Canine Pyoderma and Otitis Externa: A Retrospective Analysis of Multidrug-Resistant Bacterial Carriage in Hong Kong
Abstract
1. Introduction
2. Results
2.1. Study Population
2.2. Microbiological Results
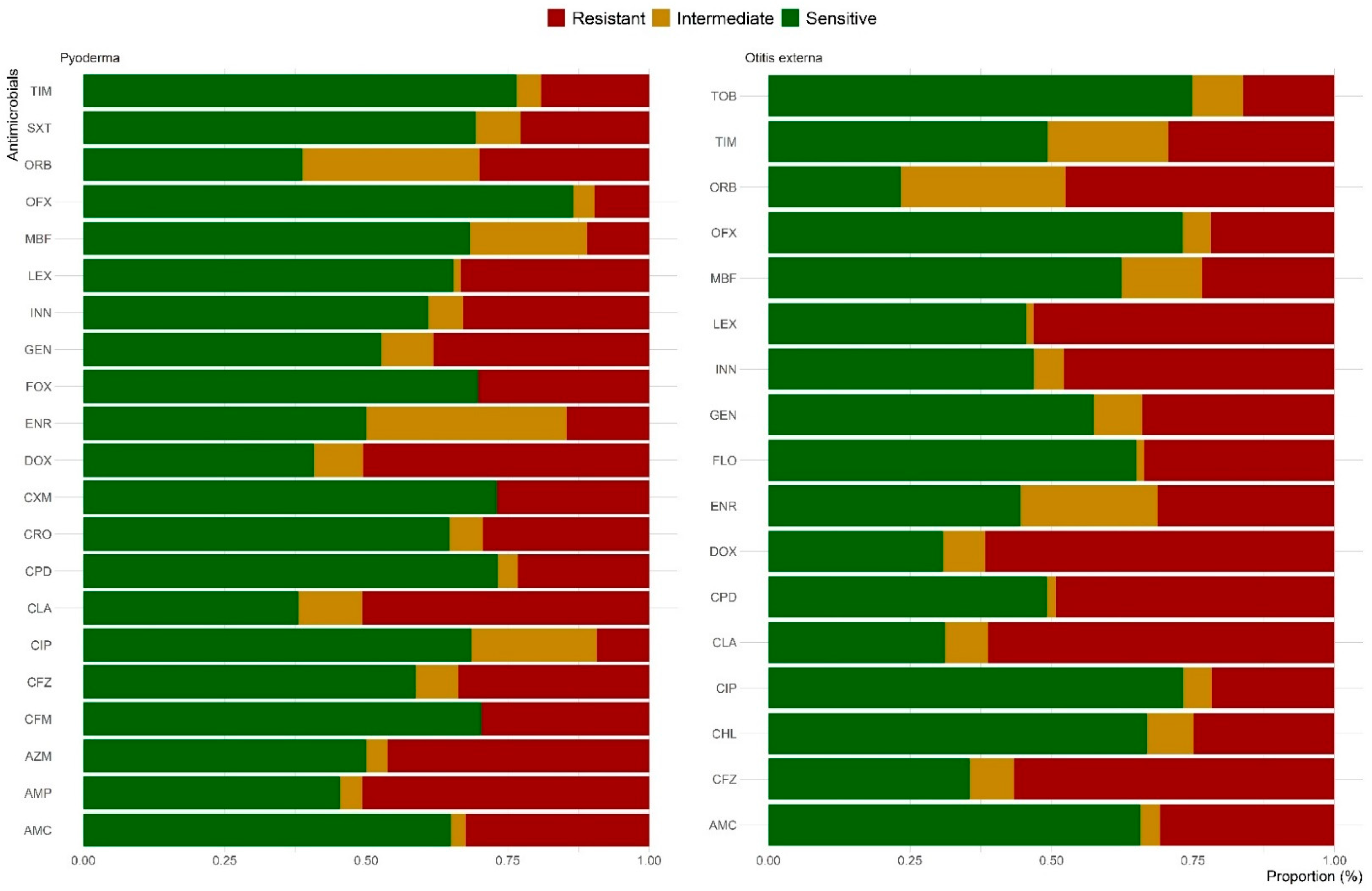
2.3. Antimicrobial Susceptibility Testing
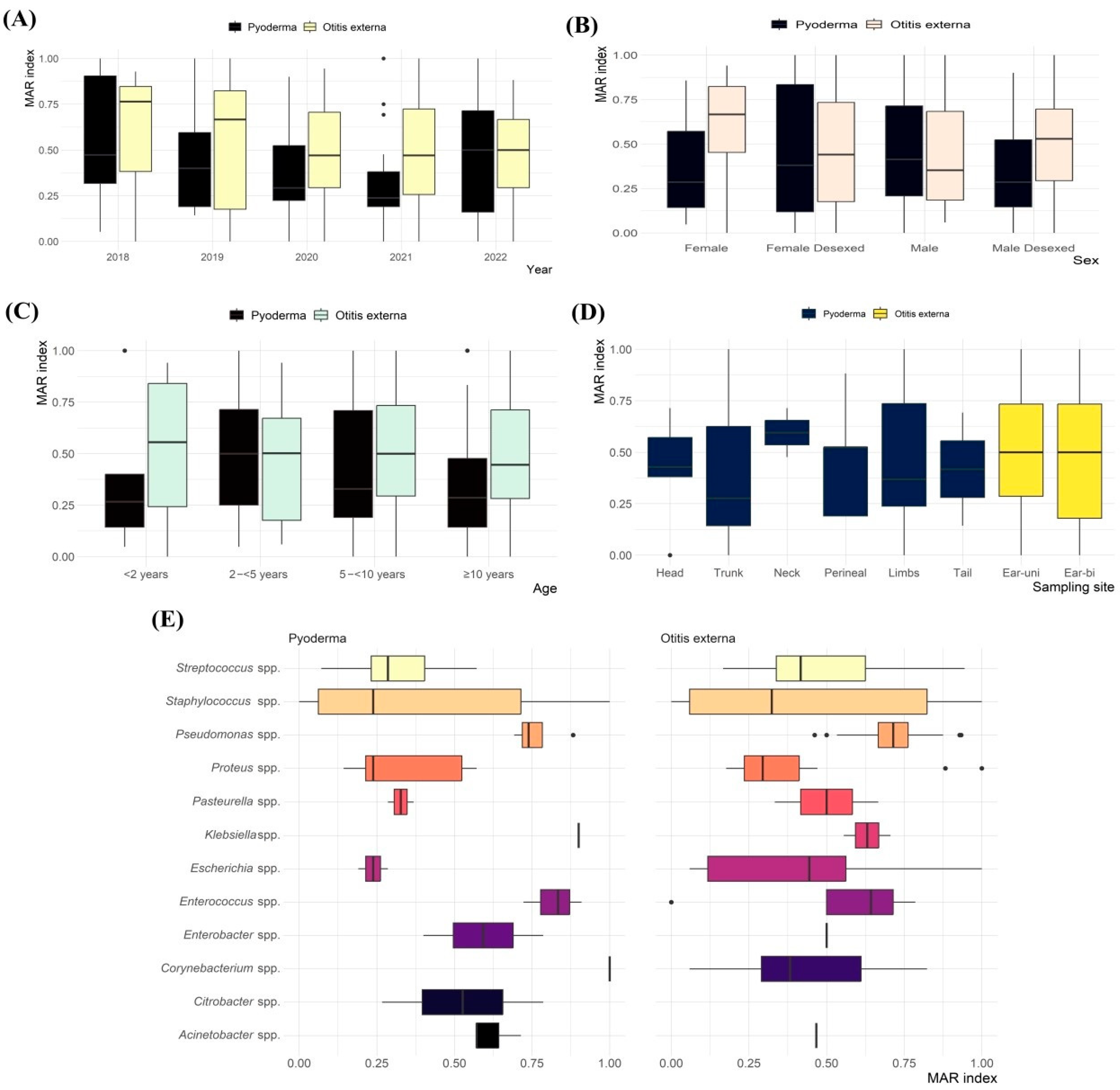
2.4. MAR Index and MDR
3. Discussion
4. Materials and Methods
4.1. Study Design and Samples
4.2. Data Collection and Management
4.3. Microbiological Examination and Identification
4.4. Antimicrobial Susceptibility Testing
4.5. Data Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AMR | Antimicrobial resistance |
| MDR | Multidrug resistance |
| MAR | Multiple antibiotic resistance index |
| MRS | Methicillin-resistant staphylococcus |
References
- Bajwa, J. Canine superficial pyoderma and therapeutic considerations. Can. Vet. J. 2016, 57, 204. [Google Scholar] [PubMed]
- Seckerdieck, F.; Mueller, R.S. Recurrent pyoderma and its underlying primary diseases: A retrospective evaluation of 157 dogs. Vet. Rec. 2018, 182, 434. [Google Scholar] [CrossRef]
- Loeffler, A.; Lloyd, D.H. What has changed in canine pyoderma? A narrative review. Vet. J. 2018, 235, 73–82. [Google Scholar] [CrossRef] [PubMed]
- Pye, C. Pseudomonas otitis externa in dogs. Can. Vet. J. 2018, 59, 1231. [Google Scholar]
- Tesin, N.; Stojanovic, D.; Stancic, I.; Kladar, N.; Ružić, Z.; Spasojevic, J.; Tomanic, D.; Kovacevic, Z. Prevalence of the microbiological causes of canine otitis externa and the antibiotic susceptibility of the isolated bacterial strains. Pol. J. Vet. Sci. 2023, 26, 449–459. [Google Scholar] [CrossRef]
- Rosser, E.J. Causes of otitis externa. Vet. Clin. Small Anim. Pract. 2004, 34, 459–468. [Google Scholar] [CrossRef]
- O’Neill, D.G.; Volk, A.V.; Soares, T.; Church, D.B.; Brodbelt, D.C.; Pegram, C. Frequency and predisposing factors for canine otitis externa in the UK–a primary veterinary care epidemiological view. Canine Med. Genet. 2021, 8, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Kwon, J.; Ko, H.J.; Yang, M.H.; Park, C.; Park, S.C. Antibiotic resistance and species profile of Enterococcus species in dogs with chronic otitis externa. Vet. Sci. 2022, 9, 592. [Google Scholar] [CrossRef]
- Kasai, T.; Fukui, Y.; Aoki, K.; Ishii, Y.; Tateda, K. Changes in the ear canal microbiota of dogs with otitis externa. J. Appl. Microbiol. 2021, 130, 1084–1091. [Google Scholar] [CrossRef]
