Postβ-Lactamase-Inhibiting Effect of Sulbactam in Combination with Ceftriaxone on Extended-Spectrum-β-Lactamase-Producing Escherichia coli
Abstract
1. Introduction
2. Results
2.1. Susceptibility of E. coli NCTC 13353 to CRO/SBT
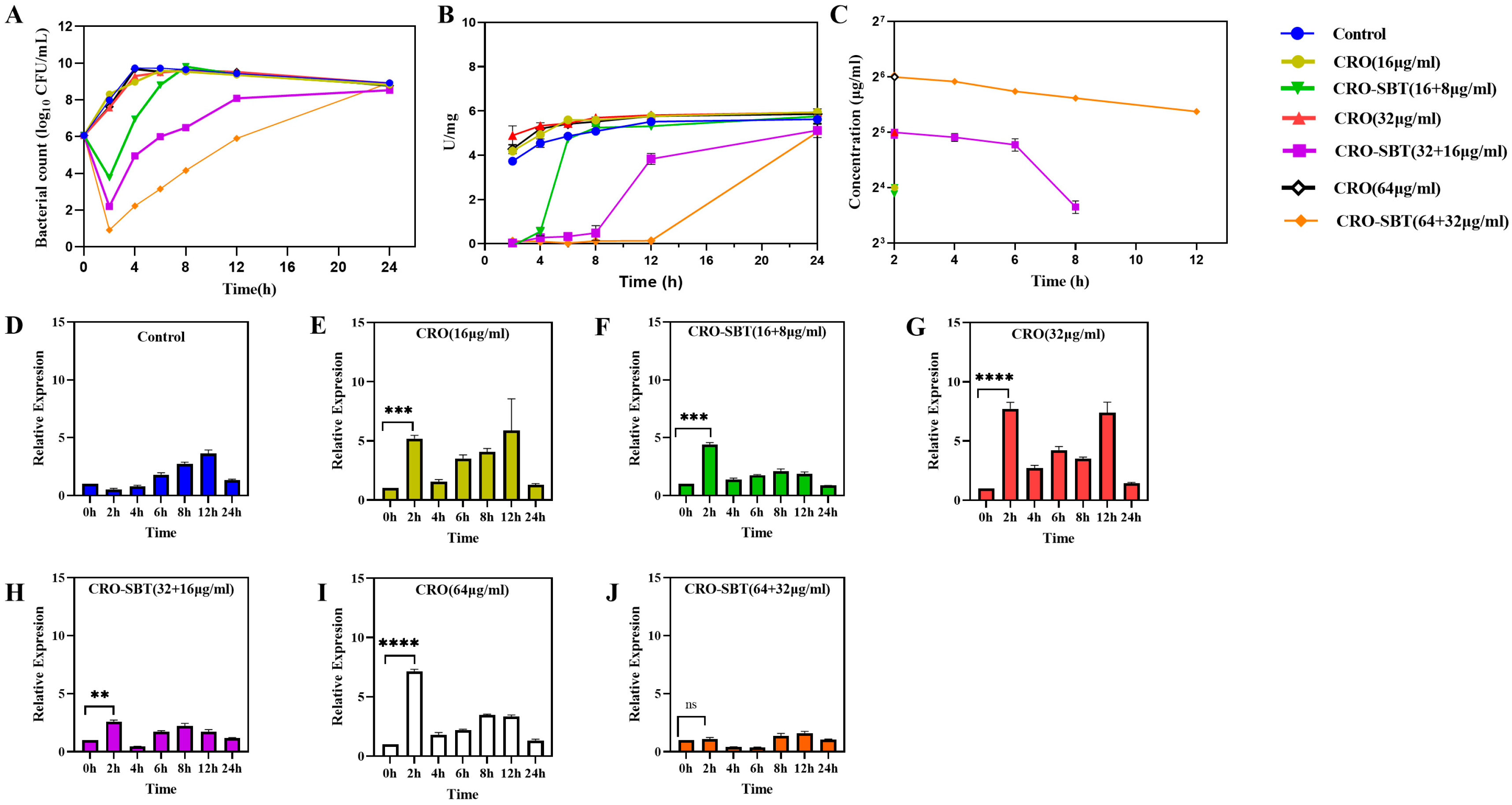
2.2. SBT Can Induce PLIE and Improve the Antibacterial Effect of CRO Under Static Conditions
2.3. Although SBT Is Eliminated from HFIM, SBT Performs PLIE, Which Continuously Inhibits Beta-Lactamase Activity
2.3.1. PK Profile Simulation in the HFIM
2.3.2. Dynamic Time-Killing Curves
2.3.3. Determination of β-Lactamase Activity and Expression Levels of the blaCTX-M-15 Gene
3. Discussion
4. Materials and Methods
4.1. Antimicrobial Agents and Bacteria
4.2. Antimicrobial Susceptibility Testing
4.3. Static Postβ-Lactamase Inhibitor Effect (PLIE)
4.3.1. Determination of Bacterial Count
4.3.2. Determination of Ceftriaxone and Sulbactam Concentrations
4.3.3. Determination of β-Lactamase Activity
4.3.4. RT-qPCR for Quantification of blaCTX-M-15
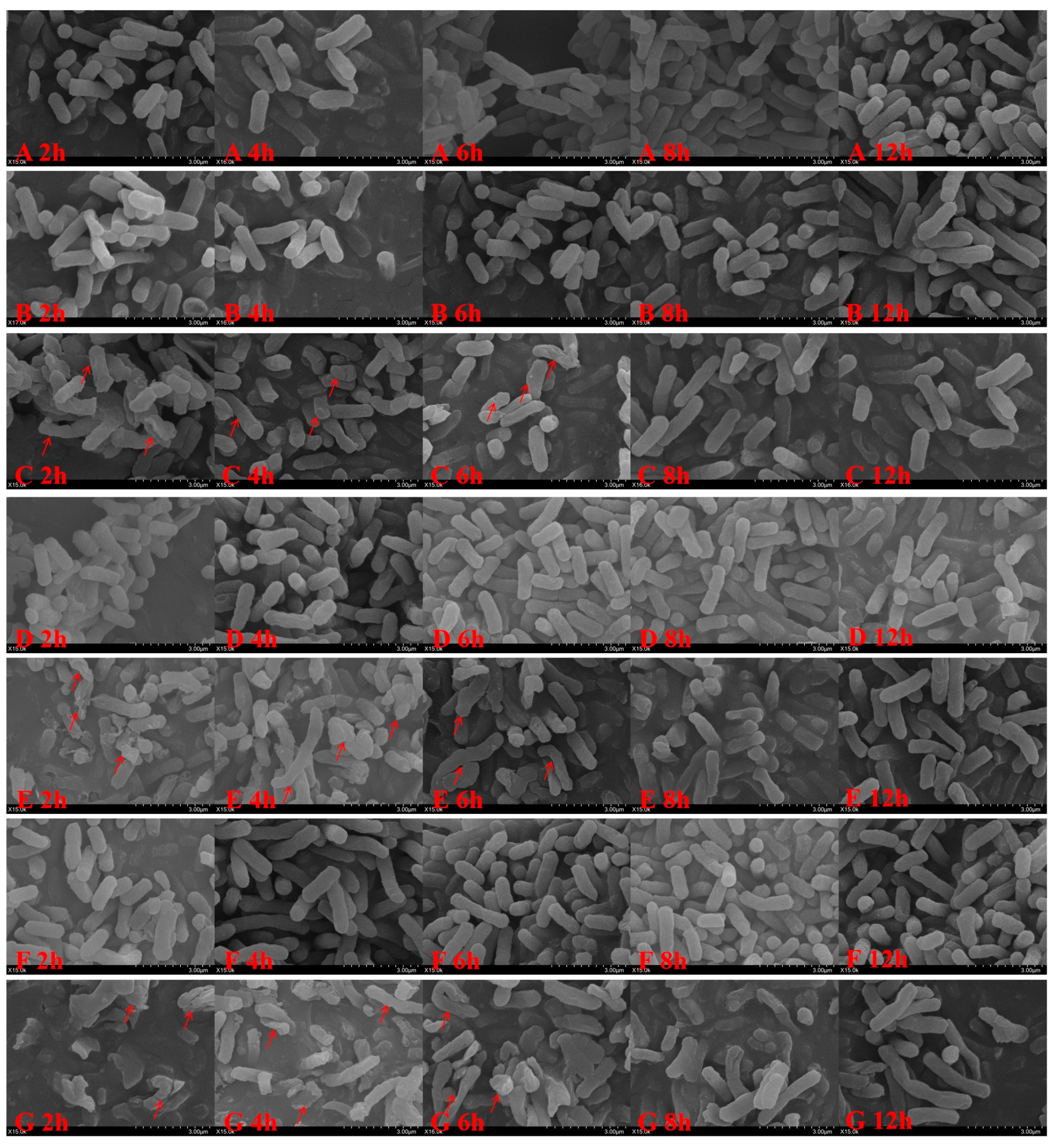
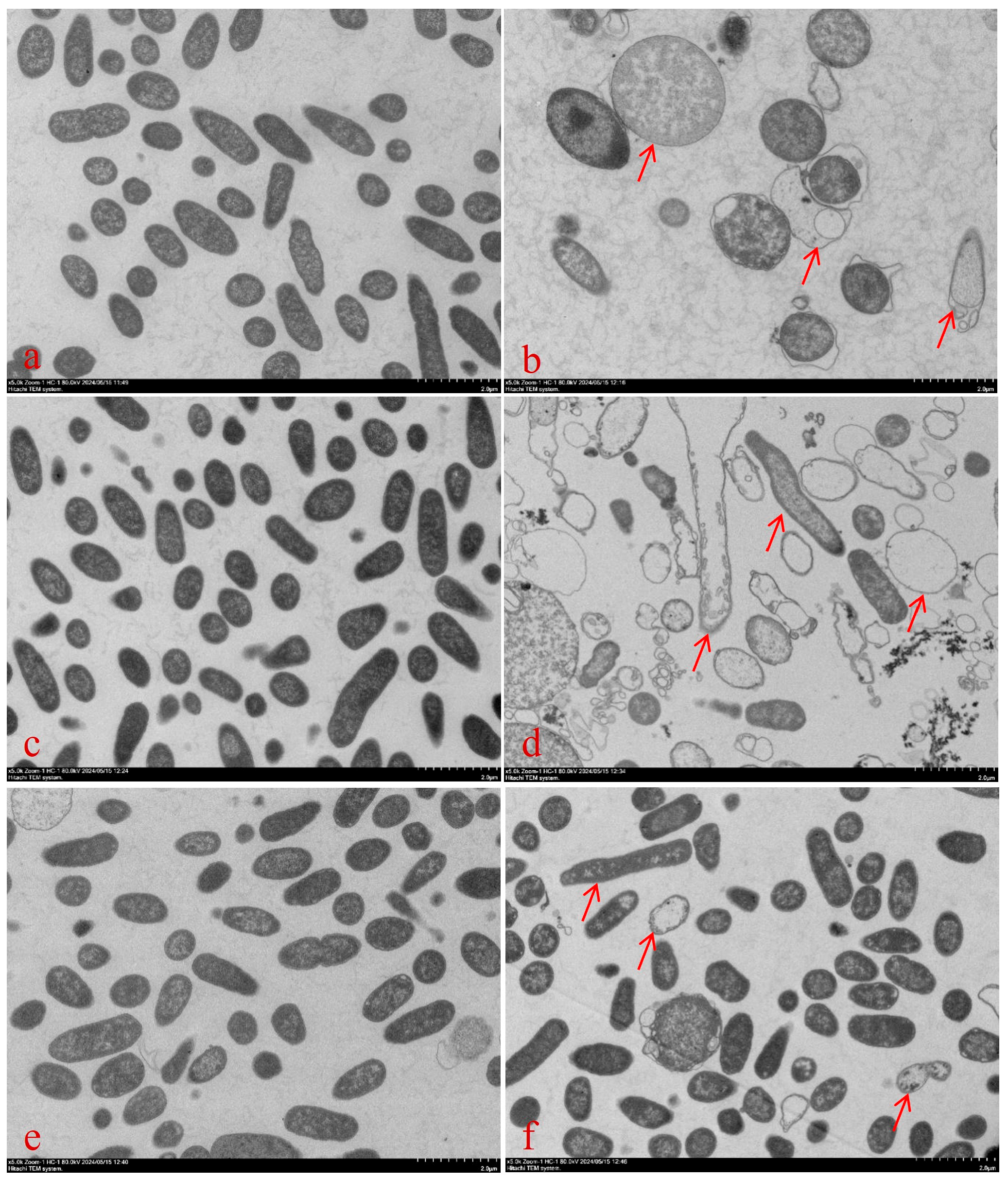
4.3.5. Bacterial Morphology
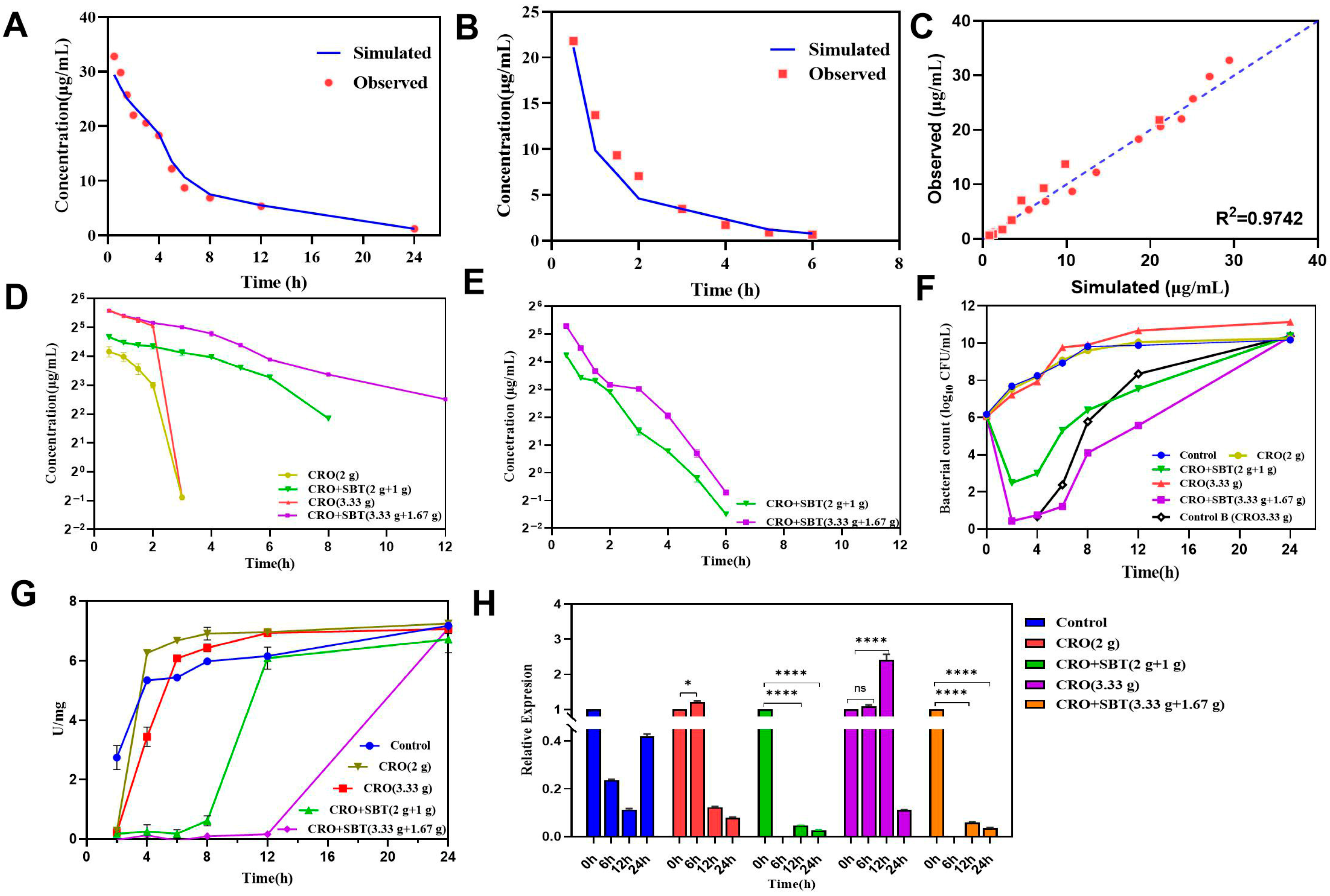
4.4. Hollow-Fiber Infection Model (HFIM) Simulating the In Vivo PK of CRO Monotherapy and CRO/SBT Combinations
4.4.1. Development of the HFIM
4.4.2. Simulated PK Profiles in HFIM
4.4.3. Determination of Bacterial Number, β-Lactamase Activity, and Expression Level of blaCTX-M-15
4.5. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Unemo, M.; Golparian, D.; Eyre, D.W. Antimicrobial Resistance in Neisseria gonorrhoeae and Treatment of Gonorrhea. Methods Mol. Biol. 2019, 1997, 37–58. [Google Scholar] [CrossRef]
