Retargeting Gram-Positive-Only Adarotene-Derived Antibacterials to Broad-Spectrum Antibiotics
Abstract
1. Introduction
2. Results and Discussion
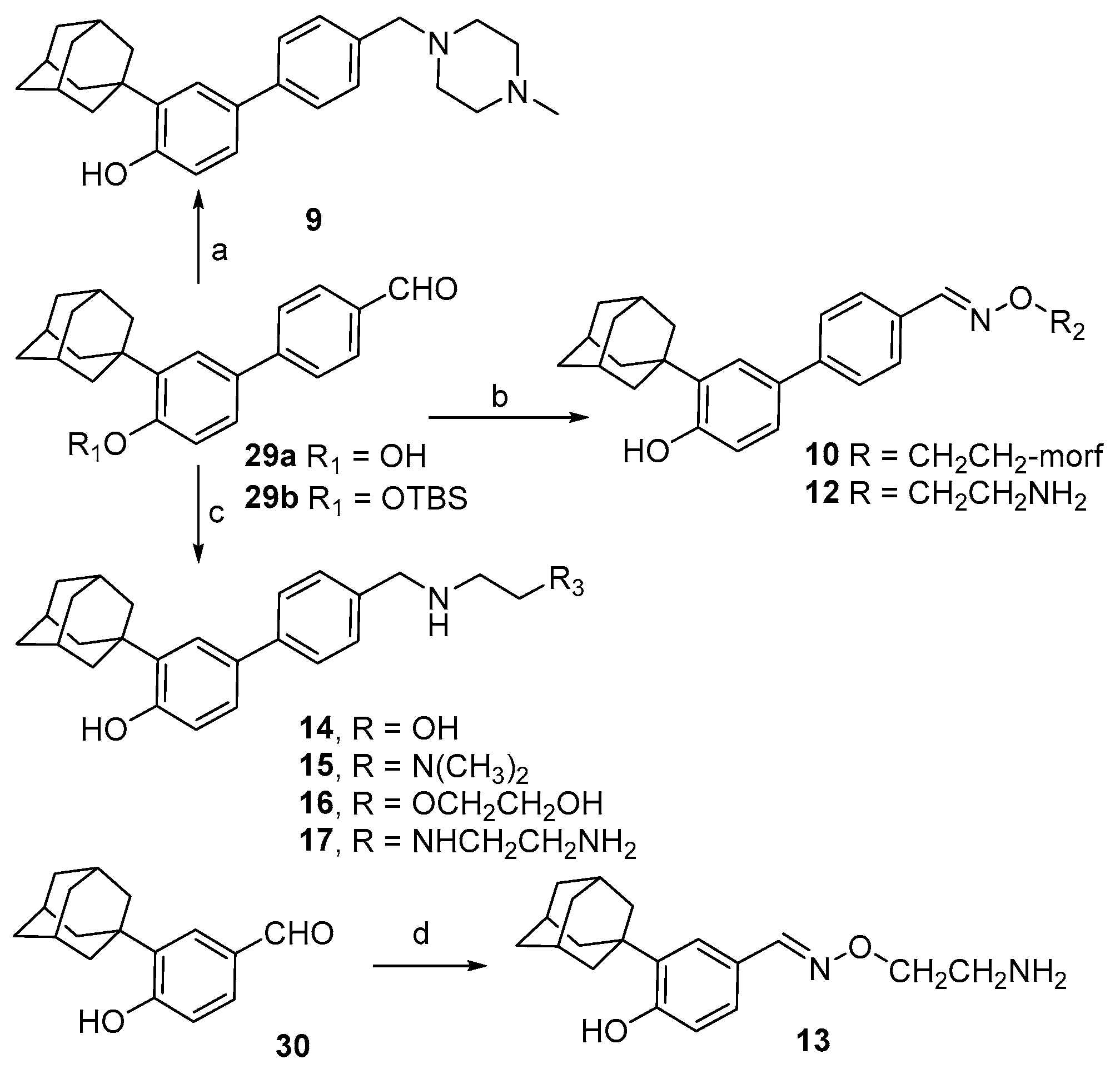
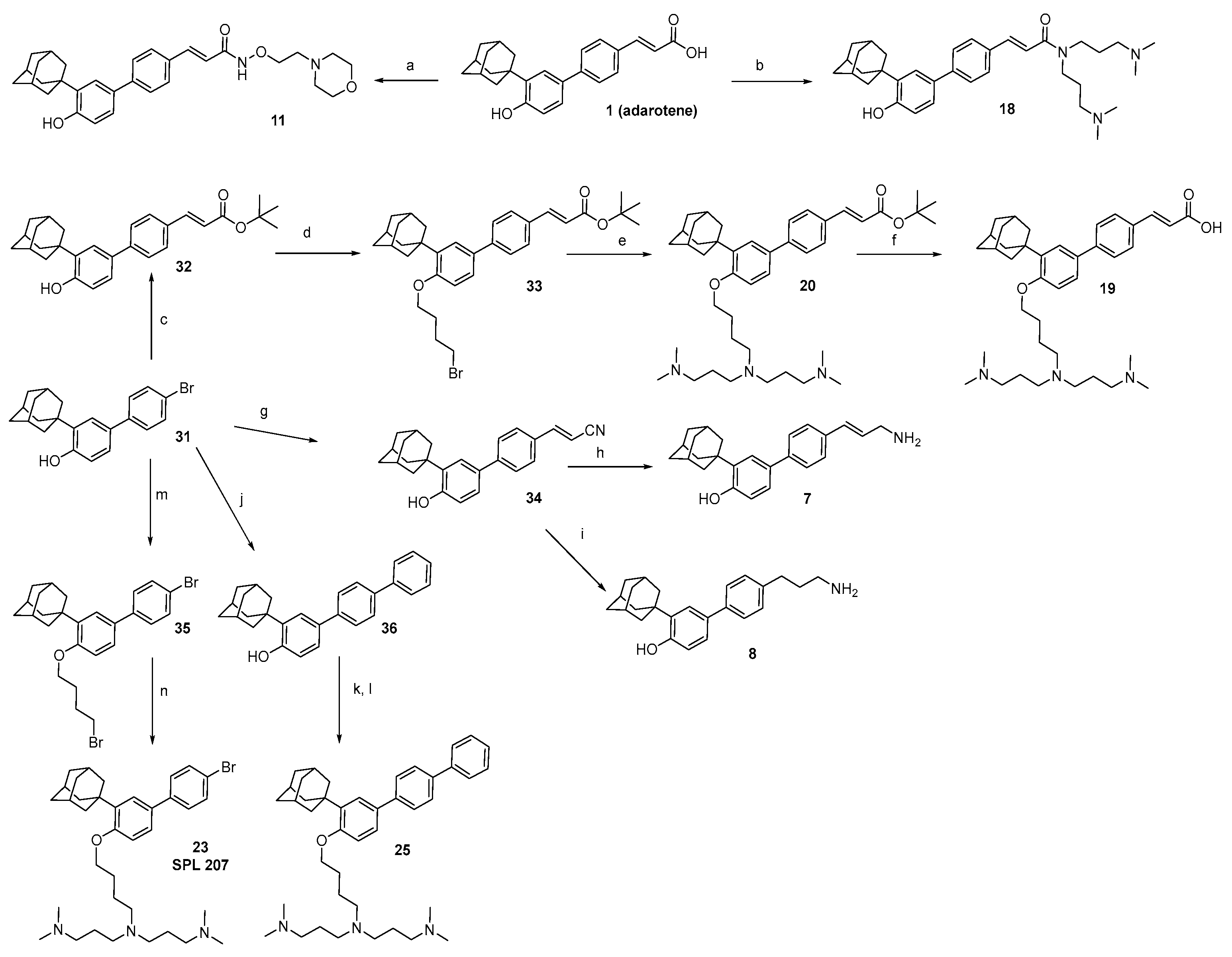
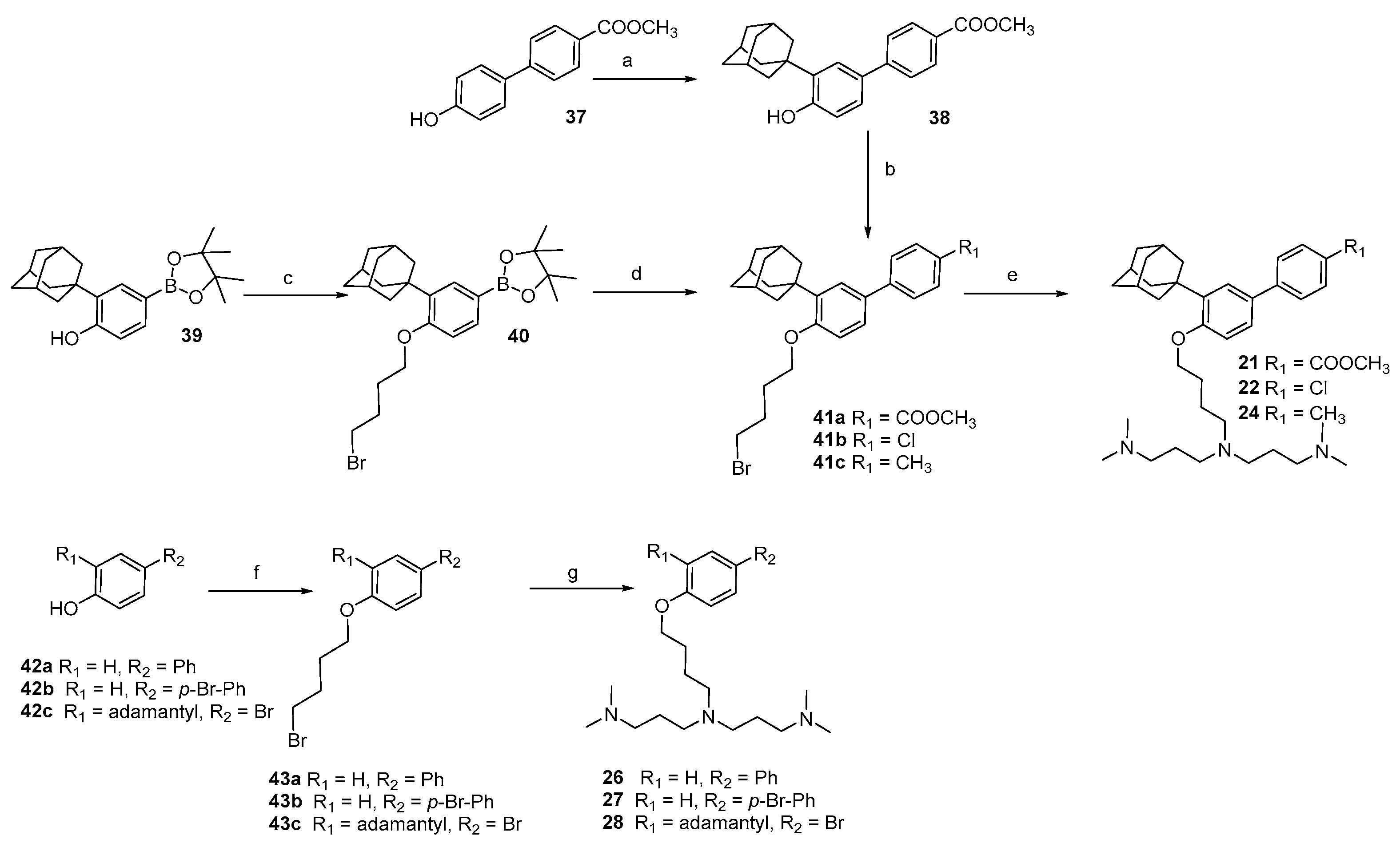
2.1. Chemistry
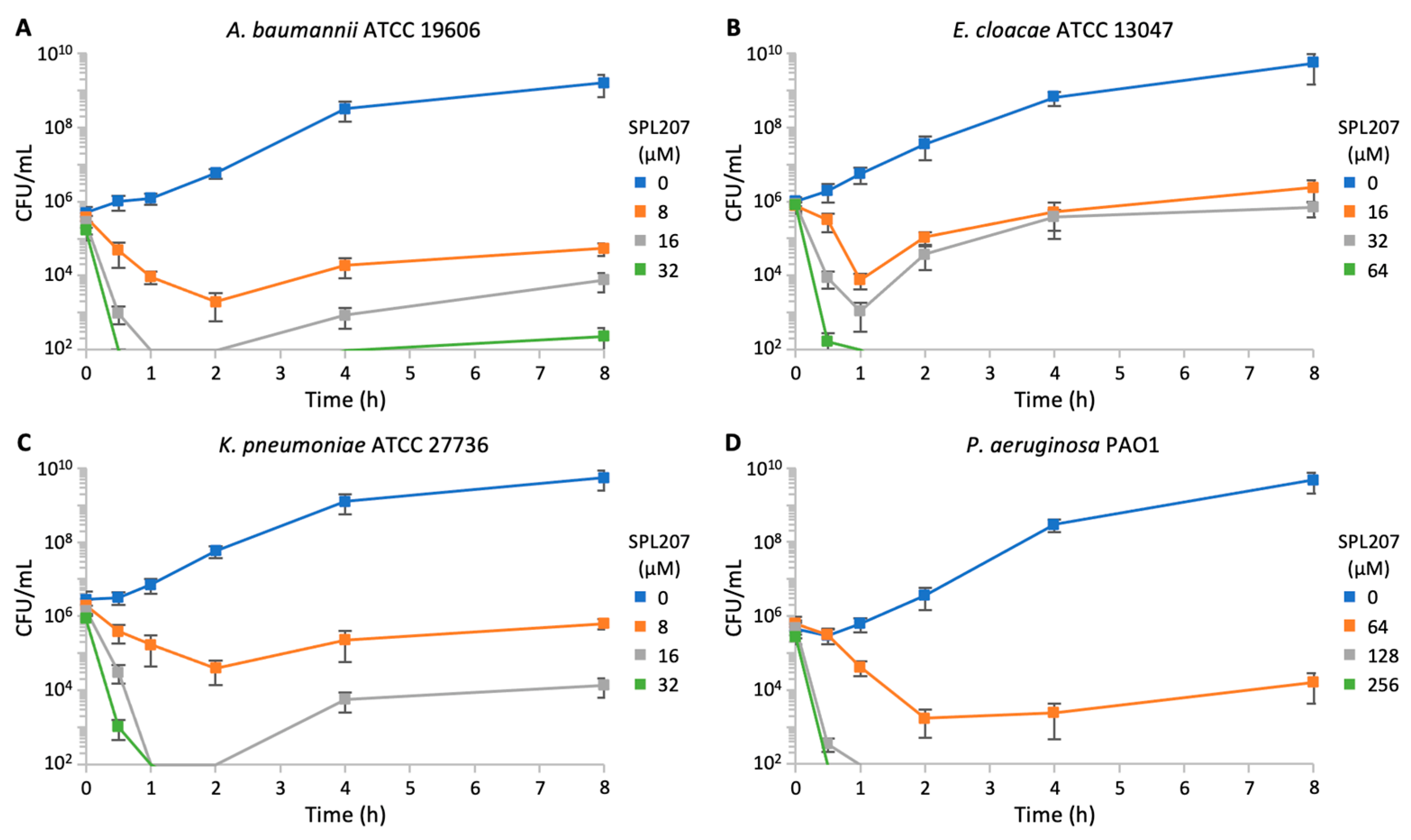
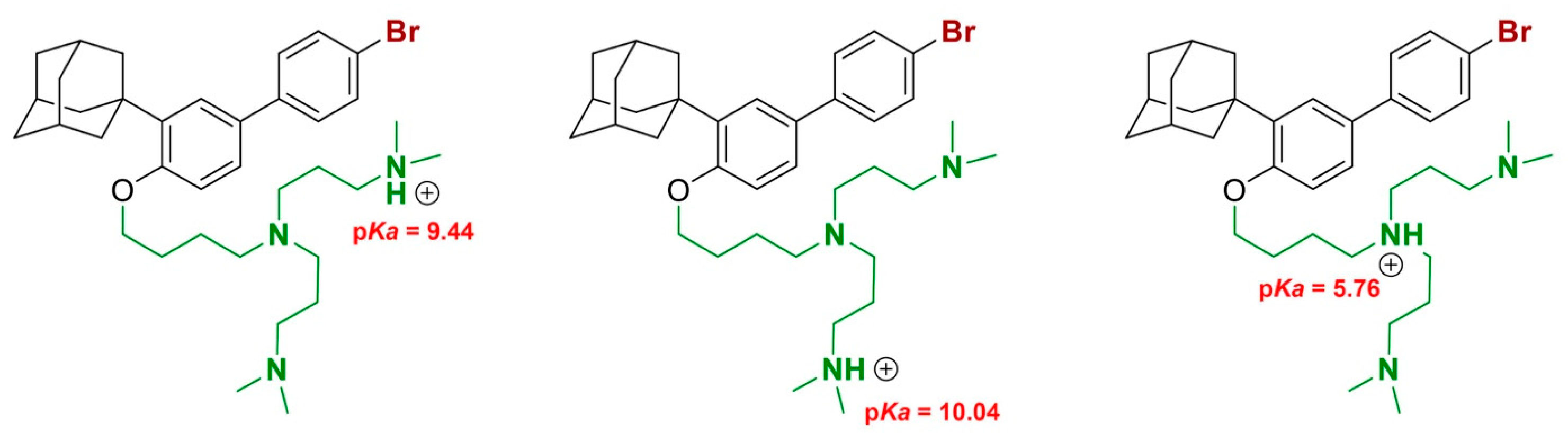
2.2. Biology and Computational Analysis
2.2.1. Investigation About P. aeruginosa Resistance to SPL207
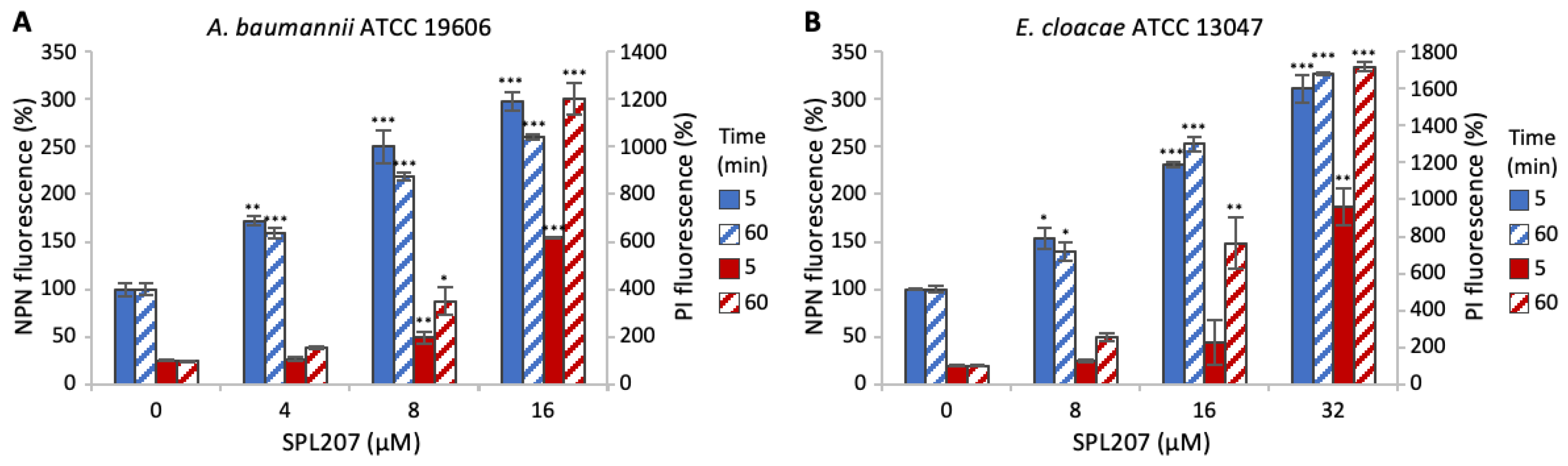
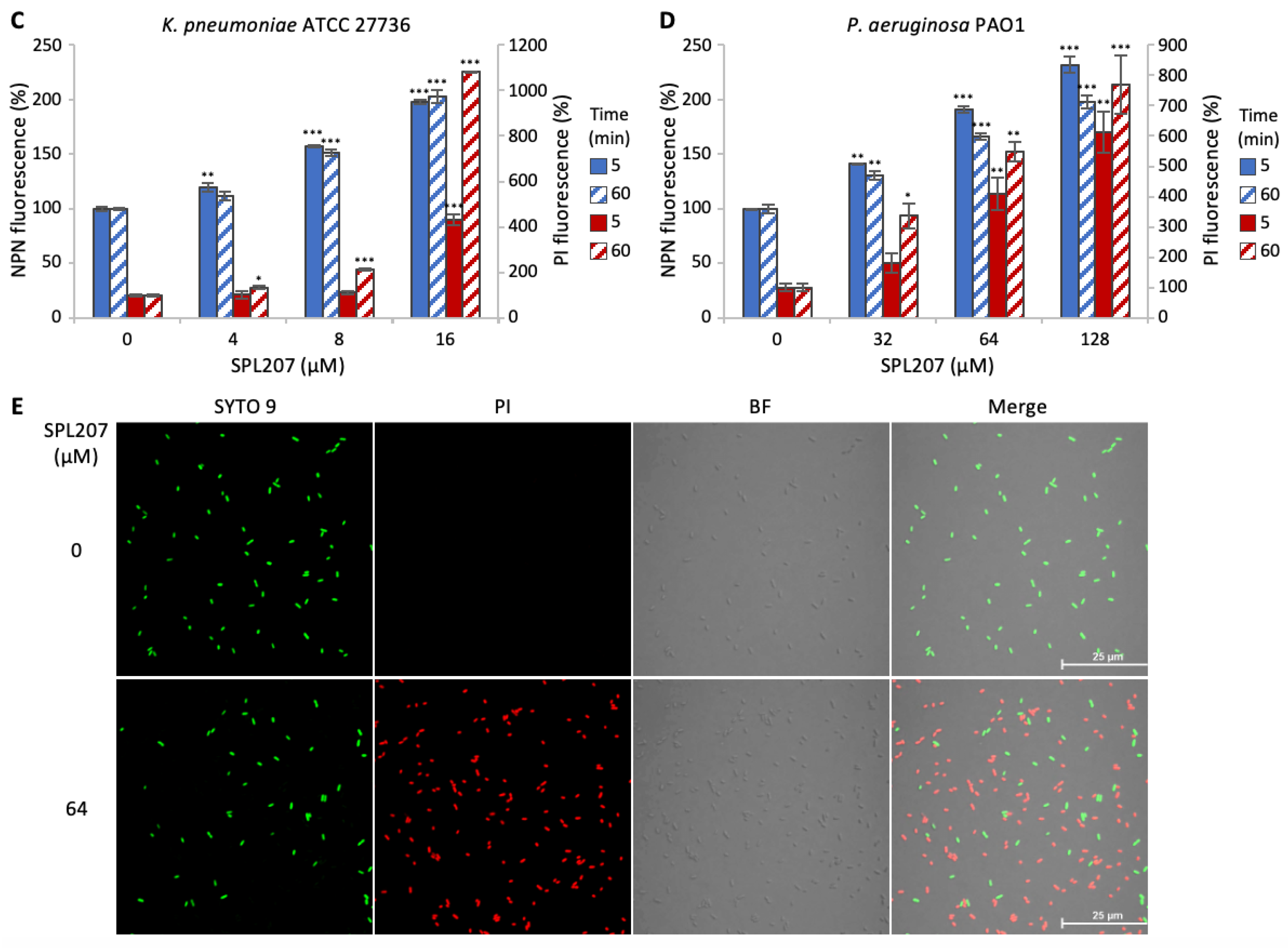
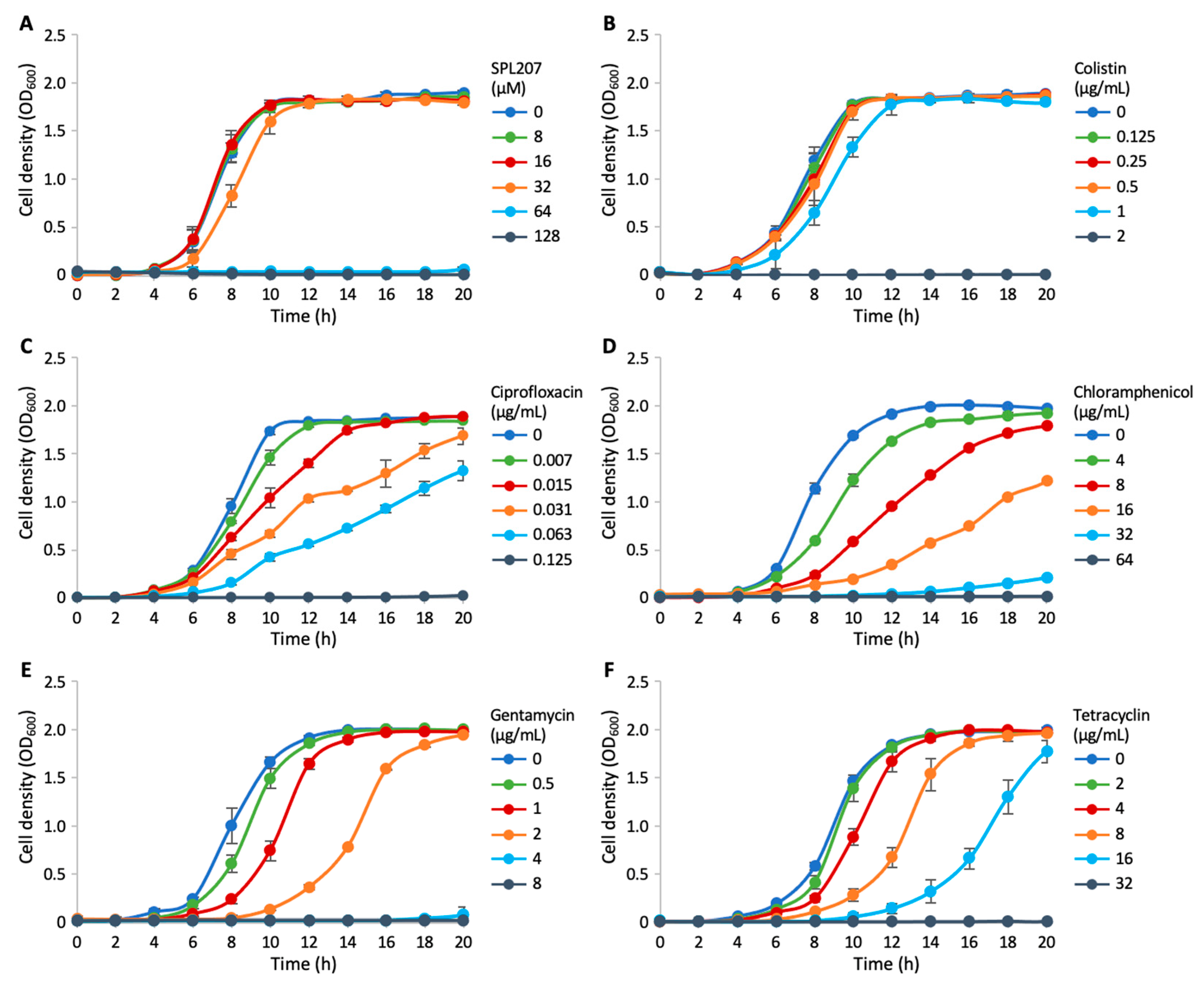
2.2.2. Investigation of SPL207 Mechanism of Action and Synergy with Colistin
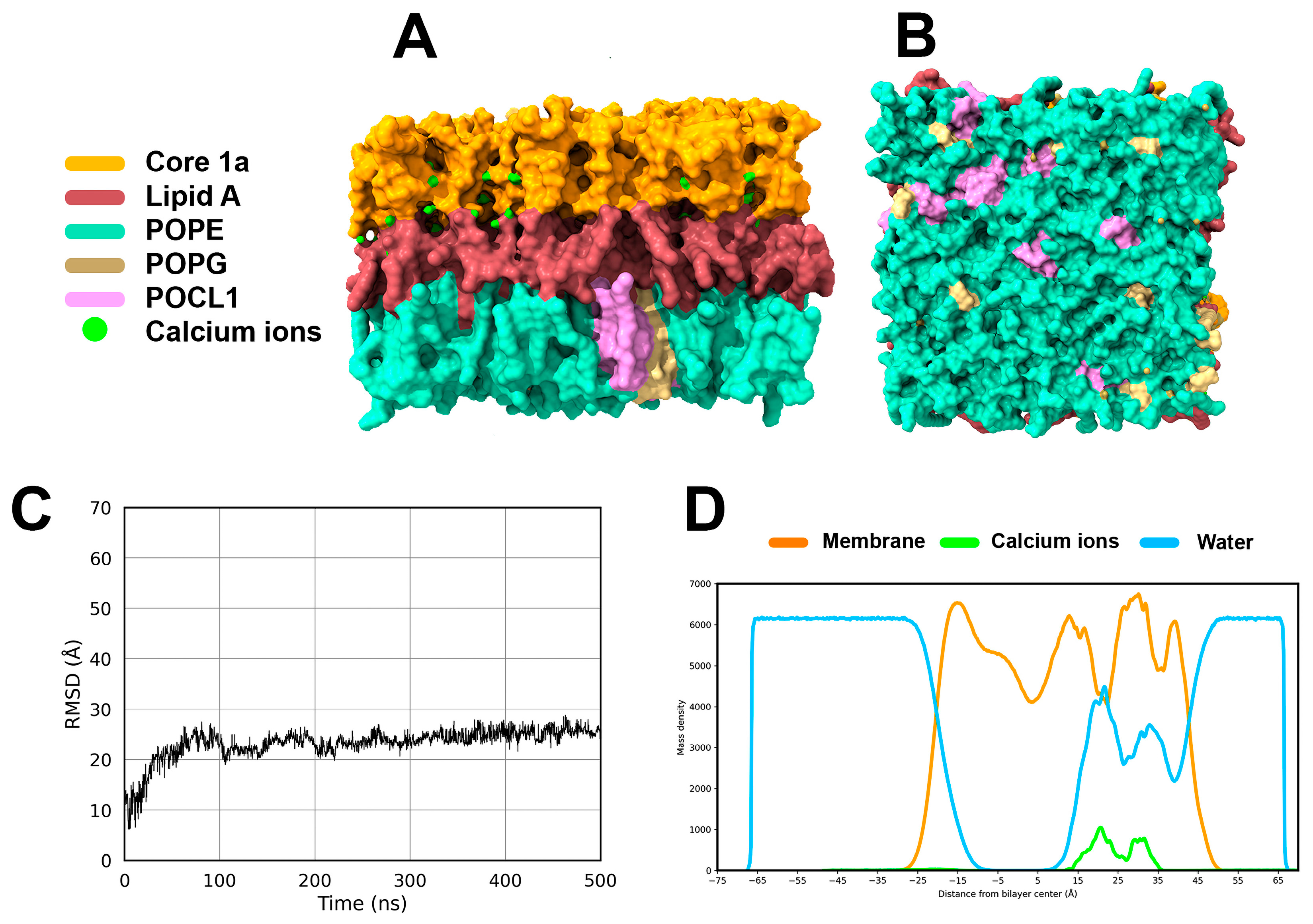
2.2.3. Small Molecule/Membrane Interaction Analysis by MD Simulations
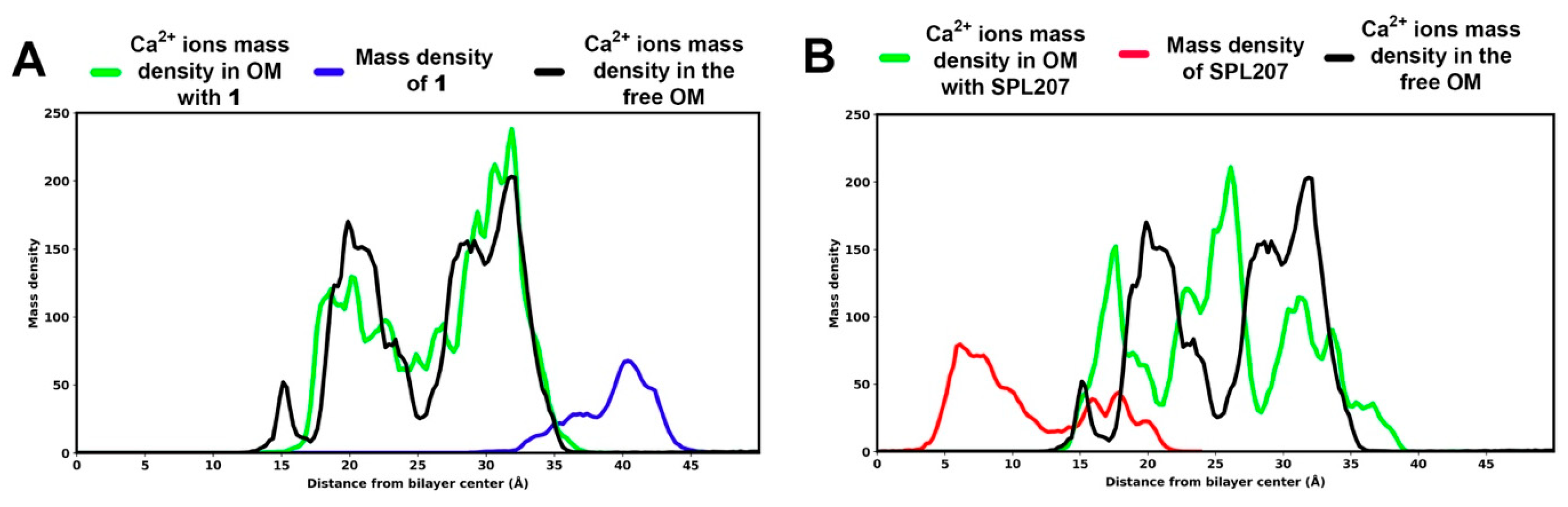
