Epidemiology of ESBL-Producing, Carbapenem-Resistant, and Carbapenemase-Producing Enterobacterales in Southern Africa
Abstract
1. Introduction
2. Results
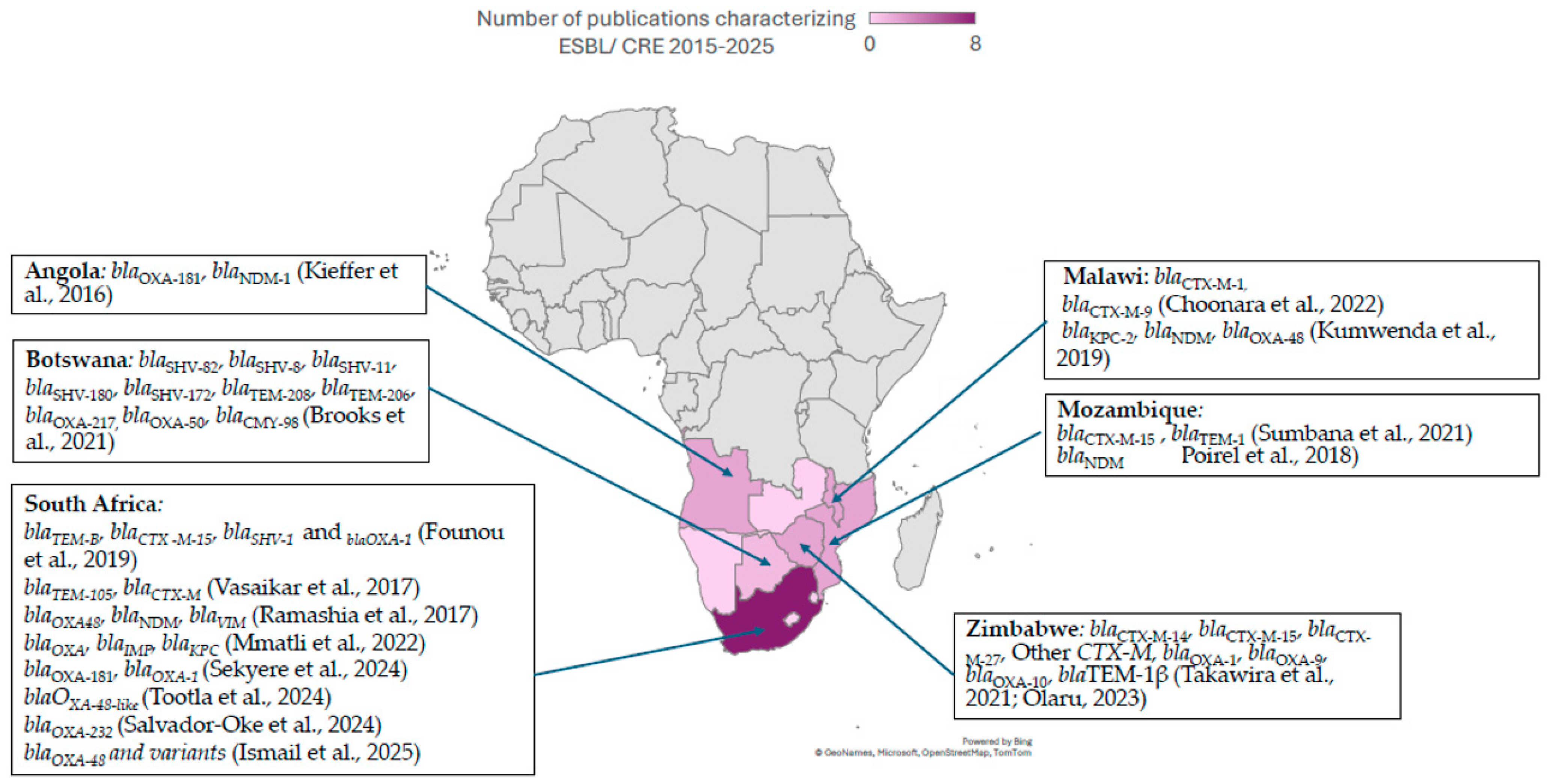
2.1. Epidemiology of ESBL-PE in Southern Africa
2.2. Epidemiology of CPE and CRE in Southern Africa
2.3. Available Molecules for Treatment of Infections Due to MDR
3. Discussion
4. Materials and Methods
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| 3GC | Third-generation cephalosporins |
| AMR | Antimicrobial resistance |
| βL/βLI | Β-lactam/β lactamase inhibitor |
| βL/nβLI | Β-lactam/non-β lactamase inhibitor |
| CRE | Carbapenem-resistant Enterobacterales |
| CPE | Carbapenemase-producing Enterobacterales |
| CTX | Cefotaximase β-lactamase |
| ESBL | Extended-spectrum β-lactamase |
| ESCrE | Extended-spectrum cephalosporin-resistant Enterobacterales |
| GAP | Global action plan |
| GES | Guiana extended-spectrum carbapenemase |
| IMI | Imipenem hydrolyzing β-lactamase |
| KPC | Klebsiella pneumoniae carbapenemase |
| MBL | Metallo-β lactamase |
| MDR | Multi-drug-resistant |
| NAP | National action plan |
| OXA | Oxacillinases |
| SHV | Sulfhydryl variable β-lactamase |
| SME | Serratia marcescens enzyme (SME) |
| TEM | Temoneira |
| TMP/SMX | Trimethoprim-sulfamethoxazole |
References
- World Health Organization. Global action plan on antimicrobial resistance. In Global Action Plan on Antimicrobial Resistance; World Health Organization: Geneva, Switzerland, 2015. [Google Scholar]
- Murray, C.J.L.; Ikuta, K.S.; Sharara, F.; Swetschinski, L.; Aguilar, G.R.; Gray, A.; Han, C.; Bisignano, C.; Rao, P.; Wool, E.; et al. Global burden of bacterial antimicrobial resistance in 2019: A systematic analysis. Lancet 2022, 399, 629–655. [Google Scholar] [CrossRef]
- GBD 2021 Antimicrobial Resistance Collaborators. Global burden of bacterial antimicrobial resistance 1990–2021: A systematic analysis with forecasts to 2050. Lancet 2024, 404, 1199–1226. [Google Scholar] [CrossRef]
- WHO. Global Database for Tracking Antimicrobial Resistance (AMR) Country Self-Assessment Survey (TrACSS); World Health Organization: Geneva, Switzerland, 2024. [Google Scholar]
- Kariuki, S.; Kering, K.; Wairimu, C.; Onsare, R.; Mbae, C. Antimicrobial Resistance Rates and Surveillance in Sub-Saharan Africa: Where Are We Now? Infect. Drug Resist. 2022, 15, 3589–3609. [Google Scholar] [CrossRef]
- Osena, G.; Kapoor, G.; Kalanxhi, E.; Ouassa, T.; Shumba, E.; Brar, S.; Alimi, Y.; Moreira, M.; Matu, M.; Sow, A.; et al. Antimicrobial resistance in Africa: A retrospective analysis of data from 14 countries, 2016–2019. PLoS Med. 2025, 22, e1004638. [Google Scholar] [CrossRef] [PubMed]
- Ndir, A.; Diop, A.; Faye, P.M.; Cissé, M.F.; Ndoye, B.; Astagneau, P. Epidemiology and Burden of Bloodstream Infections Caused by Extended-Spectrum Beta-Lactamase Producing Enterobacteriaceae in a Pediatric Hospital in Senegal. PLoS ONE 2016, 11, e0143729. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- de Jager, P.; Chirwa, T.; Naidoo, S.; Perovic, O.; Thomas, J. Nosocomial Outbreak of New Delhi Metallo-β-Lactamase-1-Producing Gram-Negative Bacteria in South Africa: A Case-Control Study. PLoS ONE 2015, 10, e0123337. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- World Bank. Drug-Resistant Infections: A Threat to Our Economic Future; World Bank: Washington, DC, USA, 2017. [Google Scholar]
- Laurence, T.; Lamberti, O.; Smith, R.; Drake, T.; McDonnell, A. The Global Direct Inpatient Cost of Antimicrobial Resistance: A Modelling Study; Center for Global Development: Washington, DC, USA, 2025; No. 712. [Google Scholar]
- Institute for Health Metrics and Evaluation (IHME). Available online: https://www.healthdata.org/research-analysis/health-topics/antimicrobial-resistance-amr (accessed on 1 June 2025).
- Africa CDC AMR Landmark Report. Available online: https://africacdc.org/download/african-union-amr-landmark-report-voicing-african-priorities-on-the-active-pandemic/ (accessed on 1 October 2025).
- Alomari, R.; Abdel-Razeq, A.; Shamiah, H. Comprehensive assessment of the global burden of antimicrobial resistance: Trends and insights from 2000 to 2023. Am. J. Biomed. 2024, 12, 151–168. [Google Scholar] [CrossRef]