- Heffernan, A.; Sime, F.; Lim, S.M.S.; Adiraju, S.; Wallis, S.; Lipman, J.; Grant, G.; Roberts, J. Pharmacodynamics of ceftriaxone for the treatment of methicillin-susceptible Staphylococcus aureus: Is it a viable treatment option? Int. J. Antimicrob. Agents 2022, 59, 106537. [Google Scholar] [CrossRef] [PubMed]
- Yetmar, Z.A.; Razi, S.; Nayfeh, T.; Gerberi, D.J.; Mahmood, M.; Abu Saleh, O.M. Ceftriaxone versus antistaphylococcal antibiotics for definitive treatment of methicillin-susceptible Staphylococcus aureus infections: A systematic review and meta-analysis. Int. J. Antimicrob. Agents 2022, 59, 106486. [Google Scholar] [CrossRef] [PubMed]
- Grégoire, M.; Dailly, E.; Le Turnier, P.; Garot, D.; Guimard, T.; Bernard, L.; Tattevin, P.; Vandamme, Y.-M.; Hoff, J.; Lemaitre, F.; et al. High-Dose Ceftriaxone for Bacterial Meningitis and Optimization of Administration Scheme Based on Nomogram. Antimicrob. Agents Chemother. 2019, 63, e00634-19. [Google Scholar] [CrossRef]
- Ganguly, A.; de la Flor, C.; Alvarez, K.; Brown, L.S.; Mang, N.S.; Smartt, J.; King, H.; Perl, T.M.; Filizola, H.; Bhavan, K.P. Safety and Efficacy of Ceftriaxone in the Treatment of Methicillin-Susceptible Staphylococcus aureus Bloodstream Infections: A Noninferiority Retrospective Cohort Study. Ann. Pharmacother. 2023, 57, 425–431. [Google Scholar] [CrossRef]
- Fraschini, F.; Braga, P.; Scarpazza, G.; Scaglione, F.; Pignataro, O.; Sambataro, G.; Mariani, C.; Roviaro, G.; Varoli, F.; Esposti, G. Human pharmacokinetics and distribution in various tissues of ceftriaxone. Chemotherapy 1986, 32, 192–199. [Google Scholar] [CrossRef]
- Comce, M.H.; Weersink, R.A.; Beuers, U.; van Hest, R.M.; Lantinga, M.A. Pharmacokinetics of ceftriaxone, gentamicin, meropenem and vancomycin in liver cirrhosis: A systematic review. J. Antimicrob. Chemother. 2024, 79, 2750–2761. [Google Scholar] [CrossRef] [PubMed]
