Antifungal Susceptibility of Malassezia pachydermatis Isolates from Companion Animals and Genomic Insights into Resistance Mechanisms
Abstract
1. Introduction
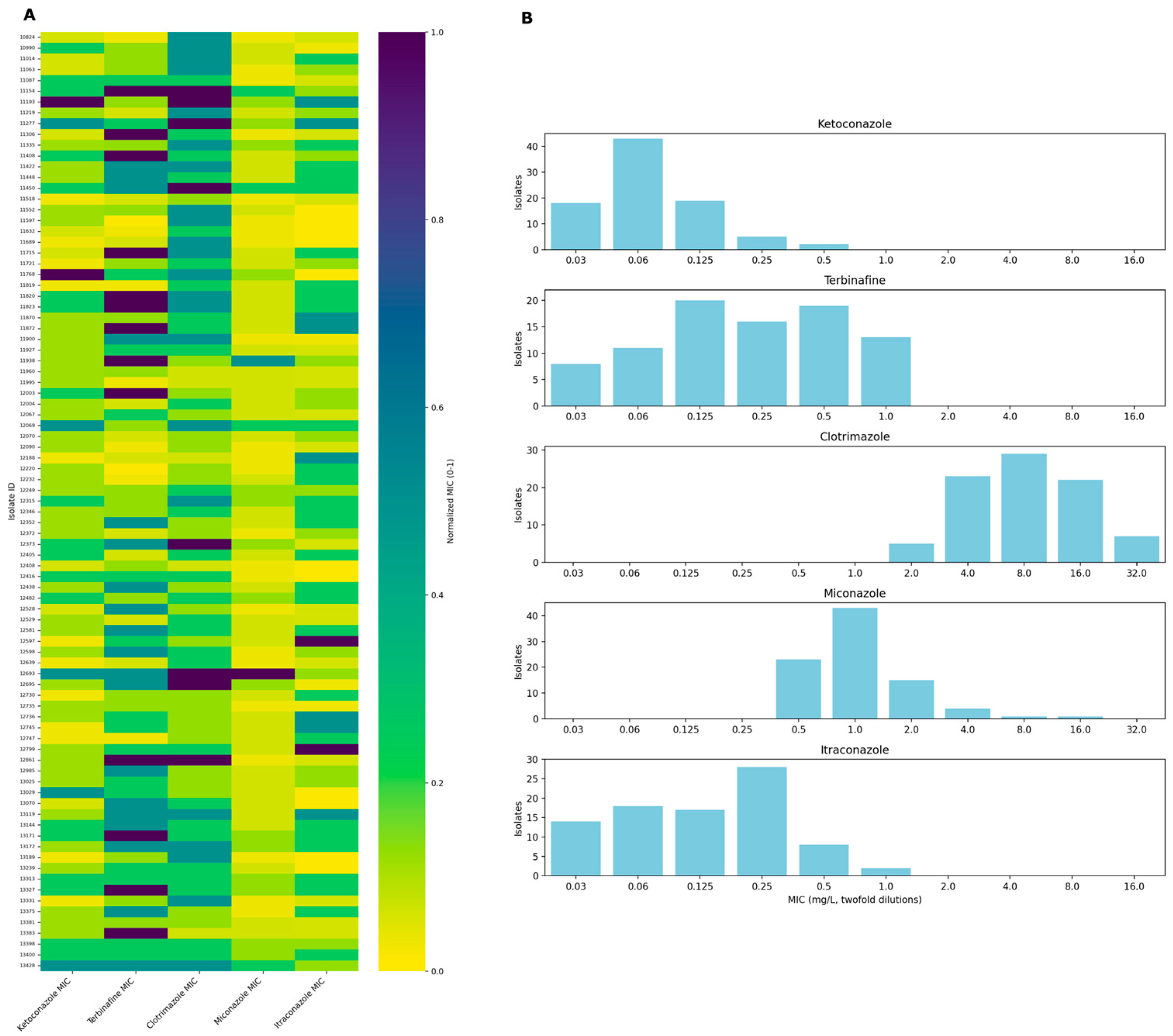
2. Results
3. Discussion
4. Materials and Methods
4.1. Isolate Collection and Species-Level Identification
4.2. Antifungal Susceptibility Testing
4.3. Genome Sequencing and Bioinformatics Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Velegraki, A.; Cafarchia, C.; Gaitanis, G.; Iatta, R.; Boekhout, T. Malassezia Infections in Humans and Animals: Pathophysiology, Detection, and Treatment. PLoS Pathog. 2015, 11, e1004523. [Google Scholar] [CrossRef]
- Chow, N.A.; Chinn, R.; Pong, A.; Schultz, K.; Kim, J.; Gade, L.; Jackson, B.R.; Beer, K.D.; Litvintseva, A.P. Use of Whole-Genome Sequencing to Detect an Outbreak of Malassezia pachydermatis Infection and Colonization in a Neonatal Intensive Care Unit—California, 2015–2016. Infect. Control Hosp. Epidemiol. 2020, 41, 851–853. [Google Scholar] [CrossRef]
- Rhimi, W.; Theelen, B.; Boekhout, T.; Otranto, D.; Cafarchia, C. Malassezia spp. Yeasts of Emerging Concern in Fungemia. Front. Cell. Infect. Microbiol. 2020, 10, 370. [Google Scholar] [CrossRef]
- Peano, A.; Johnson, E.; Chiavassa, E.; Tizzani, P.; Guillot, J.; Pasquetti, M. Antifungal Resistance Regarding Malassezia pachydermatis: Where Are We Now? J. Fungi 2020, 6, 93. [Google Scholar] [CrossRef]
- M27-A3; Reference Method for Broth Dilution Antifungal Susceptibility Testing of Yeasts. Approved Standard-Third Edition; Clinical and Laboratory Standards Institute (CLSI): Wayne, PA, USA, 2008.
- M27-S4; Reference Method for Broth Dilution Antifungal Susceptibility Testing of Yeasts. Fourth Informational Supplement 2012; Clinical and Laboratory Standards Institute (CLSI): Wayne, PA, USA, 2012.
- Cafarchia, C.; Figueredo, L.A.; Favuzzi, V.; Surico, M.R.; Colao, V.; Iatta, R.; Montagna, M.T.; Otranto, D. Assessment of the Antifungal Susceptibility of Malassezia pachydermatis in Various Media Using a CLSI Protocol. Vet. Microbiol. 2012, 159, 536–540. [Google Scholar] [CrossRef]
- Peano, A.; Pasquetti, M.; Tizzani, P.; Chiavassa, E.; Guillot, J.; Johnson, E. Methodological Issues in Antifungal Susceptibility Testing of Malassezia pachydermatis. J. Fungi 2017, 3, 37. [Google Scholar] [CrossRef]