- Ruef, M.; Emonet, S.; Merglen, A.; Dewez, J.E.; Obama, B.M.; Catho, G.; Andrey, D.O.; Kowalski, M.; Harbarth, S.; Combescure, C.; et al. Carriage of third-generation cephalosporin-resistant and carbapenem-resistant Enterobacterales among children in sub-Saharan Africa: A systematic review and meta-analysis. eClinicalMedicine 2024, 70, 102508. [Google Scholar] [CrossRef] [PubMed]
- Aiken, A.M.; Rehman, A.M.; de Kraker, M.E.A.; Madrid, L.; Kebede, M.; Labi, A.-K.; Obeng-Nkrumah, N.; Nyamwaya, B.; Kagucia, E.; Cocker, D.; et al. Mortality associated with third-generation cephalosporin resistance in Enterobacterales bloodstream infections at eight sub-Saharan African hospitals (MBIRA): A prospective cohort study. Lancet Infect. Dis. 2023, 23, 1280–1290. [Google Scholar] [CrossRef] [PubMed]
- Lester, R.; Musicha, P.; Kawaza, K.; Langton, J.; Mango, J.; Mangochi, H.; Bakali, W.; Pearse, O.; Mallewa, J.; Denis, B.; et al. Effect of resistance to third-generation cephalosporins on morbidity and mortality from bloodstream infections in Blantyre, Malawi: A prospective cohort study. Lancet Microbe 2022, 3, e922–e930. [Google Scholar] [CrossRef]
- Bush, K.; Bradford, P.A. β-Lactams and β-Lactamase Inhibitors: An Overview. Cold Spring Harb. Perspect. Med. 2016, 6, a025247. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Peirano, G.; Pitout, J.D.D. Extended-Spectrum β-Lactamase-Producing Enterobacteriaceae: Update on Molecular Epidemiology and Treatment Options. Drugs 2019, 79, 1529–1541. [Google Scholar] [CrossRef]
- Hall, B.G.; Barlow, M. Revised Ambler classification of β-lactamases. J. Antimicrob. Chemother. 2005, 55, 1050–1051. [Google Scholar] [CrossRef] [PubMed]
- Sawa, T.; Kooguchi, K.; Moriyama, K. Molecular diversity of extended-spectrum β-lactamases and carbapenemases, and antimicrobial resistance. J. Intensiv. Care 2020, 8, 13. [Google Scholar] [CrossRef]
- Saravanan, M.; Ramachandran, B.; Barabadi, H. The prevalence and drug resistance pattern of extended spectrum β–lactamases (ESBLs) producing Enterobacteriaceae in Africa. Microb. Pathog. 2018, 114, 180–192. [Google Scholar] [CrossRef]
- Fadare, F.T.; Okoh, A.I. Distribution and molecular characterization of ESBL, pAmpC β-lactamases, and non-β-lactam encoding genes in Enterobacteriaceae isolated from hospital wastewater in Eastern Cape Province, South Africa. PLoS ONE 2021, 16, e0254753. [Google Scholar] [CrossRef]
- Almogbel, M.; Altheban, A.; Alenezi, M.; Al-Motair, K.; A Menezes, G.; Elabbasy, M.; Hammam, S.; Hays, J.P.; A Khan, M. CTX-M-15 Positive Escherichia coli and Klebsiella pneumoniae Outbreak in the Neonatal Intensive Care Unit of a Maternity Hospital in Ha’il, Saudi Arabia. Infect. Drug Resist. 2021, 14, 2843–2849. [Google Scholar] [CrossRef]
- Hamprecht, A.; Sommer, J.; Willmann, M.; Brender, C.; Stelzer, Y.; Krause, F.F.; Tsvetkov, T.; Wild, F.; Riedel-Christ, S.; Kutschenreuter, J.; et al. Pathogenicity of Clinical OXA-48 Isolates and Impact of the OXA-48 IncL Plasmid on Virulence and Bacterial Fitness. Front. Microbiol. 2019, 10, 2509. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lee, H.; Shin, J.; Chung, Y.J.; Park, M.; Kang, K.J.; Baek, J.Y.; Shin, D.; Chung, D.R.; Peck, K.R.; Song, J.H.; et al. Co-introduction of plasmids harbouring the carbapenemase genes, blaNDM-1 and blaOXA-232, increases fitness and virulence of bacterial host. J. Biomed. Sci. 2020, 27, 8. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Yu, Y.; Dai, P.; Niu, M.; Han, R.; Liu, S.; Du, Y. Antimicrobial resistance, molecular characteristics, virulence and pathogenicity of blaNDM-1-positive Enterobacter cloacae. J. Med. Microbiol. 2023, 72, 001712. [Google Scholar] [CrossRef] [PubMed]
- Sattler, J.; Ernst, C.M.; Zweigner, J.; Hamprecht, A. High frequency of acquired virulence factors in carbapenemase-producing Klebsiella pneumoniae isolates from a large German university hospital, 2013–2021. Antimicrob. Agents Chemother. 2024, 68, e0060224. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Bush, K.; Jacoby, G.A. Updated Functional Classification of β-Lactamases. Antimicrob. Agents Chemother. 2010, 54, 969–976. [Google Scholar] [CrossRef] [PubMed]
- Iskandar, K.; Molinier, L.; Hallit, S.; Sartelli, M.; Hardcastle, T.C.; Haque, M.; Lugova, H.; Dhingra, S.; Sharma, P.; Islam, S.; et al. Surveillance of antimicrobial resistance in low- and middle-income countries: A scattered picture. Antimicrob. Resist. Infect. Control. 2021, 10, 63. [Google Scholar] [CrossRef]
