www.ine.es/prodyser/pubweb/anuario11/anu11_02demog.pdf (accessed on 12 November 2012).
- Iglesias, A.; Nebot, C.; Miranda, J.M.; Vázquez, B.I.; Cepeda, A. Detection and quantitative analysis of 21 veterinary drugs in river water using high-pressure liquid chromatography coupled to tandem mass spectrometry. Environ. Sci. Pollut. Res. 2012, 19, 3235–3249. [Google Scholar] [CrossRef]
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Iglesias, A.; Nebot, C.; Miranda, J.M.; Vázquez, B.I.; Abuín, C.M.F.; Cepeda, A. Determination of the Presence of Three Antimicrobials in Surface Water Collected from Urban and Rural Areas. Antibiotics 2013, 2, 46-57. https://doi.org/10.3390/antibiotics2010046
Iglesias A, Nebot C, Miranda JM, Vázquez BI, Abuín CMF, Cepeda A. Determination of the Presence of Three Antimicrobials in Surface Water Collected from Urban and Rural Areas. Antibiotics. 2013; 2(1):46-57. https://doi.org/10.3390/antibiotics2010046
Chicago/Turabian StyleIglesias, Alejandra, Carolina Nebot, Jose M. Miranda, Beatriz I. Vázquez, Carlos M. Franco Abuín, and Alberto Cepeda. 2013. "Determination of the Presence of Three Antimicrobials in Surface Water Collected from Urban and Rural Areas" Antibiotics 2, no. 1: 46-57. https://doi.org/10.3390/antibiotics2010046
APA StyleIglesias, A., Nebot, C., Miranda, J. M., Vázquez, B. I., Abuín, C. M. F., & Cepeda, A. (2013). Determination of the Presence of Three Antimicrobials in Surface Water Collected from Urban and Rural Areas. Antibiotics, 2(1), 46-57. https://doi.org/10.3390/antibiotics2010046