- Jeanvoine, A.; Bouxom, H.; Leroy, J.; Gbaguidi-Haore, H.; Bertrand, X.; Slekovec, C. Resistance to third-generation cephalosporins in Escherichia coli in the French community: The times they are a-changin? Int. J. Antimicrob. Agents 2020, 55, 105909. [Google Scholar] [CrossRef]
- Tooke, C.L.; Hinchliffe, P.; Bragginton, E.C.; Colenso, C.K.; Hirvonen, V.H.A.; Takebayashi, Y.; Spencer, J. β-Lactamases and β-Lactamase Inhibitors in the 21st Century. J. Mol. Biol. 2019, 431, 3472–3500. [Google Scholar] [CrossRef]
- Yin, L.; Gou, Y.; Dai, Y.; Wang, T.; Gu, K.; Tang, T.; Hussain, S.; Huang, X.; He, C.; Liang, X.; et al. Cinnamaldehyde Restores Ceftriaxone Susceptibility against Multidrug-Resistant Salmonella. Int. J. Mol. Sci. 2023, 24, 9288. [Google Scholar] [CrossRef]
- Paitan, Y. Current Trends in Antimicrobial Resistance of Escherichia coli. Curr. Top. Microbiol. Immunol. 2018, 416, 181–211. [Google Scholar] [CrossRef]
- Patil, S.; Chen, X.; Lian, M.; Wen, F. Phenotypic and genotypic characterization of multi-drug-resistant Escherichia coli isolates harboring blaCTX-M group extended-spectrum β-lactamases recovered from pediatric patients in Shenzhen, southern China. Infect. Drug Resist. 2019, 12, 1325–1332. [Google Scholar] [CrossRef]
- Laure, N.N.; Ahn, J. Antibiofilm Activity of β-Lactam/β-Lactamase Inhibitor Combination against Multidrug-Resistant Salmonella Typhimurium. Pathogens 2022, 11, 349. [Google Scholar] [CrossRef]
- Singh, S.; Sahu, C.; Patel, S.S.; Singh, A.; Yaduvanshi, N. A Comparative In Vitro Sensitivity Study of “Ceftriaxone-Sulbactam-EDTA” and Various Antibiotics against Gram-negative Bacterial Isolates from Intensive Care Unit. Indian J. Crit. Care Med. 2020, 24, 1213–1217. [Google Scholar] [CrossRef]
