Penicillin-Binding Proteins in Streptococcus agalactiae and Their Association with Reduced Penicillin Susceptibility: An Overview
Abstract
1. Introduction
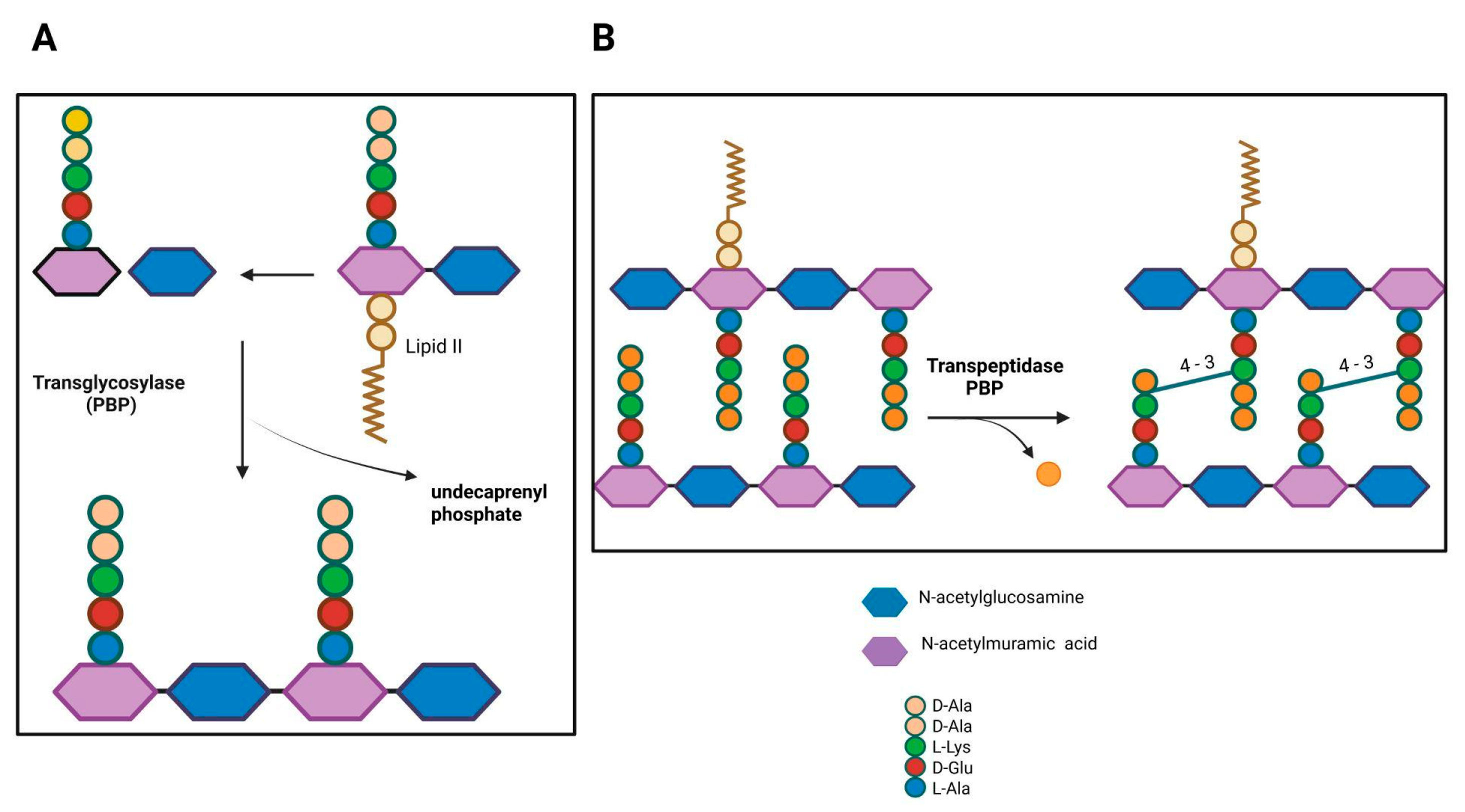
2. Penicillin-Binding Proteins (PBPs)
3. Mutations in Penicillin-Binding Proteins (PBPs)
| Author, Year [Reference] | Penicillin MIC | Mutations Identified | Country (Period) | |||
|---|---|---|---|---|---|---|
| pbp2x | pbp2a | pbp2b | pbp1a | |||
| Chu, 2007 [41] | 0.25 (n = 1) | ND | ND | ND | ND | Hong Kong (2005) |
| Kimura, 2008 [3] | 0.25 (n = 8) 0.5 (n = 5) 1.0 (n = 1) | Q557E, V405A | ND | ND | ND | Japan (1995–2005) |
| Nagano, 2008 [35] | 0.25 (n = 1) 0.5 (n = 6) 1.0 (n = 2) | S353F, A374V, F395L, V405A, A400V, R433H, H438Y, A514V, Q557E, G648A, T77I | E63K, T175I, L285F, Y236C | V80A, Y262N, G539E, T567I, G613R | L45P, N163K, Y470F, G527V, N723S | Japan (2003–2004) |
| Gaudreau, 2010 [42] | 0.25 (n = 1) | I377V, G627V, N575D | S453N, N682D | V625I, P278L | T526A | Canada (2004–2007) |
| Longtin, 2011 [43] | 0.5 (n = 1) | G371D | E636G, S644F, S676F | ND | T546P | Canada (2008) |
| Nagano, 2012 [44] | 0.25 (n = 10) | V405A, F395L, R433H, H438Y, G648A, I377V, V510I | NT | T567I | ND | Japan (2007) |
| Nagano, 2014 [45] | 0.5 (n = 2) | A400V, V405A | ND | Q557E, T567I | ND | Japan (2011–2012) |
| Seki, 2015 [46] | 0.25 (n = 19) 0.5 (n = 20) 1.0 (n = 6) | V405A, Q557E | NT | NT | NT | Japan (2012–2013) |
| Morozumi, 2016 [47] | 0.125 (n = 5) 0.25 (n = 3) 0.5 (n = 1) | K372E, I377V, G398A, V405A, Q412L, G429D, H438Y, D478A, E513Q, Q557E | NT | NT | NT | Japan (2010–2013) |
| Metcalf, 2017 [36] | 0.25 (n = 6) | I377V, G406D, Q557E | NT | NR | NR | USA (2015) |