- Bleischwitz, S.; Winkelmann, T.S.; Pfeifer, Y.; Fischer, M.A.; Pfennigwerth, N.; Hammerl, J.A.; Binsker, U.; Hans, J.B.; Gatermann, S.; Käsbohrer, A.; et al. Antimicrobial Resistance Surveillance: Data Harmonisation and Data Selection within Secondary Data Use. Antibiotics 2024, 13, 656. [Google Scholar] [CrossRef]
- World Health Organization. Antimicrobial Resistance in the WHO African Region: A Systematic Literature Review; World Health Organization: Geneva, Switzerland, 2021. [Google Scholar]
- Narendrakumar, L.; Chakraborty, M.; Kumari, S.; Paul, D.; Das, B. β-Lactam potentiators to re-sensitize resistant pathogens: Discovery, development, clinical use and the way forward. Front. Microbiol. 2023, 13, 1092556. [Google Scholar] [CrossRef]
- Abay, G.K.; Shfare, M.T.; Teklu, T.G.; Kidane, K.M.; Gebremeskel, T.K.; Kahsay, A.G.; Gezae, K.E.; Muthupandian, S.; Degene, T.A. Extended-spectrum β-lactamase production and antimicrobial resistance among Enterobacteriaceae causing clinical infections in Africa: A systematic review and meta-analysis (2012–2020). Eur. J. Med. Res. 2025, 30, 14. [Google Scholar] [CrossRef]
- Olaitan, M.O.; Orababa, O.Q.; Shittu, R.B.; Obunukwu, G.M.; Kade, A.E.; Arowolo, M.T.; Oyediran, A.A.; Yusuff, R.A. Prevalence of ESBL-producing Escherichia coli in sub-Saharan Africa: A meta-analysis using a One Health approach. One Health 2025, 20, 101090. [Google Scholar] [CrossRef]
- Takawira, F.T.; Pitout, J.D.; Thilliez, G.; Mashe, T.; Gutierrez, A.V.; Kingsley, R.A.; Peirano, G.; Matheu, J.; Midzi, S.M.; Mwamakamba, L.W.; et al. Molecular epidemiology of extended-spectrum beta-lactamase–producing extra-intestinal pathogenic Escherichia coli strains over a 2-year period (2017–2019) from Zimbabwe. Eur. J. Clin. Microbiol. Infect. Dis. 2021, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Founou, R.C.; Founou, L.L.; Allam, M.; Ismail, A.; Essack, S.Y. Whole Genome Sequencing of Extended Spectrum β-lactamase (ESBL)-producing Klebsiella pneumoniae Isolated from Hospitalized Patients in KwaZulu-Natal, South Africa. Sci. Rep. 2019, 9, 6266. [Google Scholar] [CrossRef] [PubMed]
- Haindongo, E.H.; Funtua, B.; Singu, B.; Hedimbi, M.; Kalemeera, F.; Hamman, J.; Vainio, O.; Hakanen, A.J.; Vuopio, J. Antimicrobial resistance among bacteria isolated from urinary tract infections in females in Namibia, 2016–2017. Antimicrob. Resist. Infect. Control. 2022, 11, 33. [Google Scholar] [CrossRef]
- Kaba, M.; Manenzhe, R.; Moodley, C.; Zar, H.; Nicol, M. Epidemiology of extended-spectrum beta-lactamase- and carbapenemase-producing bacteria in stool from apparently healthy children, South Africa. Int. J. Infect. Dis. 2016, 45, 96. [Google Scholar] [CrossRef]
- Mannathoko, N.; Mosepele, M.; Gross, R.; Smith, R.M.; Alby, K.; Glaser, L.; Richard-Greenblatt, M.; Dumm, R.; Sharma, A.; Jaskowiak-Barr, A.; et al. Colonization with extended-spectrum cephalosporin-resistant Enterobacterales (ESCrE) and carbapenem-resistant Enterobacterales (CRE) in healthcare and community settings in Botswana: An antibiotic resistance in communities and hospitals (ARCH) study. Int. J. Infect. Dis. 2022, 122, 313–320. [Google Scholar] [CrossRef]
- Wilmore, S.M.S.; Kranzer, K.; Williams, A.; Makamure, B.; Nhidza, A.F.; Mayini, J.; Bandason, T.; Metcalfe, J.; Nicol, M.P.; Balakrishnan, I.; et al. Carriage of extended-spectrum beta-lactamase-producing Enterobacteriaceae in HIV-infected children in Zimbabwe. J. Med. Microbiol. 2017, 66, 609–615. [Google Scholar] [CrossRef]
- Founou, R.C.; Founou, L.L.; Essack, S.Y. Extended spectrum beta-lactamase mediated resistance in carriage and clinical gram-negative ESKAPE bacteria: A comparative study between a district and tertiary hospital in South Africa. Antimicrob. Resist. Infect. Control. 2018, 7, 134. [Google Scholar] [CrossRef] [PubMed]
- Lautenbach, E.; Mosepele, M.; Smith, R.M.; Styczynski, A.; Gross, R.; Cressman, L.; Jaskowiak-Barr, A.; Alby, K.; Glaser, L.; Richard-Greenblatt, M.; et al. Risk Factors for Community Colonization With Extended-Spectrum Cephalosporin-Resistant Enterobacterales (ESCrE) in Botswana: An Antibiotic Resistance in Communities and Hospitals (ARCH) Study. Clin. Infect. Dis. 2023, 77, S89–S96. [Google Scholar] [CrossRef] [PubMed]
- Vasaikar, S.; Obi, L.; Morobe, I.; Bisi-Johnson, M. Molecular Characteristics and Antibiotic Resistance Profiles of Klebsiella Isolates in Mthatha, Eastern Cape Province, South Africa. Int. J. Microbiol. 2017, 2017, 8486742. [Google Scholar] [CrossRef]
