Blood Cultures Time-to-Positivity as an Antibiotic Stewardship Tool in Immunocompromised Children with Gram-Negative Bacteraemia
Abstract
1. Introduction
2. Results
2.1. Study Population
2.2. Microbiological Findings
2.3. Empirical Therapy and Clinical Outcomes
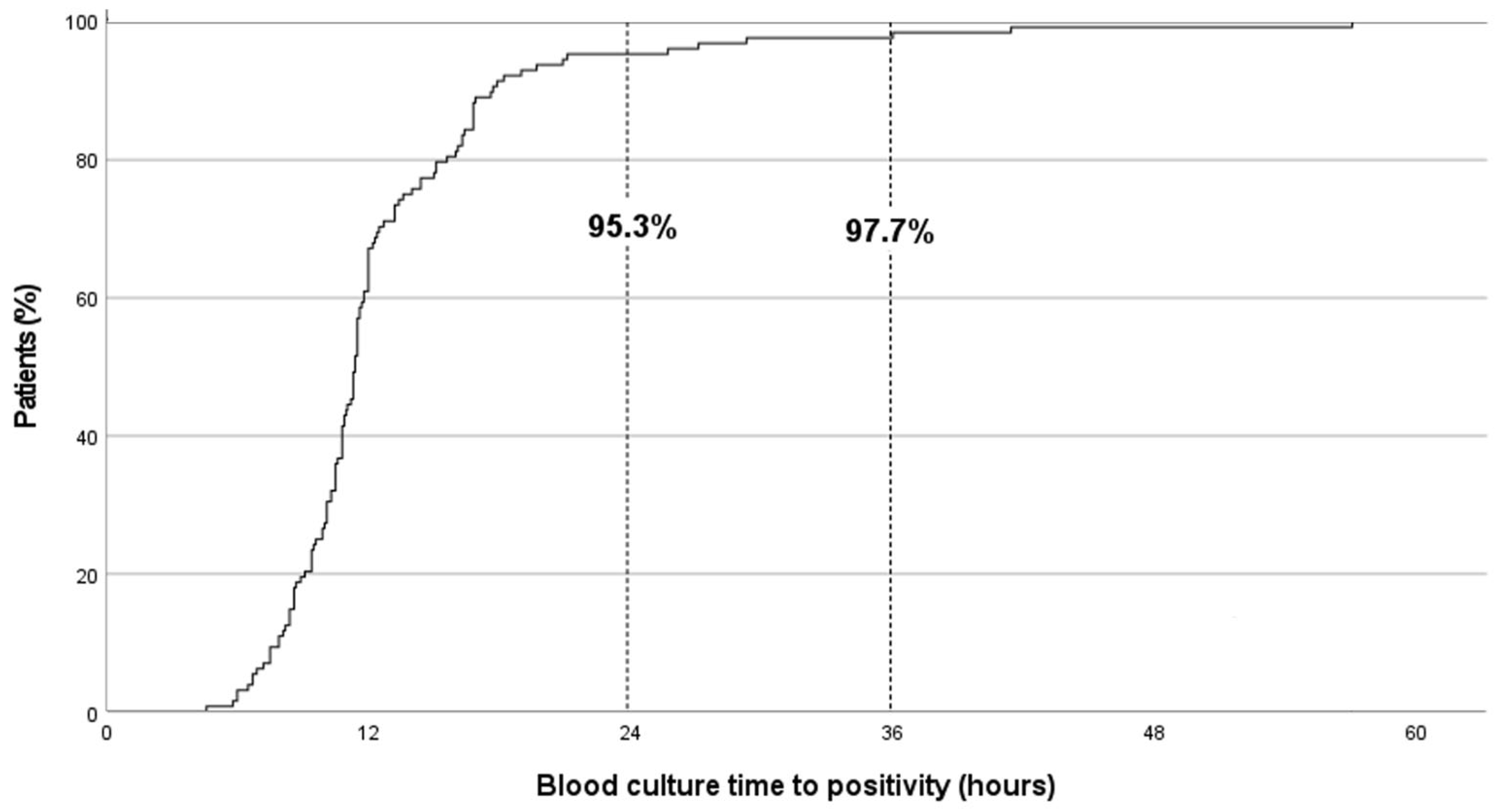
2.4. BC-TTP Distribution and Associated Clinical and Microbiological Factors
2.5. Factors Associated with TTP > 24 h
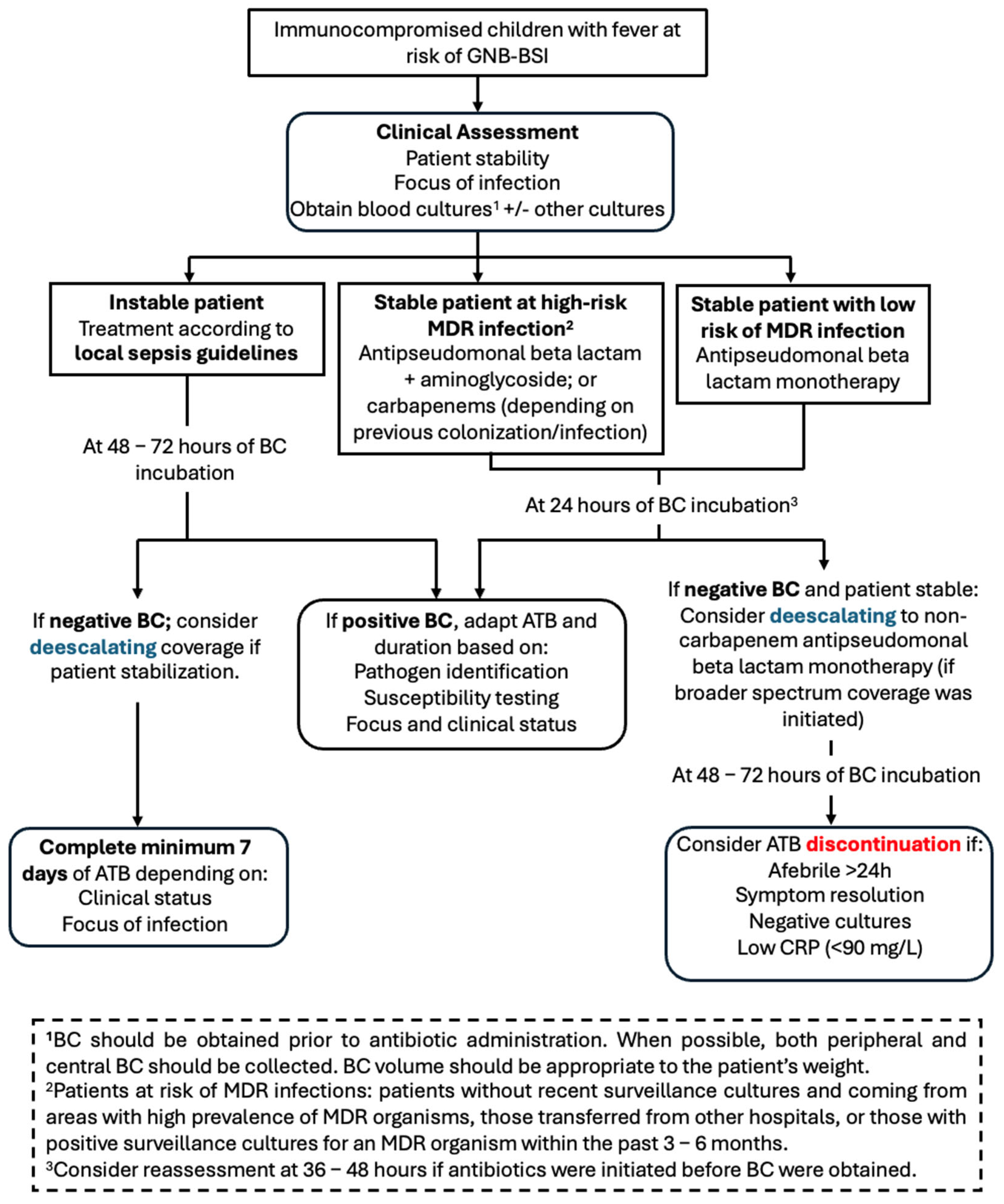
3. Discussion
4. Materials and Methods
4.1. Study Design and Setting
4.2. Study Population
4.3. Management of FN
4.4. Microbiological Procedures and Definitions
4.5. Data Collection and Outcomes
4.6. Statistical Analysis
4.7. Ethical Considerations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Maarbjerg, S.F.; Kiefer, L.V.; Albertsen, B.K.; Schrøder, H.; Wang, M. Bloodstream Infections in Children with Cancer: Pathogen Distribution and Antimicrobial Susceptibility Patterns over a 10-Year Period. J. Pediatr. Hematol. Oncol. 2022, 44, e160–e167. [Google Scholar] [CrossRef] [PubMed]
- Ammann, R.A.; Laws, H.J.; Schrey, D.; Ehlert, K.; Moser, O.; Dilloo, D.; Bode, U.; Wawer, A.; Schrauder, A.; Cario, G.; et al. Bloodstream infection in paediatric cancer centres—leukaemia and relapsed malignancies are independent risk factors. Eur. J. Pediatr. 2015, 174, 675–686. [Google Scholar] [CrossRef] [PubMed]
- Willis, D.N.; McGlynn, M.C.; Reich, P.J.; Hayashi, R.J. Mortality in pediatric oncology and stem cell transplant patients with bloodstream infections. Front. Oncol. 2023, 12, 1063253. [Google Scholar] [CrossRef]