| Sigaúque, 2018 [48] | 0.12 (n = 7) | G398A | ND | ND | ND | Mozambique (2001–2015) |
| Yi, 2019 [49] | 0.5 (n = 2) | G398A, V405A, Q557E | NT | NT | NT | South Korea (NI) |
| Van der Linden, 2020 [50] | 0.5 (n = 1) 1.0 (n = 1) | I377V, F395L, V405A, H438Y, V510I, Q557E | ND | V80A | A521V, del719–722, N723S, V726A, T526I | Germany (NI) |
| Li, 2020 * [51] | 2.0 (n = 1) | I377V, T720S | E63K | V80A, S147A, S160A | T701P V706A | Hong Kong (2014–2017) |
| McGee, 2021 [52] | 0.25 (n = 6) | I377V, G406D G398A, G627V V510I, I510V Q557E, L534S G627V | NT | NT | NT | USA (2015–2017) |
| Nishiyama, 2022 [53] | 0.25 (n = 5) 0.5 (n = 3) | G398A, V405A, Q557E, N575D, S295G, Q557E | NT | ND | G719A, R629H | Japan (2017–2018) |
| Koide, 2022 [54] | 0.25 (n = 7) 1.0 (n = 1) | V405A, G329V, G398A, G429D, I377V, F395L, R433H, H438Y, V510I, G648A, V405A, Q412L, Q557E | Defective | T567I | T587I, F524V, G719N | Japan (2008–2016) |
| Chu, 2007 [41] | 0.25 (n = 1) | ND | ND | ND | ND | Hong Kong (2005) |
| Ikebe, 2023 [55] | 0.25 (n = 6) 0.5 (n = 1) | I377V, G398A, Q412L, H438Y, V405A, Q557E, F395L, R433H, T473M, V510I, G329V, K372E, G429D | ND | T567I | T537P, F541V, N635-, G636-, A621V, A547V | Japan (2014–2021) |
| Ntozini, 2025 [56] | 0.25 (n = 1) | New types that are not yet assigned a number | ND | ND | ND | South Africa (2019–2020) |
| McGuire, 2025 [57] | 1.0 (n = 1) | I342V, V475I P160S, Y331H P140L, N540D | A27T, N741A V744A, V541I | V80A | K63E | England (2016) |
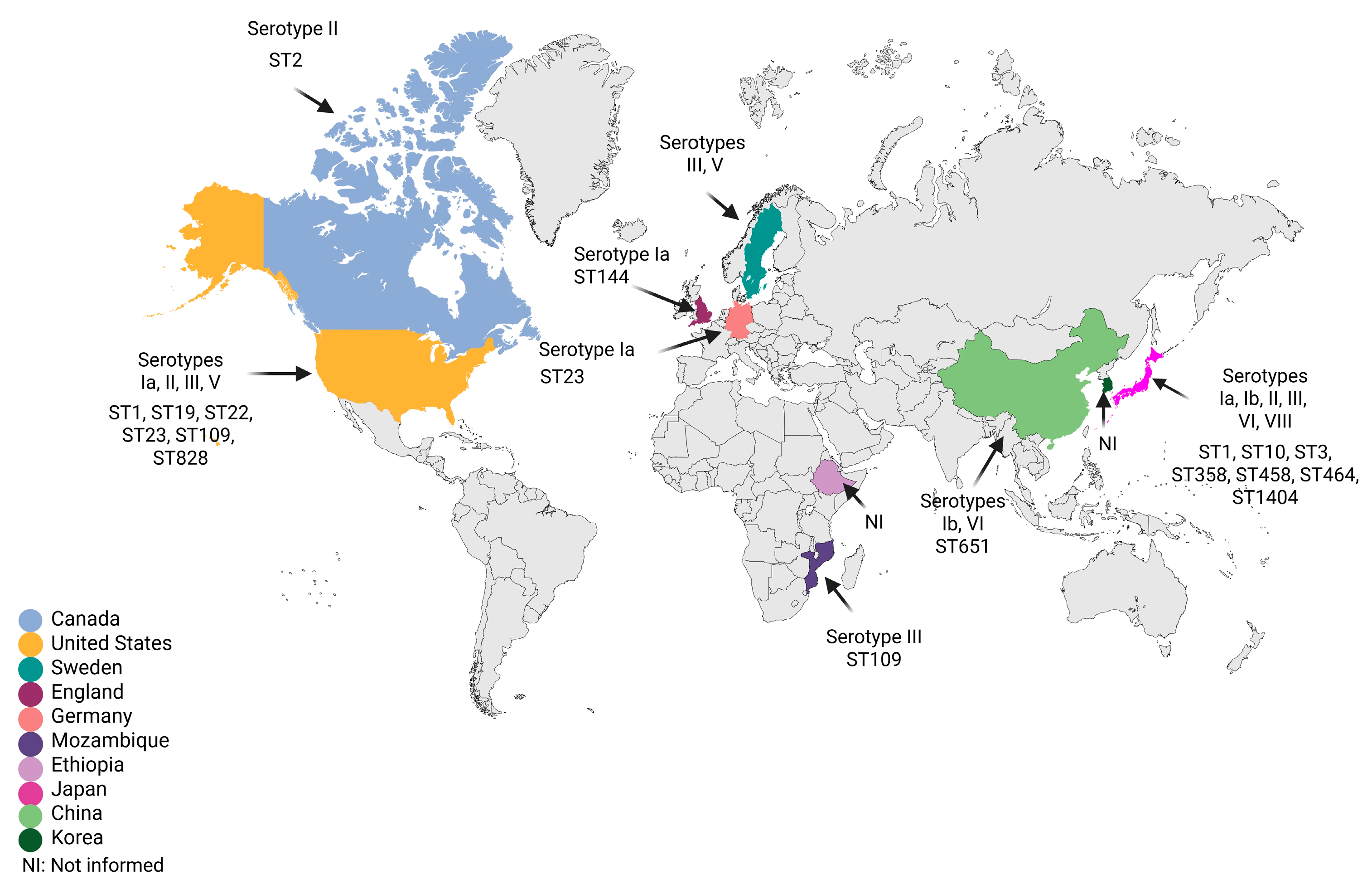
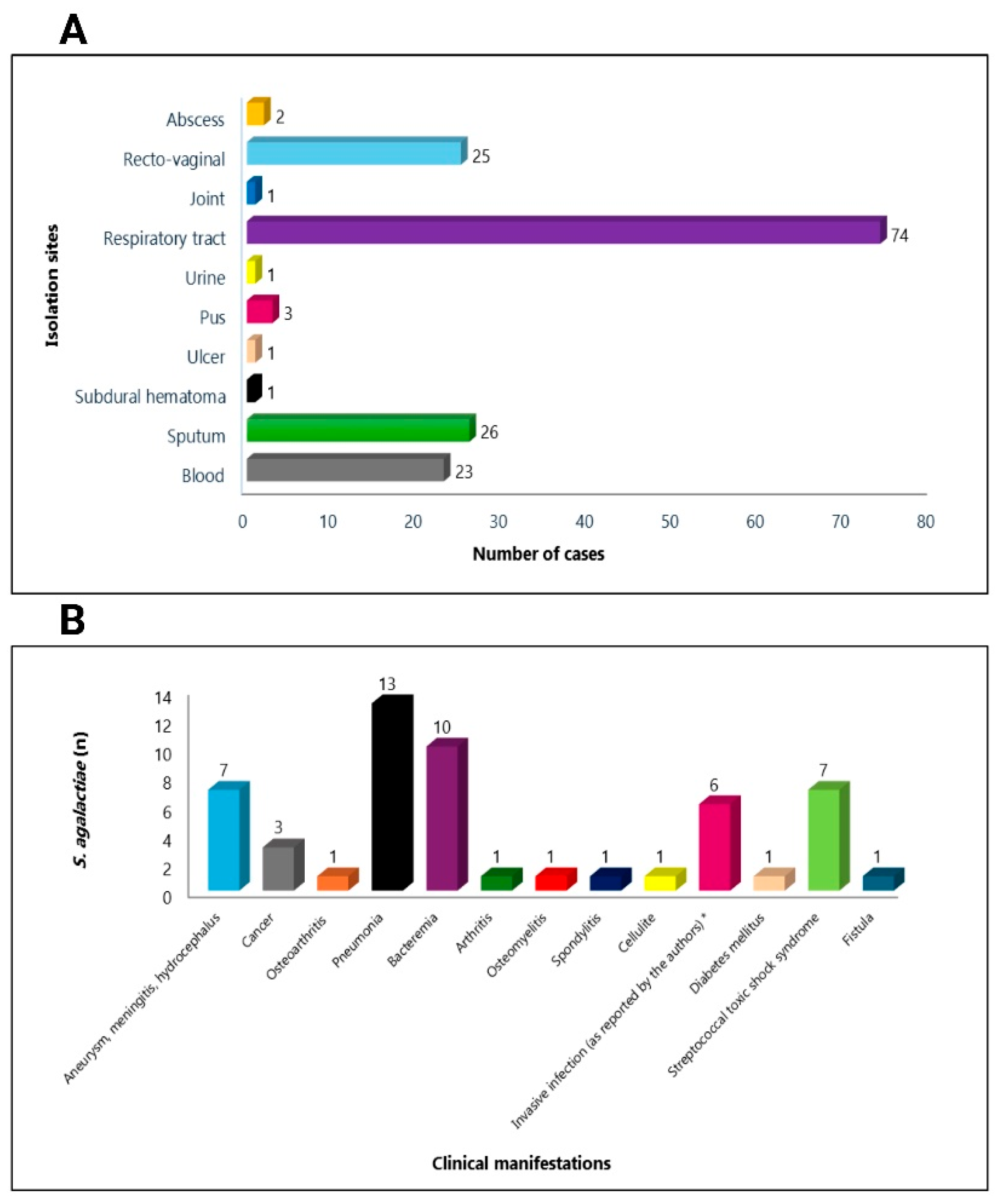
4. S. agalactiae with Reduced β-Lactam Susceptibility
5. Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| S. agalactiae | Streptococcus agalactiae |