- Brooks, K.P. Characterization of Extended-Spectrum-β Lactamases (Esbls) and Other Resistant Genes Encoding Bacteria from a Rural Community Settlement. 2021. Available online: http://repository.biust.ac.bw/handle/123456789/541 (accessed on 1 October 2025).
- Olaru, I. Understanding Gram-Negative Infections and Antimicrobial Resistance in Zimbabwe. Doctoral Dissertation, London School of Hygiene & Tropical Medicine, London, UK, 2023. [Google Scholar]
- Choonara, F.E.; Haldorsen, B.C.; Janice, J.; Mbanga, J.; Ndhlovu, I.; Saulosi, O.; Maida, T.; Lampiao, F.; Simonsen, G.S.; Essack, S.Y.; et al. Molecular Epidemiological Characterisation of ESBL- and Plasmid-Mediated AmpC-Producing Escherichia coli and Klebsiella pneumoniae at Kamuzu Central Hospital, Lilongwe, Malawi. Trop. Med. Infect. Dis. 2022, 7, 245. [Google Scholar] [CrossRef]
- Sumbana, J.J.; Santona, A.; Fiamma, M.; Taviani, E.; Deligios, M.; Zimba, T.; Lucas, G.; Sacarlal, J.; Rubino, S.; Paglietti, B. Extraintestinal Pathogenic Escherichia coli ST405 Isolate Coharboring blaNDM-5 and blaCTXM-15: A New Threat in Mozambique. Microb. Drug Resist. 2021, 27, 1633–1640. [Google Scholar] [CrossRef] [PubMed]
- Mmatli, M.; Leshaba, T.M.S.; Skosana, L.B.; Mbelle, N.M.; Sekyere, J.O. Molecular Screening of Clinical Multidrug-Resistant Gram-Negative Bacteria Shows Endemicity of Carbapenemases, Coexistence of Multiple Carbapenemases, and Rarity of mcr in South Africa. Microb. Drug Resist. 2022, 28, 1028–1036. [Google Scholar] [CrossRef]
- Ramashia, M.; Phofa, T.D.; Nkawane, G.M.; Nogbou, N.-D.; Bolukaoto, J.Y.; Nchabeleng, M.; Musyoki, A.M. Investigation of carbapenem-resistant Enterobacterales isolates at a tertiary laboratory in Pretoria, South Africa. Acta Microbiol. Immunol. Hung 2023, 70, 295–303. [Google Scholar] [CrossRef]
- Sekyere, J.O.; Mmatli, M.; Bosch, A.; Ntsoane, R.V.; Naidoo, H.; Doyisa, S.; Maningi, N.E.; Mbelle, N.M.; Said, M. Molecular epidemiology of multidrug-resistant Klebsiella pneumoniae, Enterobacter cloacae, and Escherichia coli outbreak among neonates in Tembisa hospital, South Africa. Front. Cell. Infect. Microbiol. 2024, 14, 1328123. [Google Scholar] [CrossRef]
- Kieffer, N.; Nordmann, P.; Aires-De-Sousa, M.; Poirel, L. High Prevalence of Carbapenemase-Producing Enterobacteriaceae among Hospitalized Children in Luanda, Angola. Antimicrob. Agents Chemother. 2016, 60, 6189–6192. [Google Scholar] [CrossRef]
- Ismail, H.; Zwane, T.B.C.; Du Toit, E.; da Costa, R.M.A.; Franceschi, F.; Perovic, O. Carbapenem-resistant Enterobacterales among patients with bloodstream infections in South Africa: Consolidated surveillance data, 2015–2021. PLoS ONE 2025, 20, e0324262. [Google Scholar] [CrossRef]
- Poirel, L.; Goutines, J.; Aires-De-Sousa, M.; Nordmann, P. High Rate of Association of 16S rRNA Methylases and Carbapenemases in Enterobacteriaceae Recovered from Hospitalized Children in Angola. Antimicrob. Agents Chemother. 2018, 62, e00021-18. [Google Scholar] [CrossRef] [PubMed]
- Tootla, H.D.; Prentice, E.; Moodley, C.; Marais, G.; Nyakutira, N.; Reddy, K.; Bamford, C.; Niehaus, A.; Whitelaw, A.; Brink, A. Carbapenem-resistant Enterobacterales among hospitalized patients in Cape Town, South Africa: Clinical and microbiological epidemiology. JAC-Antimicrob. Resist. 2024, 6, dlae051. [Google Scholar] [CrossRef]
- Salvador-Oke, K.T.; Pitout, J.D.D.; Peirano, G.; Strydom, K.A.; Kingsburgh, C.; Ehlers, M.M.; Ismail, A.; Takawira, F.T.; Kock, M.M. Molecular epidemiology of carbapenemase-producing Klebsiella pneumoniae in Gauteng South Africa. Sci. Rep. 2024, 14, 27337. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kumwenda, G.P.; Sugawara, Y.; Abe, R.; Akeda, Y.; Kasambara, W.; Chizani, K.; Takeuchi, D.; Sakamoto, N.; Tomono, K.; Hamada, S. First Identification and genomic characterization of multidrug-resistant carbapenemase-producing Enterobacteriaceae clinical isolates in Malawi, Africa. J. Med. Microbiol. 2019, 68, 1707–1715. [Google Scholar] [CrossRef]
- van Duin, D.; Doi, Y. The global epidemiology of carbapenemase-producing Enterobacteriaceae. Virulence 2017, 8, 460–469. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Galera, S.; Bravo-Ferrer, J.M.; Paniagua, M.; Kostyanev, T.; de Kraker, M.E.; Feifel, J.; Sojo-Dorado, J.; Schotsman, J.; Cantón, R.; Daikos, G.L.; et al. Risk factors for infections caused by carbapenem-resistant Enterobacterales: An international matched case-control-control study (EURECA). eClinicalMedicine 2023, 57, 101871. [Google Scholar] [CrossRef]