- Bery, A.; Sodhi, C.; Bhanot, R. Successful Management of Urosepsis with Ceftriaxone+Sulbactam+EDTA: A Case Report of Penem Sparing Approach. J. Clin. Diagn. Res. 2017, 11, OD18–OD19. [Google Scholar] [CrossRef]
- Mir, M.D.A.; Chaudhary, S.; Payasi, A.; Sood, R.; Mavuduru, R.S.; Shameem, M. CSE (Ceftriaxone+ Sulbactam+ Disodium EDTA) Versus Meropenem for the Treatment of Complicated Urinary Tract Infections, Including Acute Pyelonephritis: PLEA, a Double-Blind, Randomized Noninferiority Trial. Open Forum Infect. Dis. 2019, 6, ofz373. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.; Kumar, M.; Shergill, S.P.S.; Tandel, K. Evaluation of ceftriaxone-sulbactam-disodium edetate adjuvant combination against multi-drug resistant Gram-negative organisms. Afr. J. Lab. Med. 2020, 9, 991. [Google Scholar] [CrossRef]
- Sanghavi, S.; Ghoshal, U.; Poddar, S.; Satpute, M.; Sahu, C.; Pawar, D.; Sharma, A.; Vaidya, P.H. In Vitro Susceptibility of Clinical Isolates to Ceftriaxone Alone and Ceftriaxone in Combination With Sulbactam or Tazobactam: A Comparative Study of Broad-Spectrum β-Lactam Antibiotics in India. Cureus 2023, 15, e46014. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Li, X.; Xia, Y.; Chu, Y.; Zhong, H.; Li, J.; Liang, P.; Bu, Y.; Zhao, R.; Liao, Y.; et al. Recommendation of Antimicrobial Dosing Optimization During Continuous Renal Replacement Therapy. Front. Pharmacol. 2020, 11, 786. [Google Scholar] [CrossRef] [PubMed]
- Xin, X.; Jian, L.; Xia, X.; Jia, B.; Huang, W.; Li, C.; Wang, C.; Zhou, L.; Sun, X.; Tang, X.; et al. A multicentre clinical study on the injection of ceftriaxone/sulbactam compared with cefoperazone/sulbactam in the treatment of respiratory and urinary tract infections. Ann. Clin. Microbiol. Antimicrob. 2013, 12, 38. [Google Scholar] [CrossRef]