- Iatta, R.; Puttilli, M.R.; Immediato, D.; Otranto, D.; Cafarchia, C. The Role of Drug Efflux Pumps in Malassezia pachydermatis and Malassezia furfur Defence Against Azoles. Mycoses 2017, 60, 178–182. [Google Scholar] [CrossRef]
- Díaz, L.; Castellá, G.; Bragulat, M.R.; Cabañes, F.J. ERG11 Gene Variability and Azole Susceptibility in Malassezia pachydermatis. Mycopathologia 2023, 188, 21–34. [Google Scholar] [CrossRef]
- Kim, M.; Cho, Y.-J.; Park, M.; Choi, Y.; Hwang, S.Y.; Jung, W.H. Genomic Tandem Quadruplication Is Associated with Ketoconazole Resistance in Malassezia pachydermatis. J. Microbiol. Biotechnol. 2018, 28, 1937–1945. [Google Scholar] [CrossRef]
- Klobučnı́ková, V.; Kohút, P.; Leber, R.; Fuchsbichler, S.; Schweighofer, N.; Turnowsky, F.; Hapala, I. Terbinafine Resistance in a Pleiotropic Yeast Mutant Is Caused by a Single Point Mutation in the ERG1 Gene. Biochem. Biophys. Res. Commun. 2003, 309, 666–671. [Google Scholar] [CrossRef]
- Burmester, A.; Hipler, U.; Uhrlaß, S.; Nenoff, P.; Singal, A.; Verma, S.B.; Elsner, P.; Wiegand, C. Indian Trichophyton mentagrophytes Squalene Epoxidase Erg1 Double Mutants Show High Proportion of Combined Fluconazole and Terbinafine Resistance. Mycoses 2020, 63, 1175–1180. [Google Scholar] [CrossRef]
- Zhang, J.; Li, L.; Lv, Q.; Yan, L.; Wang, Y.; Jiang, Y. The Fungal CYP51s: Their Functions, Structures, Related Drug Resistance, and Inhibitors. Front. Microbiol. 2019, 10, 691. [Google Scholar] [CrossRef]
- Park, M.; Cho, Y.-J.; Lee, Y.W.; Jung, W.H. Genomic Multiplication and Drug Efflux Influence Ketoconazole Resistance in Malassezia restricta. Front. Cell. Infect. Microbiol. 2020, 10, 191. [Google Scholar] [CrossRef]
- Kim, D.; Lim, Y.-R.; Ohk, S.O.; Kim, B.J.; Chun, Y.-J. Functional Expression and Characterization of CYP51 from Dandruff-Causing Malassezia globosa: Malassezia globosa CYP51. FEMS Yeast Res. 2011, 11, 80–87. [Google Scholar] [CrossRef]
- Cafarchia, C.; Figueredo, L.A.; Iatta, R.; Montagna, M.T.; Otranto, D. In Vitro Antifungal Susceptibility of Malassezia pachydermatis from Dogs with and Without Skin Lesions. Vet. Microbiol. 2012, 155, 395–398. [Google Scholar] [CrossRef]
- Cafarchia, C.; Iatta, R.; Immediato, D.; Puttilli, M.R.; Otranto, D. Azole Susceptibility of Malassezia pachydermatis and Malassezia furfur and Tentative Epidemiological Cut-Off Values. Med. Mycol. 2015, 53, 743–748. [Google Scholar] [CrossRef]