| GBS | Group B Streptococcus |
| IAP | Intrapartum antibiotic prophylaxis |
| EUCAST | European Committee on Antimicrobial Susceptibility Testing |
| CLSI | Clinical and Laboratory Standards Institute |
| MIC | Minimum inhibitory concentration |
| PBPs | Penicillin-binding proteins |
| WHO | World Health Organization |
| HMM | High molecular weight |
| LMM | Low molecular weight |
| RPS | Reduced penicillin susceptibility |
| D-Ala | D-alanine |
| L-Lys | L-lysine |
| D-Glu | D-glutamate |
| L-Ala | L-alanine |
| PBPL | Bacterial Priority Pathogens List |
| MDR | Multidrug resistant |
| MLST | Multilocus sequence typing |
| STs | Sequence type |
| MRSA | Methicillin-resistant Staphylococcus aureus |
| KAHA | α-ketoacid-hydroxylamine |
References
- Raabe, V.N.; Shane, A.L. Group B Streptococcus (Streptococcus agalactiae). Microbiol. Spectr. 2019, 7, 1–13. [Google Scholar] [CrossRef]
- Mei, J.Y.; Silverman, N.S. Group B Streptococcus in Pregnancy. Obstet. Gynecol. Clin. N. Am. 2023, 50, 375–387. [Google Scholar] [CrossRef]
- Kimura, K.; Suzuki, S.; Wachino, J.; Kurokawa, H.; Yamane, K.; Shibata, N.; Nagano, N.; Kato, H.; Shibayama, K.; Arakawa, Y. First Molecular Characterization of Group B Streptococci with Reduced Penicillin Susceptibility. Antimicrob. Agents Chemother. 2008, 52, 2890–2897. [Google Scholar] [CrossRef]
- European Committee on Antimicrobial Susceptibility Testing Breakpoint Tables for Interpretation of MICs and Zone Diameters Version 15.0, Valid from 2025-01-01. Available online: https://aurosan.de/images/mediathek/servicematerial/EUCAST_RefStaemme_Sollwerte.pdf (accessed on 27 November 2025).
