Liposomal Fluopsin C: Physicochemical Properties, Cytotoxicity, and Antibacterial Activity In Vitro and over In Vivo MDR Klebsiella pneumoniae Bacteremia Model
Abstract
1. Introduction
2. Results
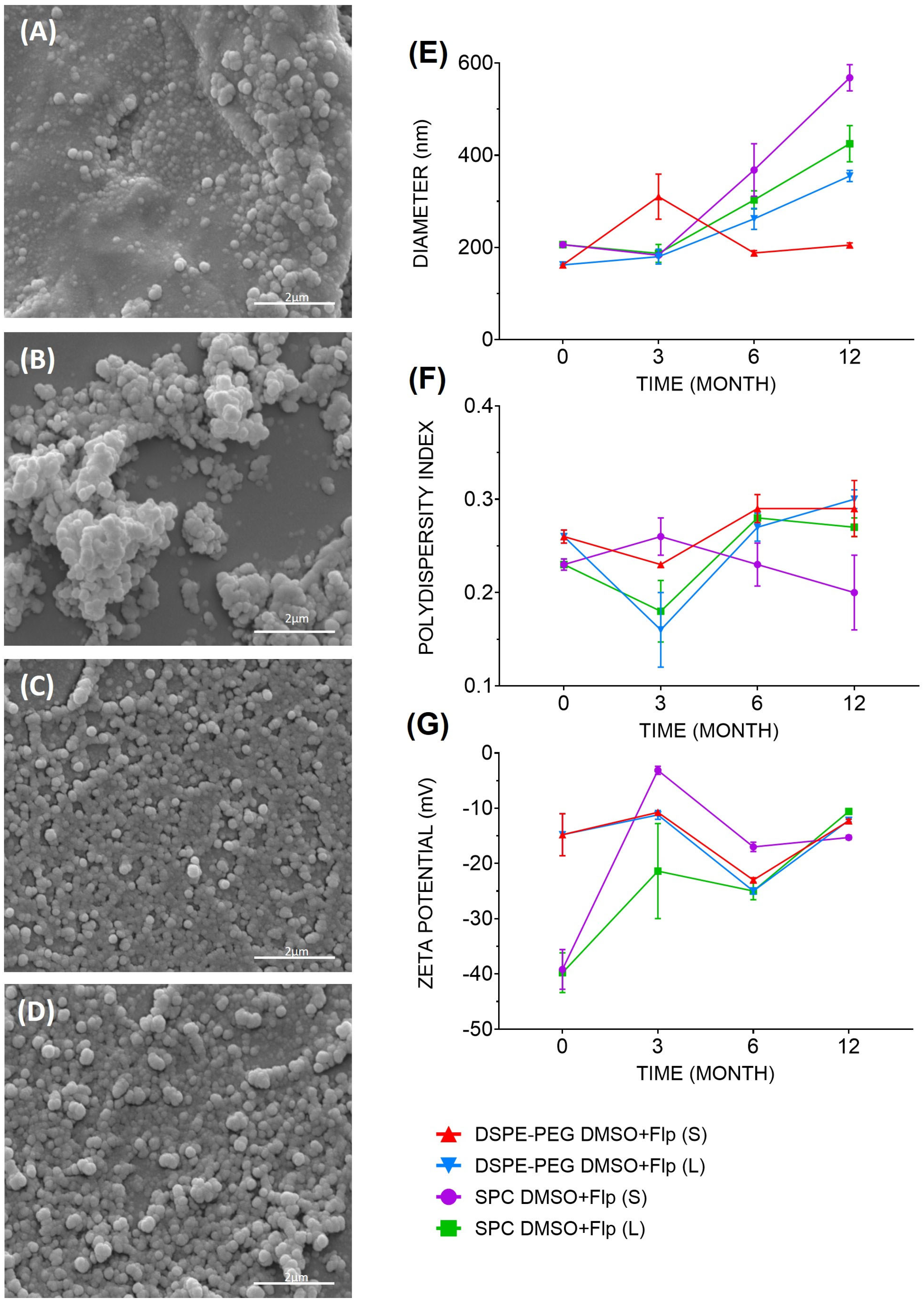
2.1. Characterization of the Fluopsin C-Containing Liposomal Formulations
2.2. Determination of In Vitro Liposomal Fluopsin C Cytotoxicity
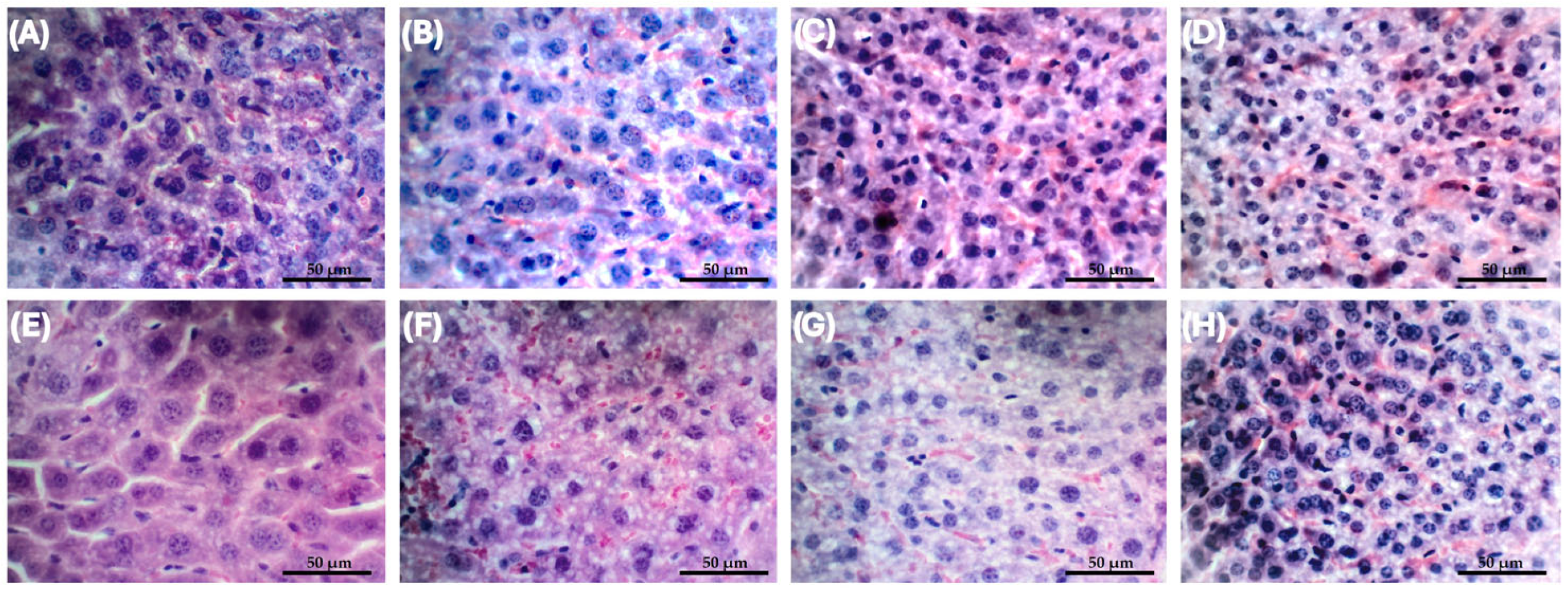
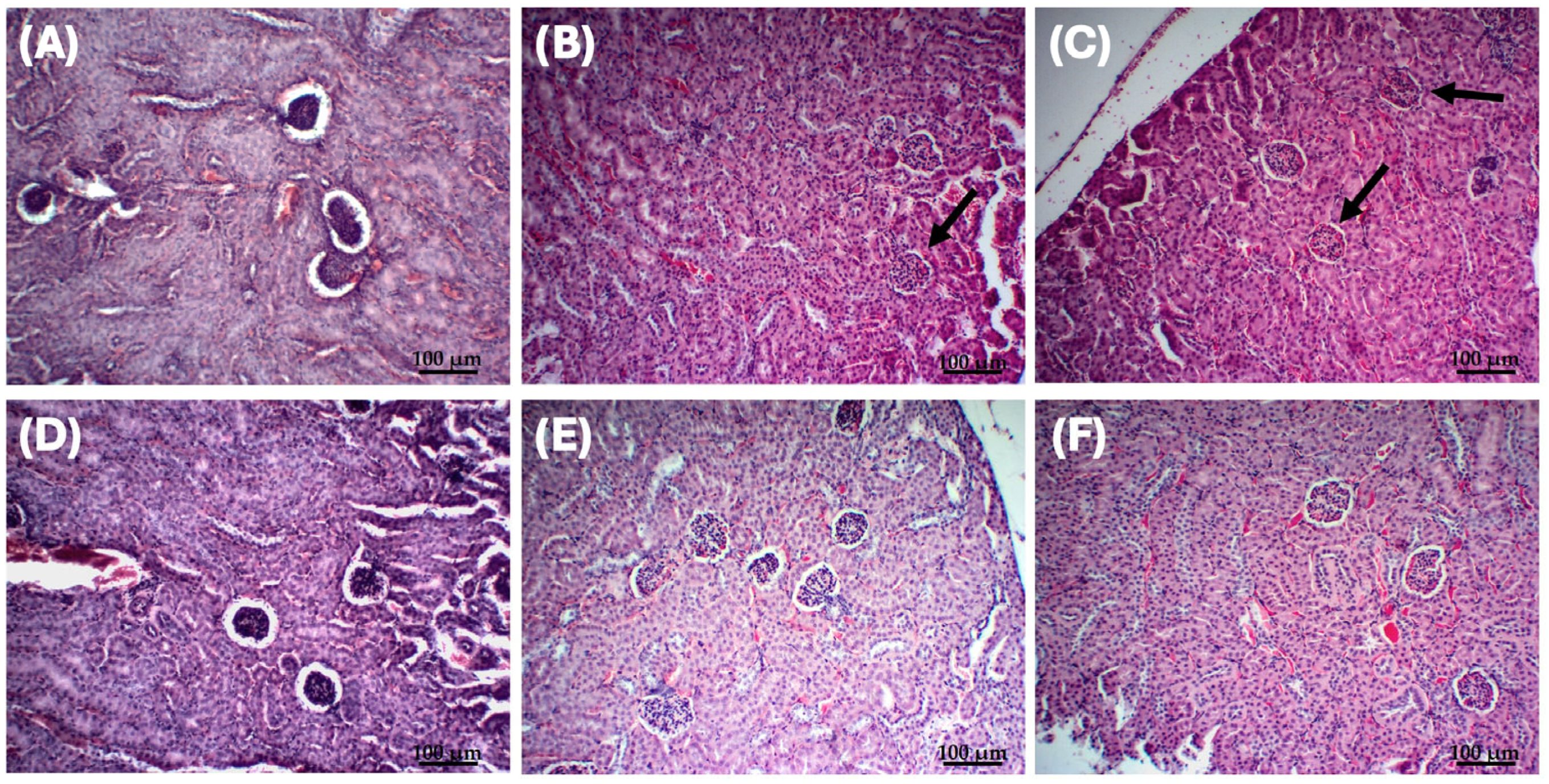
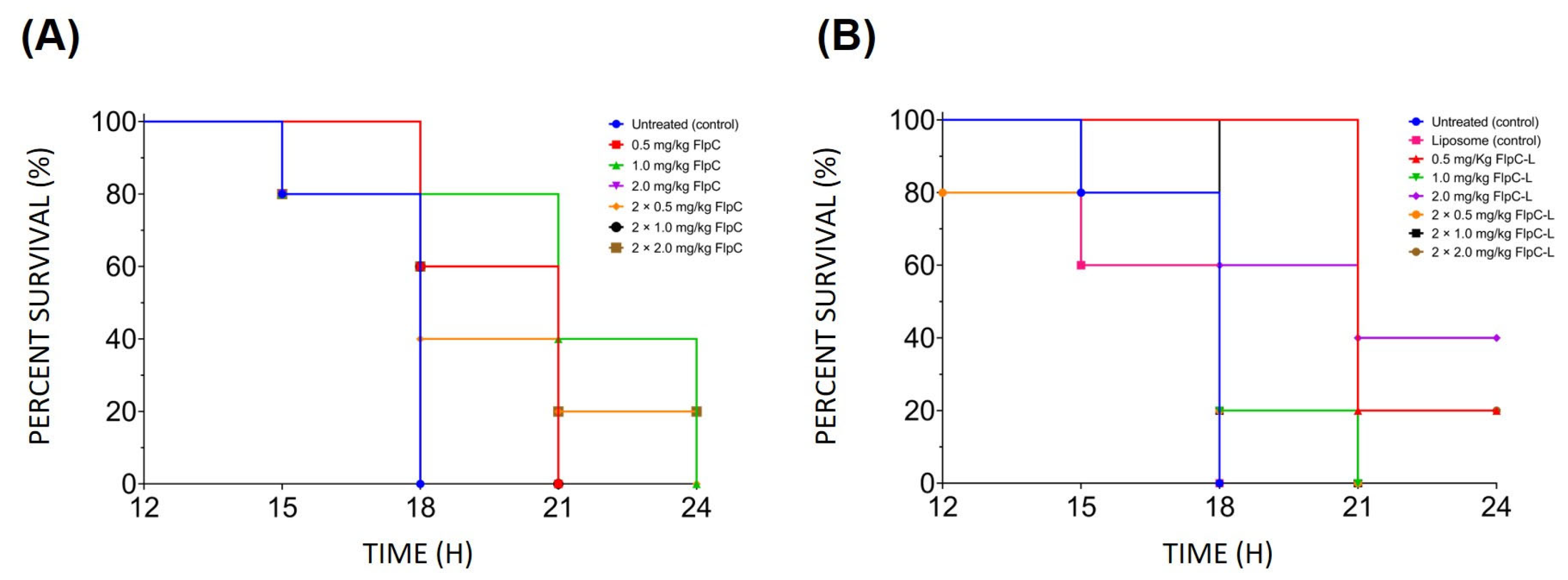
2.3. In Vivo Assessment of Liposomal Fluopsin C Toxicity
2.4. Determination of the Minimum Inhibitory Concentration (MIC) and Minimum Bactericidal Concentration (MBC) of Free and Liposomal Fluopsin C
2.5. Assessment of the Effect of Free and Liposomal Fluopsin C over the Bacteremia Model in Mice
3. Discussion
4. Materials and Methods
4.1. Microorganisms
4.2. Production and Purification of Fluopsin C
4.3. Production and Extrusion of Lipid Vesicles
4.4. Characterization of Fluopsin C-Containing Liposomes
4.4.1. Encapsulation Efficiency
4.4.2. Determination of Free and Liposomal Fluopsin C Release Curve
4.5. Analysis of Liposomal Formulations by Scanning Electron Microscopy (SEM)
4.6. Determination of In Vitro Liposomal Fluopsin C Cytotoxicity
4.7. Determination of the Minimum Inhibitory and Bactericidal Concentration of Free and Liposomal Fluopsin C
4.8. In Vivo Assessments
4.8.1. Animals
4.8.2. Lethal Inoculum of K. pneumoniae KPN-19 and Bacteremia Model Establishment
4.8.3. Lethal Dose for Fluopsin C Liposomal Formulation
4.8.4. Antibacterial Activity of Liposomal and Free Fluopsin C In Vivo
4.8.5. Histopathological Analysis of the Most Effective Dose
4.9. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A

| Liver | Acute Toxicity | Repeated Doses Toxicity | Lipossomal Control | Negative Control | ||||||||
| Time (days) | I | H | N | I | H | N | I | H | N | I | H | N |
| 1 | 1 | 0 | 1 | 1 | 0 | 1 | 1 | 0 | 1 | 0 | 0 | 2 |
| 10 | 1 | 0 | 1 | 1 | 0 | 1 | 1 | 0 | 2 | 0 | 0 | 2 |