- Peano, A.; Beccati, M.; Chiavassa, E.; Pasquetti, M. Evaluation of the Antifungal Susceptibility of Malassezia pachydermatis to Clotrimazole, Miconazole and Thiabendazole Using a Modified CLSI M27-A3 Microdilution Method. Vet. Dermatol. 2012, 23, 131. [Google Scholar] [CrossRef]
- Jerzsele, Á.; Balázs, B.; Kálmánfi, E.; Lajos, Z.; Gálfi, P.; Gyetvai, B. Kutyából és macskából izolált Malassezia pachydermatis törzsek in vitro érzékenységi vizsgálata. Magy. Állatorvosok Lapja 2013, 135, 351–356. [Google Scholar]
- Angileri, M.; Pasquetti, M.; De Lucia, M.; Peano, A. Azole Resistance of Malassezia pachydermatis Causing Treatment Failure in a Dog. Med. Mycol. Case Rep. 2019, 23, 58–61. [Google Scholar] [CrossRef]
- Kano, R.; Yokoi, S.; Kariya, N.; Oshimo, K.; Kamata, H. Multi-Azole-Resistant Strain of Malassezia pachydermatis Isolated from a Canine Malassezia Dermatitis. Med. Mycol. 2019, 57, 346–350. [Google Scholar] [CrossRef]
- Murayama, N.; Kano, R. Azole and Terbinafine Susceptibility Testing of Malassezia pachydermatis in Japan. J. Vet. Med. Sci. 2023, 85, 383–385. [Google Scholar] [CrossRef]
- Kano, R.; Aramaki, C.; Murayama, N.; Mori, Y.; Yamagishi, K.; Yokoi, S.; Kamata, H. High Multi-Azole-Resistant Malassezia pachydermatis Clinical Isolates from Canine Malassezia Dermatitis. Med. Mycol. 2019, 58, 197–200. [Google Scholar] [CrossRef]
- Domán, M.; Kaszab, E.; Laczkó, L.; Bali, K.; Makrai, L.; Kovács, R.; Majoros, L.; Bányai, K. Genomic Epidemiology of Antifungal Resistance in Human and Avian Isolates of Candida albicans: A Pilot Study from the One Health Perspective. Front. Vet. Sci. 2024, 11, 1345877. [Google Scholar] [CrossRef]
- Forche, A.; Alby, K.; Schaefer, D.; Johnson, A.D.; Berman, J.; Bennett, R.J. The Parasexual Cycle in Candida albicans Provides an Alternative Pathway to Meiosis for the Formation of Recombinant Strains. PLoS Biol. 2008, 6, e110. [Google Scholar] [CrossRef]
- Pryszcz, L.P.; Németh, T.; Saus, E.; Ksiezopolska, E.; Hegedűsová, E.; Nosek, J.; Wolfe, K.H.; Gacser, A.; Gabaldón, T. The Genomic Aftermath of Hybridization in the Opportunistic Pathogen Candida metapsilosis. PLoS Genet. 2015, 11, e1005626. [Google Scholar] [CrossRef]
- Theelen, B.; Mixão, V.; Ianiri, G.; Goh, J.P.Z.; Dijksterhuis, J.; Heitman, J.; Dawson, T.L.; Gabaldón, T.; Boekhout, T. Multiple Hybridization Events Punctuate the Evolutionary Trajectory of Malassezia furfur. mBio 2022, 13, e03853-21. [Google Scholar] [CrossRef]