- Medleau, L.; Long, R.E.; Brown, J.; Miller, W.H. Frequency and antimicrobial susceptibility of Staphylococcus species isolated from canine pyodermas. Am. J. Vet. Res. 1986, 47, 229–231. [Google Scholar] [CrossRef]
- Putriningsih, P.A.S.; Phuektes, P.; Jittimanee, S.; Kampa, J. Methicillin-resistant Staphylococci in canine pyoderma in Thailand. Vet. World 2023, 16, 2340. [Google Scholar] [CrossRef] [PubMed]
- De Martino, L.; Nocera, F.P.; Mallardo, K.; Nizza, S.; Masturzo, E.; Fiorito, F.; Iovane, G.; Catalanotti, P. An update on microbiological causes of canine otitis externa in Campania Region, Italy. Asian Pac. J. Trop. Biomed. 2016, 6, 384–389. [Google Scholar] [CrossRef]
- Dowling, P.M. Antimicrobial therapy of skin and ear infections. Can. Vet. J. 1996, 37, 695. [Google Scholar] [PubMed]
- Silva, V.; Oliveira, A.; Manageiro, V.; Caniça, M.; Contente, D.; Capita, R.; Alonso-Calleja, C.; Carvalho, I.; Capelo, J.L.; Igrejas, G. Clonal diversity and antimicrobial resistance of methicillin-resistant Staphylococcus pseudintermedius isolated from canine pyoderma. Microorganisms 2021, 9, 482. [Google Scholar] [CrossRef]
- Ganiere, J.P.; Medaille, C.; Mangion, C. Antimicrobial drug susceptibility of Staphylococcus intermedius clinical isolates from canine pyoderma. J. Vet. Med. B 2005, 52, 25–31. [Google Scholar] [CrossRef]
- Morris, D.O.; Loeffler, A.; Davis, M.F.; Guardabassi, L.; Weese, J.S. Recommendations for approaches to meticillin-resistant staphylococcal infections of small animals: Diagnosis, therapeutic considerations and preventative measures. Clinical Consensus Guidelines of the World Association for Veterinary Dermatology. Vet. Dermatol. 2017, 28, 304-e369. [Google Scholar] [CrossRef]
- Cheng, V.C.; Wong, S.C.; Ho, P.-L.; Yuen, K.-Y. Strategic measures for the control of surging antimicrobial resistance in Hong Kong and mainland of China. Emerg. Microbes Infect. 2015, 4, 1–13. [Google Scholar] [CrossRef]
- Boost, M.; So, S.; Perreten, V. Low rate of methicillin-resistant coagulase-positive staphylococcal colonization of veterinary personnel in Hong Kong. Zoonoses Public Health. 2011, 58, 36–40. [Google Scholar] [CrossRef]
- Rhouma, M.; Archambault, M.; Butaye, P. Antimicrobial use and resistance in animals from a one health perspective. Vet. Sci. 2023, 10, 319. [Google Scholar] [CrossRef]
- Yoon, J.-W.; Lee, K.-J.; Lee, S.-Y.; Chae, M.-J.; Park, J.-K.; Yoo, J.-H.; Park, H.-M. Antibiotic resistance profiles of Staphylococcus pseudintermedius isolates from canine patients in Korea. J. Microbiol. Biotechnol. 2010, 20, 1764–1768. [Google Scholar]