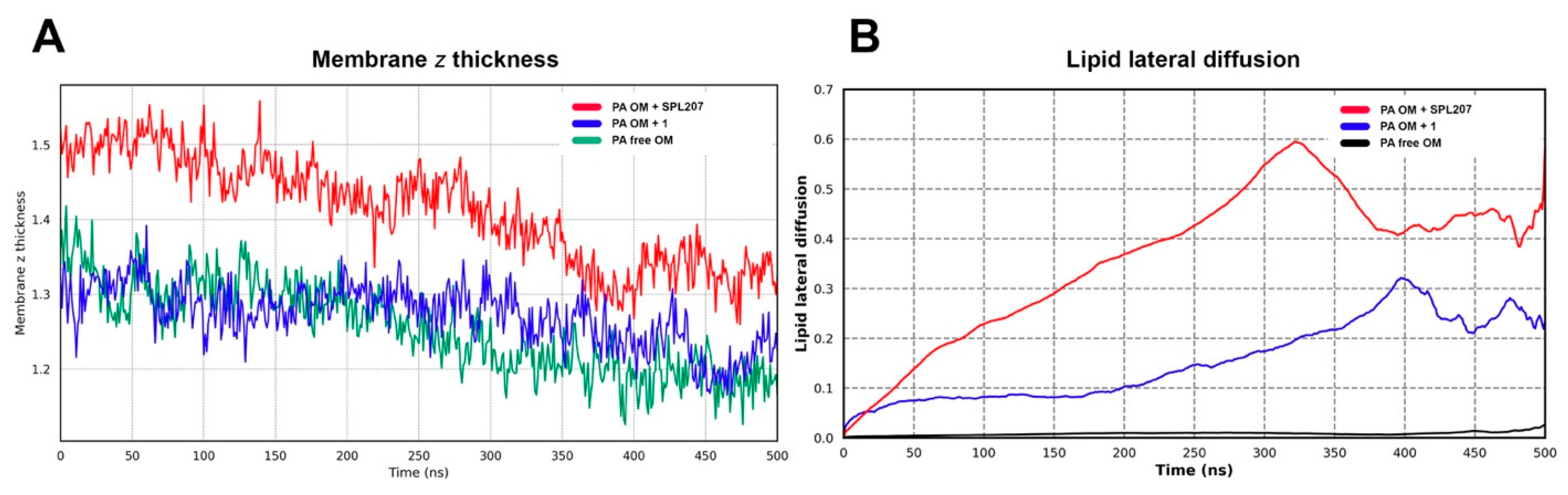
2.2.4. Displacement of Ca2+ Ions and Membrane Destabilization by SPL207
3. Materials and Methods
3.1. Chemistry
3.2. Bacterial Strains and Growth Conditions
3.3. Antimicrobial Assays
3.4. Cell Viability Assay
3.5. Fluorescent Probe-Permeability Assays
3.6. Checkerboard Assays
3.7. System Preparation for MD Simulations
3.8. MD Simulations
3.9. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| BOP | Benzotriazole-1-yl-oxy-tris-(dimethylamino)-phosphoniumhexafluorophosphate |
| CFU | colony forming units |
| DIPEA | diisopropylethylamine |
| DMF | N,N-dimethylformamide |
| EDTA | ethylenediaminetetraacetic acid |
| EtOH | ethanol |
| FICI | fractional inhibitory concentration index |
| GPO | gram-positive only |
| HBTU | N,N,N′,N′-Tetramethyl-O-(1H-benzotriazol-1-yl)uronium hexafluorophosphate |