| 20 | 0 | 0 | 2 | 0 | 0 | 1 | 1 | 0 | 1 | 0 | 0 | 1 |
| 40 | 0 | 0 | 2 | 0 | 0 | 1 | 0 | 0 | 1 | 0 | 0 | 2 |
| Kidney | Acute Toxicity | Repeated Dose Toxicity | Lipossomal Control | Negative Control | ||||||||
| Time (days) | I | H | N | I | H | N | I | H | N | I | H | N |
| 1 | 1 | 1 | 1 | 0 | 0 | 1 | 0 | 0 | 1 | 0 | 0 | 2 |
| 10 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 1 | 0 | 0 | 2 |
| 20 | 0 | 0 | 1 | 0 | 0 | 2 | 0 | 0 | 1 | 0 | 0 | 2 |
| 40 | 0 | 0 | 2 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 2 |

References
- World Health Organization (WHO). WHO Bacterial Priority Pathogens List, 2024: Bacterial Pathogens of Public Health Importance to Guide Research, Development and Strategies to Prevent and Control Antimicrobial Resistance; World Health Organization: Geneva, Switzerland, 2024; ISBN 978-92-4-010230-9. [Google Scholar]
- World Health Organization (WHO). Global Priority List of Antibiotic-Resistant Bacteria to Guide Research, Discovery, and Development of New Antibiotics; World Health Organization: Geneva, Switzerland, 2017; ISBN 9789240026438. [Google Scholar]
- Kochan, T.J.; Nozick, S.H.; Medernach, R.L.; Cheung, B.H.; Gatesy, S.W.M.; Lebrun-Corbin, M.; Mitra, S.D.; Khalatyan, N.; Fiorella, K.; Chao, Q.; et al. Genomic surveillance for multidrug-resistant or hypervirulent Klebsiella pneumoniae among United States bloodstream isolates. BMC Infect. Dis. 2022, 22, 603. [Google Scholar] [CrossRef]
- Jacobs, D.M.; Safir, M.C.; Huang, D.; Minhaj, F.; Parker, A.; Rao, G.G. Triple Combination Antibiotic Therapy for Carbapenemase-Producing Klebsiella pneumoniae: A Systematic Review. Ann. Clin. Microbiol. Antimicrob. 2017, 16, 76. [Google Scholar] [CrossRef]
- Sheu, C.C.; Chang, Y.T.; Lin, S.Y.; Chen, Y.H.; Hsueh, P.R. Infections Caused by Carbapenem-Resistant Enterobacteriaceae: An Update on Therapeutic Options. Front. Microbiol. 2019, 10, 80. [Google Scholar] [CrossRef]