- Clinical and Laboratory Standards Institute (CLSI). Performance Standards for Antimicrobial Susceptibility Testing, 35th ed.; CLSI Supplement M100; CLSI: Wayne, PA, USA, 2025. [Google Scholar]
- Laible, G.; Spratt, B.G.; Hakenbeck, R. Interspecies Recombinational Events during the Evolution of Altered PBP 2x Genes in Penicillin-resistant Clinical Isolates of Streptococcus pneumoniae. Mol. Microbiol. 1991, 5, 1993–2002. [Google Scholar] [CrossRef]
- Calvez, P.; Breukink, E.; Roper, D.I.; Dib, M.; Contreras-Martel, C.; Zapun, A. Substitutions in PBP2b from β-Lactam-Resistant Streptococcus pneumoniae Have Different Effects on Enzymatic Activity and Drug Reactivity. J. Biol. Chem. 2017, 292, 2854–2865. [Google Scholar] [CrossRef] [PubMed]
- Goffin, C.; Ghuysen, J.-M. Multimodular Penicillin-Binding Proteins: An Enigmatic Family of Orthologs and Paralogs. Microbiol. Mol. Biol. Rev. 1998, 62, 1079–1093. [Google Scholar] [CrossRef]
- Sauvage, E.; Terrak, M. Glycosyltransferases and Transpeptidases/Penicillin-Binding Proteins: Valuable Targets for New Antibacterials. Antibiotics 2016, 5, 12. [Google Scholar] [CrossRef] [PubMed]
- Pazos, M.; Peters, K. Peptidoglycan; Elsevier: Amsterdam, The Netherlands, 2019; pp. 127–168. [Google Scholar]
- Miyachiro, M.M.; Contreras-Martel, C.; Dessen, A. Penicillin-Binding Proteins (PBPs) and Bacterial Cell Wall Elongation Complexes; Springer: Berlin/Heidelberg, Germany, 2019; pp. 273–289. [Google Scholar]
- Shalaby, M.-A.W.; Dokla, E.M.E.; Serya, R.A.T.; Abouzid, K.A.M. Penicillin Binding Protein 2a: An Overview and a Medicinal Chemistry Perspective. Eur. J. Med. Chem. 2020, 199, 112312. [Google Scholar] [CrossRef] [PubMed]
- Martin, C.; Sibold, C.; Hakenbeck, R. Relatedness of Penicillin-Binding Protein 1a Genes from Different Clones of Penicillin-Resistant Streptococcus pneumoniae Isolated in South Africa and Spain. EMBO J. 1992, 11, 3831–3836. [Google Scholar] [CrossRef]
- Smith, A.M.; Klugman, K.P. Alterations in Penicillin-Binding Protein 2B from Penicillin-Resistant Wild-Type Strains of Streptococcus pneumoniae. Antimicrob. Agents Chemother. 1995, 39, 859–867. [Google Scholar] [CrossRef]
- Dowson, C.G.; Hutchison, A.; Spratt, B.G. Extensive Re-modelling of the Transpeptidase Domain of Penicillin-binding Protein 2B of a Penicillin-resistant South African Isolate of Streptococcus pneumoniae. Mol. Microbiol. 1989, 3, 95–102. [Google Scholar] [CrossRef] [PubMed]
- Persson, J.; Beall, B.; Linse, S.; Lindahl, G. Extreme Sequence Divergence but Conserved Ligand-Binding Specificity in Streptococcus pyogenes M Protein. PLoS Pathog. 2006, 2, e47. [Google Scholar] [CrossRef]
- Zhu, L.; Yerramilli, P.; Pruitt, L.; Mishra, A.; Olsen, R.J.; Beres, S.B.; Waller, A.S.; Musser, J.M. Functional Insights into the High-Molecular-Mass Penicillin-Binding Proteins of Streptococcus agalactiae Revealed by Gene Deletion and Transposon Mutagenesis Analysis. J. Bacteriol. 2021, 203, 10–1128. [Google Scholar] [CrossRef]
- Chi, F.; Nolte, O.; Bergmann, C.; Ip, M.; Hakenbeck, R. Crossing the Barrier: Evolution and Spread of a Major Class of Mosaic Pbp2x in Streptococcus pneumoniae, S. mitis and S. oralis. Int. J. Med. Microbiol. 2007, 297, 503–512. [Google Scholar] [CrossRef] [PubMed]
- David, B.; Duchêne, M.-C.; Haustenne, G.L.; Pérez-Núñez, D.; Chapot-Chartier, M.-P.; De Bolle, X.; Guédon, E.; Hols, P.; Hallet, B. PBP2b Plays a Key Role in Both Peripheral Growth and Septum Positioning in Lactococcus lactis. PLoS ONE 2018, 13, e0198014. [Google Scholar] [CrossRef] [PubMed]
- Tsui, H.T.; Boersma, M.J.; Vella, S.A.; Kocaoglu, O.; Kuru, E.; Peceny, J.K.; Carlson, E.E.; VanNieuwenhze, M.S.; Brun, Y.V.; Shaw, S.L.; et al. Pbp2x Localizes Separately from Pbp2band Other Peptidoglycan Synthesis Proteins during Later Stages of Cell Division of Sreptococcus pneumoniae D39. Mol. Microbiol. 2014, 94, 21–40. [Google Scholar] [CrossRef]
- Musser, J.M.; Beres, S.B.; Zhu, L.; Olsen, R.J.; Vuopio, J.; Hyyryläinen, H.-L.; Gröndahl-Yli-Hannuksela, K.; Kristinsson, K.G.; Darenberg, J.; Henriques-Normark, B.; et al. Reduced In Vitro Susceptibility of Streptococcus pyogenes to β-Lactam Antibiotics Associated with Mutations in the Pbp2x Gene Is Geographically Widespread. J. Clin. Microbiol. 2020, 58, e01993-19. [Google Scholar] [CrossRef]
