Cold Plasma as a Revolutionary Antimicrobial Modality: A Multifaceted Weapon Against Antibiotic Resistance
Abstract
1. Introduction
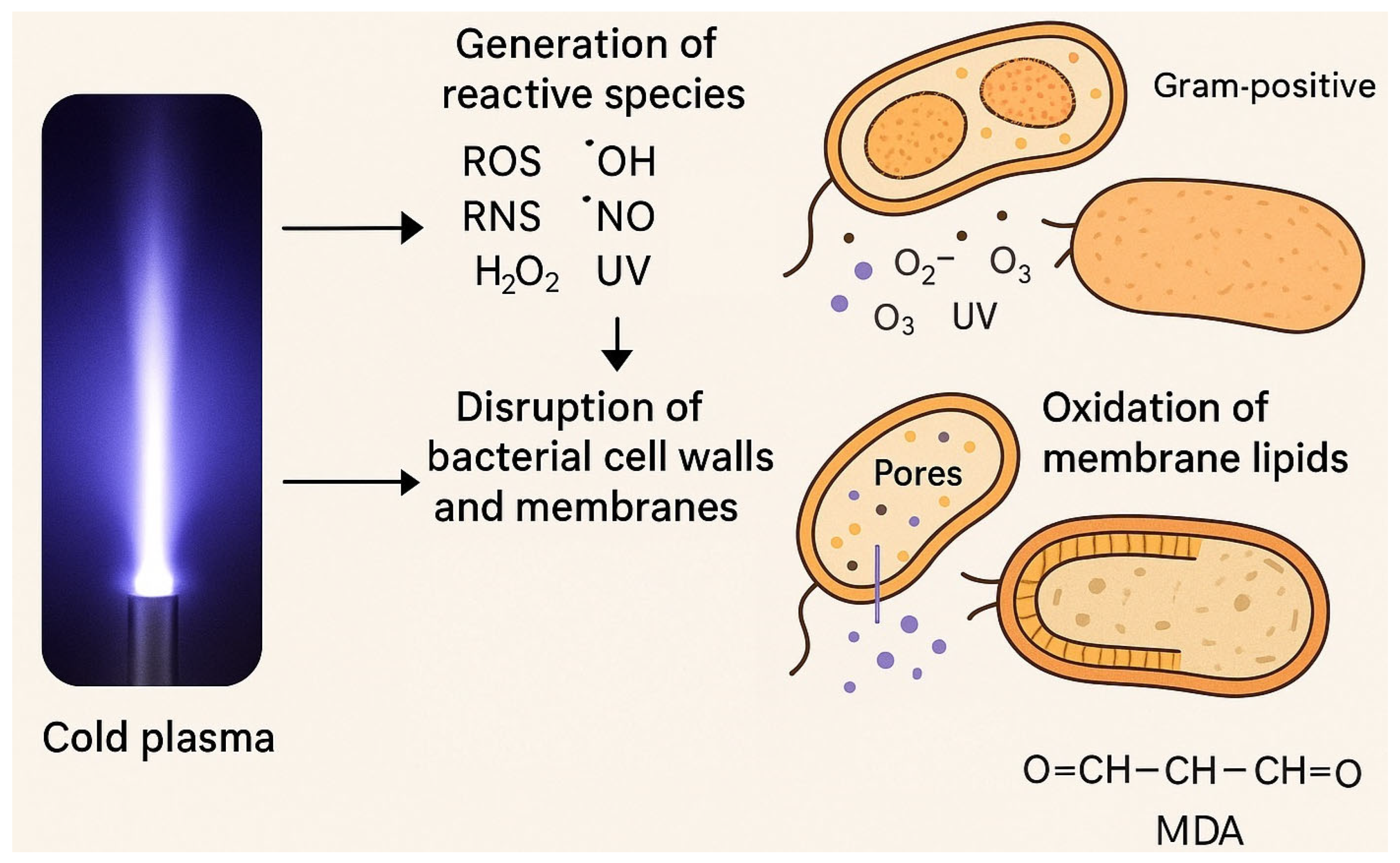
2. Mechanisms of Cold Plasma Action on Bacteria
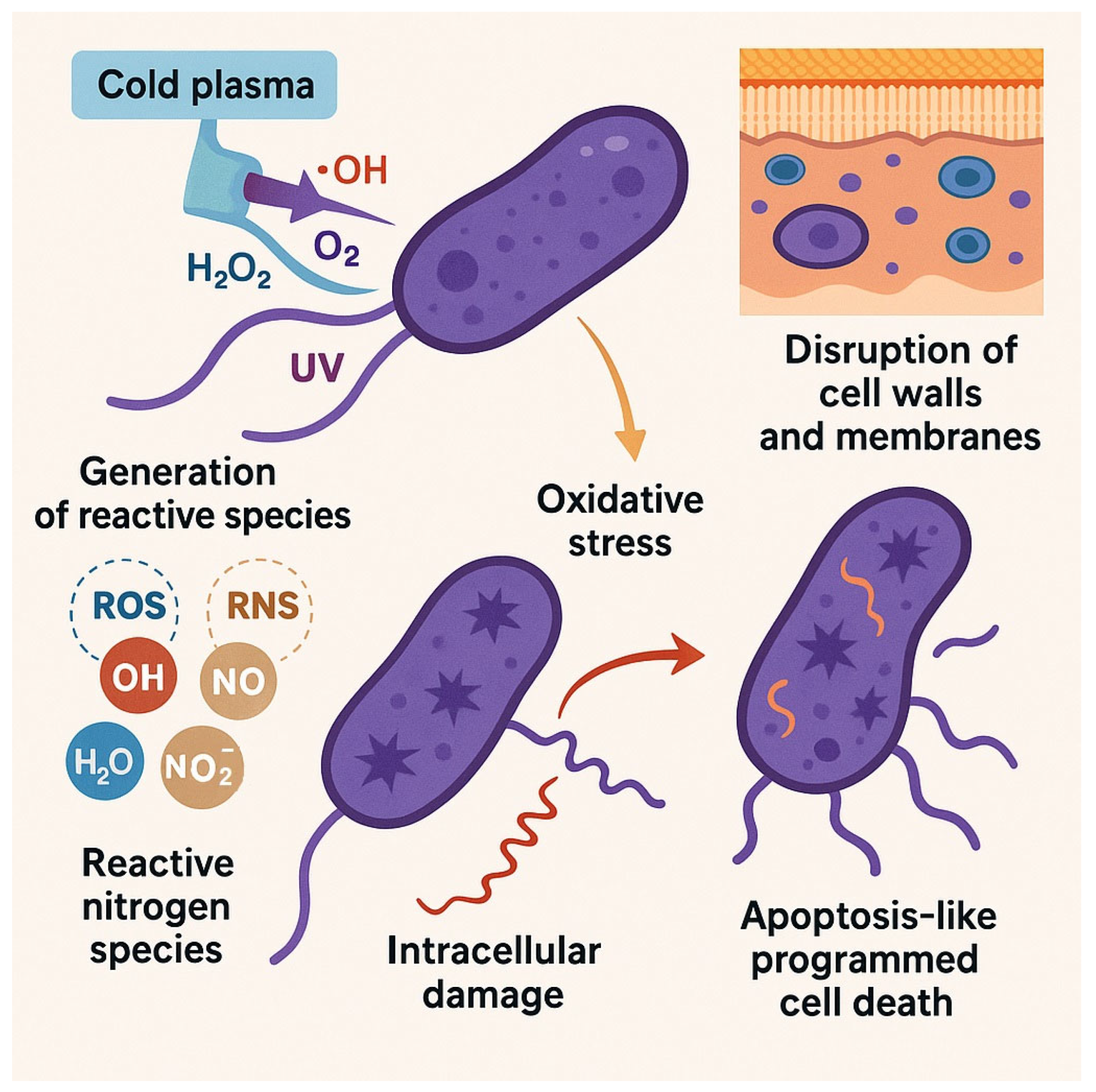
2.1. Generation of Reactive Species
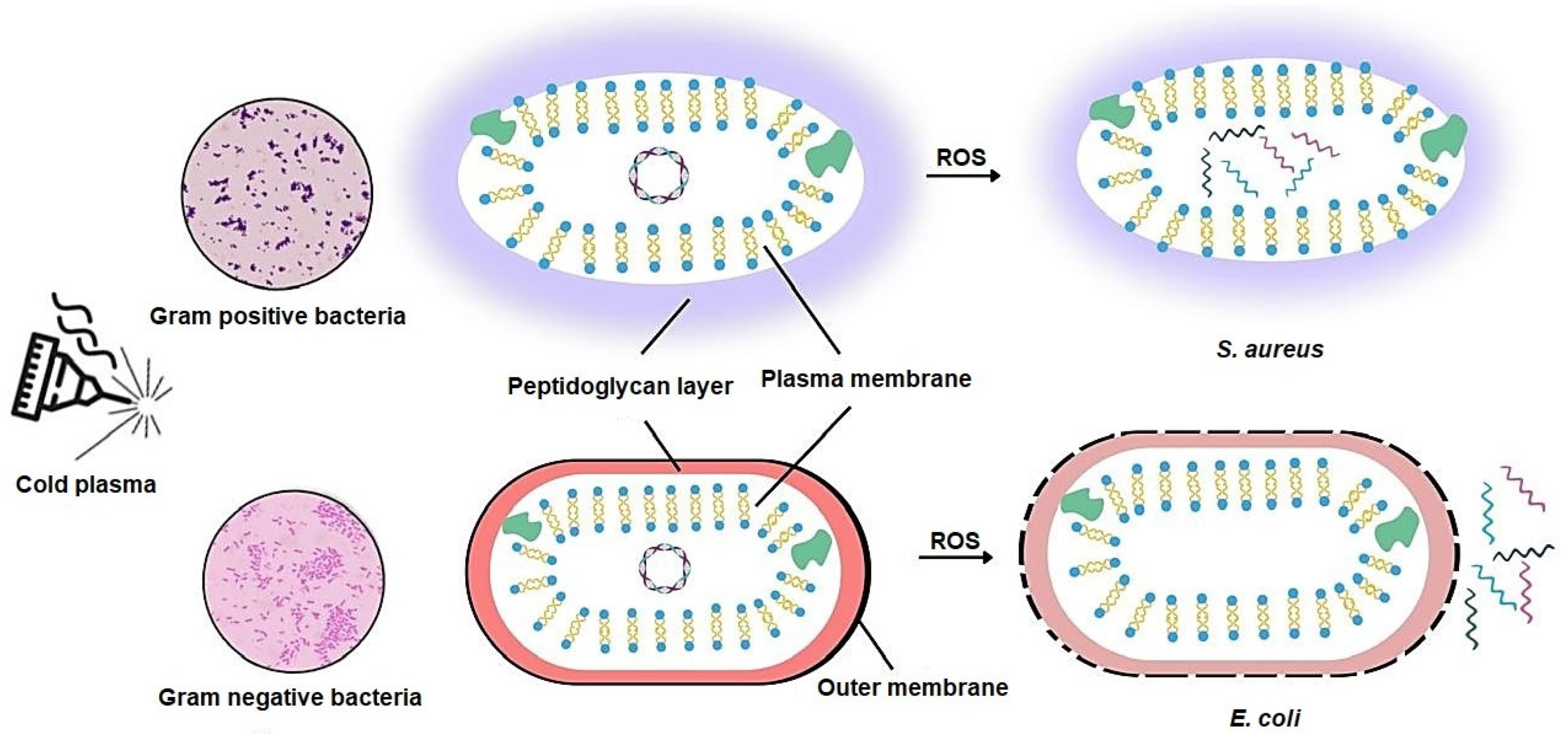
2.2. Disruption of Bacterial Cell Walls and Membranes
2.3. Oxidative Stress and Intracellular Damage
2.4. Effects of Cold Plasma on Bacterial DNA and Proteins
2.5. Induction of Apoptosis-like Cell Death in Bacteria
2.6. Clarification of Certainty in Mechanisms
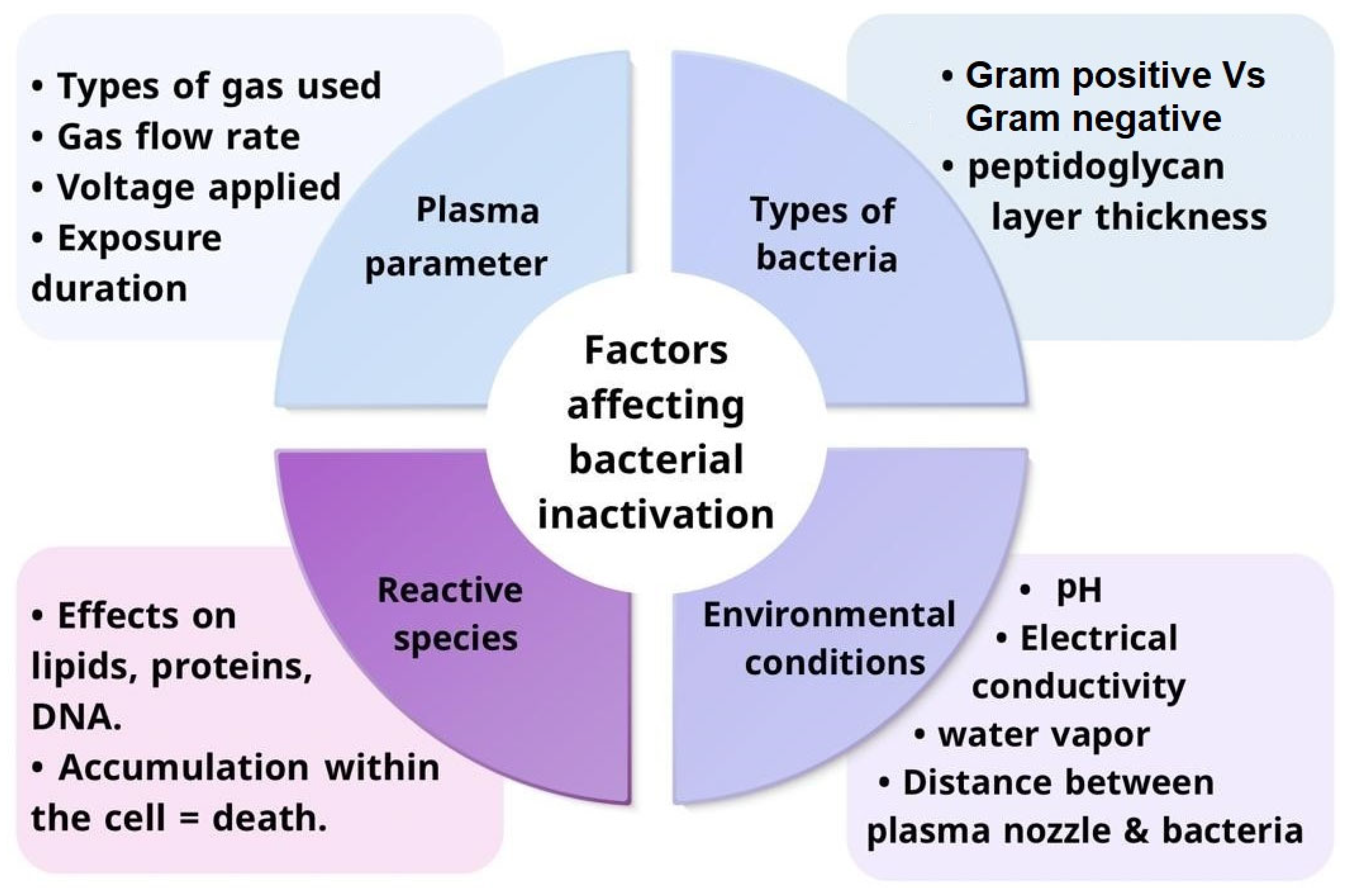
3. Factors Affecting Bacterial Inactivation by Cold Plasma
3.1. Primary Factors
3.1.1. Plasma Parameters and Their Influence on Bacterial Inactivation
3.1.2. Role of Reactive Species in Bacterial Inactivation
3.2. Secondary Factors
3.2.1. Bacterial Types and Their Response to Cold Plasma
3.2.2. Impact of Environmental Conditions on Cold Plasma Efficiency
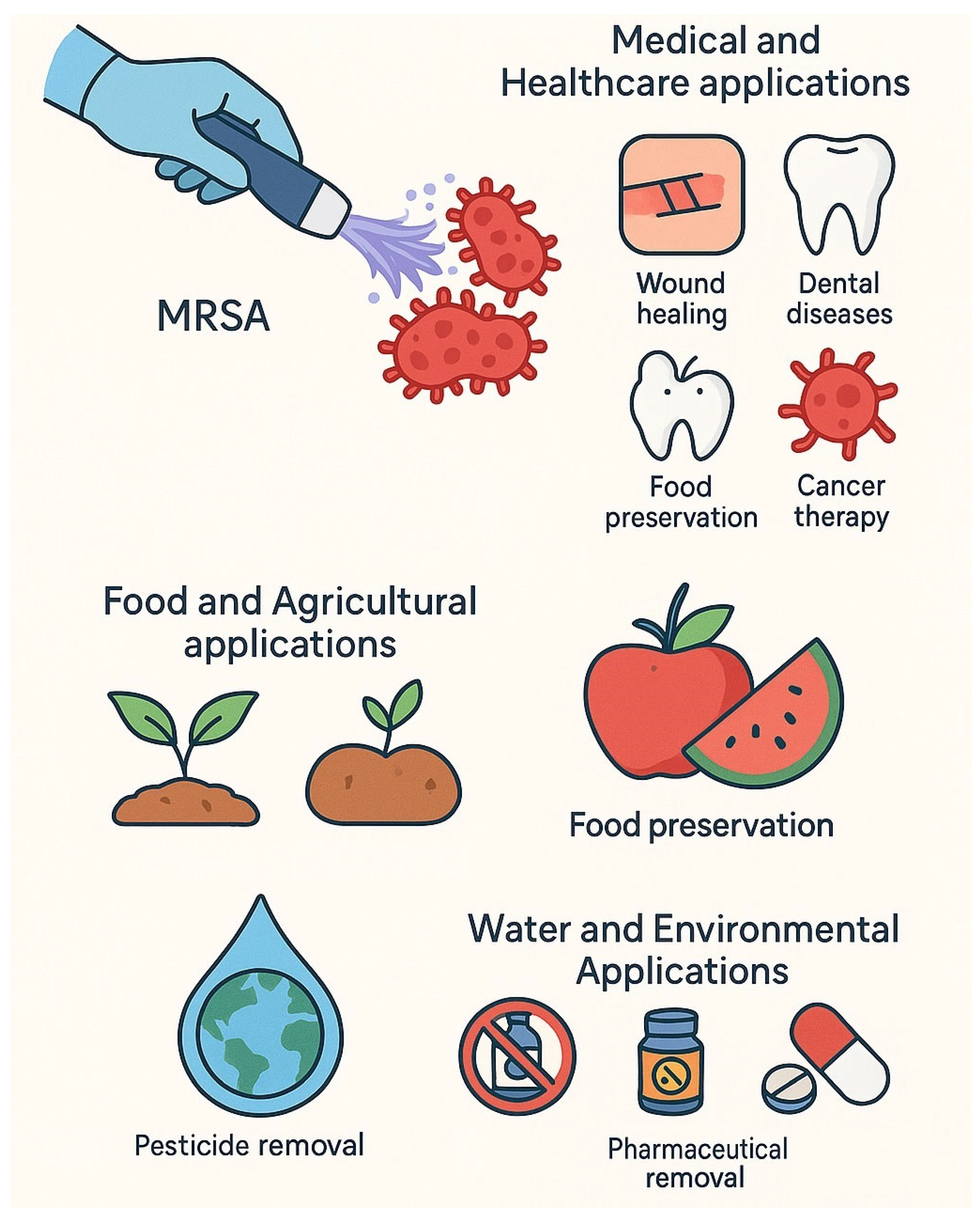
4. Applications of Cold Plasma in Bacterial Treatment
4.1. Medical and Healthcare Applications
4.2. Food and Agricultural Applications
4.3. Water and Environmental Applications
5. Cold Plasma in Combination with Other Antimicrobial Strategies
5.1. Synergistic Effects with Antibiotics
5.2. Enhancement of Bacteriophage Therapy
5.3. Cold Plasma and Nanotechnology-Based Antibacterial Agents
5.4. Use in Combination with UV or Chemical Disinfectants
5.5. Critical Comparison of Combination Strategies
- CAP with Antibiotics: Because of the increased reactive species produced, there is a higher chance of tissue injury when CAP and antibiotics are used together. CAP can decrease the bacterial load and increase the effectiveness of antibiotics, but its use must be carefully managed to avoid cytotoxicity in nearby healthy tissues [124]
- CAP with Bacteriophages: Bacteriophages are known to eliminate unwanted effects on human cells due to their specificity to bacteria. However, due to its strong bactericidal action and the generation of reactive species, the synergistic usage of CAP may still present an indirect cytotoxicity risk. Phage resistance and the immunological response to phage therapy are additional issues that require more research [125,126].
- CAP with Antibacterial Agents Based on Nanotechnology: Although nanoparticles provide improved penetration and targeted distribution, their size, shape, and surface chemistry can all increase their risk of cytotoxicity. For instance, because silver nanoparticles can interact with biological components and produce ionic forms, they can cause cytotoxicity and genotoxicity [127]. When combined with CAP, a thorough assessment of the behavior of the nanoparticles under conditions of oxidative stress generated by the plasma is necessary [128].
- CAP using Chemical or UV Disinfectants: Although these combinations provide better disinfection, there is a chance that the materials will deteriorate and leave behind residues that could harm the environment and human health. Strict safety precautions are required because the oxidative effects of CAP combined with UV or chemical agents can increase these dangers [124].
6. Conclusions
7. Future Prospects
- Standardization of treatment protocols across different plasma sources, treatment parameters, and application fields to ensure reproducibility, comparability between studies, and safety benchmarks.