- Laws, M.; Shaaban, A.; Rahman, K.M. Antibiotic Resistance Breakers: Current Approaches and Future Directions. FEMS Microbiol. Rev. 2019, 43, 490–516. [Google Scholar] [CrossRef]
- Da Silva, A.C.P.; Velasquez, P.A.G. Perfil De Resistência De Klebsiella pneumoniae Isoladas De Pacientes Da Unidade De Terapia Intensiva De Um Hospital No Sudoeste Do Paraná. Discip. Sci.|Saúde 2018, 18, 259–270. [Google Scholar]
- Centers for Disease Control and Prevention. Antibiotic Resistence Threats in the United States; CDC: Atlanta, GA, USA, 2013; 114p.
- Afonso, L.; Grzegorczyk, K.G.; Salomão, J.M.; Basso, K.R.; Alves, L.C.; Silva, M.C.D.; Chryssafidis, A.L.; Gionco-Cano, B.; Yamada-Ogatta, S.F.; Andrade, G. Fluopsin C Promotes Biofilm Removal of XDR Acinetobacter baumannii and Presents an Additive Effect with Polymyxin B on Planktonic Cells. Antibiotics 2024, 13, 875. [Google Scholar] [CrossRef]
- Navarro, M.O.P.; Simionato, A.S.; Pérez, J.C.B.; Barazetti, A.R.; Emiliano, J.; Niekawa, E.T.G.; Andreata, M.F.L.; Modolon, F.; Dealis, M.L.; Araújo, E.J.A.; et al. Fluopsin C for Treating Multidrug-resistant Infections: In vitro Activity against Clinically Important Strains and in vivo Efficacy Against Carbapenemase-producing Kleb pneumoniae. Front. Microbiol. 2019, 10, 2431. [Google Scholar] [CrossRef] [PubMed]
- Kerbauy, G.; Vivian, A.C.P.; Glenda, S.C.; Simionato, A.S.; Pelisson, M.; Vespero, E.; Costa, S.F.; Andrade, C.G.T.J.; Barbieri, D.M.; Mello, J.C.P.; et al. Effect of a Metalloantibiotic Produced by Pseudomonas aeruginosa on Klebsiella pneumoniae Carbapenemase (KPC)-producing K. pneumoniae. Curr. Pharm. Biotechnol. 2016, 17, 389–397. [Google Scholar] [CrossRef]
- Cardozo, V.F.; Oliveira, A.G.; Nishio, E.K.; Perugini, M.R.E.; Andrade, C.G.T.J.; Silveira, W.D.; Durán, N.; Andrade, G.; Kobayashi, R.K.T.; Nakazato, G. Antibacterial Activity of Extracellular Compounds Produced by a Pseudomonas Strain against Methicillin-resistant Staphylococcus aureus (MRSA) strains. Ann. Clin. Microbiol. Antimicrob. 2013, 12, 12. [Google Scholar] [CrossRef] [PubMed]