- Stamsås, G.A.; Restelli, M.; Ducret, A.; Freton, C.; Garcia, P.S.; Håvarstein, L.S.; Straume, D.; Grangeasse, C.; Kjos, M. A CozE Homolog Contributes to Cell Size Homeostasis of Streptococcus pneumoniae. mBio 2020, 11, e02461-20. [Google Scholar] [CrossRef]
- Hamilton, A.; Popham, D.L.; Carl, D.J.; Lauth, X.; Nizet, V.; Jones, A.L. Penicillin-Binding Protein 1a Promotes Resistance of Group B Streptococcus to Antimicrobial Peptides. Infect. Immun. 2006, 74, 6179–6187. [Google Scholar] [CrossRef]
- Jones, A.L.; Mertz, R.H.; Carl, D.J.; Rubens, C.E. A Streptococcal Penicillin-Binding Protein Is Critical for Resisting Innate Airway Defenses in the Neonatal Lung. J. Immunol. 2007, 179, 3196–3202. [Google Scholar] [CrossRef]
- Jones, A.L.; Knoll, K.M.; Rubens, C.E. Identification of Streptococcus Agalactiae Virulence Genes in the Neonatal Rat Sepsis Model Using Signature-tagged Mutagenesis. Mol. Microbiol. 2000, 37, 1444–1455. [Google Scholar] [CrossRef]
- Boes, A.; Kerff, F.; Herman, R.; Touze, T.; Breukink, E.; Terrak, M. The Bacterial Cell Division Protein Fragment EFtsN Binds to and Activates the Major Peptidoglycan Synthase PBP1b. J. Biol. Chem. 2020, 295, 18256–18265. [Google Scholar] [CrossRef]
- Lim, D.; Strynadka, N.C.J. Structural Basis for the β Lactam Resistance of PBP2a from Methicillin-Resistant Staphylococcus aureus. Nat. Struct. Biol. 2002, 9, 870–876. [Google Scholar] [CrossRef]
- Adedeji-Olulana, A.F.; Wacnik, K.; Lafage, L.; Pasquina-Lemonche, L.; Tinajero-Trejo, M.; Sutton, J.A.F.; Bilyk, B.; Irving, S.E.; Portman Ross, C.J.; Meacock, O.J.; et al. Two Codependent Routes Lead to High-Level MRSA. Science 2024, 386, 573–580. [Google Scholar] [CrossRef]
- Sjodt, M.; Brock, K.; Dobihal, G.; Rohs, P.D.A.; Green, A.G.; Hopf, T.A.; Meeske, A.J.; Srisuknimit, V.; Kahne, D.; Walker, S.; et al. Structure of the Peptidoglycan Polymerase RodA Resolved by Evolutionary Coupling Analysis. Nature 2018, 556, 118–121. [Google Scholar] [CrossRef]
- Rohs, P.D.A.; Buss, J.; Sim, S.I.; Squyres, G.R.; Srisuknimit, V.; Smith, M.; Cho, H.; Sjodt, M.; Kruse, A.C.; Garner, E.C.; et al. A Central Role for PBP2 in the Activation of Peptidoglycan Polymerization by the Bacterial Cell Elongation Machinery. PLoS Genet. 2018, 14, e1007726. [Google Scholar] [CrossRef] [PubMed]
- Nishimoto, A.T.; Dao, T.H.; Jia, Q.; Ortiz-Marquez, J.C.; Echlin, H.; Vogel, P.; van Opijnen, T.; Rosch, J.W. Interspecies Recombination, Not de Novo Mutation, Maintains Virulence after β-Lactam Resistance Acquisition in Streptococcus pneumoniae. Cell Rep. 2022, 41, 111835. [Google Scholar] [CrossRef] [PubMed]
- Zapun, A.; Contreras-Martel, C.; Vernet, T. Penicillin-Binding Proteins and β-Lactam Resistance. FEMS Microbiol. Rev. 2008, 32, 361–385. [Google Scholar] [CrossRef] [PubMed]
- Sauvage, E.; Kerff, F.; Terrak, M.; Ayala, J.A.; Charlier, P. The Penicillin-Binding Proteins: Structure and Role in Peptidoglycan Biosynthesis. FEMS Microbiol. Rev. 2008, 32, 234–258. [Google Scholar] [CrossRef]
- Nagano, N.; Kimura, K.; Nagano, Y.; Yakumaru, H.; Arakawa, Y. Molecular Characterization of Group B Streptococci with Reduced Penicillin Susceptibility Recurrently Isolated from a Sacral Decubitus Ulcer. J. Antimicrob. Chemother. 2009, 64, 1326–1328. [Google Scholar] [CrossRef]
- Nagano, N.; Nagano, Y.; Kimura, K.; Tamai, K.; Yanagisawa, H.; Arakawa, Y. Genetic Heterogeneity in Pbp Genes among Clinically Isolated Group B Streptococci with Reduced Penicillin Susceptibility. Antimicrob. Agents Chemother. 2008, 52, 4258–4267. [Google Scholar] [CrossRef]
- Metcalf, B.J.; Chochua, S.; Gertz, R.E.; Hawkins, P.A.; Ricaldi, J.; Li, Z.; Walker, H.; Tran, T.; Rivers, J.; Mathis, S.; et al. Short-Read Whole Genome Sequencing for Determination of Antimicrobial Resistance Mechanisms and Capsular Serotypes of Current Invasive Streptococcus agalactiae Recovered in the USA. Clin. Microbiol. Infect. 2017, 23, 574.e7–574.e14. [Google Scholar] [CrossRef] [PubMed]
- Kimura, K.; Nagano, N.; Nagano, Y.; Suzuki, S.; Wachino, J.; Shibayama, K.; Arakawa, Y. High Frequency of Fluoroquinolone- and Macrolide-Resistant Streptococci among Clinically Isolated Group B Streptococci with Reduced Penicillin Susceptibility. J. Antimicrob. Chemother. 2013, 68, 539–542. [Google Scholar] [CrossRef] [PubMed]