- Wang, Y.; Yang, J.; Logue, C.; Liu, K.; Cao, X.; Zhang, W.; Shen, J.; Wu, C. Methicillin-resistant Staphylococcus pseudintermedius isolated from canine pyoderma in North China. J. Appl. Microbiol. 2012, 112, 623–630. [Google Scholar] [CrossRef]
- Tanveer, M.; Ntakiyisumba, E.; Hirwa, F.; Yoon, H.; Oh, S.-I.; Kim, C.; Kim, M.H.; Yoon, J.-S.; Won, G. Prevalence of bacterial pathogens isolated from canines with pyoderma and otitis externa in Korea: A systematic review and meta-analysis. Vet. Sci. 2024, 11, 656. [Google Scholar] [CrossRef] [PubMed]
- Rosales, R.S.; Ramírez, A.S.; Moya-Gil, E.; de la Fuente, S.N.; Suárez-Pérez, A.; Poveda, J.B. Microbiological survey and evaluation of antimicrobial susceptibility patterns of microorganisms obtained from suspect cases of canine otitis externa in Gran Canaria, Spain. Animals 2024, 14, 742. [Google Scholar] [CrossRef] [PubMed]
- Chehida, F.B.; Tombari, W.; Gharsa, H.; Rabia, Y.; Ferhi, S.; Jrad, M.; Messadi, L. New insights into molecular characterization, antimicrobial resistance and virulence factors of methicillin-sensitive coagulase-positive Staphylococcus spp. from dogs with pyoderma and otitis externa. Microbiol. Res. 2024, 15, 1208. [Google Scholar] [CrossRef]
- Ludwig, C.; De Jong, A.; Moyaert, H.; El Garch, F.; Janes, R.; Klein, U.; Morrissey, I.; Thiry, J.; Youala, M. Antimicrobial susceptibility monitoring of dermatological bacterial pathogens isolated from diseased dogs and cats across Europe (ComPath results). J. Appl. Microbiol. 2016, 121, 1254–1267. [Google Scholar] [CrossRef] [PubMed]
- Pinchbeck, L.R.; Cole, L.K.; Hillier, A.; Kowalski, J.J.; Rajala-Schultz, P.J.; Bannerman, T.L.; York, S. Genotypic relatedness of staphylococcal strains isolated from pustules and carriage sites in dogs with superficial bacterial folliculitis. Am. J. Vet. Res. 2006, 67, 1337–1346. [Google Scholar] [CrossRef]
- Arais, L.R.; Barbosa, A.V.; Carvalho, C.A.; Cerqueira, A.M. Antimicrobial resistance, integron carriage, and gyrA and gyrB mutations in Pseudomonas aeruginosa isolated from dogs with otitis externa and pyoderma in Brazil. Vet. Dermatol. 2016, 27, e113–e131. [Google Scholar] [CrossRef]
- Garcias, B.; Batalla, M.; Vidal, A.; Durán, I.; Darwich, L. Trends in antimicrobial resistance of canine otitis pathogens in the Iberian Peninsula (2010–2021). Antibiotics 2025, 14, 328. [Google Scholar] [CrossRef]