- Thorburn, C.E.; Molesworth, S.J.; Sutherland, R.; Rittenhouse, S. Postantibiotic and post-beta-lactamase inhibitor effects of amoxicillin plus clavulanate. Antimicrob. Agents Chemother. 1996, 40, 2796–2801. [Google Scholar] [CrossRef] [PubMed]
- Sader, H.S.; Rhomberg, P.R.; Jones, R.N. Post-β-lactamase-inhibitor effect of tazobactam in combination with ceftolozane on extended-spectrum-β-lactamase-producing strains. Antimicrob. Agents Chemother. 2014, 58, 2434–2437. [Google Scholar] [CrossRef] [PubMed]
- Lavigne, J.-P.; Bonnet, R.; Michaux-Charachon, S.; Jourdan, J.; Caillon, J.; Sotto, A. Post-antibiotic and post-beta-lactamase inhibitor effects of ceftazidime plus sulbactam on extended-spectrum beta-lactamase-producing Gram-negative bacteria. J. Antimicrob. Chemother. 2004, 53, 616–619. [Google Scholar] [CrossRef]
- Pillar, C.M.; Stoneburner, A.; Shinabarger, D.L.; Krause, K.M.; Nichols, W.W. The postantibiotic effect and post-β-lactamase-inhibitor effect of ceftazidime, ceftaroline and aztreonam in combination with avibactam against target Gram-negative bacteria. Lett. Appl. Microbiol. 2016, 63, 96–102. [Google Scholar] [CrossRef] [PubMed]
- Baker, S.; Thomson, N.; Weill, F.-X.; Holt, K.E. Genomic insights into the emergence and spread of antimicrobial-resistant bacterial pathogens. Science 2018, 360, 733–738. [Google Scholar] [CrossRef]
- Castanheira, M.; Simner, P.J.; Bradford, P.A. Extended-spectrum β-lactamases: An update on their characteristics, epidemiology and detection. JAC Antimicrob. Resist. 2021, 3, dlab092. [Google Scholar] [CrossRef]