- Chesnel, L.; Pernot, L.; Lemaire, D.; Champelovier, D.; Croizé, J.; Dideberg, O.; Vernet, T.; Zapun, A. The Structural Modifications Induced by the M339F Substitution in PBP2x from Streptococcus pneumoniae Further Decreases the Susceptibility to β-Lactams of Resistant Strains. J. Biol. Chem. 2003, 278, 44448–44456. [Google Scholar] [CrossRef]
- Mouz, N.; Gordon, E.; Di Guilmi, A.-M.; Petit, I.; Pétillot, Y.; Dupont, Y.; Hakenbeck, R.; Vernet, T.; Dideberg, O. Identification of a Structural Determinant for Resistance to β-Lactam Antibiotics in Gram-Positive Bacteria. Proc. Natl. Acad. Sci. USA 1998, 95, 13403–13406. [Google Scholar] [CrossRef]
- Kimura, K.; Nagano, N.; Arakawa, Y. Classification of Group B Streptococci with Reduced β-Lactam Susceptibility (GBS-RBS) Based on the Amino Acid Substitutions in PBPs. J. Antimicrob. Chemother. 2015, 70, 1601–1603. [Google Scholar] [CrossRef]
- Chu, Y.W.; Tse, C.; Tsang, G.K.-L.; So, D.K.-S.; Fung, J.T.-L.; Lo, J.Y.-C. Invasive Group B Streptococcus Isolates Showing Reduced Susceptibility to Penicillin in Hong Kong. J. Antimicrob. Chemother. 2007, 60, 1407–1409. [Google Scholar] [CrossRef]
- Gaudreau, C.; Lecours, R.; Ismail, J.; Gagnon, S.; Jette, L.; Roger, M. Prosthetic Hip Joint Infection with a Streptococcus agalactiae Isolate Not Susceptible to Penicillin G and Ceftriaxone. J. Antimicrob. Chemother. 2010, 65, 594–595. [Google Scholar] [CrossRef]
- Longtin, J.; Vermeiren, C.; Shahinas, D.; Tamber, G.S.; McGeer, A.; Low, D.E.; Katz, K.; Pillai, D.R. Novel Mutations in a Patient Isolate of Streptococcus agalactiae with Reduced Penicillin Susceptibility Emerging after Long-Term Oral Suppressive Therapy. Antimicrob. Agents Chemother. 2011, 55, 2983–2985. [Google Scholar] [CrossRef]
- Nagano, N.; Nagano, Y.; Toyama, M.; Kimura, K.; Tamura, T.; Shibayama, K.; Arakawa, Y. Nosocomial Spread of Multidrug-Resistant Group B Streptococci with Reduced Penicillin Susceptibility Belonging to Clonal Complex 1. J. Antimicrob. Chemother. 2012, 67, 849–856. [Google Scholar] [CrossRef] [PubMed]
- Nagano, N.; Nagano, Y.; Toyama, M.; Kimura, K.; Shibayama, K.; Arakawa, Y. Penicillin-Susceptible Group B Streptococcal Clinical Isolates with Reduced Cephalosporin Susceptibility. J. Clin. Microbiol. 2014, 52, 3406–3410. [Google Scholar] [CrossRef] [PubMed]
- Seki, T.; Kimura, K.; Reid, M.E.; Miyazaki, A.; Banno, H.; Jin, W.; Wachino, J.; Yamada, K.; Arakawa, Y. High Isolation Rate of MDR Group B Streptococci with Reduced Penicillin Susceptibility in Japan. J. Antimicrob. Chemother. 2015, 70, 2725–2728. [Google Scholar] [CrossRef] [PubMed]
- Morozumi, M.; Wajima, T.; Takata, M.; Iwata, S.; Ubukata, K. Molecular Characteristics of Group B Streptococci Isolated from Adults with Invasive Infections in Japan. J. Clin. Microbiol. 2016, 54, 2695–2700. [Google Scholar] [CrossRef]
- Sigaúque, B.; Kobayashi, M.; Vubil, D.; Nhacolo, A.; Chaúque, A.; Moaine, B.; Massora, S.; Mandomando, I.; Nhampossa, T.; Bassat, Q.; et al. Invasive Bacterial Disease Trends and Characterization of Group B Streptococcal Isolates among Young Infants in Southern Mozambique, 2001–2015. PLoS ONE 2018, 13, e0191193. [Google Scholar] [CrossRef]
- Yi, A.; Kim, C.-K.; Kimura, K.; Arakawa, Y.; Hur, M.; Yun, Y.-M.; Moon, H.-W. First Case in Korea of Group B Streptococcus With Reduced Penicillin Susceptibility Harboring Amino Acid Substitutions in Penicillin-Binding Protein 2X. Ann. Lab. Med. 2019, 39, 414–416. [Google Scholar] [CrossRef]
- van der Linden, M.; Mamede, R.; Levina, N.; Helwig, P.; Vila-Cerqueira, P.; Carriço, J.A.; Melo-Cristino, J.; Ramirez, M.; Martins, E.R. Heterogeneity of Penicillin-Non-Susceptible Group B Streptococci Isolated from a Single Patient in Germany. J. Antimicrob. Chemother. 2020, 75, 296–299. [Google Scholar] [CrossRef]
- Li, C.; Sapugahawatte, D.; Yang, Y.; Wong, K.; Lo, N.; Ip, M. Multidrug-Resistant Streptococcus agalactiae Strains Found in Human and Fish with High Penicillin and Cefotaxime Non-Susceptibilities. Microorganisms 2020, 8, 1055. [Google Scholar] [CrossRef]