- Bourély, C.; Cazeau, G.; Jarrige, N.; Leblond, A.; Madec, J.; Haenni, M.; Gay, E. Antimicrobial resistance patterns of bacteria isolated from dogs with otitis. Epidemiol. Infect. 2019, 147, e121. [Google Scholar] [CrossRef]
- Huerta, B.; Maldonado, A.; Ginel, P.J.; Tarradas, C.; Gómez-Gascón, L.; Astorga, R.J.; Luque, I. Risk factors associated with the antimicrobial resistance of staphylococci in canine pyoderma. Vet. Microbiol. 2011, 150, 302–308. [Google Scholar] [CrossRef][Green Version]
- Dinkova, V.; Rusenova, N. A retrospective study (2019–2023) on the prevalence and antimicrobial resistance of isolates from canine clinical samples submitted to the University Veterinary Hospital in Stara Zagora, Bulgaria. Microorganisms 2024, 12, 1670. [Google Scholar] [CrossRef]
- Ravens, P.; Vogelnest, L.; Ewen, E.; Bosward, K.; Norris, J. Canine superficial bacterial pyoderma: Evaluation of skin surface sampling methods and antimicrobial susceptibility of causal Staphylococcus isolates. Aust. Vet. J. 2014, 92, 149–155. [Google Scholar] [CrossRef] [PubMed]
- Srednik, M.E.; Perea, C.A.; Giacoboni, G.I.; Hicks, J.A.; Foxx, C.L.; Harris, B.; Schlater, L.K. Genomic features of antimicrobial resistance in Staphylococcus pseudintermedius isolated from dogs with pyoderma in Argentina and the United States: A comparative study. Int. J. Mol. Sci. 2023, 24, 11361. [Google Scholar] [CrossRef] [PubMed]
- Calabro, C.; Sadhu, R.; Xu, Y.; Aprea, M.; Guarino, C.; Cazer, C.L. Longitudinal antimicrobial susceptibility trends of canine Staphylococcus pseudintermedius. Prev. Vet. Med. 2024, 226, 106170. [Google Scholar] [CrossRef]
- Kawakami, T.; Shibata, S.; Murayama, N.; Nagata, M.; Nishifuji, K.; Iwasaki, T.; Fukata, T. Antimicrobial susceptibility and methicillin resistance in Staphylococcus pseudintermedius and Staphylococcus schleiferi subsp. coagulans isolated from dogs with pyoderma in Japan. J. Vet. Med. Sci. 2010, 72, 1615–1619. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Chen, S.; Ma, S.; Jiao, Y.; Hong, H.; Wang, S.; Huang, W.; An, Q.; Song, Y.; Dang, X. Antimicrobial resistance and risk factors of canine bacterial skin infections. Pathogens 2025, 14, 309. [Google Scholar] [CrossRef]
- Petrov, V.; Zhelev, G.; Marutsov, P.; Koev, K.; Georgieva, S.; Toneva, I.; Urumova, V. Microbiological and antibacterial resistance profile in canine otitis externa--a comparative analysis. Bulg. J. Vet. Med. 2019, 22, 447–456. [Google Scholar] [CrossRef]
- Breidenstein, E.B.; de la Fuente-Núñez, C.; Hancock, R.E. Pseudomonas aeruginosa: All roads lead to resistance. Trends Microbiol. 2011, 19, 419–426. [Google Scholar] [CrossRef]