- Egawa, Y.; Umino, K.; Awataguchi, S.; Kawano, Y.; Okuda, T. Antibiotic YC 73 of Pseudomonas origin. I. Production, Isolation and Properties. J. Antibiot 1970, 23, 267–270. [Google Scholar] [CrossRef]
- Sharma, D. Fluopsin C: A potential candidate against the deadly drug-resistant microbial infections in humans. Future Microbiol. 2020, 15, 381–384. [Google Scholar] [CrossRef] [PubMed]
- Catalano, A.; Iacopetta, D.; Ceramella, J.; Scumaci, D.; Giuzio, F.; Saturnino, C.; Stefano, A.; Rosano, C.; Sinicropi, M.S. Multidrug resistance (MDR): A widespread phenomenon in pharmacological therapies. Molecules 2022, 27, 616. [Google Scholar] [CrossRef] [PubMed]
- Alarfaj, R.E.; Alkhulaifi, M.M.; Al-Fahad, A.J.; Aljihani, S.; Yassin, A.E.B.; Alghoribi, M.F.; Halwani, M.A. Antibacterial efficacy of liposomal formulations containing tobramycin and N-acetylcysteine against tobramycin-resistant Escherichia coli, Klebsiella pneumoniae, and Acinetobacter baumannii. Pharmaceutics 2022, 14, 130. [Google Scholar] [CrossRef] [PubMed]
- Alavi, S.E.; Esfahani, M.K.M.; Raza, A.; Adelnia, H.; Shahmabadi, H.E. PEG-grafted liposomes for enhanced antibacterial and antibiotic activities: An in vivo study. NanoImpact 2022, 25, 100384. [Google Scholar] [CrossRef]
- Khan, A.A.; Allemailem, K.S.; Almatroodi, S.A.; Almatroudi, A.; Rahmani, A.H. Recent strategies towards the surface modification of liposomes: An innovative approach for different clinical applications. 3 Biotech. 2020, 10, 163. [Google Scholar] [CrossRef]
- Li, M.; Du, C.; Guo, N.; Teng, Y.; Meng, X.; Sun, H.; Li, S.; Yu, P.; Galons, H. Composition design and medical application of liposomes. Eur. J. Med. Chem. 2019, 164, 640–653. [Google Scholar] [CrossRef]
- Agência Nacional de Vigilância Sanitária (ANVISA). Guia Para a Condução de Estudos Não Clínicos de Toxicologia e Segurança Farmacológica Necessários ao Desenvolvimento de Medicamentos, 2nd ed.; Gerência de Avaliação de Segurança e Eficácia: Brasília, Brasil, 2013; 48p.