- Mechanistic studies at the molecular level, particularly regarding the interaction of reactive species with bacterial biomolecules, biofilm matrix components, and potential resistance pathways, to better predict efficacy and limitations.
- Development of portable, cost-effective, and scalable plasma devices for deployment in hospitals, food-processing facilities, agricultural settings, and remote or resource-limited environments, addressing current scalability challenges.
- Assessment of long-term safety and cytotoxicity, especially in applications involving human tissues, medical devices, and food products, with particular emphasis on possible impacts on sensory qualities in food and host microbiota balance in clinical use.
- Clinical trials with larger patient cohorts, as current studies are limited by small sample sizes and short follow-up periods, which restrict generalizability and regulatory approval.
- Regulatory considerations and approval frameworks: collaborative efforts with health and food safety authorities (e.g., FDA, EFSA, Codex) are necessary to establish guidelines for plasma-based technologies to transition from experimental to mainstream adoption.
- Optimization of plasma treatment in food and water applications, addressing current gaps such as variability in microbial inactivation across different matrices and potential effects on nutritional and organoleptic properties of foods.
- Integration with complementary technologies (e.g., antimicrobials, coatings, nanomaterials) to enhance efficacy and reduce variability in outcomes across clinical, industrial, and environmental contexts.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Vaňková, E.; Julák, J.; Machková, A.; Obrová, K.; Klančnik, A.; Smole Možina, S.; Scholtz, V. Overcoming Antibiotic Resistance: Non-Thermal Plasma and Antibiotics Combination Inhibits Important Pathogens. Pathog. Dis. 2024, 82, ftae007. [Google Scholar] [CrossRef]
- Salam, M.d.A.; Al-Amin, M.d.Y.; Salam, M.T.; Pawar, J.S.; Akhter, N.; Rabaan, A.A.; Alqumber, M.A.A. Antimicrobial Resistance: A Growing Serious Threat for Global Public Health. Healthcare 2023, 11, 1946. [Google Scholar] [CrossRef]
- Adelman, M.W.; Connor, A.A.; Hsu, E.; Saharia, A.; Mobley, C.M.; Victor, D.W.; Hobeika, M.J.; Lin, J.; Grimes, K.A.; Ramos, E.; et al. Bloodstream Infections after Solid Organ Transplantation: Clinical Epidemiology and Antimicrobial Resistance (2016–21). JAC-Antimicrob. Resist. 2023, 6, dlad158. [Google Scholar] [CrossRef]
- Ahmed, S.K.; Hussein, S.; Qurbani, K.; Ibrahim, R.H.; Fareeq, A.; Mahmood, K.A.; Mohamed, M.G. Antimicrobial Resistance: Impacts, Challenges, and Future Prospects. J. Med. Surg. Public Health 2024, 2, 100081. [Google Scholar] [CrossRef]
- Kermanshah, H.; Saeedi, R.; Ahmadi, E.; Ranjbar Omrani, L. Efficacy of Cavity Liners with/without Atmospheric Cold Helium Plasma Jet for Dentin Remineralization. Biomater. Investig. Dent. 2020, 7, 120–125. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Zhang, C.; Han, Q. Mechanisms of Bacterial Inhibition and Tolerance around Cold Atmospheric Plasma. Appl. Microbiol. Biotechnol. 2023, 107, 5301–5316. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Xu, Z.; Cheng, C.; Wei, J.; Lan, Y.; Ni, G.; Sun, Q.; Qian, S.; Zhang, H.; Xia, W.; et al. Bactericidal Effects of Plasma Induced Reactive Species in Dielectric Barrier Gas–Liquid Discharge. Plasma Chem. Plasma Process. 2017, 37, 415–431. [Google Scholar] [CrossRef]