- Pang, Z.; Raudonis, R.; Glick, B.R.; Lin, T.-J.; Cheng, Z. Antibiotic resistance in Pseudomonas aeruginosa: Mechanisms and alternative therapeutic strategies. Biotechnol. Adv. 2019, 37, 177–192. [Google Scholar] [CrossRef]
- Płókarz, D.; Czopowicz, M.; Bierowiec, K.; Rypuła, K. Virulence genes as markers for Pseudomonas aeruginosa biofilm formation in dogs and cats. Animals 2022, 12, 422. [Google Scholar] [CrossRef]
- Meroni, G.; Soares Filipe, J.F.; Drago, L.; Martino, P.A. Investigation on antibiotic-resistance, biofilm formation and virulence factors in multi drug resistant and non multi drug resistant Staphylococcus pseudintermedius. Microorganisms 2019, 7, 702. [Google Scholar] [CrossRef] [PubMed]
- Proietti, P.C.; Stefanetti, V.; Hyatt, D.R.; Marenzoni, M.L.; Capomaccio, S.; Coletti, M.; Bietta, A.; Franciosini, M.P.; Passamonti, F. Phenotypic and genotypic characterization of canine pyoderma isolates of Staphylococcus pseudintermedius for biofilm formation. J. Vet. Med. Sci. 2015, 77, 945–951. [Google Scholar] [CrossRef]
- Griffeth, G.C.; Morris, D.O.; Abraham, J.L.; Shofer, F.S.; Rankin, S.C. Screening for skin carriage of methicillin-resistant coagulase-positive staphylococci and Staphylococcus schleiferi in dogs with healthy and inflamed skin. Vet. Dermatol. 2008, 19, 142–149. [Google Scholar] [CrossRef] [PubMed]
- Grönthal, T.; Eklund, M.; Thomson, K.; Piiparinen, H.; Sironen, T.; Rantala, M. Antimicrobial resistance in Staphylococcus pseudintermedius and the molecular epidemiology of methicillin-resistant S. pseudintermedius in small animals in Finland. J. Antimicrob. Chemother. 2017, 72, 1021–1030. [Google Scholar] [CrossRef]
- Scherer, C.B.; Botoni, L.S.; Coura, F.M.; Silva, R.O.; Santos, R.D.d.; Heinemann, M.B.; Costa-Val, A.P. Frequency and antimicrobial susceptibility of Staphylococcus pseudintermedius in dogs with otitis externa. Ciência Rural. 2018, 48, e20170738. [Google Scholar] [CrossRef]
- Lyskova, P.; Vydrzalova, M.; Mazurova, J. Identification and antimicrobial susceptibility of bacteria and yeasts isolated from healthy dogs and dogs with otitis externa. J. Vet. Med. A 2007, 54, 559–563. [Google Scholar] [CrossRef]
- Dégi, J.; Moțco, O.-A.; Dégi, D.M.; Suici, T.; Mareș, M.; Imre, K.; Cristina, R.T. Antibiotic susceptibility profile of Pseudomonas aeruginosa canine isolates from a multicentric study in Romania. Antibiotics 2021, 10, 846. [Google Scholar] [CrossRef] [PubMed]