- Cannon, R.D.; Lamping, E.; Holmes, A.R.; Niimi, K.; Baret, P.V.; Keniya, M.V.; Tanabe, K.; Niimi, M.; Goffeau, A.; Monk, B.C. Efflux-Mediated Antifungal Drug Resistance. Clin. Microbiol. Rev. 2009, 22, 291–321. [Google Scholar] [CrossRef]
- Kong, X.; Tang, C.; Singh, A.; Ahmed, S.A.; Al-Hatmi, A.M.S.; Chowdhary, A.; Nenoff, P.; Gräser, Y.; Hainsworth, S.; Zhan, P.; et al. Antifungal Susceptibility and Mutations in the Squalene Epoxidase Gene in Dermatophytes of the Trichophyton mentagrophytes Species Complex. Antimicrob. Agents Chemother. 2021, 65, e00056-21. [Google Scholar] [CrossRef]
- Martel, C.M.; Parker, J.E.; Bader, O.; Weig, M.; Gross, U.; Warrilow, A.G.S.; Rolley, N.; Kelly, D.E.; Kelly, S.L. Identification and Characterization of Four Azole-Resistant Erg3 Mutants of Candida albicans. Antimicrob. Agents Chemother. 2010, 54, 4527–4533. [Google Scholar] [CrossRef]
- Branco, J.; Ola, M.; Silva, R.M.; Fonseca, E.; Gomes, N.C.; Martins-Cruz, C.; Silva, A.P.; Silva-Dias, A.; Pina-Vaz, C.; Erraught, C.; et al. Impact of ERG3 Mutations and Expression of Ergosterol Genes Controlled by UPC2 and NDT80 in Candida parapsilosis Azole Resistance. Clin. Microbiol. Infect. 2017, 23, 575.e1–575.e8. [Google Scholar] [CrossRef]
- Schoch, C.L.; Seifert, K.A.; Huhndorf, S.; Robert, V.; Spouge, J.L.; Levesque, C.A.; Chen, W.; Fungal Barcoding Consortium; Fungal Barcoding Consortium Author List; Bolchacova, E.; et al. Nuclear Ribosomal Internal Transcribed Spacer (ITS) Region as a Universal DNA Barcode Marker for Fungi. Proc. Natl. Acad. Sci. USA 2012, 109, 6241–6246. [Google Scholar] [CrossRef]
- Domán, M.; Makrai, L.; Lengyel, G.; Kovács, R.; Majoros, L.; Bányai, K. Molecular Diversity and Genetic Relatedness of Candida albicans Isolates from Birds in Hungary. Mycopathologia 2021, 186, 237–244. [Google Scholar] [CrossRef]
- Cafarchia, C.; Gasser, R.B.; Figueredo, L.A.; Latrofa, M.S.; Otranto, D. Advances in the Identification of Malassezia. Mol. Cell. Probes 2011, 25, 1–7. [Google Scholar] [CrossRef]
- Tamura, K.; Stecher, G.; Kumar, S. MEGA11: Molecular Evolutionary Genetics Analysis Version 11. Mol. Biol. Evol. 2021, 38, 3022–3027. [Google Scholar] [CrossRef]
- Caesar Labs, Inc. 2025. Julius (June 19 Version) [Large Language Model]. Available online: https://julius.ai/ (accessed on 19 June 2025).