- Barjasteh, A.; Kaushik, N.; Choi, E.H.; Kaushik, N.K. Cold Atmospheric Pressure Plasma: A Growing Paradigm in Diabetic Wound Healing—Mechanism and Clinical Significance. Int. J. Mol. Sci. 2023, 24, 16657. [Google Scholar] [CrossRef]
- Gao, X.; Shang, J.; Zhang, M.; Yan, H.; Yang, K.; Wang, Y. Mechanisms Underlying the Antimicrobial Effects of Cold Plasma on Kazachstania Bulderi in Bayberry Juice through Transcriptomic Analysis. LWT 2025, 222, 117336. [Google Scholar] [CrossRef]
- Lunder, M.; Dahle, S.; Fink, R. Cold Atmospheric Plasma for Surface Disinfection: A Promising Weapon against Deleterious Meticillin-Resistant Staphylococcus aureus Biofilms. J. Hosp. Infect. 2024, 143, 64–75. [Google Scholar] [CrossRef]
- Cherif, M.M.; Assadi, I.; Khezami, L.; Ben Hamadi, N.; Assadi, A.A.; Elfalleh, W. Review on Recent Applications of Cold Plasma for Safe and Sustainable Food Production: Principles, Implementation, and Application Limits. Appl. Sci. 2023, 13, 2381. [Google Scholar] [CrossRef]
- Harikrishna, S.; Anil, P.P.; Shams, R.; Dash, K.K. Cold Plasma as an Emerging Nonthermal Technology for Food Processing: A Comprehensive Review. J. Agric. Food Res. 2023, 14, 100747. [Google Scholar] [CrossRef]
- Dharini, M.; Jaspin, S.; Mahendran, R. Cold Plasma Reactive Species: Generation, Properties, and Interaction with Food Biomolecules. Food Chem. 2023, 405, 134746. [Google Scholar] [CrossRef]
- Fitzpatrick, R. Plasma Physics: An Introduction, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2022. [Google Scholar] [CrossRef]
- Mai-Prochnow, A.; Clauson, M.; Hong, J.; Murphy, A.B. Gram Positive and Gram Negative Bacteria Differ in Their Sensitivity to Cold Plasma. Sci. Rep. 2016, 6, 38610. [Google Scholar] [CrossRef] [PubMed]
- Hasirci, V.; Hasirci, N. Control of Polymeric Biomaterial Surfaces. In Surfaces and Interfaces for Biomaterials; Elsevier: Amsterdam, The Netherlands, 2005; pp. 29–59. [Google Scholar] [CrossRef]
- Braný, D.; Dvorská, D.; Halašová, E.; Škovierová, H. Cold Atmospheric Plasma: A Powerful Tool for Modern Medicine. Int. J. Mol. Sci. 2020, 21, 2932. [Google Scholar] [CrossRef] [PubMed]
- Hnatiuc, E.; Astanei, D.; Ursache, M.; Hnatiuc, B.; Brisset, J. A Review over the Cold Plasma Reactors and Their Applications. In Proceedings of the 2012 International Conference and Exposition on Electrical and Power Engineering, Iasi, Romania, 25–27 October 2012; pp. 497–502. [Google Scholar] [CrossRef]
- Lata, S.; Chakravorty, S.; Mitra, T.; Pradhan, P.K.; Mohanty, S.; Patel, P.; Jha, E.; Panda, P.K.; Verma, S.K.; Suar, M. Aurora Borealis in Dentistry: The Applications of Cold Plasma in Biomedicine. Mater. Today Bio 2022, 13, 100200. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.J.; Lim, D.J.; Lee, M.Y.; Lee, W.J.; Chang, S.E.; Won, C.H. Prospective, Comparative Clinical Pilot Study of Cold Atmospheric Plasma Device in the Treatment of Atopic Dermatitis. Sci. Rep. 2021, 11, 14461. [Google Scholar] [CrossRef]
- Laroussi, M. Cold Plasma in Medicine and Healthcare: The New Frontier in Low Temperature Plasma Applications. Front. Phys. 2020, 8, 74. [Google Scholar] [CrossRef]
- Stryczewska, H.D.; Jakubowski, T.; Kalisiak, S.; Giżewski, T.; Pawłat, J. Power Systems of Plasma Reactors for Non-Thermal Plasma Generation. J. Adv. Oxid. Technol. 2013, 16. [Google Scholar] [CrossRef]