- Wang, D.Y.; Van der Mei, H.C.; Ren, Y.; Busscher, H.J.; Shi, L. Lipid-based antimicrobial delivery-systems for the treatment of bacterial infections. Front. Chem. 2020, 7, 872. [Google Scholar] [CrossRef]
- Eleraky, N.E.; Allam, A.; Hassan, S.B.; Omar, M.M. Nanomedicine fight against antibacterial resistance: An overview of the recent pharmaceutical innovations. Pharmaceutics 2020, 12, 142. [Google Scholar] [CrossRef]
- Weaver, E.; Macartney, R.A.; Irwin, R.; Uddin, S.; Hooker, A.; Burke, G.A.; Wylie, M.P.; Lamprou, D.A. Liposomal encapsulation of amoxicillin via microfluidics with subsequent investigation of the significance of PEGylated therapeutics. Int. J. Pharm. 2024, 650, 123710. [Google Scholar] [CrossRef]
- Shah, V.M.; Nguyen, D.X.; Patel, P.; Cote, B.; Al-Fatease, A.; Pham, Y.; Huynh, M.G.; Woo, Y.; Alani, A.W.G. Liposomes produced by microfluidics and extrusion: A comparison for scale-up purposes. Nanomed. Nanotechnol. Biol. Med. 2019, 8, 146–156. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, L.; Chi, H.; Wang, S. An alternative choice of lidocaine-loaded liposomes: Lidocaine-loaded lipid–polymer hybrid nanoparticles for local anesthetic therapy. Drug Deliv. 2016, 23, 1254–1260. [Google Scholar] [CrossRef]
- Gonzalez Gomez, A.; Hosseinidoust, Z. Liposomes for antibiotic encapsulation and delivery. ACS Infect. Dis. 2020, 6, 896–908. [Google Scholar] [CrossRef] [PubMed]
- Nsairat, H.; Khater, D.; Sayed, U.; Odeh, F.; Al Bawab, A.; Alshaer, W. Liposomes: Structure, composition, types, and clinical applications. Heliyon 2022, 8, e09394. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Grainger, D.W. Lyophilized liposome-based parenteral drug development: Reviewing complex product design strategies and current regulatory environments. Adv. Drug Deliv. Rev. 2019, 151, 56–71. [Google Scholar] [CrossRef]