| IM | inner membrane |
| LPS | lipopolysaccharide |
| MeOH | methanol |
| MD | molecular dynamics |
| MDR | multidrug-resistant |
| MIC | minimum inhibitory concentration |
| MHB-II | Muller-Hinton II (broth) |
| NPN | N-phenyl-1-naphthylamine |
| OD | optical density |
| OM | outer membrane |
| PAO1 | Pseudomonas aeruginosa (strain) |
| PI | propidium iodide |
| POPE | 3-palmitoyl-2-oleoyl-D-glycero-1-Phosphatidylethanolamine |
| POPG | 3-palmitoyl-2-oleoyl-D-glycero-1-Phosphatidylglycerol |
| POCL1 | POPG + POPG cardiolipin with head group charge = −1 |
| RMSD | root mean square deviation |
| rt | room temperature |
| SAR | structure-activity relationship |
| SPU | self-promoted uptake |
| TEA | triethylamine |
| TFA | trifluoroacetic acid |
| THF | tetrahydrofuran |
References
- Willyard, C. The Drug-Resistant Bacteria That Pose the Greatest Health Threats. Nature 2017, 543, 15. [Google Scholar] [CrossRef]
- Naghavi, M.; Vollset, S.E.; Ikuta, K.S.; Swetschinski, L.R.; Gray, A.P.; Wool, E.E.; Robles Aguilar, G.; Mestrovic, T.; Smith, G.; Han, C.; et al. Global Burden of Bacterial Antimicrobial Resistance 1990–2021: A Systematic Analysis with Forecasts to 2050. Lancet 2024, 404, 1199–1226. [Google Scholar] [CrossRef]
- Tang, K.W.K.; Millar, B.C.; Moore, J.E. Antimicrobial Resistance (AMR). Br. J. Biomed. Sci. 2023, 80, 11387. [Google Scholar] [CrossRef]
- OECD Antimicrobial Resistance Tackling the Burden in the European Union Briefing Note for EU/EEA Countries Contents; 2019. Available online: https://www.oecd.org/en/publications/antimicrobial-resistance-tackling-the-burden-in-the-european-union_33cbfc1c-en.html (accessed on 16 June 2025).