- Shahid, M.; Singhai, M.; Malik, A.; Shukla, I.; Khan, H.M.; Shujatullah, F.; Tahira, F. In vitro efficacy of ceftriaxone/sulbactam against Escherichia coli isolates producing CTX-M-15 extended-spectrum beta-lactamase. J. Antimicrob. Chemother. 2007, 60, 187–188. [Google Scholar] [CrossRef][Green Version]
- Spratt, B.G. Distinct penicillin binding proteins involved in the division, elongation, and shape of Escherichia coli K12. Proc. Natl. Acad. Sci. USA 1975, 72, 2999–3003. [Google Scholar] [CrossRef]
- Kocaoglu, O.; Carlson, E.E. Profiling of β-lactam selectivity for penicillin-binding proteins in Escherichia coli strain DC2. Antimicrob. Agents Chemother. 2015, 59, 2785–2790. [Google Scholar] [CrossRef]
- Seyfinejad, B.; Ozkan, S.A.; Jouyban, A. Recent advances in the determination of unbound concentration and plasma protein binding of drugs: Analytical methods. Talanta 2021, 225, 122052. [Google Scholar] [CrossRef]
- Wong, G.; Briscoe, S.; Adnan, S.; McWhinney, B.; Ungerer, J.; Lipman, J.; Roberts, J.A. Protein binding of β-lactam antibiotics in critically ill patients: Can we successfully predict unbound concentrations? Antimicrob. Agents Chemother. 2013, 57, 6165–6170. [Google Scholar] [CrossRef] [PubMed]
- Ji, X.-W.; Zhu, X.; Li, Y.; Xue, F.; Kuan, I.H.S.; He, Q.-F.; Meng, X.-R.; Xiang, X.-Q.; Cui, Y.-M.; Zheng, B. Model-Informed Drug Development of New Cefoperazone Sodium and Sulbactam Sodium Combination (3:1): Pharmacokinetic/Pharmacodynamic Analysis and Antibacterial Efficacy Against Enterobacteriaceae. Front. Pharmacol. 2022, 13, 856792. [Google Scholar] [CrossRef]
- Tam, V.H.; Merlau, P.R.; Hudson, C.S.; Kline, E.G.; Eales, B.M.; Smith, J.; Sofjan, A.K.; Shields, R.K. Optimal ceftazidime/avibactam dosing exposure against KPC-producing Klebsiella pneumoniae. J. Antimicrob. Chemother. 2022, 77, 3130–3137. [Google Scholar] [CrossRef]