- Scriboni, A.B.; Couto, V.M.; Ribeiro, L.N.D.M.; Freires, I.A.; Groppo, F.C.; De Paula, E.; Franz-Montan, M.; Cogo-Müller, K. Fusogenic liposomes increase the antimicrobial activity of vancomycin against Staphylococcus aureus biofilm. Front. Pharmacol. 2019, 10, 1401. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.; Chen, G.; Zhang, J. A review of liposomes as a drug delivery system: Current status of approved products, regulatory environments, and future perspectives. Molecules 2022, 27, 1372. [Google Scholar] [CrossRef]
- Werner, J.; Umstätter, F.; Hertlein, T.; Beijer, B.; Kleist, C.; Mühlberg, E.; Zimmermann, S.; Haberkorn, U.; Ohlsen, K.; Fricker, G.; et al. Improved pharmacokinetics and enhanced efficacy of the vancomycin derivative FU002 using a liposomal nanocarrier. Nanomed. Nanotechnol. Biol. Med. 2024, 56, 102731. [Google Scholar] [CrossRef]
- Pushparaj Selvadoss, P.; Nellore, J.; Balaraman Ravindrran, M.; Sekar, U. Novel pyochelin-based PEGylated liposomes for enhanced delivery of antibiotics against resistant clinical isolates of Pseudomonas aeruginosa. Artif. Cells Nanomed. Biotechnol. 2017, 46, 2043–2053. [Google Scholar] [CrossRef]
- Briuglia, M.L.; Rotella, C.; McFarlane, A.; Lamprou, D.A. Influence of cholesterol on liposome stability and on in vitro drug release. Drug Deliv. Transl. Res. 2015, 5, 231–242. [Google Scholar] [CrossRef]
- Fan, X.; Fan, J.; Wang, X.; Wu, P.; Wu, G. S-thanatin functionalized liposome potentially targeting on Klebsiella pneumoniae and its application in sepsis mouse model. Front. Pharmacol. 2015, 6, 249. [Google Scholar] [CrossRef]
- Simionato, A.S.; Cano, B.G.; Navarro, M.O.P.; Tavares, E.R.; Ribeiro, R.A.; Hungria, M.; Yamauchi, L.M.; Yamada-Ogatta, S.F.; Andrade, G. Whole-Genome Sequence of Bioactive Compound-Producing Pseudomonas aeruginosa Strain LV. Microbiol. Resour. Announc. 2021, 10, e01120-20. [Google Scholar] [CrossRef] [PubMed]
- Rampazo, L.G.L. Evaluation of Biological Agents and their Products on the Incidence of Citrus Canker Foliar Lesions. Master’s Thesis, Universidade Estadual de Londrina, Londrina, Brasil, 2004. [Google Scholar]