- Niedźwiedź, I.; Waśko, A.; Pawłat, J.; Polak-Berecka, M. The State of Research on Antimicrobial Activity of Cold Plasma. Pol. J. Microbiol. 2019, 68, 153–164. [Google Scholar] [CrossRef]
- Talukdar, P.; Gohain, R.B.; Bharadwaj, P.; Thakur, D.; Biswas, S. Inactivation of Candida albicans, Staphylococcus aureus and Multidrug-Resistant Escherichia coli with Dielectric Barrier Discharged Cold Atmospheric Plasma: A Comparative Study with Antimicrobial Drugs. J. Med. Microbiol. 2025, 74. [Google Scholar] [CrossRef] [PubMed]
- Nicol, M.J.; Brubaker, T.R.; Honish, B.J.; Simmons, A.N.; Kazemi, A.; Geissel, M.A.; Whalen, C.T.; Siedlecki, C.A.; Bilén, S.G.; Knecht, S.D.; et al. Antibacterial Effects of Low-Temperature Plasma Generated by Atmospheric-Pressure Plasma Jet Are Mediated by Reactive Oxygen Species. Sci. Rep. 2020, 10, 3066. [Google Scholar] [CrossRef] [PubMed]
- Murali, R.; Singh, P.; Ragunathan, D.; Damarla, R.; Kichenaradjou, D.; Surriyanarayanan, K.M.; Jayaram, S.K.; Chandramoorthy, H.C.; Kumar, A.; Krishnan, M.E.G.; et al. Antimicrobial Activity of Cold Atmospheric Plasma on Bacterial Strains Derived from Patients with Diabetic Foot Ulcers. J. Microbiol. Biotechnol. 2024, 34, 2353–2361. [Google Scholar] [CrossRef] [PubMed]
- Von Woedtke, T.; Emmert, S.; Metelmann, H.-R.; Rupf, S.; Weltmann, K.-D. Perspectives on Cold Atmospheric Plasma (CAP) Applications in Medicine. Phys. Plasmas 2020, 27, 070601. [Google Scholar] [CrossRef]
- Kondeti, V.S.S.K.; Phan, C.Q.; Wende, K.; Jablonowski, H.; Gangal, U.; Granick, J.L.; Hunter, R.C.; Bruggeman, P.J. Long-Lived and Short-Lived Reactive Species Produced by a Cold Atmospheric Pressure Plasma Jet for the Inactivation of Pseudomonas aeruginosa and Staphylococcus aureus. Free Radic. Biol. Med. 2018, 124, 275–287. [Google Scholar] [CrossRef]
- Hu, J.; Zhang, Y.; Pan, W.; Han, Q.; Wei, Y.; Li, Y.; Hu, Y.; Ying, X.; Armani, A.; Guidi, A.; et al. Antibacterial Mechanism of Atmospheric Cold Plasma against Pseudomonas fluorescens and Pseudomonas putida and Its Preservation Application on In-Packaged Red Shrimp Paste. Food Chem. 2025, 464, 141590. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, Y.; Zhao, Y.; Sun, Y.; Duan, M.; Wang, H.; Dai, R.; Liu, Y.; Li, X.; Jia, F. Bactericidal Efficacy Difference between Air and Nitrogen Cold Atmospheric Plasma on Bacillus cereus: Inactivation Mechanism of Gram-Positive Bacteria at the Cellular and Molecular Level. Food Res. Int. 2023, 173, 113204. [Google Scholar] [CrossRef]
- Lv, X.; Cheng, J.-H. Evaluation of the Effects of Cold Plasma on Cell Membrane Lipids and Oxidative Injury of Salmonella typhimurium. Molecules 2022, 27, 640. [Google Scholar] [CrossRef]
- Han, L.; Patil, S.; Boehm, D.; Milosavljević, V.; Cullen, P.J.; Bourke, P. Mechanisms of Inactivation by High-Voltage Atmospheric Cold Plasma Differ for Escherichia coli and Staphylococcus aureus. Appl. Environ. Microbiol. 2016, 82, 450–458. [Google Scholar] [CrossRef]
- Dahle, S.; Žigon, J.; Fink, R. Cold Plasma for Sustainable Control of Hygienically Relevant Biofilms. The Interaction of Plasma Distance and Exposure Time. Int. J. Environ. Health Res. 2024, 34, 340–354. [Google Scholar] [CrossRef]
- Sizar, O.; Leslie, S.W.; Unakal, C.G. Gram-Positive Bacteria. In StatPearls; StatPearls Publishing: St. Petersburg, FL, USA, 2025. Available online: http://www.ncbi.nlm.nih.gov/books/NBK470553/ (accessed on 2 July 2025).