- WHO TEAM WHO Bacterial Priority Pathogens List, 2024: Bacterial Pathogens of Public Health Importance to Guide Research, Development and Strategies to Prevent and Control Antimicrobial Resistance; 2024. Available online: https://www.who.int/publications/i/item/9789240093461 (accessed on 16 June 2025).
- Richter, M.F.; Hergenrother, P.J. The Challenge of Converting Gram-positive-only Compounds into Broad-spectrum Antibiotics. Ann. N. Y Acad. Sci. 2019, 1435, 18–38. [Google Scholar] [CrossRef]
- Perlmutter, S.J.; Geddes, E.J.; Drown, B.S.; Motika, S.E.; Lee, M.R.; Hergenrother, P.J. Compound Uptake into E. Coli Can Be Facilitated by N-Alkyl Guanidiniums and Pyridiniums. ACS Infect. Dis. 2021, 7, 162–173. [Google Scholar] [CrossRef]
- Dupuy, F.G.; Pagano, I.; Andenoro, K.; Peralta, M.F.; Elhady, Y.; Heinrich, F.; Tristram-Nagle, S. Selective Interaction of Colistin with Lipid Model Membranes. Biophys. J. 2018, 114, 919–928. [Google Scholar] [CrossRef]
- McCreary, E.K.; Heil, E.L.; Tamma, P.D. New Perspectives on Antimicrobial Agents: Cefiderocol. Antimicrob. Agents Chemother. 2021, 65, e0217120. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.; Miller, P.A.; Miller, M.J. Conjugation of Aztreonam, a Synthetic Monocyclic β-Lactam Antibiotic, to a Siderophore Mimetic Significantly Expands Activity Against Gram-Negative Bacteria. ACS Infect. Dis. 2021, 7, 2979–2986. [Google Scholar] [CrossRef]
- Li, H.; Liu, J.; Liu, C.-F.; Li, H.; Luo, J.; Fang, S.; Chen, Y.; Zhong, R.; Liu, S.; Lin, S. Design, Synthesis, and Biological Evaluation of Membrane-Active Bakuchiol Derivatives as Effective Broad-Spectrum Antibacterial Agents. J. Med. Chem. 2021, 64, 5603–5619. [Google Scholar] [CrossRef] [PubMed]
- Kim, W.; Zhu, W.; Hendricks, G.L.; Van Tyne, D.; Steele, A.D.; Keohane, C.E.; Fricke, N.; Conery, A.L.; Shen, S.; Pan, W.; et al. A New Class of Synthetic Retinoid Antibiotics Effective against Bacterial Persisters. Nature 2018, 556, 103–107. [Google Scholar] [CrossRef] [PubMed]
- Princiotto, S.; Mazzini, S.; Musso, L.; Arena, F.; Dallavalle, S.; Pisano, C. New Antimicrobials Based on the Adarotene Scaffold with Activity against Multi-Drug Resistant Staphylococcus Aureus and Vancomycin-Resistant Enterococcus. Antibiotics 2021, 10, 126. [Google Scholar] [CrossRef]
- Princiotto, S.; Casciaro, B.; Temprano, A.G.; Musso, L.; Sacchi, F.; Loffredo, M.R.; Cappiello, F.; Sacco, F.; Raponi, G.; Fernandez, V.P.; et al. The Antimicrobial Potential of Adarotene Derivatives against Staphylococcus Aureus Strains. Bioorg. Chem. 2024, 145, 107227. [Google Scholar] [CrossRef]
- Long, Q.; Zhou, W.; Zhou, H.; Tang, Y.; Chen, W.; Liu, Q.; Bian, X. Polyamine-Containing Natural Products: Structure, Bioactivity, and Biosynthesis. Nat. Prod. Rep. 2024, 41, 525–564. [Google Scholar] [CrossRef]
- Chen, H.; Zhong, L.; Zhou, H.; Bai, X.; Sun, T.; Wang, X.; Zhao, Y.; Ji, X.; Tu, Q.; Zhang, Y.; et al. Biosynthesis and Engineering of the Nonribosomal Peptides with a C-Terminal Putrescine. Nat. Commun. 2023, 14, 6619. [Google Scholar] [CrossRef] [PubMed]
- Cincinelli, R.; Dallavalle, S.; Nannei, R.; Carella, S.; De Zani, D.; Merlini, L.; Penco, S.; Garattini, E.; Giannini, G.; Pisano, C.; et al. Synthesis and Structure−Activity Relationships of a New Series of Retinoid-Related Biphenyl-4-Ylacrylic Acids Endowed with Antiproliferative and Proapoptotic Activity. J. Med. Chem. 2005, 48, 4931–4946. [Google Scholar] [CrossRef]
- Cincinelli, R.; Musso, L.; Guglielmi, M.B.; La Porta, I.; Fucci, A.; Luca D’Andrea, E.; Cardile, F.; Colelli, F.; Signorino, G.; Darwiche, N.; et al. Novel Adamantyl Retinoid-Related Molecules with POLA1 Inhibitory Activity. Bioorg. Chem. 2020, 104, 104253. [Google Scholar] [CrossRef]
- Lorusso, A.B.; Carrara, J.A.; Barroso, C.D.N.; Tuon, F.F.; Faoro, H. Role of Efflux Pumps on Antimicrobial Resistance in Pseudomonas Aeruginosa. Int. J. Mol. Sci. 2022, 23, 15779. [Google Scholar] [CrossRef]
- Nikaido, H.; Vaara, M. Molecular Basis of Bacterial Outer Membrane Permeability. Microbiol. Rev. 1985, 49, 1–32. [Google Scholar] [CrossRef] [PubMed]
- Hancock, R.E.W. Alterations in outer membrane permeability. Annu. Rev. Microbiol. 1984, 38, 237–264. [Google Scholar] [CrossRef] [PubMed]
- Poole, K. Pseudomonas Aeruginosa: Resistance to the Max. Front. Microbiol. 2011, 2, 65. [Google Scholar] [CrossRef]
- Morita, Y.; Sobel, M.L.; Poole, K. Antibiotic Inducibility of the MexXY Multidrug Efflux System of Pseudomonas Aeruginosa: Involvement of the Antibiotic-Inducible PA5471 Gene Product. J. Bacteriol. 2006, 188, 1847–1855. [Google Scholar] [CrossRef] [PubMed]
- Rampioni, G.; Pillai, C.R.; Longo, F.; Bondì, R.; Baldelli, V.; Messina, M.; Imperi, F.; Visca, P.; Leoni, L. Effect of Efflux Pump Inhibition on Pseudomonas Aeruginosa Transcriptome and Virulence. Sci. Rep. 2017, 7, 11392. [Google Scholar] [CrossRef]
- Fernández-Piñar, R.; Lo Sciuto, A.; Rossi, A.; Ranucci, S.; Bragonzi, A.; Imperi, F. In Vitro and in Vivo Screening for Novel Essential Cell-Envelope Proteins in Pseudomonas Aeruginosa. Sci. Rep. 2015, 5, 17593. [Google Scholar] [CrossRef]