- Andrade, G. Processo de Produção, Purificação e Obtenção de Substâncias Com Atividades Antibióticas Para o Controle de Doenças Causadas Por Bactérias Em Plantas. Br. Patent PI 0803350-1, 10 September 2008. [Google Scholar]
- Bedoya, J.C.; Dealis, M.L.; Silva, C.S.; Niekawa, E.T.G.; Navarro, M.O.P.; Simionato, A.S.; Modolon, F.; Chryssafidis, A.L.; Andrade, G. Enhanced production of target bioactive metabolites produced by Pseudomonas aeruginosa LV strain. Biocatal. Agric. Biotechnol. 2019, 17, 653–664. [Google Scholar] [CrossRef]
- Ribeiro, L.N.M.; de Paula, E.; Rossi, D.A.; Monteiro, G.P.; Júnior, E.C.V.; Silva, R.R.; Franco, R.R.; Espíndola, F.S.; Goulart, L.R.; Fonseca, B.B. Hybrid pectin-liposome formulation against multi-resistant bacterial strains. Pharmaceutics 2020, 12, 769. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.G.; Jeong, J.H.; Lee, K.M.; Jeong, K.H.; Yang, H.; Kim, M.; Jung, H.; Lee, S.; Choi, Y.W. Nanostructured lipid carrier-loaded hyaluronic acid microneedles for controlled dermal delivery of a lipophilic molecule. Int. J. Nanomed. 2014, 9, 289–299. [Google Scholar] [CrossRef]
- Chierrito, D.; Villas-Boas, C.B.; Tonin, R.S.; Fernandez-Limos, F.; Sanches, A.C.C.; Mello, J.C.P. Using cell cultures for the investigation of treatments for Attention Deficit Hyperactivity Disorder: Systematic review. Curr. Neuropharmacol. 2019, 17, 916–925. [Google Scholar] [CrossRef]
- Clinical and Laboratory Standards Institute (CLSI). Performance Standards for Antimicrobial Susceptibility Testing, 31st ed; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2021. [Google Scholar]


| Formulations | EE (%) |
|---|---|
| SPC+Flp | 66.04 ± 8.19 |
| SPC DMSO+Flp | 83.75 ± 1.95 |
| DSPE-PEG+Flp | 78.04 ± 3.58 |
| DSPE-PEG DMSO+Flp | 83.67 ± 2.06 |
| Formulation | CC50 (µg/mL) | CC90 (µg/mL) |
|---|---|---|
| DSPE-PEG DMSO+Flp | 1.14 | 1.26 |
| DSPE-PEG DMSO | >32.00 | >32.00 |
| SPC DMSO+Flp | 1.26 | 1.60 |
| SPC DMSO | 22.62 | 79.07 |
| Fluopsin C | 0.74 | 0.85 |
| Formulation | SPC 1 (mM) | Cholesterol (mM) | DSPE-PEG 2 (mM) | Fluopsin C (mM) | Phosphate Buffer: DMSO 3 (%) |