- Dezest, M.; Bulteau, A.-L.; Quinton, D.; Chavatte, L.; Le Bechec, M.; Cambus, J.P.; Arbault, S.; Nègre-Salvayre, A.; Clément, F.; Cousty, S. Oxidative Modification and Electrochemical Inactivation of Escherichia coli upon Cold Atmospheric Pressure Plasma Exposure. PLOS ONE 2017, 12, e0173618. [Google Scholar] [CrossRef]
- Patange, A.; O’Byrne, C.; Boehm, D.; Cullen, P.J.; Keener, K.; Bourke, P. The Effect of Atmospheric Cold Plasma on Bacterial Stress Responses and Virulence Using Listeria monocytogenes Knockout Mutants. Front. Microbiol. 2019, 10, 2841. [Google Scholar] [CrossRef]
- Fasnacht, M.; Polacek, N. Oxidative Stress in Bacteria and the Central Dogma of Molecular Biology. Front. Mol. Biosci. 2021, 8, 671037. [Google Scholar] [CrossRef] [PubMed]
- Sies, H. Oxidative Stress. In Stress: Physiology, Biochemistry, and Pathology; Elsevier: Amsterdam, The Netherlands, 2019; pp. 153–163. [Google Scholar] [CrossRef]
- Ibrahim Ahmed, D.; Tawfik Kashef, M.; Nabil Mohamed El-Tayeb, W.; El-Dien Mahmoud Shawky Hosny, A. Variable Bacterial Responses to Oxidative Stress in Different Bacterial Species. Al-Azhar Med. J. 2022, 51, 1825–1836. [Google Scholar] [CrossRef]
- Seixas, A.F.; Quendera, A.P.; Sousa, J.P.; Silva, A.F.Q.; Arraiano, C.M.; Andrade, J.M. Bacterial Response to Oxidative Stress and RNA Oxidation. Front. Genet. 2022, 12, 821535. [Google Scholar] [CrossRef] [PubMed]
- Vaishampayan, A.; Grohmann, E. Antimicrobials Functioning through ROS-Mediated Mechanisms: Current Insights. Microorganisms 2021, 10, 61. [Google Scholar] [CrossRef]
- Choudhary, D.; Foster, K.R.; Uphoff, S. The Master Regulator OxyR Orchestrates Bacterial Oxidative Stress Response Genes in Space and Time. Cell Syst. 2024, 15, 1033–1045.e6. [Google Scholar] [CrossRef]
- Yuan, F.; Yin, S.; Xu, Y.; Xiang, L.; Wang, H.; Li, Z.; Fan, K.; Pan, G. The Richness and Diversity of Catalases in Bacteria. Front. Microbiol. 2021, 12, 645477. [Google Scholar] [CrossRef]
- Broxton, C.N.; Culotta, V.C. SOD Enzymes and Microbial Pathogens: Surviving the Oxidative Storm of Infection. PLOS Pathog. 2016, 12, e1005295. [Google Scholar] [CrossRef]
- Lu, H.; Patil, S.; Keener, K.M.; Cullen, P.J.; Bourke, P. Bacterial Inactivation by High-Voltage Atmospheric Cold Plasma: Influence of Process Parameters and Effects on Cell Leakage and DNA. J. Appl. Microbiol. 2014, 116, 784–794. [Google Scholar] [CrossRef]
- Patenall, B.L.; Hathaway, H.J.; Laabei, M.; Young, A.E.; Thet, N.T.; Jenkins, A.T.A.; Short, R.D.; Allinson, S.L. Assessment of Mutations Induced by Cold Atmospheric Plasma Jet Treatment Relative to Known Mutagens in Escherichia coli. Mutagenesis 2021, 36, 380–387. [Google Scholar] [CrossRef]
- Coutinho, N.M.; Silveira, M.R.; Rocha, R.S.; Moraes, J.; Ferreira, M.V.S.; Pimentel, T.C.; Freitas, M.Q.; Silva, M.C.; Raices, R.S.L.; Ranadheera, C.S.; et al. Cold Plasma Processing of Milk and Dairy Products. Trends Food Sci. Technol. 2018, 74, 56–68. [Google Scholar] [CrossRef]
- Smith, B.T.; Grossman, A.D.; Walker, G.C. Localization of UvrA and Effect of DNA Damage on the Chromosome of Bacillus Subtilis. J. Bacteriol. 2002, 184, 488–493. [Google Scholar] [CrossRef] [PubMed]
- Mohseni, P.; Ghorbani, A.; Fariborzi, N. Exploring the Potential of Cold Plasma Therapy in Treating Bacterial Infections in Veterinary Medicine: Opportunities and Challenges. Front. Vet. Sci. 2023, 10, 1240596. [Google Scholar] [CrossRef]
- Abbasi, E.; Mehrabadi, J.F.; Nourani, M.; Nazar Namini, Y.; Mohammadi, S.; Esmaeili, D.; Abbasi, A. Evaluation of Cold Atmospheric-Pressure Plasma against Burn Wound Infections and Gene Silencing. Iran. J. Microbiol. 2021. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Tang, W.; Zhang, X.; Song, Z.; Tong, T. An Overview of Stimuli-Responsive Intelligent Antibacterial Nanomaterials. Pharmaceutics 2023, 15, 2113. [Google Scholar] [CrossRef] [PubMed]
- Bárdos, L.; Baránková, H. Cold Atmospheric Plasma: Sources, Processes, and Applications. Thin Solid Films 2010, 518, 6705–6713. [Google Scholar] [CrossRef]
- Ding, T.; Cullen, P.J.; Yan, W. (Eds.) Applications of Cold Plasma in Food Safety; Springer Singapore: Singapore, 2022; ISBN 978-981-16-1826-0. [Google Scholar]
- Hori, M. Radical-Controlled Plasma Processes. Rev. Mod. Plasma Phys. 2022, 6, 36. [Google Scholar] [CrossRef]
- Xu, Y.; Yuan, H.; Wang, H.; Lu, K.; Yang, D. Effectiveness of Noble Gas Addition for Plasma Synthesis of Ammonia in a Dielectric Barrier Discharge Reactor. Appl. Sci. 2024, 14, 3001. [Google Scholar] [CrossRef]
- Das, S.; Gajula, V.P.; Mohapatra, S.; Singh, G.; Kar, S. Role of Cold Atmospheric Plasma in Microbial Inactivation and the Factors Affecting Its Efficacy. Health Sci. Rev. 2022, 4, 100037. [Google Scholar] [CrossRef]
- Kwun, M.S.; Lee, D.G. Bacterial Apoptosis-Like Death through Accumulation of Reactive Oxygen Species by Quercetin in Escherichia coli. J. Microbiol. Biotechnol. 2024, 34, 1395–1400. [Google Scholar] [CrossRef]
- Rout, S.; Panda, P.K.; Dash, P.; Srivastav, P.P.; Hsieh, C.-T. Cold Plasma-Induced Modulation of Protein and Lipid Macromolecules: A Review. Int. J. Mol. Sci. 2025, 26, 1564. [Google Scholar] [CrossRef]
- Ding, H.; Wang, T.; Sun, Y.; Zhang, Y.; Wei, J.; Cai, R.; Guo, C.; Yuan, Y.; Yue, T. Role and Mechanism of Cold Plasma in Inactivating Alicyclobacillus acidoterrestris in Apple Juice. Foods 2023, 12, 1531. [Google Scholar] [CrossRef]
- Barjasteh, A.; Kaushik, N.; Choi, E.H.; Kaushik, N.K. Cold Atmospheric Pressure Plasma Solutions for Sustainable Food Packaging. Int. J. Mol. Sci. 2024, 25, 6638. [Google Scholar] [CrossRef]
- Pankaj, S.K.; Keener, K.M. Cold Plasma: Background, Applications and Current Trends. Curr. Opin. Food Sci. 2017, 16, 49–52. [Google Scholar] [CrossRef]
- Seong, M.-J.; Park, K.-R.; Kim, S.J.; Joh, H.-M.; Moon, H.; Chung, T.H. Characterization and Bacterial Inactivation Application of Multiple Pin-to-Plate Electrode-Based DBD Plasma Driven by Nanosecond-Pulsed High Voltage. Phys. Plasmas 2025, 32, 023504. [Google Scholar] [CrossRef]
- Ikawa, S.; Tani, A.; Nakashima, Y.; Kitano, K. Physicochemical Properties of Bactericidal Plasma-Treated Water. J. Phys. Appl. Phys. 2016, 49, 425401. [Google Scholar] [CrossRef]
- Deepak, G.D. Atul. Biomedical Applications of Cold Plasma. J. Clin. Diagn. Res. 2022. [Google Scholar] [CrossRef]
- Chen, Z.; Chen, G.; Obenchain, R.; Zhang, R.; Bai, F.; Fang, T.; Wang, H.; Lu, Y.; Wirz, R.E.; Gu, Z. Cold Atmospheric Plasma Delivery for Biomedical Applications. Mater. Today 2022, 54, 153–188. [Google Scholar] [CrossRef]
- Lunov, O.; Zablotskii, V.; Churpita, O.; Jäger, A.; Polívka, L.; Syková, E.; Dejneka, A.; Kubinová, Š. The Interplay between Biological and Physical Scenarios of Bacterial Death Induced by Non-Thermal Plasma. Biomaterials 2016, 82, 71–83. [Google Scholar] [CrossRef] [PubMed]
- Avellar, H.K.; Williams, M.R.; Brandão, J.; Narayanan, S.; Ramachandran, A.; Holbrook, T.C.; Schoonover, M.J.; Bailey, K.L.; Payton, M.E.; Pai, K.K.; et al. Safety and Efficacy of Cold Atmospheric Plasma for the Sterilization of a Pasteurella multocida–Contaminated Subcutaneously Implanted Foreign Body in Rabbits. Am. J. Vet. Res. 2021, 82, 118–124. [Google Scholar] [CrossRef] [PubMed]