| Isolate | Position | Substitution | MIC KTZ (mg/L) | MIC TER (mg/L) | MIC CLT (mg/L) | MIC MCZ (mg/L) | MIC ITR (mg/L) |
|---|---|---|---|---|---|---|---|
| 11938 | 33 | V → I | 0.06 | 1 | 4 | 8 | 0.125 |
| 141 | K → R | ||||||
| 181 | E → Q | ||||||
| 12069 | 25 | I → I/S | 0.25 | 0.125 | 16 | 4 | 0.25 |
| 52 | W → W/L | ||||||
| 84 | R → R/K | ||||||
| 86 | L → L/F | ||||||
| 181 | E → Q | ||||||
| 212 | N → N/S | ||||||
| 290 | E → D | ||||||
| 352 | Y → Y/F | ||||||
| 399 | H → H/R | ||||||
| 446 | K → K/R | ||||||
| 12693 | 25 | I → S | 0.25 | 0.5 | >32 | 16 | 0.125 |
| 52 | W → L | ||||||
| 84 | R → K | ||||||
| 86 | L → F | ||||||
| 181 | E → Q | ||||||
| 212 | N → S | ||||||
| 290 | E → D | ||||||
| 352 | Y → F | ||||||
| 399 | H → R | ||||||
| 446 | K → R | ||||||
| 13172 | 17 | A → T | 0.06 | 0.5 | 16 | 2 | 0.25 |
| 175 | R → H | ||||||
| 181 | E → Q | ||||||
| 11154 | 25 | I → S | 0.125 | 1 | 32 | 4 | 0.125 |
| 52 | W → L | ||||||
| 84 | R → K | ||||||
| 86 | L → F | ||||||
| 181 | E → Q | ||||||
| 212 | N → S | ||||||
| 290 | E → D | ||||||
| 352 | Y → F | ||||||
| 399 | H → R | ||||||
| 446 | K → R | ||||||
| 11193 | 17 | A → A/T | 0.5 | 0.125 | 32 | 2 | 0.5 |
| 25 | I → I/S | ||||||
| 52 | W → W/L | ||||||
| 84 | R → K | ||||||
| 86 | L → L/F | ||||||
| 175 | R → R/H | ||||||
| 178 | Q → Q/R | ||||||
| 181 | E → Q | ||||||
| 212 | N → S | ||||||
| 290 | E → E/D | ||||||
| 352 | Y → Y/F | ||||||
| 399 | H → H/R | ||||||
| 446 | K → K/R | ||||||
| 11768 | 25 | I → S | 0.5 | 0.25 | 16 | 2 | ≤0.03 |
| 84 | R → K | ||||||
| 86 | L → F | ||||||
| 181 | E → Q | ||||||
| 290 | E → D | ||||||
| 399 | H → R | ||||||
| 11277 | - | - | 0.25 | 0.25 | 32 | 2 | 0.5 |
| 11450 | - | - | 0.125 | 0.5 | 32 | 4 | 0.25 |
| 12747 | - | - | ≤0.03 | 0.03 | 4 | 1 | 0.25 |
| Isolate | Position | Substitution | MIC KTZ (mg/L) | MIC TER (mg/L) | MIC CLT (mg/L) | MIC MCZ (mg/L) | MIC ITR (mg/L) |
|---|---|---|---|---|---|---|---|
| 11938 | 153 | P → R | 0.06 | 1 | 4 | 8 | 0.125 |
| 12069 | 131 | R → R/G | 0.25 | 0.125 | 16 | 4 | 0.25 |
| 193 | P → P/S | ||||||
| 196 | R → R/C | ||||||
| 364 | M → M/I | ||||||
| 456 | S → S/A | ||||||
| 480 | V → V/A | ||||||
| 493 | I → I/V | ||||||
| 511 | Y → Y/H | ||||||
| 527 | N → N/D | ||||||
| 12693 | 131 | R → G | 0.25 | 0.5 | >32 | 16 | 0.125 |
| 364 | M → I | ||||||
| 456 | S → A | ||||||
| 480 | V → A | ||||||