- Lo Sciuto, A.; Martorana, A.M.; Fernández-Piñar, R.; Mancone, C.; Polissi, A.; Imperi, F. Pseudomonas Aeruginosa LptE Is Crucial for LptD Assembly, Cell Envelope Integrity, Antibiotic Resistance and Virulence. Virulence 2018, 9, 1718–1733. [Google Scholar] [CrossRef]
- Vaara, M. Agents That Increase the Permeability of the Outer Membrane. Microbiol. Rev. 1992, 56, 395–411. [Google Scholar] [CrossRef]
- Loh, B.; Grant, C.; Hancock, R.E. Use of the Fluorescent Probe 1-N-Phenylnaphthylamine to Study the Interactions of Aminoglycoside Antibiotics with the Outer Membrane of Pseudomonas Aeruginosa. Antimicrob. Agents Chemother. 1984, 26, 546–551. [Google Scholar] [CrossRef]
- Kwon, J.Y.; Kim, M.K.; Mereuta, L.; Seo, C.H.; Luchian, T.; Park, Y. Mechanism of Action of Antimicrobial Peptide P5 Truncations against Pseudomonas Aeruginosa and Staphylococcus Aureus. AMB Express 2019, 9, 122. [Google Scholar] [CrossRef] [PubMed]
- D’Agostino, I.; Ardino, C.; Poli, G.; Sannio, F.; Lucidi, M.; Poggialini, F.; Visaggio, D.; Rango, E.; Filippi, S.; Petricci, E.; et al. Antibacterial Alkylguanidino Ureas: Molecular Simplification Approach, Searching for Membrane-Based MoA. Eur. J. Med. Chem. 2022, 231, 114158. [Google Scholar] [CrossRef] [PubMed]
- Nang, S.C.; Li, M.; Harper, M.; Mandela, E.; Bergen, P.J.; Rolain, J.-M.; Zhu, Y.; Velkov, T.; Li, J. Polymyxin Causes Cell Envelope Remodelling and Stress Responses in Mcr-1-Harbouring Escherichia Coli. Int. J. Antimicrob. Agents 2022, 59, 106505. [Google Scholar] [CrossRef]
- Doern, C.D. When Does 2 Plus 2 Equal 5? A Review of Antimicrobial Synergy Testing. J. Clin. Microbiol. 2014, 52, 4124–4128. [Google Scholar] [CrossRef]
- Lo Sciuto, A.; Imperi, F. Aminoarabinosylation of Lipid A Is Critical for the Development of Colistin Resistance in Pseudomonas Aeruginosa. Antimicrob. Agents Chemother. 2018, 62, e01820-17. [Google Scholar] [CrossRef]
- EUCAST Clinical Breakpoints-Breakpoints and Guidance. Available online: https://www.eucast.org/clinical_breakpoints (accessed on 5 June 2025).
- de Sanchez Blas, B.; Temprano, A.G.; Cives-Losada, C.; Briz, O.; Lozano, E.; Martinez-Chantar, M.L.; Avila, M.A.; Mori, M.; Ghallab, A.; Hengstler, J.G.; et al. A Novel Noninvasive Test Based on Near-Infrared Fluorescent Cholephilic Probes for Hepatobiliary Secretory Function Assessment. Biomed. Pharmacother. 2025, 187, 118074. [Google Scholar] [CrossRef]
- Jiang, X.; Sun, Y.; Yang, K.; Yuan, B.; Velkov, T.; Wang, L.; Li, J. Coarse-Grained Simulations Uncover Gram-Negative Bacterial Defense against Polymyxins by the Outer Membrane. Comput. Struct. Biotechnol. J. 2021, 19, 3885–3891. [Google Scholar] [CrossRef]
- Clifton, L.A.; Skoda, M.W.A.; Le Brun, A.P.; Ciesielski, F.; Kuzmenko, I.; Holt, S.A.; Lakey, J.H. Effect of Divalent Cation Removal on the Structure of Gram-Negative Bacterial Outer Membrane Models. Langmuir 2015, 31, 404–412. [Google Scholar] [CrossRef]
- Li, J.; Beuerman, R.; Verma, C.S. Dissecting the Molecular Mechanism of Colistin Resistance in Mcr-1 Bacteria. J. Chem. Inf. Model. 2020, 60, 4975–4984. [Google Scholar] [CrossRef] [PubMed]
- Milletti, F.; Storchi, L.; Sforna, G.; Cruciani, G. New and Original PKa Prediction Method Using Grid Molecular Interaction Fields. J. Chem. Inf. Model. 2007, 47, 2172–2781. [Google Scholar] [CrossRef] [PubMed]
- Maginn, E.J.; Messerly, R.A.; Carlson, D.J.; Roe, D.R.; Elliot, J.R. Best Practices for Computing Transport Properties 1. Self-Diffusivity and Viscosity from Equilibrium Molecular Dynamics [Article v1.0]. Living J. Comput. Mol. Sci. 2018, 1, 6324. [Google Scholar] [CrossRef]
- Yeh, I.-C.; Hummer, G. System-Size Dependence of Diffusion Coefficients and Viscosities from Molecular Dynamics Simulations with Periodic Boundary Conditions. J. Phys. Chem. B 2004, 108, 15873–15879. [Google Scholar] [CrossRef]
- von Bülow, S.; Bullerjahn, J.T.; Hummer, G. Systematic Errors in Diffusion Coefficients from Long-Time Molecular Dynamics Simulations at Constant Pressure. J. Chem. Phys. 2020, 153, 021101. [Google Scholar] [CrossRef] [PubMed]
- Ren, W.; Liu, Y.; Wu, G.; Liu, J.; Lu, X. Stereoregular Polycarbonate Synthesis: Alternating Copolymerization of CO2 with Aliphatic Terminal Epoxides Catalyzed by Multichiral Cobalt(III) Complexes. J. Polym. Sci. A Polym. Chem. 2011, 49, 4894–4901. [Google Scholar] [CrossRef]
- Cincinelli, R.; Musso, L.; Giannini, G.; Zuco, V.; De Cesare, M.; Zunino, F.; Dallavalle, S. Influence of the Adamantyl Moiety on the Activity of Biphenylacrylohydroxamic Acid-Based HDAC Inhibitors. Eur. J. Med. Chem. 2014, 79, 251–259. [Google Scholar] [CrossRef]
- Giannini, G.; Brunetti, T.; Battistuzzi, G.; Alloatti, D.; Quattrociocchi, G.; Cima, M.G.; Merlini, L.; Dallavalle, S.; Cincinelli, R.; Nannei, R.; et al. New Retinoid Derivatives as Back-Ups of Adarotene. Bioorg. Med. Chem. 2012, 20, 2405–2415. [Google Scholar] [CrossRef] [PubMed]
- Kong, L.; Qi, T.; Ren, Z.; Jin, Y.; Li, Y.; Cheng, Y.; Xiao, F. High-Performance Intrinsic Low-k Polymer via the Synergistic Effect of Its Three Units: Adamantyl, Perfluorocyclobutylidene and Benzocyclobutene. RSC Adv. 2016, 6, 68560–68567. [Google Scholar] [CrossRef]
- CLSI M07-A9; Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria That Grow Aerobically; Approved Standard—Ninth Edition. Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2012.