- Nakae, T.; Nakajima, A.; Ono, T.; Saito, K.; Yoneyama, H. Resistance to beta-lactam antibiotics in Pseudomonas aeruginosa due to interplay between the MexAB-OprM efflux pump and beta-lactamase. Antimicrob. Agents Chemother. 1999, 43, 1301–1303. [Google Scholar] [CrossRef]
- Clinical and Laboratory Standards Institute. Performance Standards for Antimicrobial Susceptibility Testing; Twenty-third Informational Supplement M100-Ed32; CLSI: Wayne, PA, USA, 2022. [Google Scholar]
- Singh, R.; Almutairi, M.; AAlm, R.; Lahiri, S.D.; Martin, M.S.; Chen, A.; Ambler, E.J. Ceftaroline efficacy against high-MIC clinical Staphylococcus aureus isolates in an in vitro hollow-fibre infection model. J. Antimicrob. Chemother. 2017, 72, 2796–2803. [Google Scholar] [CrossRef] [PubMed]
- Kesisoglou, I.; Eales, B.M.; Ledesma, K.R.; Merlau, P.R.; Tam, V.H.; Wang, W.; Nikolaou, M. Simultaneous in vitro simulation of multiple antimicrobial agents with different elimination half-lives in a pre-clinical infection model. Comput. Chem. Eng. 2021, 155, 107540. [Google Scholar] [CrossRef] [PubMed]

| Drug | Quantitative Ion Pair (m/z) | Qualitative Ion Pair (m/z) | Declustering Potential (DP/V) | Collision Energy (U/V) |
|---|---|---|---|---|
| Ceftriaxone | 555.1 > 396.0 | 555.1 > 396.0 | 110 | 30 |
| 555.1 > 324.0 | ||||
| Sulbactam | 232.1 > 64.0 | 232.1 > 64.0 | 18 | |
| 232.1 > 140.1 |
| Group | Parameters | Calculation Equation | Value |
|---|---|---|---|
| CRO-SBT | V | - | 500.0 mL |
| FCE | FCE = V × kSBT | 4.1 r/min | |
| FDC | FDC = V × kCRO | 1.0 r/min | |
| FDS/FSC | FDS = FCE − FDC | 3.1 r/min | |