- Marais, G.; Moodley, C.; Claassen-Weitz, S.; Patel, F.; Prentice, E.; Tootla, H.; Nyakutira, N.; Lennard, K.; Reddy, K.; Bamford, C.; et al. Carbapenem-resistant Klebsiella pneumoniae among hospitalized patients in Cape Town, South Africa: Molecular epidemiology and characterization. JAC-Antimicrob. Resist. 2024, 6, dlae050. [Google Scholar] [CrossRef]
- Manenzhe, R.I.; Zar, H.J.; Nicol, M.P.; Kaba, M. The spread of carbapenemase-producing bacteria in Africa: A systematic review. J. Antimicrob. Chemother. 2014, 70, 23–40. [Google Scholar] [CrossRef] [PubMed]
- Macesic, N.; Hawkey, J.; Vezina, B.; Wisniewski, J.A.; Cottingham, H.; Blakeway, L.V.; Harshegyi, T.; Pragastis, K.; Badoordeen, G.Z.; Dennison, A.; et al. Genomic dissection of endemic carbapenem resistance reveals metallo-beta-lactamase dissemination through clonal, plasmid and integron transfer. Nat. Commun. 2023, 14, 4764. [Google Scholar] [CrossRef]
- Bassetti, M.; Garau, J. Current and future perspectives in the treatment of multidrug-resistant Gram-negative infections. J. Antimicrob. Chemother. 2021, 76, iv23–iv37. [Google Scholar] [CrossRef]
- Matlock, A.; Garcia, J.A.; Moussavi, K.; Long, B.; Liang, S.Y.-T. Advances in novel antibiotics to treat multidrug-resistant gram-negative bacterial infections. Intern. Emerg. Med. 2021, 16, 2231–2241. [Google Scholar] [CrossRef]
- Lagacé-Wiens, P.; Walkty, A.; Karlowsky, J. Ceftazidime–avibactam: An evidence-based review of its pharmacology and potential use in the treatment of Gram-negative bacterial infections. Core Évid. 2014, 9, 13–25. [Google Scholar] [CrossRef]
- Huang, Y.-S.; Zhou, H. Breakthrough Advances in Beta-Lactamase Inhibitors: New Synthesized Compounds and Mechanisms of Action Against Drug-Resistant Bacteria. Pharmaceuticals 2025, 18, 206. [Google Scholar] [CrossRef]
- Gaibani, P.; Giani, T.; Bovo, F.; Lombardo, D.; Amadesi, S.; Lazzarotto, T.; Coppi, M.; Rossolini, G.M.; Ambretti, S. Resistance to Ceftazidime/Avibactam, Meropenem/Vaborbactam and Imipenem/Relebactam in Gram-Negative MDR Bacilli: Molecular Mechanisms and Susceptibility Testing. Antibiotics 2022, 11, 628. [Google Scholar] [CrossRef]
- Zasowski, E.J.; Rybak, J.M.; Rybak, M.J. The β-lactams strike back: Ceftazidime-avibactam. Pharmacother. J. Hum. Pharmacol. Drug Ther. 2015, 35, 755–770. [Google Scholar] [CrossRef] [PubMed]
- Duda-Madej, A.; Viscardi, S.; Topola, E. Meropenem/Vaborbactam: β-Lactam/β-Lactamase Inhibitor Combination, the Future in Eradicating Multidrug Resistance. Antibiotics 2023, 12, 1612. [Google Scholar] [CrossRef]
- Sun, D.; Rubio-Aparicio, D.; Nelson, K.; Dudley, M.N.; Lomovskaya, O. Meropenem-Vaborbactam Resistance Selection, Resistance Prevention, and Molecular Mechanisms in Mutants of KPC-Producing Klebsiella pneumoniae. Antimicrob. Agents Chemother. 2017, 61, e01694-17. [Google Scholar] [CrossRef] [PubMed]
- Yu, W.; Zhang, H.; Xu, Y.; Zhu, Y.; Jia, P.; Kang, Y.; Yang, Q. In vitro activity of ceftolozane/tazobactam against ESBL-producing enterobacterales in China: SMART 2016–2019. J. Glob. Antimicrob. Resist. 2025, 42, 161–166. [Google Scholar] [CrossRef]
- Jousset, A.B.; Bernabeu, S.; Emeraud, C.; Bonnin, R.A.; Lomont, A.; Zahar, J.R.; Merens, A.; Isnard, C.; Soismier, N.; Farfour, E.; et al. Evaluation of ceftolozane-tazobactam susceptibility on a French nationwide collection of Enterobacterales. J. Glob. Antimicrob. Resist. 2023, 32, 78–84. [Google Scholar] [CrossRef]
- Serio, A.W.; Keepers, T.; Krause, K.M. Plazomicin Is Active Against Metallo-β-Lactamase-Producing Enterobacteriaceae. Open Forum Infect. Dis. 2019, 6, ofz123. [Google Scholar] [CrossRef]
- Livermore, D.M.; Mushtaq, S.; Warner, M.; Zhang, J.C.; Maharjan, S.; Doumith, M.; Woodford, N. Activity of aminoglycosides, including ACHN-490, against carbapenem-resistant Enterobacteriaceae isolates. J. Antimicrob. Chemother. 2010, 66, 48–53. [Google Scholar] [CrossRef]