- Nam, S.-H.; Kim, M.-K.; Choi, J.-O.; Kim, G.-C. No-Ozone Cold Atmospheric Plasma Makes Distilled Water an Effective Tooth Bleaching Gel. Appl. Sci. 2024, 14, 5896. [Google Scholar] [CrossRef]
- Wagner, G.; Eggers, B.; Duddeck, D.; Kramer, F.-J.; Bourauel, C.; Jepsen, S.; Deschner, J.; Nokhbehsaim, M. Influence of Cold Atmospheric Plasma on Dental Implant Materials — an in Vitro Analysis. Clin. Oral Investig. 2022, 26, 2949–2963. [Google Scholar] [CrossRef] [PubMed]
- Gao, R.; He, H.; Yang, X.; Wang, W.; Gao, J.; Yang, C. Cold Atmospheric Plasma and Skin Wound Healing: Influence on Microbial Diversity and Composition. BMC Microbiol. 2025, 25, 260. [Google Scholar] [CrossRef]
- Jensen, J.-O.; Schulz, L.; Schleusser, S.; Matzkeit, N.; Stang, F.H.; Mailaender, P.; Kraemer, R.; Kleemann, M.; Deichmann, H.; Kisch, T. The Repetitive Application of Cold Atmospheric Plasma (CAP) Improves Microcirculation Parameters in Chronic Wounds. Microvasc. Res. 2021, 138, 104220. [Google Scholar] [CrossRef]
- Desai, M.; Chandel, A.; Chauhan, O.P.; Semwal, A.D. Uses and Future Prospects of Cold Plasma in Agriculture. Food Humanity 2024, 2, 100262. [Google Scholar] [CrossRef]
- Sharma, R.; Nath, P.C.; Rustagi, S.; Sharma, M.; Inbaraj, B.S.; Dikkala, P.K.; Nayak, P.K.; Sridhar, K. Cold Plasma—A Sustainable Energy-Efficient Low-Carbon Food Processing Technology: Physicochemical Characteristics, Microbial Inactivation, and Industrial Applications. Int. J. Food Sci. 2025, 2025, 4166141. [Google Scholar] [CrossRef]
- Bahreini, M.; Anvar, S.A.; Nowruzi, B.; Sari, A. Effects of the Cold Atmospheric Plasma Treatment Technology on Staphylococcus aureus and Escherichia coli Populations in Raw Milk. J. Nutr. Health 2021, 9. [Google Scholar] [CrossRef]
- Aguilar Uscanga, B.R.; Calderón Santoyo, M.; Ragazzo Sánchez, J.A.; Alemán Duarte, M.I.; Pérez Montaño, J.A.; Balcázar-López, E.; Solís Pacheco, J.R. Effect of the Application of Cold Plasma Energy on the Inactivation of Microorganisms, Proteins, and Lipids Deterioration in Adobera Cheese. J. Food Qual. 2022, 2022, 1–9. [Google Scholar] [CrossRef]
- Bagheri, H.; Abbaszadeh, S. Effect of Cold Plasma on Quality Retention of Fresh-Cut Produce. J. Food Qual. 2020, 2020, 1–8. [Google Scholar] [CrossRef]
- Ben Amor, I.; Hemmami, H.; Zeghoud, S.; Zahnit, W.; Nedjoud, G.; Ferhat, M.F.; Messaoudi, M. Cold Plasma Technology in Wastewater Treatment: Mechanisms, Applications, and Key Challenges for Organic Pollutant Removal. Int. J. Environ. Sci. Technol. 2025, 22, 10895–10912. [Google Scholar] [CrossRef]
- Nguyen, D.V.; Ho, N.M.; Hoang, K.D.; Le, T.V.; Le, V.H. An Investigation on Treatment of Groundwater with Cold Plasma for Domestic Water Supply. Groundw. Sustain. Dev. 2020, 10, 100309. [Google Scholar] [CrossRef]
- Rashmei, Z.; Bornasi, H.; Ghoranneviss, M. Evaluation of Treatment and Disinfection of Water Using Cold Atmospheric Plasma. J. Water Health 2016, 14, 609–616. [Google Scholar] [CrossRef] [PubMed]
- Maybin, J.-A.; McClenaghan, L.A.; Gilmore, B.F.; Thompson, T.P. Cold Plasma for Enhanced Water Purification. Sustain. Microbiol. 2024, 1, qvae032. [Google Scholar] [CrossRef]
- Kumar, A.; Škoro, N.; Gernjak, W.; Puač, N. Cold Atmospheric Plasma Technology for Removal of Organic Micropollutants from Wastewater—a Review. Eur. Phys. J. D 2021, 75, 283. [Google Scholar] [CrossRef]
- Wypart-Pawul, A.; Neczaj, E.; Grosser, A.; Grobelak, A. Assessment of the Effectiveness of Atmospheric Plasma on the Removal of Selected Pharmaceuticals from Water. Desalination Water Treat. 2024, 320, 100600. [Google Scholar] [CrossRef]
- Wielogorska, E.; Flynn, P.B.; Meneely, J.; Thompson, T.P.; Graham, W.G.; Gilmore, B.F.; Elliott, C.T. Assessment of Cold Atmospheric Pressure Plasma (CAPP) Treatment for Degradation of Antibiotic Residues in Water. Antibiotics 2023, 12, 1115. [Google Scholar] [CrossRef]
- Moldgy, A.; Nayak, G.; Aboubakr, H.A.; Goyal, S.M.; Bruggeman, P.J. Inactivation of Virus and Bacteria Using Cold Atmospheric Pressure Air Plasmas and the Role of Reactive Nitrogen Species. J. Phys. Appl. Phys. 2020, 53, 434004. [Google Scholar] [CrossRef]
- Xiang, Q.; Fan, L.; Li, Y.; Dong, S.; Li, K.; Bai, Y. A Review on Recent Advances in Plasma-Activated Water for Food Safety: Current Applications and Future Trends. Crit. Rev. Food Sci. Nutr. 2022, 62, 2250–2268. [Google Scholar] [CrossRef]
- Yang, L.; Niyazi, G.; Qi, Y.; Yao, Z.; Huang, L.; Wang, Z.; Guo, L.; Liu, D. Plasma-Activated Saline Promotes Antibiotic Treatment of Systemic Methicillin-Resistant Staphylococcus aureus Infection. Antibiotics 2021, 10, 1018. [Google Scholar] [CrossRef]
- Rapacka-Zdonczyk, A.; Wozniak, A.; Nakonieczna, J.; Grinholc, M. Development of Antimicrobial Phototreatment Tolerance: Why the Methodology Matters. Int. J. Mol. Sci. 2021, 22, 2224. [Google Scholar] [CrossRef]
- Charoux, C.M.G.; Patange, A.D.; Hinds, L.M.; Simpson, J.C.; O’Donnell, C.P.; Tiwari, B.K. Antimicrobial Effects of Airborne Acoustic Ultrasound and Plasma Activated Water from Cold and Thermal Plasma Systems on Biofilms. Sci. Rep. 2020, 10, 17297. [Google Scholar] [CrossRef] [PubMed]
- Shabani, H.; Dezhpour, A.; Jafari, S.; Moghaddam, M.J.M.; Nilkar, M. Antimicrobial Activity of Cold Atmospheric-Pressure Argon Plasma Combined with Chicory (Cichorium Intybus L.) Extract against P. aeruginosa and E. coli Biofilms. Sci. Rep. 2023, 13, 9441. [Google Scholar] [CrossRef] [PubMed]
- Prasad, K.; Sasi, S.; Weerasinghe, J.; Levchenko, I.; Bazaka, K. Enhanced Antimicrobial Activity through Synergistic Effects of Cold Atmospheric Plasma and Plant Secondary Metabolites: Opportunities and Challenges. Molecules 2023, 28, 7481. [Google Scholar] [CrossRef] [PubMed]
- Xiao, G.; Li, J.; Sun, Z. The Combination of Antibiotic and Non-Antibiotic Compounds Improves Antibiotic Efficacy against Multidrug-Resistant Bacteria. Int. J. Mol. Sci. 2023, 24, 15493. [Google Scholar] [CrossRef]
- Xia, Y.; Wei, X.; Gao, P.; Wang, C.; De Jong, A.; Chen, J.H.K.; Rodríguez-Sánchez, M.J.; Rodríguez-Nogales, A.; Diez-Echave, P.; Gálvez, J.; et al. Bismuth-Based Drugs Sensitize Pseudomonas aeruginosa to Multiple Antibiotics by Disrupting Iron Homeostasis. Nat. Microbiol. 2024, 9, 2600–2613. [Google Scholar] [CrossRef]
- Hwang, J.; Barman, S.; Gao, R.; Yang, X.; O’Malley, A.; Nagarkatti, P.; Nagarkatti, M.; Chruszcz, M.; Tang, C. Membrane-Active Metallopolymers: Repurposing and Rehabilitating Antibiotics to Gram-Negative Superbugs. Adv. Healthc. Mater. 2023, 12, 2301764. [Google Scholar] [CrossRef]
- Bagheri, M.; Von Kohout, M.; Zoric, A.; Fuchs, P.C.; Schiefer, J.L.; Opländer, C. Can Cold Atmospheric Plasma Be Used for Infection Control in Burns? A Preclinical Evaluation. Biomedicines 2023, 11, 1239. [Google Scholar] [CrossRef]
- Baz, A.; Bakri, A.; Butcher, M.; Short, B.; Ghimire, B.; Gaur, N.; Jenkins, T.; Short, R.D.; Riggio, M.; Williams, C.; et al. Staphylococcus aureus Strains Exhibit Heterogenous Tolerance to Direct Cold Atmospheric Plasma Therapy. Biofilm 2023, 5, 100123. [Google Scholar] [CrossRef]