- McGee, L.; Chochua, S.; Li, Z.; Mathis, S.; Rivers, J.; Metcalf, B.; Ryan, A.; Alden, N.; Farley, M.M.; Harrison, L.H.; et al. Multistate, Population-Based Distributions of Candidate Vaccine Targets, Clonal Complexes, and Resistance Features of Invasive Group B Streptococci Within the United States, 2015–2017. Clin. Infect. Dis. 2021, 72, 1004–1013. [Google Scholar] [CrossRef] [PubMed]
- Nishiyama, N.; Kinjo, T.; Uechi, K.; Parrott, G.; Nakamatsu, M.; Tateyama, M.; Fujita, J. Clinical and Bacterial Features of Group B Streptococci with Reduced Penicillin Susceptibility from Respiratory Specimens: A Case–Control Study. Eur. J. Clin. Microbiol. Infect. Dis. 2022, 41, 1–8. [Google Scholar] [CrossRef]
- Koide, S.; Nagano, Y.; Takizawa, S.; Sakaguchi, K.; Soga, E.; Hayashi, W.; Tanabe, M.; Denda, T.; Kimura, K.; Arakawa, Y.; et al. Genomic Traits Associated with Virulence and Antimicrobial Resistance of Invasive Group B Streptococcus Isolates with Reduced Penicillin Susceptibility from Elderly Adults. Microbiol. Spectr. 2022, 10, e00568-22. [Google Scholar] [CrossRef]
- Ikebe, T.; Okuno, R.; Uchitani, Y.; Takano, M.; Yamaguchi, T.; Otsuka, H.; Kazawa, Y.; Fujita, S.; Kobayashi, A.; Date, Y.; et al. Serotype Distribution and Antimicrobial Resistance of Streptococcus agalactiae Isolates in Nonpregnant Adults with Streptococcal Toxic Shock Syndrome in Japan in 2014 to 2021. Microbiol. Spectr. 2023, 11, e04987-22. [Google Scholar] [CrossRef]
- Ntozini, B.; Walaza, S.; Metcalf, B.; Hazelhurst, S.; de Gouveia, L.; Meiring, S.; Mogale, D.; Mtshali, S.; Ismail, A.; Ndlangisa, K.; et al. Molecular Epidemiology of Invasive Group B Streptococcus in South Africa, 2019–2020. J. Infect. Dis. 2025, 231, e697–e707. [Google Scholar] [CrossRef]
- McGuire, E.; Ready, D.; Ellaby, N.; Potterill, I.; Pike, R.; Hopkins, K.L.; Guy, R.L.; Lamagni, T.; Mack, D.; Scobie, A.; et al. A Case of Penicillin-Resistant Group B Streptococcus Isolated from a Patient in the UK. J. Antimicrob. Chemother. 2025, 80, 399–404. [Google Scholar] [CrossRef]
- World Health Organization. World Health Organization WHO Bacterial Priority List, 2024: Bacterial Pathogens of Public Health Importance to Guide Research, Development and Strategies to Prevent and Control Antimicrobial Resistance; World Health Organization: Geneva, Switzerland, 2024.
- Sati, H.; Carrara, E.; Savoldi, A.; Hansen, P.; Garlasco, J.; Campagnaro, E.; Boccia, S.; Castillo-Polo, J.A.; Magrini, E.; Garcia-Vello, P.; et al. The WHO Bacterial Priority Pathogens List 2024: A Prioritisation Study to Guide Research, Development, and Public Health Strategies against Antimicrobial Resistance. Lancet Infect. Dis. 2025, 25, 1033–1043. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, J. Group B Streptococcus: Virulence Factors and Pathogenic Mechanism. Microorganisms 2022, 10, 2483. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, M.; McGee, L.; Chochua, S.; Apostol, M.; Alden, N.B.; Farley, M.M.; Harrison, L.H.; Lynfield, R.; Vagnone, P.S.; Smelser, C.; et al. Low but Increasing Prevalence of Reduced Beta-Lactam Susceptibility Among Invasive Group B Streptococcal Isolates, US Population-Based Surveillance, 1998–2018. Open Forum. Infect. Dis. 2021, 8, ofaa634. [Google Scholar] [CrossRef] [PubMed]
- Alemayehu, G.; Geteneh, A.; Dessale, M.; Ayalew, E.; Demeke, G.; Reta, A.; Kiros, M. High Prevalence of Penicillin-Resistant Group B Streptococcus among Pregnant Women in Northwest Ethiopia. Sci. Rep. 2025, 15, 30047. [Google Scholar] [CrossRef]
- Shin, S.; Whang, D.H.; Um, T.-H.; Cho, C.R.; Chang, J. Increase in Penicillin Non-Susceptibility in Group B Streptococci Alongside Rising Isolation Rates—Based on 24 Years of Clinical Data from a Single University Hospital. Antibiotics 2025, 14, 928. [Google Scholar] [CrossRef]
- Park, J.; So, M.-K.; Kim, Y.-H.; Chung, H.-S. Characterization of Penicillin-Non-Susceptible, Multidrug-Resistant Streptococcus agalactiae Using Whole-Genome Sequencing. Curr. Microbiol. 2025, 82, 344. [Google Scholar] [CrossRef]
- Persson, E.; Berg, S.; Bergseng, H.; Bergh, K.; Valsö-lyng, R.; Trollfors, B. Antimicrobial Susceptibility of Invasive Group B Streptococcal Isolates from South-West Sweden 1988–2001. Scand. J. Infect. Dis. 2008, 40, 308–313. [Google Scholar] [CrossRef] [PubMed]