- Solomkin, J.; Evans, D.; Slepavicius, A.; Lee, P.; Marsh, A.; Tsai, L.; Sutcliffe, J.A.; Horn, P. Assessing the Efficacy and Safety of Eravacycline vs Ertapenem in Complicated Intra-abdominal Infections in the Investigating Gram-Negative Infections Treated With Eravacycline (IGNITE 1) Trial. JAMA Surg. 2017, 152, 224–232. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Wei, X.; Jin, Y.; Bai, F.; Cheng, Z.; Chen, S.; Pan, X.; Wu, W. Development of Resistance to Eravacycline by Klebsiella pneumoniae and Collateral Sensitivity-Guided Design of Combination Therapies. Microbiol. Spectr. 2022, 10, e0139022. [Google Scholar] [CrossRef]
- Zhanel, G.G.; Mansour, C.; Mikolayanko, S.; Lawrence, C.K.; Zelenitsky, S.; Ramirez, D.; Schweizer, F.; Bay, D.; Adam, H.; Lagacé-Wiens, P.; et al. Cefepime–taniborbactam: A novel cephalosporin/β-lactamase inhibitor combination. Drugs 2024, 84, 1219–1250. [Google Scholar] [CrossRef]
- Vázquez-Ucha, J.C.; Lasarte-Monterrubio, C.; Guijarro-Sánchez, P.; Oviaño, M.; Álvarez-Fraga, L.; Alonso-García, I.; Arca-Suárez, J.; Bou, G.; Beceiro, A. Assessment of Activity and Resistance Mechanisms to Cefepime in Combination with the Novel β-Lactamase Inhibitors Zidebactam, Taniborbactam, and Enmetazobactam against a Multicenter Collection of Carbapenemase-Producing Enterobacterales. Antimicrob. Agents Chemother. 2022, 66, e0167621. [Google Scholar] [CrossRef] [PubMed]
- Fratoni, A.J.; Berry, A.V.; Liu, X.; Chen, X.; Wu, Y.; Nicolau, D.P.; Abdelraouf, K. Imipenem/funobactam (formerly XNW4107) in vivo pharmacodynamics against serine carbapenemase-producing Gram-negative bacteria: A novel modelling approach for time-dependent killing. J. Antimicrob. Chemother. 2023, 78, 2343–2353. [Google Scholar] [CrossRef] [PubMed]
- Zhanel, G.G.; Golden, A.R.; Zelenitsky, S.; Wiebe, K.; Lawrence, C.K.; Adam, H.J.; Idowu, T.; Domalaon, R.; Schweizer, F.; Zhanel, M.A.; et al. Cefiderocol: A Siderophore Cephalosporin with Activity Against Carbapenem-Resistant and Multidrug-Resistant Gram-Negative Bacilli. Drugs 2019, 79, 271–289. [Google Scholar] [CrossRef]
- Salleh, M.Z. Addressing antimicrobial resistance: Structural insights into cefiderocol’s mode of action and emerging resistance mechanisms. J. Infect. Public Health 2025, 18, 102871. [Google Scholar] [CrossRef]
- Campanella, T.A.; Gallagher, J.C. A Clinical Review and Critical Evaluation of Imipenem-Relebactam: Evidence to Date. Infect. Drug Resist. 2020, 13, 4297–4308. [Google Scholar] [CrossRef]
- Schmidt-Malan, S.M.; Mishra, A.J.; Mushtaq, A.; Brinkman, C.L.; Patel, R. In Vitro Activity of Imipenem-Relebactam and Ceftolozane-Tazobactam against Resistant Gram-Negative Bacilli. Antimicrob. Agents Chemother. 2018, 62, e02563-18. [Google Scholar] [CrossRef]
- Al Musawa, M.; Bleick, C.R.; Herbin, S.R.; Caniff, K.E.; Van Helden, S.R.; Rybak, M.J. Aztreonam–avibactam: The dynamic duo against multidrug-resistant gram-negative pathogens. Pharmacother. J. Hum. Pharmacol. Drug Ther. 2024, 44, 927–938. [Google Scholar] [CrossRef]
- Wu, S.; Ma, K.; Feng, Y.; Zong, Z. Resistance to aztreonam-avibactam due to a mutation of SHV-12 in Enterobacter. Ann. Clin. Microbiol. Antimicrob. 2023, 22, 49. [Google Scholar] [CrossRef]
- Sader, H.S.; Mendes, R.E.; Duncan, L.R.; Carvalhaes, C.G.; Castanheria, M. Antimicrobial activity of cefepime/zidebactam (WCK 5222), a β-lactam/β-lactam enhancer combination, against clinical isolates of Gram-negative bacteria collected worldwide (2018–19). J. Antimicrob. Chemother. 2022, 77, 2642–2649. [Google Scholar] [CrossRef] [PubMed]
- Mushtaq, S.; Garello, P.; Vickers, A.; Woodford, N.; Livermore, D.M. Activity of cefepime/zidebactam (WCK 5222) against ‘problem’ antibiotic-resistant Gram-negative bacteria sent to a national reference laboratory. J. Antimicrob. Chemother. 2021, 76, 1511–1522. [Google Scholar] [CrossRef] [PubMed]
- Brink, A.J.; Coetzee, J.; Richards, G.A.; Feldman, C.; Lowman, W.; Tootla, H.D.; Miller, M.G.; Niehaus, A.J.; Wasserman, S.; Perovic, O.; et al. Best practices: Appropriate use of the new β-lactam/β-lactamase inhibitor combinations, ceftazidime-avibactam and ceftolozane-tazobactam in South Africa. S. Afr. J. Infect. Dis. 2022, 37, 10. [Google Scholar] [CrossRef] [PubMed]
- Ouchar Mahamat, O.; Kempf, M.; Lounnas, M.; Tidjani, A.; Hide, M.; Benavides, J.A.; Carrière, C.; Bañuls, A.L.; Jean-Pierre, H.; Ouedraogo, A.S.; et al. Epidemiology and prevalence of extended-spectrum β-lactamase- and carbapenemase-producing Enterobacteriaceae in humans, animals and the environment in West and Central Africa. Int. J. Antimicrob. Agents 2021, 57, 106203. [Google Scholar] [CrossRef] [PubMed]