|---|---|---|---|---|---|
| DSPE-PEG+Flp | 0.54 | 0.41 | 0.05 | 1 | 100:0 |
| DSPE-PEG DMSO+Flp | 0.54 | 0.41 | 0.05 | 1 | 90:10 |
| SPC+Flp | 0.7 | 0.3 | - | 1 | 100:0 |
| SPC DMSO+Flp | 0.7 | 0.3 | - | 1 | 90:10 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dealis Gomes, M.L.; Afonso, L.; Basso, K.R.; Alves, L.C.; Macías, E.J.N.; Yamada-Ogatta, S.F.; Guidi, A.C.; de Mello, J.C.P.; Andrade, F.G.; Cabeça, L.F.; et al. Liposomal Fluopsin C: Physicochemical Properties, Cytotoxicity, and Antibacterial Activity In Vitro and over In Vivo MDR Klebsiella pneumoniae Bacteremia Model. Antibiotics 2025, 14, 948. https://doi.org/10.3390/antibiotics14090948
Dealis Gomes ML, Afonso L, Basso KR, Alves LC, Macías EJN, Yamada-Ogatta SF, Guidi AC, de Mello JCP, Andrade FG, Cabeça LF, et al. Liposomal Fluopsin C: Physicochemical Properties, Cytotoxicity, and Antibacterial Activity In Vitro and over In Vivo MDR Klebsiella pneumoniae Bacteremia Model. Antibiotics. 2025; 14(9):948. https://doi.org/10.3390/antibiotics14090948
Chicago/Turabian StyleDealis Gomes, Mickely Liuti, Leandro Afonso, Kawany Roque Basso, Leonardo Cruz Alves, Enri Josué Navia Macías, Sueli Fumie Yamada-Ogatta, Ana Carolina Guidi, João Carlos Palazzo de Mello, Fábio Goulart Andrade, Luís Fernando Cabeça, and et al. 2025. "Liposomal Fluopsin C: Physicochemical Properties, Cytotoxicity, and Antibacterial Activity In Vitro and over In Vivo MDR Klebsiella pneumoniae Bacteremia Model" Antibiotics 14, no. 9: 948. https://doi.org/10.3390/antibiotics14090948
APA StyleDealis Gomes, M. L., Afonso, L., Basso, K. R., Alves, L. C., Macías, E. J. N., Yamada-Ogatta, S. F., Guidi, A. C., de Mello, J. C. P., Andrade, F. G., Cabeça, L. F., Cely, M. V. T., & Andrade, G. (2025). Liposomal Fluopsin C: Physicochemical Properties, Cytotoxicity, and Antibacterial Activity In Vitro and over In Vivo MDR Klebsiella pneumoniae Bacteremia Model. Antibiotics, 14(9), 948. https://doi.org/10.3390/antibiotics14090948