- White, S.D.; Cole, L.K. Pyoderma, Otitis Externa and Otitis Media. In Greene’s Infectious Diseases of the Dog and Cat; WB Saunders: Philadelphia, PA, USA, 2021; pp. 1551–1568. [Google Scholar]
- Xin, C.; Hill, F.; Elsohaby, I. Retrospective analysis of antimicrobial resistance in bacterial pathogens from pet rabbits in Hong Kong, 2019–2022. J. Vet. Diagn. Investig. 2024, 36, 711–718. [Google Scholar] [CrossRef]
- Clinical and Laboratory Standards Institute (CLSI). Performance Standards for Antimicrobial Susceptibility Testing, 35th ed.; CLSI supplement M100; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2025. [Google Scholar]
- Clinical and Laboratory Standards Institute (CLSI). Performance Standards for Antimicrobial Disk and Dilution Susceptibility Tests for Bacteria Isolated from Animals, 7th ed.; CLSI Report VET01S; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2025. [Google Scholar]
- Krumperman, P.H. Multiple antibiotic resistance indexing of Escherichia coli to identify high-risk sources of fecal contamination of foods. Appl. Environ. Microbiol. 1983, 46, 165–170. [Google Scholar] [CrossRef]
- Magiorakos, A.-P.; Srinivasan, A.; Carey, R.B.; Carmeli, Y.; Falagas, M.; Giske, C.; Harbarth, S.; Hindler, J.; Kahlmeter, G.; Olsson-Liljequist, B. Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: An international expert proposal for interim standard definitions for acquired resistance. Clin. Microbiol. Infect. 2012, 18, 268–281. [Google Scholar] [CrossRef]
- Gu, Z.; Eils, R.; Schlesner, M. Complex heatmaps reveal patterns and correlations in multidimensional genomic data. Bioinformatics 2016, 32, 2847–2849. [Google Scholar] [CrossRef] [PubMed]
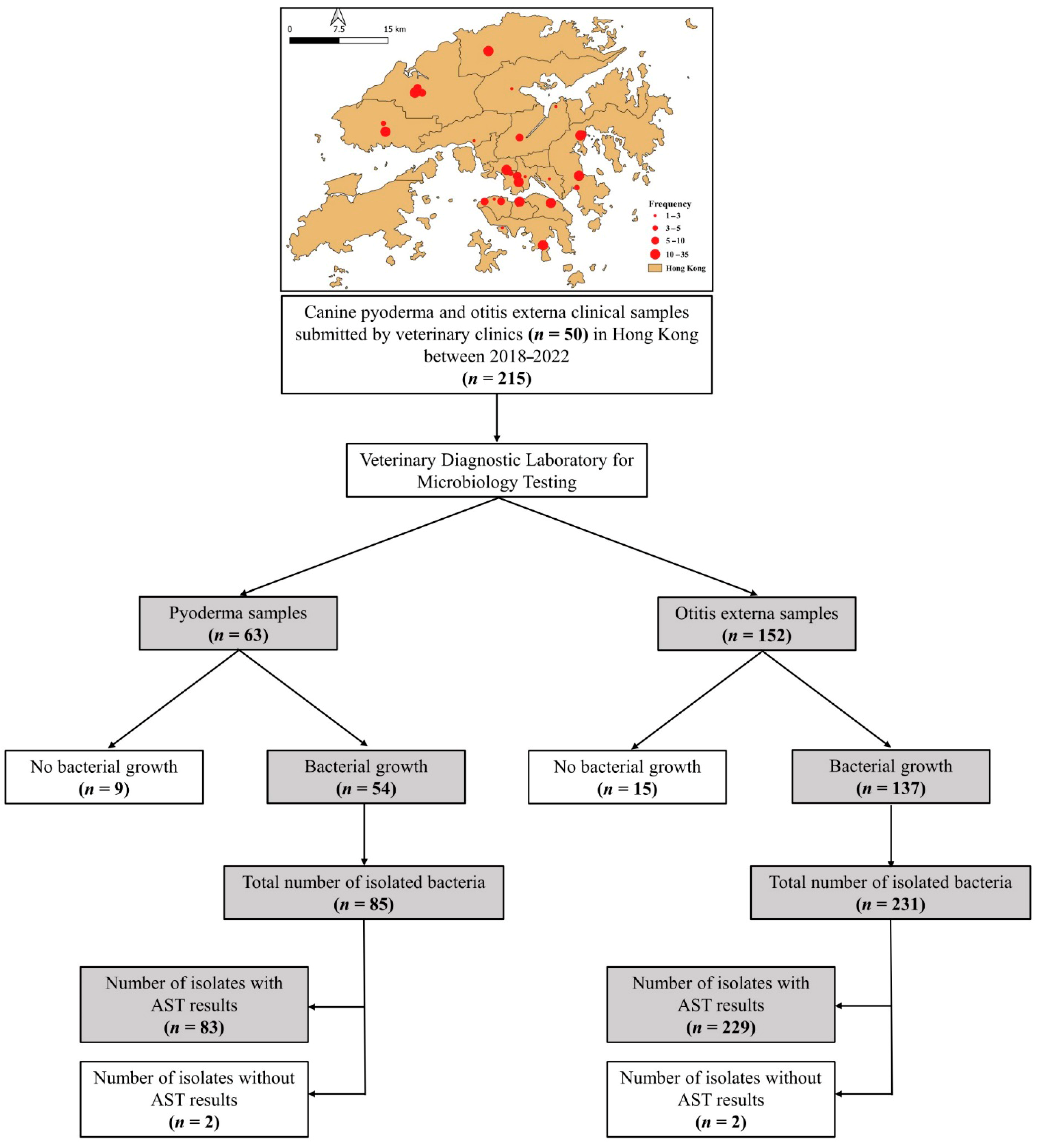
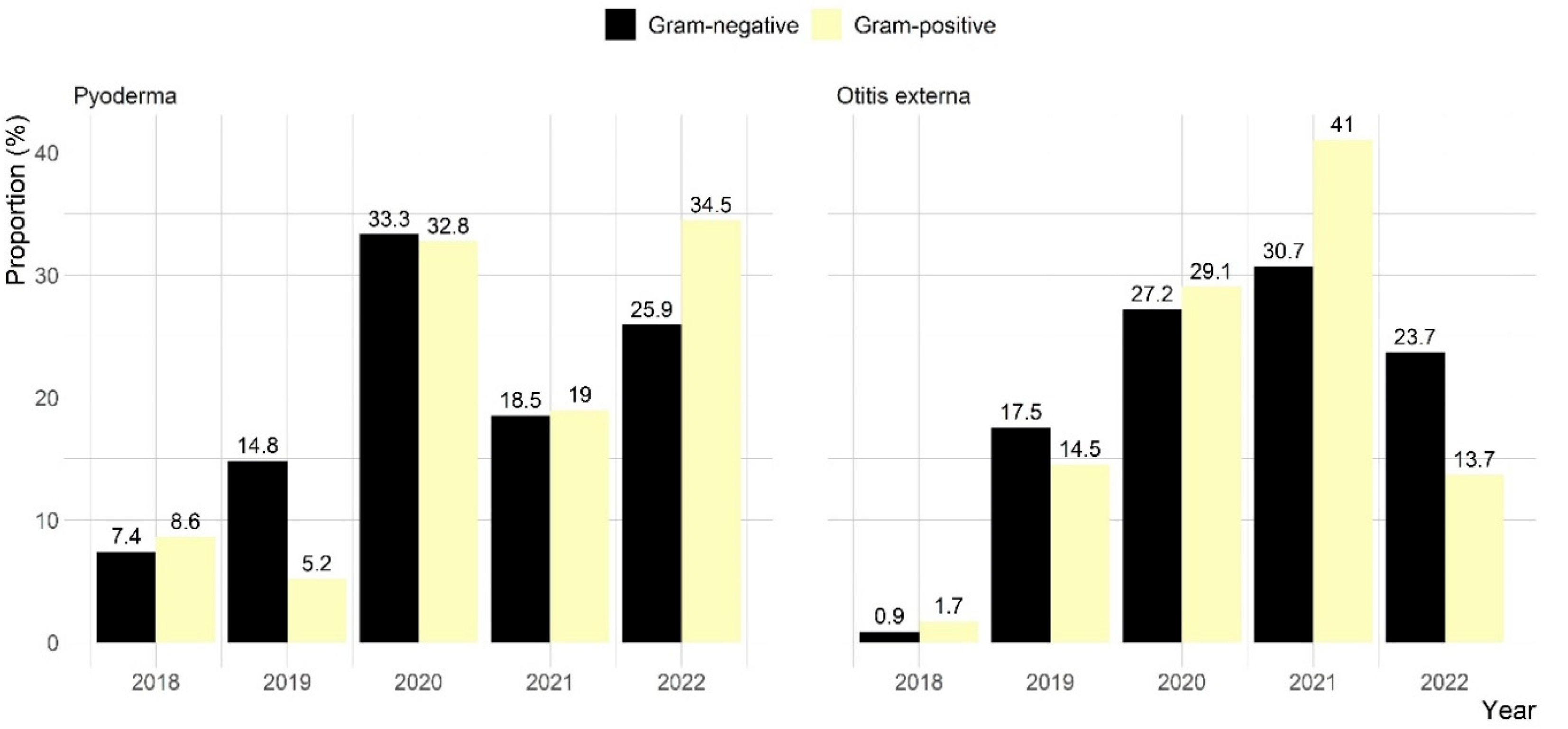
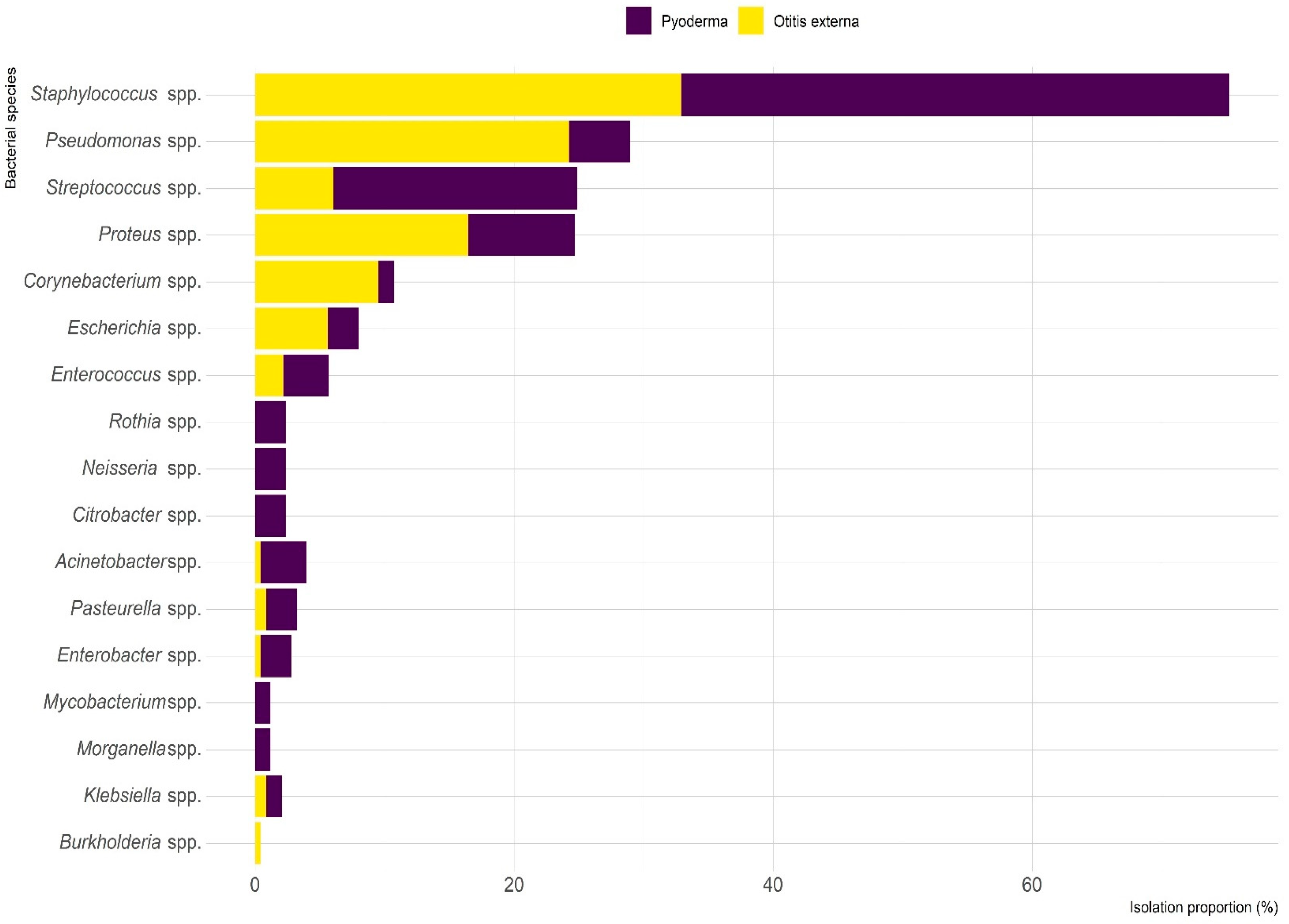
| Characteristics | Number (%) of Dogs with | Total (n = 215) | |
|---|---|---|---|
| Pyoderma (n = 63) | Otitis Externa (n = 152) | ||
| Sampling years | |||
| 2018 | 3 (4.8) | 3 (2.0) | 6 (2.8) |
| 2019 | 6 (9.5) | 26 (17.1) | 32 (14.9) |
| 2020 | 21 (33.3) | 47 (30.9) | 68 (31.6) |
| 2021 | 13 (20.6) | 49 (32.2) | 62 (28.8) |
| 2022 | 20 (31.8) | 27 (17.8) | 47 (21.9) |
| Breed | |||
| Purebred | 59 (93.6) | 149 (98.0) | 208 (96.7) |
| Mixed | 4 (6.4) | 3 (2.0) | 7 (3.3) |
| Sex | |||
| Male | 43 (68.2) | 82 (54.3) | 125 (58.4) |
| Female | 20 (31.8) | 69 (45.7) | 89 (41.6) |
| Reproductive Status | |||
| Intact | 35 (55.6) | 48 (31.6) | 83 (38.6) |