- Collalto, D.; Fortuna, A.; Visca, P.; Imperi, F.; Rampioni, G.; Leoni, L. Synergistic Activity of Colistin in Combination with Clofoctol against Colistin Resistant Gram-Negative Pathogens. Microbiol. Spectr. 2023, 11, e0427522. [Google Scholar] [CrossRef] [PubMed]
- Cervoni, M.; Lo Sciuto, A.; Bianchini, C.; Mancone, C.; Imperi, F. Exogenous and Endogenous Phosphoethanolamine Transferases Differently Affect Colistin Resistance and Fitness in Pseudomonas Aeruginosa. Front. Microbiol. 2021, 12, 778968. [Google Scholar] [CrossRef]
- Li, Y.; Liu, J.; Gumbart, J.C. Preparing Membrane Proteins for Simulation Using CHARMM-GUI. In Methods in Molecular Biology; Springer: Berlin/Heidelberg, Germany, 2021; Volume 2302. [Google Scholar]
- Jo, S.; Kim, T.; Iyer, V.G.; Im, W. CHARMM-GUI: A Web-Based Graphical User Interface for CHARMM. J. Comput. Chem. 2008, 29, 1859–1865. [Google Scholar] [CrossRef] [PubMed]
- Jefferies, D.; Hsu, P.C.; Khalid, S. Through the Lipopolysaccharide Glass: A Potent Antimicrobial Peptide Induces Phase Changes in Membranes. Biochemistry 2017, 56, 1672–1679. [Google Scholar] [CrossRef]
- Zhu, Y.; Lu, J.; Han, M.L.; Jiang, X.; Azad, M.A.K.; Patil, N.A.; Lin, Y.W.; Zhao, J.; Hu, Y.; Yu, H.H.; et al. Polymyxins Bind to the Cell Surface of Unculturable Acinetobacter Baumannii and Cause Unique Dependent Resistance. Adv. Sci. 2020, 7, 2000704. [Google Scholar] [CrossRef]
- Khondker, A.; Dhaliwal, A.K.; Saem, S.; Mahmood, A.; Fradin, C.; Moran-Mirabal, J.; Rheinstädter, M.C. Membrane Charge and Lipid Packing Determine Polymyxin-Induced Membrane Damage. Commun. Biol. 2019, 2, 67. [Google Scholar] [CrossRef]
- Stead, C.M.; Zhao, J.; Raetz, C.R.H.; Trent, M.S. Removal of the Outer Kdo from Helicobacter Pylori Lipopolysaccharide and Its Impact on the Bacterial Surface. Mol. Microbiol. 2010, 78, 837–852. [Google Scholar] [CrossRef]
- Berglund, N.A.; Piggot, T.J.; Jefferies, D.; Sessions, R.B.; Bond, P.J.; Khalid, S. Interaction of the Antimicrobial Peptide Polymyxin B1 with Both Membranes of E. Coli: A Molecular Dynamics Study. PLoS Comput. Biol. 2015, 11, e1004180. [Google Scholar] [CrossRef] [PubMed]
- Loutet, S.A.; Flannagan, R.S.; Kooi, C.; Sokol, P.A.; Valvano, M.A. A Complete Lipopolysaccharide Inner Core Oligosaccharide Is Required for Resistance of Burkholderia Cenocepacia to Antimicrobial Peptides and Bacterial Survival in Vivo. J. Bacteriol. 2006, 188, 2073–2080. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.; Yang, K.; Han, M.L.; Yuan, B.; Li, J.; Gong, B.; Velkov, T.; Schreiber, F.; Wang, L.; Li, J. Outer Membranes of Polymyxin-Resistant Acinetobacter Baumannii with Phosphoethanolamine-Modified Lipid A and Lipopolysaccharide Loss Display Different Atomic-Scale Interactions with Polymyxins. ACS Infect. Dis. 2020, 6, 2698–2708. [Google Scholar] [CrossRef]
- Vergalli, J.; Bodrenko, I.V.; Masi, M.; Moynié, L.; Acosta-Gutiérrez, S.; Naismith, J.H.; Davin-Regli, A.; Ceccarelli, M.; van den Berg, B.; Winterhalter, M.; et al. Porins and Small-Molecule Translocation across the Outer Membrane of Gram-Negative Bacteria. Nat. Rev. Microbiol 2020, 18, 164–176. [Google Scholar] [CrossRef]
- López, C.A.; Zgurskaya, H.; Gnanakaran, S. Molecular Characterization of the Outer Membrane of Pseudomonas Aeruginosa. Biochim. Biophys. Acta Biomembr. 2020, 1862, 183151. [Google Scholar] [CrossRef]
- Rice, A.; Wereszczynski, J. Atomistic Scale Effects of Lipopolysaccharide Modifications on Bacterial Outer Membrane Defenses. Biophys. J. 2018, 114, 1389–1399. [Google Scholar] [CrossRef]
- Akhoundsadegh, N.; Belanger, C.R.; Hancock, R.E.W. Outer Membrane Interaction Kinetics of New Polymyxin B Analogs in Gram-Negative Bacilli. Antimicrob. Agents Chemother. 2019, 63, e00935-19. [Google Scholar] [CrossRef]
- Jiang, X.; Yang, K.; Yuan, B.; Han, M.; Zhu, Y.; Roberts, K.D.; Patil, N.A.; Li, J.; Gong, B.; Hancock, R.E.W.; et al. Molecular Dynamics Simulations Informed by Membrane Lipidomics Reveal the Structure-Interaction Relationship of Polymyxins with the Lipid A-Based Outer Membrane of Acinetobacter Baumannii. J. Antimicrob. Chemother. 2020, 75, 3534–3543. [Google Scholar] [CrossRef]
- PICTO, version 4.5.4.1; OpenEye, Cadence Molecular Sciences: Santa Fe, NM, USA. Available online: http://www.eyesopen.com (accessed on 23 January 2025).
- OMEGA, version 4.2.0.1; OpenEye, Cadence Molecular Sciences: Santa Fe, NM, USA. Available online: http://www.eyesopen.com (accessed on 23 January 2025).
- Hawkins, P.C.D.; Skillman, A.G.; Warren, G.L.; Ellingson, B.A.; Stahl, M.T. Conformer Generation with OMEGA: Algorithm and Validation Using High Quality Structures from the Protein Databank and Cambridge Structural Database. J. Chem. Inf. Model. 2010, 50, 572–584. [Google Scholar] [CrossRef] [PubMed]
- Milletti, F.; Vulpetti, A. Tautomer Preference in PDB Complexes and Its Impact on Structure-Based Drug Discovery. J. Chem. Inf. Model. 2010, 50, 1062–1074. [Google Scholar] [CrossRef]
- Milletti, F.; Storchi, L.; Sfoma, G.; Cross, S.; Cruciani, G. Tautomer Enumeration and Stability Prediction for Virtual Screening on Large Chemical Databases. J. Chem. Inf. Model. 2009, 49, 68–75. [Google Scholar] [CrossRef]
- SZYBKI, version 2.5.0.1. OpenEye, Cadence Molecular Sciences: Santa Fe, NM, USA. Available online: http://www.eyesopen.com (accessed on 23 January 2025).
- Kim, S.; Lee, J.; Jo, S.; Brooks, C.L.; Lee, H.S.; Im, W. CHARMM-GUI Ligand Reader and Modeler for CHARMM Force Field Generation of Small Molecules. J. Comput. Chem. 2017, 38, 1879–1886. [Google Scholar] [CrossRef]
- Kern, N.R. CHARMM-GUI Multicomponent Assembler for Modeling and Simulation of Complex Heterogeneous Biomolecular Systems. Biophys. J. 2019, 116, 290A. [Google Scholar] [CrossRef]
- Kern, N.R.; Lee, J.; Kyo Choi, Y.; Im, W. CHARMM-GUI Multicomponent Assembler for Modeling and Simulation of Complex Multicomponent Systems. Biophys. J. 2022, 121, 529A. [Google Scholar] [CrossRef]
- Lee, J.; Cheng, X.; Swails, J.M.; Yeom, M.S.; Eastman, P.K.; Lemkul, J.A.; Wei, S.; Buckner, J.; Jeong, J.C.; Qi, Y.; et al. CHARMM-GUI Input Generator for NAMD, GROMACS, AMBER, OpenMM, and CHARMM/OpenMM Simulations Using the CHARMM36 Additive Force Field. J. Chem. Theory Comput. 2016, 12, 405–413. [Google Scholar] [CrossRef]
- Brooks, B.R.; Brooks, C.L.; Mackerell, A.D.; Nilsson, L.; Petrella, R.J.; Roux, B.; Won, Y.; Archontis, G.; Bartels, C.; Boresch, S.; et al. CHARMM: The Biomolecular Simulation Program. J. Comput. Chem. 2009, 30, 1545–1614. [Google Scholar] [CrossRef] [PubMed]
- Salomon-Ferrer, R.; Case, D.A.; Walker, R.C. An Overview of the Amber Biomolecular Simulation Package. Wiley Interdiscip. Rev. Comput. Mol. Sci. 2013, 3, 198–210. [Google Scholar] [CrossRef]
- Case, D.A.; Cheatham, T.E.; Darden, T.; Gohlke, H.; Luo, R.; Merz, K.M.; Onufriev, A.; Simmerling, C.; Wang, B.; Woods, R.J. The Amber Biomolecular Simulation Programs. J. Comput. Chem. 2005, 26, 1668–1688. [Google Scholar] [CrossRef] [PubMed]
- Roe, D.R.; Cheatham, T.E. PTRAJ and CPPTRAJ: Software for Processing and Analysis of Molecular Dynamics Trajectory Data. J. Chem. Theory Comput. 2013, 9, 3084–3095. [Google Scholar] [CrossRef]
- Smith, P.; Lorenz, C.D. LiPyphilic: A Python Toolkit for the Analysis of Lipid Membrane Simulations. J. Chem. Theory Comput. 2021, 17, 5907–5919. [Google Scholar] [CrossRef]
- Gowers, R.; Linke, M.; Barnoud, J.; Reddy, T.; Melo, M.; Seyler, S.; Domański, J.; Dotson, D.; Buchoux, S.; Kenney, I.; et al. MDAnalysis: A Python Package for the Rapid Analysis of Molecular Dynamics Simulations. In Proceedings of the 15th Python in Science Conference, Austin, TX, USA, 11–17 July 2016. [Google Scholar] [CrossRef]
- Michaud-Agrawal, N.; Denning, E.J.; Woolf, T.B.; Beckstein, O. MDAnalysis: A Toolkit for the Analysis of Molecular Dynamics Simulations. J. Comput. Chem. 2011, 32, 2319–2327. [Google Scholar] [CrossRef] [PubMed]
- Delano, W.L. The PyMOL Molecular Graphics System. CCP4 Newsl. Protein Crystallogr. 2002, 40. Available online: https://legacy.ccp4.ac.uk/newsletters/newsletter40/11_pymol.pdf (accessed on 31 January 2025).