| 493 | I → V | ||||||
| 511 | Y → H | ||||||
| 527 | N → D | ||||||
| 13172 | 96 | V → A | 0.06 | 0.5 | 16 | 2 | 0.25 |
| 131 | R → G | ||||||
| 364 | M → I | ||||||
| 456 | S → A | ||||||
| 480 | V → A | ||||||
| 492 | C → Y | ||||||
| 493 | I → V | ||||||
| 500 | L → I | ||||||
| 527 | N → D | ||||||
| 544 | V → L | ||||||
| 11154 | 131 | R → G | 0.125 | 1 | 32 | 4 | 0.125 |
| 165 | A → T | ||||||
| 364 | M → I | ||||||
| 369 | A → T | ||||||
| 456 | S → A | ||||||
| 480 | V → A | ||||||
| 493 | I → V | ||||||
| 511 | Y → H | ||||||
| 527 | N → D | ||||||
| 11193 | 131 | R → G | 0.5 | 0.125 | 32 | 2 | 0.5 |
| 196 | R → R/C | ||||||
| 364 | M → I | ||||||
| 456 | S → A | ||||||
| 480 | V → A | ||||||
| 492 | C → C/Y | ||||||
| 493 | I → V | ||||||
| 500 | L → L/I | ||||||
| 511 | Y → Y/H | ||||||
| 527 | N → D | ||||||
| 544 | V → V/L | ||||||
| 11768 | 131 | R → G | 0.5 | 0.25 | 16 | 2 | ≤0.03 |
| 364 | M → I | ||||||
| 456 | S → A | ||||||
| 480 | V → A | ||||||
| 493 | I → V | ||||||
| 511 | Y → H | ||||||
| 527 | N → D | ||||||
| 11277 | - | - | 0.25 | 0.25 | 32 | 2 | 0.5 |
| 11450 | - | - | 0.125 | 0.5 | 32 | 4 | 0.25 |
| 12747 | - | - | ≤0.03 | 0.03 | 4 | 1 | 0.25 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Domán, M.; Első, D.; Pintér, K.; Wehmann, E.; Fehér, E.; Magyar, T. Antifungal Susceptibility of Malassezia pachydermatis Isolates from Companion Animals and Genomic Insights into Resistance Mechanisms. Antibiotics 2025, 14, 902. https://doi.org/10.3390/antibiotics14090902
Domán M, Első D, Pintér K, Wehmann E, Fehér E, Magyar T. Antifungal Susceptibility of Malassezia pachydermatis Isolates from Companion Animals and Genomic Insights into Resistance Mechanisms. Antibiotics. 2025; 14(9):902. https://doi.org/10.3390/antibiotics14090902
Chicago/Turabian StyleDomán, Marianna, Dávid Első, Krisztina Pintér, Enikő Wehmann, Enikő Fehér, and Tibor Magyar. 2025. "Antifungal Susceptibility of Malassezia pachydermatis Isolates from Companion Animals and Genomic Insights into Resistance Mechanisms" Antibiotics 14, no. 9: 902. https://doi.org/10.3390/antibiotics14090902
APA StyleDomán, M., Első, D., Pintér, K., Wehmann, E., Fehér, E., & Magyar, T. (2025). Antifungal Susceptibility of Malassezia pachydermatis Isolates from Companion Animals and Genomic Insights into Resistance Mechanisms. Antibiotics, 14(9), 902. https://doi.org/10.3390/antibiotics14090902