- Duffy, N.; Karlsson, M.; Reses, H.E.; Campbell, D.; Daniels, J.; Stanton, R.A.; Janelle, S.J.; Schutz, K.; Bamberg, W.; Rebolledo, P.A.; et al. Epidemiology of extended-spectrum β-lactamase-producing Enterobacterales in five US sites participating in the Emerging Infections Program, 2017. Infect. Control. Hosp. Epidemiol. 2022, 43, 1586–1594. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ejaz, H.; Qamar, M.U.; Farhana, A.; Younas, S.; Batool, A.; Lone, D.; Atif, M.; Alruways, M.W.; Alruwaili, M.; Hamad, I.; et al. The Rising Tide of Antibiotic Resistance: A Study on Extended-Spectrum Beta-Lactamase and Carbapenem-Resistant Escherichia coli and Klebsiella pneumoniae. J. Clin. Lab. Anal. 2024, 38, e25081. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Alraey, Y.; Assiry, M.M.; Ahmad, I.; Alqahtani, A.; Basheer, N.; AlAsiri, M.A.M.; Alshehri, S.A.M.; Alhamhhum, S.M.S.; Alhefdi, S.M.; Khan, M.S.; et al. Antimicrobial resistance and beta-lactamase gene distribution among clinical isolates: A two-year cohort study. Sci. Rep. 2025, 15, 23951. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- UK Health Security Agency. Available online: https://www.gov.uk/government/publications/carbapenemase-producing-gram-negative-bacteria-laboratory-surveillance/carbapenemase-producing-gram-negative-organisms-in-england-since-october-2020-quarterly-update-q4-2024#geographical-differences-in-carbapenemase-family-distribution (accessed on 29 November 2025).
- Wang, Z.; Lu, Q.; Mao, X.; Li, L.; Dou, J.; He, Q.; Shao, H.; Luo, Q. Prevalence of Extended-Spectrum β-Lactamase-Resistant Genes in Escherichia coli Isolates from Central China during 2016–2019. Animals 2022, 12, 3191. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
| Country | Leading Pathogen–Drug Combination (Enterobacterales) and Attributed Mortality |
|---|---|
| Angola | Aminoglycosides-resistant K. pneumoniae (206, UI 131–281) Fluoroquinolones-resistant K. pneumoniae (151, UI 86–217) |
| Botswana | TMP/SMX-resistant K. pneumoniae (11, UI 5–17) 3GC-resistant E. coli (9, UI 3–15) TMP/SMX-resistant E. coli (8, UI 5–12) |
| Eswatini | 3GC-resistant K. pneumoniae (10, UI 5–14) Aminoglycosides-resistant K. pneumoniae (7, UI 4–10) Fluoroquinolone-resistant K. pneumoniae (7, UI 4–10) Fluoroquinolone-resistant E. coli (5, UI 2–8) |
| Namibia | TMP/SMX-resistant K. pneumoniae (16, UI 8–25) βL/βLI-resistant K. pneumoniae (10, UI 2–19) βL/βLI-resistant E. coli (10, UI 3–17) TMP/SMX-resistant E. coli (9, UI 5–12) |
| Lesotho | Fluoroquinolone-resistant K. pneumoniae (21, UI 12–30) TMP/SMX-resistant K. pneumoniae (17, UI 9–26) |
| South Africa | Carbapenem-resistant K. pneumoniae (447, UI 351–544) Fluoroquinolone-resistant K. pneumoniae (301, UI 208–394) Aminoglycoside-resistant K. pneumoniae (285, UI 201–370) TMP/SMX-resistant K. pneumoniae (253, UI 129–376) |
| Malawi | 3GC-resistant-K. pneumoniae (200, UI 113–286) Carbapenem-resistant K. pneumoniae (145, UI 101–190) Aminoglycoside-resistant K. pneumoniae (133, UI 90–176) Fluoroquinolone-resistant K. pneumoniae (114, UI 70–158) TMP/SMX-resistant K. pneumoniae (110, UI 55–165) 3GC-resistant E. coli (99, UI 35–162) |
| Mozambique | 3GC-resistant K. pneumoniae (358, UI 197–519) Fluoroquinolone-resistant K. pneumoniae (260, UI 157–362) TMP/SMX-resistant K. pneumoniae (219, UI 106–332) TMP/SMX-resistant E. coli (185, UI 124–247) Aminoglycoside-resistant K. pneumoniae (148, UI 92–203) |
| Zambia | 3GC-resistant K. pneumoniae (219, UI 115–324) Fluoroquinolone-resistant K. pneumoniae (132, UI 79–184) 3GC-resistant E. coli (120, UI 53–187) TMP/SMX-resistant K. pneumoniae (114, 55–173) TMP/SMX-resistant E. coli (102, 67–137) Fluoroquinolone-resistant E. coli (98, 49–146) |
| Zimbabwe | 3GC-resistant K. pneumoniae (182, UI 106–258) TMP/SMX-resistant K. pneumoniae (158, UI 80–237) Carbapenem-resistant K. pneumoniae (96, UI 69–123) Aminoglycoside-resistant K. pneumoniae (84, 54–114) |
| Ambler Classification | Functional Classification (Substrates in Parentheses) | Enzymes |
|---|---|---|
| Class A | Group 2 serine β-lactamases 2a (penicillin) 2b (penicillin) 2be (extended-spectrum cephalosporins, monobactams) 2br (penicillin) 2ber (extended-spectrum cephalosporins, monobactam) 2c (carbenicillin) 2ce (carbenicillin, cefepime) 2e (extended-spectrum cephalosporins) 2f (carbapenems) | TEM-1 TEM-2 SHV-1 TEM-3 SHV-2 CTX-M-15 TEM-30 SHV-10 TEM-50 KPC-2 IMI-1 SME-1 |