| Desexed | 28 (44.4) | 104 (68.4) | 132 (61.4) |
| Age (years) | |||
| <2 | 8 (12.7) | 10 (6.6) | 18 (8.4) |
| 2 to <5 | 15 (23.8) | 27 (17.7) | 42 (19.5) |
| 5 to <10 | 29 (46.0) | 72 (47.4) | 101 (47.0) |
| ≥10 | 11 (17.5) | 43 (28.3) | 54 (25.1) |
| Sampling site | |||
| Head | 4 (6.4) | -- | 4 (1.9) |
| Neck | 4 (6.4) | -- | 4 (1.9) |
| Trunk | 32 (50.7) | -- | 32 (14.9) |
| Limbs | 16 (25.4) | -- | 16 (7.4) |
| Perineal | 4 (6.4) | -- | 4 (1.9) |
| Tail | 3 (4.7) | -- | 3 (1.4) |
| Ear (Unilateral) | -- | 127 (83.6) | 127 (59.0) |
| Ear (Bilateral) | -- | 25 (16.4) | 25 (11.6) |
| Bacterial infection | |||
| Yes | 51 (80.9) | 133 (87.5) | 184 (85.6) |
| No | 12 (19.1) | 19 (12.5) | 31 (14.4) |
| Bacterial Isolates | No. of Resistances 1 | MDR 2 n (%) | Average MAR Index (Range) 3 | |||||
|---|---|---|---|---|---|---|---|---|
| Total N | 0 n (%) | 1 n (%) | 2 n (%) | 3 n (%) | >3 n (%) | |||
| I. Pyoderma | ||||||||
| Staphylococcus spp. | 35 | 5 (14.3) | 6 (17.1) | 3 (8.6) | 2 (5.7) | 19 (54.3) | 15 (42.9) | 0.37 (0.05–1.00) |
| Streptococcus spp. | 16 | -- | 1 (6.3) | 1 (6.3) | 1 (6.3) | 13 (81.3) | 12 (75) | 0.32 (0.07–0.57) |
| Proteus spp. | 7 | -- | -- | -- | 1 (14.3) | 6 (85.7) | 6 (85.7) | 0.35 (0.14–0.57) |
| Pseudomonas spp. | 4 | -- | -- | -- | -- | 4 (100) | 4 (100) | 0.76 (0.69–0.88) |
| II. Otitis externa | ||||||||
| Staphylococcus spp. | 76 | 13 (17.1) | 8 (10.5) | 7 (9.2) | 5 (6.6) | 43 (56.6) | 35 (46.1) | 0.49 (0.05–1.00) |
| Pseudomonas spp. | 56 | -- | -- | -- | -- | 56 (100) | 55 (98.2) | 0.75 (0.58–0.94) |
| Proteus spp. | 38 | -- | -- | -- | 4 (10.5) | 34 (89.5) | 23 (60.5) | 0.38 (0.18–1.00) |
| Corynebacterium spp. | 20 | -- | 3 (15) | 1 (4.5) | -- | 16 (80) | 13 (65) | 0.42 (0.06–0.82) |
| Streptococcus spp. | 14 | -- | -- | -- | 1 (7.1) | 13 (92.9) | 9 (64.3) | 0.48 (0.17–0.94) |
| Escherichia spp. | 13 | -- | 2 (15.4) | 2 (15.4) | 1 (7.7) | 8 (61.5) | 7 (53.8) | 0.40 (0.06–1.00) |
| Enterococcus spp. | 5 | 1 (20.0) | -- | -- | -- | 4 (80.0) | 4 (80.0) | 0.72 (0.59–0.82) |
| Factor | Pyoderma | Otitis Externa | |||||
|---|---|---|---|---|---|---|---|
| MDR (%) | OR (95% CI) 1 | p-Value | MDR (%) | OR (95% CI) 1 | p-Value | ||
| Sampling years | |||||||
| 2018 | 57.1 | Ref. | 0.807 | 66.7 | Ref. | 0.390 | |
| 2019 | 100 | -- | -- | 62.2 | 0.82 (0.07–9.9) | 0.877 | |
| 2020 | 71.4 | 1.88 (0.34–10.3) | 0.470 | 64.6 | 0.91 (0.08–10.6) | 0.942 | |
| 2021 | 60.0 | 1.13 (0.18–6.9) | 0.899 | 63.9 | 0.88 (0.08–10.2) | 0.921 | |