- Burcham, L.R.; Spencer, B.L.; Keeler, L.R.; Runft, D.L.; Patras, K.A.; Neely, M.N.; Doran, K.S. Determinants of Group B streptococcal virulence potential amongst vaginal clinical isolates from pregnant women. PLoS ONE 2019, 14, e0226699. [Google Scholar] [CrossRef]
- Gao, K.; Deng, Q.; Huang, L.; Chang, C.-Y.; Zhong, H.; Xie, Y.; Guan, X.; Liu, H. Diagnostic Performance of Various Methodologies for Group B Streptococcus Screening in Pregnant Woman in China. Front. Cell Infect. Microbiol. 2021, 11, 651968. [Google Scholar] [CrossRef]
- Pimentel, B.A.D.S.; Lannes-Costa, P.S.; Viana, A.S.; Santos, G.D.S.; Leobons, M.B.G.P.; Ferreira-Carvalho, B.T.; Nagao, P.E. Molecular Characterization, Antimicrobial Resistance and Invasion of Epithelial Cells by Streptococcus agalactiae Strains Isolated from Colonized Pregnant Women and Newborns in Rio de Janeiro, Brazil. J. Appl. Microbiol. 2024, 135, lxae200. [Google Scholar] [CrossRef]
- Ghia, C.; Rambhad, G. Disease Burden Due to Group B Streptococcus in the Indian Population and the Need for a Vaccine—A Narrative Review. Ther. Adv. Infect. Dis. 2021, 8, 20499361211045253. [Google Scholar] [CrossRef] [PubMed]
- Contreras-Martel, C.; Amoroso, A.; Woon, E.C.Y.; Zervosen, A.; Inglis, S.; Martins, A.; Verlaine, O.; Rydzik, A.M.; Job, V.; Luxen, A.; et al. Structure-Guided Design of Cell Wall Biosynthesis Inhibitors That Overcome β-Lactam Resistance in Staphylococcus aureus (MRSA). ACS Chem. Biol. 2011, 6, 943–951. [Google Scholar] [CrossRef]
- Sun, D.; Tsivkovski, R.; Pogliano, J.; Tsunemoto, H.; Nelson, K.; Rubio-Aparicio, D.; Lomovskaya, O. Intrinsic Antibacterial Activity of Xeruborbactam In Vitro: Assessing Spectrum and Mode of Action. Antimicrob. Agents Chemother. 2022, 66, e00879-22. [Google Scholar] [CrossRef] [PubMed]
- O’Daniel, P.I.; Peng, Z.; Pi, H.; Testero, S.A.; Ding, D.; Spink, E.; Leemans, E.; Boudreau, M.A.; Yamaguchi, T.; Schroeder, V.A.; et al. Discovery of a New Class of Non-β-Lactam Inhibitors of Penicillin-Binding Proteins with Gram-Positive Antibacterial Activity. J. Am. Chem. Soc. 2014, 136, 3664–3672. [Google Scholar] [CrossRef]
- Stepek, I.A.; Cao, T.; Koetemann, A.; Shimura, S.; Wollscheid, B.; Bode, J.W. Antibiotic Discovery with Synthetic Fermentation: Library Assembly, Phenotypic Screening, and Mechanism of Action of β-Peptides Targeting Penicillin-Binding Proteins. ACS Chem. Biol. 2019, 14, 1030–1040. [Google Scholar] [CrossRef]
- Chandrakala, B.; Shandil, R.K.; Mehra, U.; Ravishankar, S.; Kaur, P.; Usha, V.; Joe, B.; deSousa, S.M. High-Throughput Screen for Inhibitors of Transglycosylase and/or Transpeptidase Activities of Escherichia coli Penicillin Binding Protein 1b. Antimicrob. Agents Chemother. 2004, 48, 30–40. [Google Scholar] [CrossRef] [PubMed]
- Wan, H.; Ben Othman, R.; Le Corre, L.; Poinsot, M.; Oliver, M.; Amoroso, A.; Joris, B.; Touzé, T.; Auger, R.; Calvet-Vitale, S.; et al. New MraYAA Inhibitors with an Aminoribosyl Uridine Structure and an Oxadiazole. Antibiotics 2022, 11, 1189. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2026 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license.
Share and Cite
Nagao Ferreira, L.; Pimentel Hecht, B.A.; dos Santos, L.S.; Nagao, P.E. Penicillin-Binding Proteins in Streptococcus agalactiae and Their Association with Reduced Penicillin Susceptibility: An Overview. Antibiotics 2026, 15, 31. https://doi.org/10.3390/antibiotics15010031
Nagao Ferreira L, Pimentel Hecht BA, dos Santos LS, Nagao PE. Penicillin-Binding Proteins in Streptococcus agalactiae and Their Association with Reduced Penicillin Susceptibility: An Overview. Antibiotics. 2026; 15(1):31. https://doi.org/10.3390/antibiotics15010031
Chicago/Turabian StyleNagao Ferreira, Leonardo, Bruna Alves Pimentel Hecht, Louisy Sanches dos Santos, and Prescilla Emy Nagao. 2026. "Penicillin-Binding Proteins in Streptococcus agalactiae and Their Association with Reduced Penicillin Susceptibility: An Overview" Antibiotics 15, no. 1: 31. https://doi.org/10.3390/antibiotics15010031
APA StyleNagao Ferreira, L., Pimentel Hecht, B. A., dos Santos, L. S., & Nagao, P. E. (2026). Penicillin-Binding Proteins in Streptococcus agalactiae and Their Association with Reduced Penicillin Susceptibility: An Overview. Antibiotics, 15(1), 31. https://doi.org/10.3390/antibiotics15010031