- Bakker, O.; Smits, P.; Van Weersch, C.; Quaaden, M.; Bruls, E.; Van Loon, A.; Van Der Kleij, J. Improved Wound Healing by Direct Cold Atmospheric Plasma Once or Twice a Week: A Randomized Controlled Trial on Chronic Venous Leg Ulcers. Adv. Wound Care 2025, 14, 1–13. [Google Scholar] [CrossRef]
- Rafiee, A.; Memarpour, M.; Najibi, Y.; Khalvati, B.; Kianpour, S.; Morowvat, M.H. Antimicrobial Efficacy of a Novel Antibiotic-Eluting Injectable Platelet-Rich Fibrin Scaffold against a Dual-Species Biofilm in an Infected Immature Root Canal Model. BioMed Res. Int. 2020, 2020, 6623830. [Google Scholar] [CrossRef]
- Asnaashari, M.; Mehrabinia, P.; Yadegari, Z.; Hoseini, H.; Sadafi, M.; Shojaeian, S. Evaluation of Antibacterial Effects of Cold Atmospheric Plasma, Calcium Hydroxide, and Triple Antibiotic Paste on Enterococcus faecalis Biofilm in the Root Canal System: An In Vitro Study. J. Lasers Med. Sci. 2022, 13, e50. [Google Scholar] [CrossRef]
- Liu, H.; Liang, X.; Teng, M.; Li, Z.; Peng, Y.; Wang, P.; Chen, H.; Cheng, H.; Liu, G. Cold Atmospheric Plasma: An Emerging Immunomodulatory Therapy. Adv. Ther. 2024, 7, 2300399. [Google Scholar] [CrossRef]
- Luo, J.; Xie, L.; Yang, M.; Liu, M.; Li, Q.; Wang, P.; Fan, J.; Jin, J.; Luo, C. Synergistic Antibacterial Effect of Phage pB3074 in Combination with Antibiotics Targeting Cell Wall against Multidrug-Resistant Acinetobacter baumannii In Vitro and Ex Vivo. Microbiol. Spectr. 2023, 11, e00341-23. [Google Scholar] [CrossRef] [PubMed]
- He, Z.; Liu, K.; Scally, L.; Manaloto, E.; Gunes, S.; Ng, S.W.; Maher, M.; Tiwari, B.; Byrne, H.J.; Bourke, P.; et al. Cold Atmospheric Plasma Stimulates Clathrin-Dependent Endocytosis to Repair Oxidised Membrane and Enhance Uptake of Nanomaterial in Glioblastoma Multiforme Cells. Sci. Rep. 2020, 10, 6985. [Google Scholar] [CrossRef]
- Kupke, L.S.; Arndt, S.; Lenzer, S.; Metz, S.; Unger, P.; Zimmermann, J.L.; Bosserhoff, A.-K.; Gruber, M.; Karrer, S. Cold Atmospheric Plasma Promotes the Immunoreactivity of Granulocytes In Vitro. Biomolecules 2021, 11, 902. [Google Scholar] [CrossRef] [PubMed]
- Stacey, H.J.; De Soir, S.; Jones, J.D. The Safety and Efficacy of Phage Therapy: A Systematic Review of Clinical and Safety Trials. Antibiotics 2022, 11, 1340. [Google Scholar] [CrossRef]
- Nale, J.Y.; Clokie, M.R. Preclinical Data and Safety Assessment of Phage Therapy in Humans. Curr. Opin. Biotechnol. 2021, 68, 310–317. [Google Scholar] [CrossRef]
- Pirnay, J.-P.; Verbeken, G. Magistral Phage Preparations: Is This the Model for Everyone? Clin. Infect. Dis. 2023, 77, S360–S369. [Google Scholar] [CrossRef]
- Raza, S.; Matuła, K.; Karoń, S.; Paczesny, J. Resistance and Adaptation of Bacteria to Non-Antibiotic Antibacterial Agents: Physical Stressors, Nanoparticles, and Bacteriophages. Antibiotics 2021, 10, 435. [Google Scholar] [CrossRef]
- Wahab, M.A.; Luming, L.; Matin, M.A.; Karim, M.R.; Aijaz, M.O.; Alharbi, H.F.; Abdala, A.; Haque, R. Silver Micro-Nanoparticle-Based Nanoarchitectures: Synthesis Routes, Biomedical Applications, and Mechanisms of Action. Polymers 2021, 13, 2870. [Google Scholar] [CrossRef]
- Haddaji, N.; Bahloul, B.; Bahia, W.; Bechambi, O.; Mahdhi, A. Development of Nanotechnology-Based Drug Delivery Systems for Controlling Clinical Multidrug-Resistant Staphylococcus aureus and Escherichia coli Associated with Aerobic Vaginitis. Pharmaceutics 2023, 15, 2133. [Google Scholar] [CrossRef]
- Krishnamoorthy, R.; Athinarayanan, J.; Periyasamy, V.S.; Alshuniaber, M.A.; Alshammari, G.; Hakeem, M.J.; Ahmed, M.A.; Alshatwi, A.A. Antibacterial Mechanisms of Zinc Oxide Nanoparticle against Bacterial Food Pathogens Resistant to Beta-Lactam Antibiotics. Molecules 2022, 27, 2489. [Google Scholar] [CrossRef] [PubMed]
- Al Hagbani, T.; Rizvi, S.; Hussain, T.; Mehmood, K.; Rafi, Z.; Moin, A.; Abu Lila, A.; Alshammari, F.; Khafagy, E.-S.; Rahamathulla, M.; et al. Cefotaxime Mediated Synthesis of Gold Nanoparticles: Characterization and Antibacterial Activity. Polymers 2022, 14, 771. [Google Scholar] [CrossRef] [PubMed]
- Ouyang, B.; Wei, D.; Wu, B.; Yan, L.; Gang, H.; Cao, Y.; Chen, P.; Zhang, T.; Wang, H. In the View of Electrons Transfer and Energy Conversion: The Antimicrobial Activity and Cytotoxicity of Metal-Based Nanomaterials and Their Applications. Small 2024, 20, 2303153. [Google Scholar] [CrossRef] [PubMed]
- Fujimoto, N.; Nagaoka, K.; Tatsuno, I.; Oishi, H.; Tomita, M.; Hasegawa, T.; Tanaka, Y.; Matsumoto, T. Wavelength Dependence of Ultraviolet Light Inactivation for SARS-CoV-2 Omicron Variants. Sci. Rep. 2023, 13, 9706. [Google Scholar] [CrossRef]
- Yan, D.; Malyavko, A.; Wang, Q.; Ostrikov, K.; Sherman, J.H.; Keidar, M. Multi-Modal Biological Destruction by Cold Atmospheric Plasma: Capability and Mechanism. Biomedicines 2021, 9, 1259. [Google Scholar] [CrossRef]
- Abdelshafy, A.M.; Hu, Q.; Luo, Z.; Ban, Z.; Li, L. Hydrogen Peroxide from Traditional Sanitizer to Promising Disinfection Agent in Food Industry. Food Rev. Int. 2024, 40, 658–690. [Google Scholar] [CrossRef]
- She, R.C.; Chen, D.; Pak, P.; Armani, D.K.; Schubert, A.; Armani, A.M. Lightweight UV-C Disinfection System. Biomed. Opt. Express 2020, 11, 4326. [Google Scholar] [CrossRef]
- Arcos-Limiñana, V.; Maestre-Pérez, S.; Prats-Moya, M.S. A Comprehensive Review on Ultraviolet Disinfection of Spices and Culinary Seeds and Its Effect on Quality. Compr. Rev. Food Sci. Food Saf. 2025, 24, e70076. [Google Scholar] [CrossRef]
- Islam, R.; Sun, L.; Zhang, L. Biomedical Applications of Chinese Herb-Synthesized Silver Nanoparticles by Phytonanotechnology. Nanomaterials 2021, 11, 2757. [Google Scholar] [CrossRef]
- Modi, S.; Inwati, G.K.; Gacem, A.; Saquib Abullais, S.; Prajapati, R.; Yadav, V.K.; Syed, R.; Alqahtani, M.S.; Yadav, K.K.; Islam, S.; et al. Nanostructured Antibiotics and Their Emerging Medicinal Applications: An Overview of Nanoantibiotics. Antibiotics 2022, 11, 708. [Google Scholar] [CrossRef]
- Abd El-Ghany, W.A.; Shaalan, M.; Salem, H.M. Nanoparticles Applications in Poultry Production: An Updated Review. Worlds Poult. Sci. J. 2021, 77, 1001–1025. [Google Scholar] [CrossRef]
- Kitsiou, M.; Wantock, T.; Sandison, G.; Harle, T.; Gutierrez-Merino, J.; Klymenko, O.V.; Karatzas, K.A.; Velliou, E. Determination of the Combined Effect of Grape Seed Extract and Cold Atmospheric Plasma on Foodborne Pathogens and Their Environmental Stress Knockout Mutants. Appl. Environ. Microbiol. 2024. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, W.; Mushtaq, A.; Ali, S.; Khan, N.; Liang, Y.; Duan, L. Engineering Approaches for Exosome Cargo Loading and Targeted Delivery: Biological versus Chemical Perspectives. ACS Biomater. Sci. Eng. 2024, 10, 5960–5976. [Google Scholar] [CrossRef] [PubMed]
- Javanmard, Z.; Pourhajibagher, M.; Bahador, A. Advancing Anti-Biofilm Strategies: Innovations to Combat Biofilm-Related Challenges and Enhance Efficacy. J. Basic Microbiol. 2024, 64, e2400271. [Google Scholar] [CrossRef]
- Hong, G.; Chang, J.-E. Enhancing Cancer Treatment Through Combined Approaches: Photodynamic Therapy in Concert with Other Modalities. Pharmaceutics 2024, 16, 1420. [Google Scholar] [CrossRef]