| VS | VS = FM/N/kCRO | 1577.6 mL | |
| ACRO | ACRO = Cmax,CRO × V | 15.0 mg | |
| ASBT | ASBT = Cmax,SBT × V | 10.6 mg | |
| AS,CRO | AS,CRO = ACRO × (kSBT/kCRO − 1) | 46.4 mg | |
| RCRO | RCRO = ACRO/T | 0.5 mg/min | |
| RSBT | RSBT = ACRO/T | 0.35 mg/min | |
| CRO | V | - | 500.0 mL |
| FDC | FDC = V × kCRO | 1.0 r/min | |
| FCE | FCE = V × kCRO | 1.0 r/min | |
| ACRO | ACRO = Cmax,CRO × V | 15.0 mg | |
| RateCRO | RCRO = ACRO/T | 0.5 mg/min |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, R.; Mi, K.; Lu, A.; Zhang, C.; Sun, L.; Chen, Y.; Pan, Y.; Tao, Y.; Huang, L. Postβ-Lactamase-Inhibiting Effect of Sulbactam in Combination with Ceftriaxone on Extended-Spectrum-β-Lactamase-Producing Escherichia coli. Antibiotics 2025, 14, 915. https://doi.org/10.3390/antibiotics14090915
Wang R, Mi K, Lu A, Zhang C, Sun L, Chen Y, Pan Y, Tao Y, Huang L. Postβ-Lactamase-Inhibiting Effect of Sulbactam in Combination with Ceftriaxone on Extended-Spectrum-β-Lactamase-Producing Escherichia coli. Antibiotics. 2025; 14(9):915. https://doi.org/10.3390/antibiotics14090915
Chicago/Turabian StyleWang, Ru, Kun Mi, Aihua Lu, Chengyang Zhang, Lei Sun, Yuxiang Chen, Yuanhu Pan, Yanfei Tao, and Lingli Huang. 2025. "Postβ-Lactamase-Inhibiting Effect of Sulbactam in Combination with Ceftriaxone on Extended-Spectrum-β-Lactamase-Producing Escherichia coli" Antibiotics 14, no. 9: 915. https://doi.org/10.3390/antibiotics14090915
APA StyleWang, R., Mi, K., Lu, A., Zhang, C., Sun, L., Chen, Y., Pan, Y., Tao, Y., & Huang, L. (2025). Postβ-Lactamase-Inhibiting Effect of Sulbactam in Combination with Ceftriaxone on Extended-Spectrum-β-Lactamase-Producing Escherichia coli. Antibiotics, 14(9), 915. https://doi.org/10.3390/antibiotics14090915