| 2022 | 61.5 | 1.20 (0.30–5.9) | 0.833 | 80.8 | 2.1 (0.17–25.7) | 0.574 | |
| Breed | |||||||
| Mixed | 42.9 | Ref. | 100 | Ref. | |||
| Purebred | 69.7 | 3.10 (0.64–14.8) | 0.162 | 65.8 | -- | -- | |
| Sex | |||||||
| Male | 62.5 | Ref. | 62.6 | Ref. | |||
| Female | 69.5 | 1.37 (0.51–3.7) | 0.538 | 71.4 | 1.49 (0.85–2.6) | 0.160 | |
| Reproductive Status | |||||||
| Desexed | 70.0 | Ref. | 62.8 | Ref. | |||
| Intact | 66.0 | 0.83 (0.32–2.2) | 0.711 | 75.0 | 1.78 (0.96–3.3) | 0.065 | |
| Age (years) | |||||||
| <2 | 66.7 | Ref. | 0.993 | 60.0 | Ref. | 0.599 | |
| 2 to <5 | 65.2 | 0.94 (0.18–48) | 0.938 | 72.2 | 1.73 (0.49–6.1) | 0.394 | |
| 5 to <10 | 68.4 | 1.08 (0.23–5.1) | 0.919 | 69.0 | 1.48 (0.50–4.5) | 0.486 | |
| ≥10 | 69.2 | 1.13 (0.18–6.9) | 0.899 | 61.3 | 1.06 (0.33–3.3) | 0.927 | |
| Sampling site | |||||||
| Head | 80.0 | Ref. | 0.675 | -- | -- | -- | |
| Neck | 100.0 | -- | -- | -- | -- | -- | |
| Trunk | 59.5 | 0.37 (0.04–3.6) | 0.389 | -- | -- | -- | |
| Limbs | 70.4 | 0.59 (0.06–6.2) | 0.663 | -- | -- | -- | |
| Perineal | 100.0 | -- | -- | -- | -- | -- | |
| Tail | 50.0 | 0.25 (0.01–8.6) | 0.442 | -- | -- | -- | |
| Ear (Unilateral) | -- | -- | -- | 66.8 | Ref. | ||
| Ear (Bilateral) | -- | -- | -- | 66.7 | 0.99 (0.45–2.3) | 0.986 | |
| Bacterial species | |||||||
| Gram-positive | 56.1 | Ref. | 53.1 | Ref. | |||
| Gram-negative | 92.3 | 9.4 (2.0–43.5) | 0.004 | 80.7 | 3.70 (2.0–6.7) | <0.001 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chan, W.Y.; Hobi, S.; Ferguson, A.; Elsohaby, I. Canine Pyoderma and Otitis Externa: A Retrospective Analysis of Multidrug-Resistant Bacterial Carriage in Hong Kong. Antibiotics 2025, 14, 685. https://doi.org/10.3390/antibiotics14070685
Chan WY, Hobi S, Ferguson A, Elsohaby I. Canine Pyoderma and Otitis Externa: A Retrospective Analysis of Multidrug-Resistant Bacterial Carriage in Hong Kong. Antibiotics. 2025; 14(7):685. https://doi.org/10.3390/antibiotics14070685
Chicago/Turabian StyleChan, Wing Yu, Stefan Hobi, Andrew Ferguson, and Ibrahim Elsohaby. 2025. "Canine Pyoderma and Otitis Externa: A Retrospective Analysis of Multidrug-Resistant Bacterial Carriage in Hong Kong" Antibiotics 14, no. 7: 685. https://doi.org/10.3390/antibiotics14070685
APA StyleChan, W. Y., Hobi, S., Ferguson, A., & Elsohaby, I. (2025). Canine Pyoderma and Otitis Externa: A Retrospective Analysis of Multidrug-Resistant Bacterial Carriage in Hong Kong. Antibiotics, 14(7), 685. https://doi.org/10.3390/antibiotics14070685