- Kerlikowski, A.; Matthes, R.; Pink, C.; Steffen, H.; Schlüter, R.; Holtfreter, B.; Weltmann, K.-D.; von Woedtke, T.; Kocher, T.; Jablonowski, L. Effects of Cold Atmospheric Pressure Plasma and Disinfecting Agents on Candida albicans in Root Canals of Extracted Human Teeth. J. Biophotonics 2020, 13, e202000221. [Google Scholar] [CrossRef]
- Mitropoulou, G.; Koutsokera, A.; Csajka, C.; Blanchon, S.; Sauty, A.; Brunet, J.-F.; Garnier, C.v.; Resch, G.; Guery, B. Phage Therapy for Pulmonary Infections: Lessons from Clinical Experiences and Key Considerations. Eur. Respir. Rev. 2022, 31. [Google Scholar] [CrossRef]
- Venkataraman, S.; Shahgolzari, M.; Yavari, A.; Hefferon, K. Bacteriophages as Targeted Therapeutic Vehicles: Challenges and Opportunities. Bioengineering 2025, 12, 469. [Google Scholar] [CrossRef]
- Sun, J.; Wan, J.; Zhai, X.; Wang, J.; Liu, Z.; Tian, H.; Xin, L. Silver Nanoparticles: Correlating Particle Size and Ionic Ag Release with Cytotoxicity, Genotoxicity, and Inflammatory Responses in Human Cell Lines. Toxicol. Ind. Health 2021, 37, 198–209. [Google Scholar] [CrossRef]
- Tirumala, M.G.; Anchi, P.; Raja, S.; Rachamalla, M.; Godugu, C. Novel Methods and Approaches for Safety Evaluation of Nanoparticle Formulations: A Focus Towards In Vitro Models and Adverse Outcome Pathways. Front. Pharmacol. 2021, 12. [Google Scholar] [CrossRef]
- Kapoor, A.; Mudaliar, S.B.; Bhat, V.G.; Chakraborty, I.; Prasad, A.S.B.; Mazumder, N. Phage Therapy: A Novel Approach against Multidrug-Resistant Pathogens. 3 Biotech 2024, 14, 256. [Google Scholar] [CrossRef]
- Kaur, S.; Kumari, A.; Kumari Negi, A.; Galav, V.; Thakur, S.; Agrawal, M.; Sharma, V. Nanotechnology Based Approaches in Phage Therapy: Overcoming the Pharmacological Barriers. Front. Pharmacol. 2021, 12. [Google Scholar] [CrossRef]
| Bacterial Species | Exposure Time | Main Observed Effects | Representative Studies |
|---|---|---|---|
| P. aeruginosa | Not specified | Oxidation of lipid membrane; disruption of intracellular ion balance; alteration of membrane permeability; morphological deformation; bacterial inactivation | [30] |
| E. coli NCTC 12900 | 1, 3, and 5 min | Reduction: 2 log (1 min + 24 h storage); 3.6 log (3 min direct); 2.3 log (3 min indirect); 6 log (5 min direct); 8 log (5 min indirect). Cell wall disrupted by ROS; cleavage of C–C, C–O, and C–N bonds in LPS and peptidoglycan; lipid peroxidation; collapse of cell envelope | [33] |
| S. aureus ATCC 25923 | 1, 3, and 5 min | Complete elimination after 1–5 min with 24 h storage (direct or indirect); complete elimination after 3–5 min + 1 h storage. Without storage: 1 min = 1.8 log reduction, 5 min = 6.1 log reduction. ROS penetrated cell wall, accumulated inside, internal components damaged; higher intracellular ROS than E. coli | [33] |
| Bacillus cereus | Not specified | Breakdown of cell membrane; ROS penetrated cell and attacked vital macromolecules; damage to cellular homeostasis; cell death | [31] |
| E. coli CIP 53126 | 10 min | Pore formation inside cell membrane; complete membrane disruption; morphological alterations; bacterial inactivation | [36] |
| Reactive Species | Rate Flow Effect | Bacterial Response | References |
|---|---|---|---|
| Reactive oxygen species | Target protein, lipid, DNA. | Severe damage to bacterial cells. | [55,60] |
| Free radicals | Breaking peptide bonds and unfolding of proteins in bacterial cell wall. | Change in protein three dimensions. | [56,60] |
| Reactive oxygen species | Excessive oxidative stress. | Programed cell death and necrosis. | [60] |
| Singlet oxygen | React with internal component. | Cell damaging. | [59] |
| Domain | Microbial Target | Study Type | Outcomes | Limitations | Representative Study |
|---|---|---|---|---|---|
| Sterilization of surgical implants | Pasteurella multocida | In vivo [on rabbits] | Complete inactivation of bacteria without any visible tissue damage or erythema in the epidermis, dermis, subcutis, or muscle layers | Not tested on other bacterial species | [68] |
| Environmental decontamination [hospitals] | S. aureus and MRSA biofilms | In vitro [lab experiment] | Complete disruption of MRSA biofilms | Conducted in lab, not in real hospital environments | [10] |
| Dentistry [bleaching agent] | NA | In vitro | Treated water with cold plasma exhibited strong bleaching effect without affecting mineral content or microhardness of dental hard tissues | In vitro only, no in vivo evaluation of safety and efficiency | [69] |
| Dentistry [Dental implant treatment] | NA | In vitro | Cold plasma did not affect chemical composition of implant materials and enhanced cell adhesion and proliferation on implant surfaces | No in vivo testing | [69] |
| Chronic wound treatment | Not specified | Clinical study | Multiple exposures of cold plasma improved oxygenation of tissues and enhanced capillary blood flow in tissues | No mention of long-term effects of cold plasma use in wound treatment | [71] |
| Combination Strategy | Partner Modality | Target Organisms [Species] | Study Type | Outcomes | Limitations | References |
|---|---|---|---|---|---|---|
| CAP with Antibiotics | Antibiotics | Listeria monocytogenes, Escherichia coli | In vitro/In vivo | Enhanced antibacterial efficacy, synergy against bacteria | Potential for tissue damage, limited human studies | [120] |
| CAP with Nanotechnology-based Agents | Nanoparticles | Mixed bacterial biofilms | In vitro/In vivo | Increased biofilm disruption, targeted antimicrobial delivery | Nanoparticle toxicity, regulatory challenges | [121,122] |
| CAP with UV or Chemical Disinfectants | UV/Chemical disinfectants | Broad spectrum, environmental surface pathogens | In vitro | Enhanced decontamination, reduced pathogen numbers | Potential material degradation, environmental impact | [120,122] |
| CAP and Bacteriophage Therapy | Bacteriophages | Bacterial pathogens | In vitro/In vivo | Synergistic bactericidal effects, improved phage function | Specificity of phages, potential genetic adaptation by targets | [120,123] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mahmoud, Y.A.-G.; Elkaliny, N.E.; Elshikh, F.M.; Ashraf, Y.; Metwally, K.; Yahya, G.; Sherif, S. Cold Plasma as a Revolutionary Antimicrobial Modality: A Multifaceted Weapon Against Antibiotic Resistance. Antibiotics 2025, 14, 930. https://doi.org/10.3390/antibiotics14090930
Mahmoud YA-G, Elkaliny NE, Elshikh FM, Ashraf Y, Metwally K, Yahya G, Sherif S. Cold Plasma as a Revolutionary Antimicrobial Modality: A Multifaceted Weapon Against Antibiotic Resistance. Antibiotics. 2025; 14(9):930. https://doi.org/10.3390/antibiotics14090930
Chicago/Turabian StyleMahmoud, Yehia A.-G., Nehal E. Elkaliny, Farah M. Elshikh, Yara Ashraf, Kamel Metwally, Galal Yahya, and Sameha Sherif. 2025. "Cold Plasma as a Revolutionary Antimicrobial Modality: A Multifaceted Weapon Against Antibiotic Resistance" Antibiotics 14, no. 9: 930. https://doi.org/10.3390/antibiotics14090930
APA StyleMahmoud, Y. A.-G., Elkaliny, N. E., Elshikh, F. M., Ashraf, Y., Metwally, K., Yahya, G., & Sherif, S. (2025). Cold Plasma as a Revolutionary Antimicrobial Modality: A Multifaceted Weapon Against Antibiotic Resistance. Antibiotics, 14(9), 930. https://doi.org/10.3390/antibiotics14090930