- Lehrnbecher, T.; Averbuch, D.; Castagnola, E.; Cesaro, S.; Ammann, R.A.; Garcia-Vidal, C.; Kanerva, J.; Lanternier, F.; Mesini, A.; Mikulska, M.; et al. 8th European Conference on Infections in Leukaemia. 2020 guidelines for the use of antibiotics in paediatric patients with cancer or post-haematopoietic cell transplantation. Lancet Oncol. 2021, 22, e270–e280. [Google Scholar] [CrossRef]
- Pezzani, M.D.; Arieti, F.; Rajendran, N.B.; Barana, B.; Cappelli, E.; De Rui, M.E.; Galia, L.; Hassoun-Kheir, N.; Argante, L.; Schmidt, J.; et al. Frequency of bloodstream infections caused by six key antibiotic-resistant pathogens for prioritization of research and discovery of new therapies in Europe: A systematic review. Clin. Microbiol. Infect. 2024, 30 (Suppl. S1), S4–S13. [Google Scholar] [CrossRef]
- Sallah, Y.H.; Bratti, V.F.; Rafinejad-Farahani, B.; Jayasekar Zurn, S.; Johnson, S.; Crestani, A.S.; Dacoregio, M.I.; Majeed, H.; Fazelzad, R.; Pabani, A.; et al. Antimicrobial resistance in patients with haematological malignancies: A scoping review. Lancet Oncol. 2025, 26, e242–e252. [Google Scholar] [CrossRef]
- Kazmierczak, K.M.; de Jonge, B.L.M.; Stone, G.G.; Sahm, D.F. Longitudinal analysis of ESBL and carbapenemase carriage among Enterobacterales and Pseudomonas aeruginosa isolates collected in Europe as part of the International Network for Optimal Resistance Monitoring (INFORM) global surveillance programme, 2013–2017. J. Antimicrob. Chemother. 2020, 75, 1165–1173. [Google Scholar] [CrossRef]