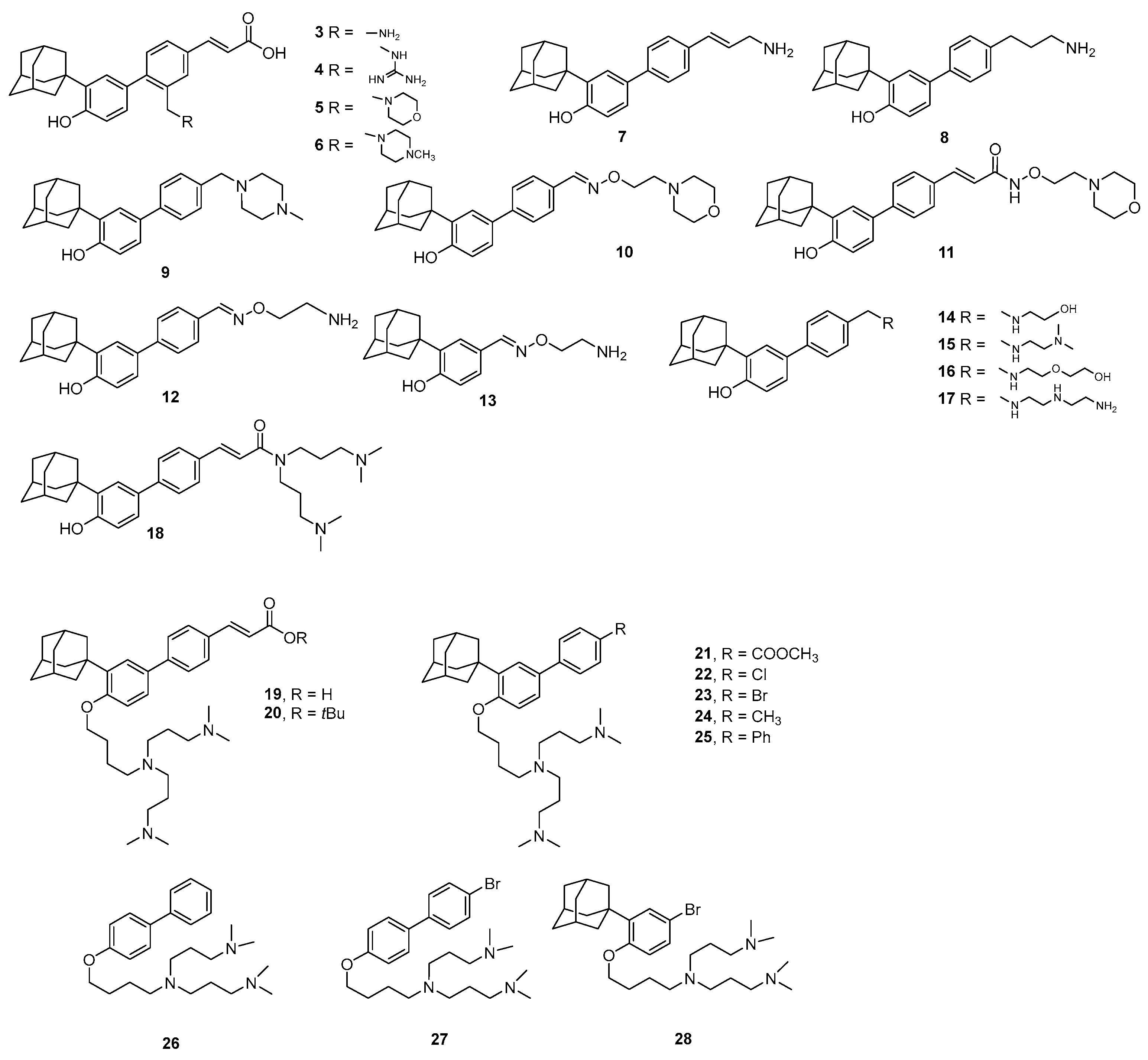

| Compound | MIC (µM) * | |
|---|---|---|
| S. aureus ATCC 25923 | E. coli ATCC 25922 | |
| 2 | 4 ** | >128 ** |
| 3 | >128 | >128 |
| 4 | 64 | >128 |
| 5 | 32 | >128 |
| 6 | 32 | >128 |
| 7 | 4 | >128 |
| 8 | 8 | >128 |
| 9 | >128 | >128 |
| 10 | >128 | >128 |
| 11 | 32 | >128 |
| 12 | 4 | >128 |
| 13 | 64 | >128 |
| 14 | 8 | >128 |
| 15 | 64 | >128 |
| 16 | 64 | >128 |
| 17 | 16 | 32 |
| 18 | 8 | 32 |
| 19 | 8 | 16 |
| 20 | 8 | 64 |
| 21 | 4 | 8 |
| 22 | 4 | 4 |
| 23 | 4 | 4 |
| 24 | 4 | 8 |
| 25 | 4 | 128 |
| 26 | 32 | 128 |
| 27 | 64 | 64 |
| 28 | 8 | 8 |
| MIC (µM) * | |
|---|---|
| Compound | P. aeruginosa ATCC 27853 |
| 18 | >128 |
| 19 | 128 |
| 20 | 128 |
| 21 | 64 |
| 22 | 128 |
| 23 | 32 |
| 24 | 128 |
| 25 | >128 |
| 26 | >128 |
| 27 | >128 |
| 28 | 64 |
| Strain | MIC SPL207 (µM) * |
|---|---|
| A. baumannii ATCC 19606 | 8 |
| A. baumannii ACICU | 8 |
| E. cloacae ATCC 13047 | 16 |
| E. coli MG1655 | 8 |
| K. pneumoniae ATCC 27736 | 8 |
| P. aeruginosa PAO1 | 64 |
| Strain | Growth Medium Supplemented with | MIC SPL207 (µM) * |
|---|---|---|
| PAO1-KP | - | 64 |
| PAO1-KP ∆efflux | - | 64 |
| PAO1 lptE | 0.002% arabinose | 16 |
| 0.5% arabinose | 64 | |
| PAO1 lptH | 0.125% arabinose | 16 |
| 0.5% arabinose | 64 | |
| PAO1 | - | 64 |
| 0.4 mM EDTA | 32 | |
| 0.8 mM EDTA | 16 |
| Strain | Colistin MIC (µg/mL) at SPL207 conc. (µM) of: | Maximum Fold Change a | ColSPL207 b MIC | SPL207Col c MIC | FICI d | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 0 | 2 | 4 | 8 | 16 | 32 | 64 | |||||
| PAO1 | 1 | 1 | 0.5 | 0.5 | 0.25 | 0.25 | 0.25 | 4 | 0.25 | 16 | 0.5 |
| PAO1 colR1 | 64 | 8 | 8 | 4 | 2 | 2 | 2 | 32 | 2 | 16 | 0.094 |
| PAO1 colR3 | 64 | 4 | 2 | 2 | 2 | 2 | 2 | 32 | 2 | 4 | 0.047 |
| PAO1 colR5 | 8 | 8 | 1 | 0.5 | 0.5 | 0.5 | 0.5 | 16 | 0.5 | 8 | 0.094 |
| Diffusion Coefficient [10−7 cm2/s] | |||
|---|---|---|---|
| System | xy Plane | z Axis | Total |
| PA free OM | 1.36 ± 0.032 | 0.38 ± 0.025 | 1.03 ± 0.051 |
| PA–OM + 1 | 2.90 ± 0.005 | 0.65 ± 0.049 | 2.15 ± 0.091 |
| PA-OM + SPL207 | 4.74 ± 1.132 | 0.84 ± 0.057 | 4.73 ± 0.474 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Princiotto, S.; Cutarella, L.; Fortuna, A.; Mellini, M.; Casciaro, B.; Loffredo, M.R.; Temprano, A.G.; Cappiello, F.; Leoni, L.; Mangoni, M.L.; et al. Retargeting Gram-Positive-Only Adarotene-Derived Antibacterials to Broad-Spectrum Antibiotics. Antibiotics 2025, 14, 956. https://doi.org/10.3390/antibiotics14090956
Princiotto S, Cutarella L, Fortuna A, Mellini M, Casciaro B, Loffredo MR, Temprano AG, Cappiello F, Leoni L, Mangoni ML, et al. Retargeting Gram-Positive-Only Adarotene-Derived Antibacterials to Broad-Spectrum Antibiotics. Antibiotics. 2025; 14(9):956. https://doi.org/10.3390/antibiotics14090956
Chicago/Turabian StylePrinciotto, Salvatore, Luigi Cutarella, Alessandra Fortuna, Marta Mellini, Bruno Casciaro, Maria Rosa Loffredo, Alvaro G. Temprano, Floriana Cappiello, Livia Leoni, Maria Luisa Mangoni, and et al. 2025. "Retargeting Gram-Positive-Only Adarotene-Derived Antibacterials to Broad-Spectrum Antibiotics" Antibiotics 14, no. 9: 956. https://doi.org/10.3390/antibiotics14090956
APA StylePrinciotto, S., Cutarella, L., Fortuna, A., Mellini, M., Casciaro, B., Loffredo, M. R., Temprano, A. G., Cappiello, F., Leoni, L., Mangoni, M. L., Mori, M., Musso, L., Sacchi, F., Pinna, C., Rampioni, G., Dallavalle, S., & Pisano, C. (2025). Retargeting Gram-Positive-Only Adarotene-Derived Antibacterials to Broad-Spectrum Antibiotics. Antibiotics, 14(9), 956. https://doi.org/10.3390/antibiotics14090956