| Class B | Group 3 metallo-βlactamases and substrates 3a (carbapenems) 3b (carbapenems) | IMP-1 VIM-1 IND-1 SFH-1 |
| Class C | Group 1 cephalosporinases and substrates 1 (cephalosporins) 1e (cephalosporins) | AmpC CMY-2 CMY-37 |
| Class D | Group 2 serine β-lactamases and substrates 2d (cloxacillin) 2de (extended-spectrum cephalosporins) 2df (carbapenems) | OXA-1 OXA-10 OXA-11 OXA-15 OXA-23 OXA-48 |
| Novel Antimicrobial Agent | Spectrum of Activity | Resistance |
|---|---|---|
| Ceftazidime/avibactam | Class A, class C, class D carbapenemase-producing Enterobacterales [67]. | Cases of resistance conferred by blaKPC mutations have been reported in Enterobacterales [67]. |
| Meropenem/vaborbactam | Activity against class A and class C β-lactamases [68]. | Resistance conferred by increased blaKPC copy number and mutations in the ompK36 porin in K. pneumoniae [69]. |
| Ceftolozane/tazobactam | Active against ESBL producers and class A lactamases [70]. | Resistance observed in KPC- and MBL-producing Enterobacterales [71]. |
| Plazomicin | Broad spectrum activity against β-lactamase- and carbapenemase-producing Enterobacterales [72]. | Possible resistance described in NDM-1-producing CRE strains, conferred by co-expression of 16S ribosomal plazomicin-inactivating methyltransferases in Enterobacterales [73]. |
| Eravacycline | Broad spectrum of activity including in CRE and bacteria resistant to β-lactam/β-lactamase inhibitors [74]. Activity against class A, class B, and class D β-lactamases [75] | Resistance mediated by mutations in the Lon protease, upregulation of AcrAB-TolC, and porins OmpA and OmpU in Enterobacterales [76]. |
| Cefepime/taniborbactam | Inhibits classes A, C, and D and MBLs, including NDM and VIM, but not IMP [77]. | Resistance in Enterobacterales arises due to the concurrent presence of multiple resistance mechanisms. For example, production of IMP, alterations in PBP3, permeability defects, and upregulation of efflux pumps [78]. |
| Imipenem/funobactam | Active against serine β-lactamases (class A, class C, and class D) [79]. | No resistance mechanisms reported to date. |
| Cefiderocol | Highly active against all classes of carbapenemase. Class A, class B, class C, and class D β-lactamases [80]. | Resistance in carbapenem-resistant Enterobacterales is mediated by different mechanisms. For example, in carbapenem-resistant K. pneumoniae, is mediated by CirA deficiency and the presence of metallo- or serine β-lactamases while in E. coli, it is due to mutations in PBP3 [81]. |
| Imipenem/relebactam | Primarily restores clinical activity of imipenem against imipenem-resistant isolates Enterobacteriaceae. Inhibits Class A and B β-lactamases [82] | Does not restore susceptibility in Enterobacterales-producing OXA-48 carbapenemases, VIM, IMP, and NDM β-lactamases [83]. |
| Aztreonam/avibactam | Active against Enterobacterales that produce MBLs [84] | Resistance may be conferred by unique mutations in β-lactamases, for example, Arg244gly substitution in blaSHV12 [85]. |
| Cefepime/zidebactam | Active against β-lactamase producing Enterobacterales including isolates that produce MBLs and Class D β-lactamases [86] | Possible resistance in ST14, OXA-232 producing K. pneumoniae [87]. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2026 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license.
Share and Cite
Ntshonga, P.; Paganotti, G.M.; Gaibani, P. Epidemiology of ESBL-Producing, Carbapenem-Resistant, and Carbapenemase-Producing Enterobacterales in Southern Africa. Antibiotics 2026, 15, 69. https://doi.org/10.3390/antibiotics15010069
Ntshonga P, Paganotti GM, Gaibani P. Epidemiology of ESBL-Producing, Carbapenem-Resistant, and Carbapenemase-Producing Enterobacterales in Southern Africa. Antibiotics. 2026; 15(1):69. https://doi.org/10.3390/antibiotics15010069
Chicago/Turabian StyleNtshonga, Pearl, Giacomo Maria Paganotti, and Paolo Gaibani. 2026. "Epidemiology of ESBL-Producing, Carbapenem-Resistant, and Carbapenemase-Producing Enterobacterales in Southern Africa" Antibiotics 15, no. 1: 69. https://doi.org/10.3390/antibiotics15010069
APA StyleNtshonga, P., Paganotti, G. M., & Gaibani, P. (2026). Epidemiology of ESBL-Producing, Carbapenem-Resistant, and Carbapenemase-Producing Enterobacterales in Southern Africa. Antibiotics, 15(1), 69. https://doi.org/10.3390/antibiotics15010069