- Stergiotis, M.; Ammann, R.A.; Droz, S.; Koenig, C.; Agyeman, P.K.A. Pediatric fever in neutropenia with bacteremia-Pathogen distribution and in vitro antibiotic susceptibility patterns over time in a retrospective single-center cohort study. PLoS ONE 2021, 16, e0246654. [Google Scholar] [CrossRef] [PubMed]
- Lehrnbecher, T.; Robinson, P.D.; Ammann, R.A.; Fisher, B.; Patel, P.; Phillips, R.; Beauchemin, M.P.; Carlesse, F.; Castagnola, E.; Davis, B.L.; et al. Guideline for the Management of Fever and Neutropenia in Pediatric Patients With Cancer and Hematopoietic Cell Transplantation Recipients: 2023 Update. J. Clin. Oncol. 2023, 41, 1774–1785. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Castagnola, E.; Bagnasco, F.; Mesini, A.; Agyeman, P.K.A.; Ammann, R.A.; Carlesse, F.; Santolaya de Pablo, M.E.; Groll, A.H.; Haeusler, G.M.; Lehrnbecher, T.; et al. Antibiotic Resistant Bloodstream Infections in Pediatric Patients Receiving Chemotherapy or Hematopoietic Stem Cell Transplant: Factors Associated with Development of Resistance, Intensive Care Admission and Mortality. Antibiotics 2021, 10, 266. [Google Scholar] [CrossRef] [PubMed]
- Barlam, T.F.; Cosgrove, S.E.; Abbo, L.M.; MacDougall, C.; Schuetz, A.N.; Septimus, E.J.; Srinivasan, A.; Dellit, T.H.; Falck-Ytter, Y.T.; Fishman, N.O.; et al. Implementing an Antibiotic Stewardship Program: Guidelines by the Infectious Diseases Society of America and the Society for Healthcare Epidemiology of America. Clin. Infect. Dis. 2016, 62, e51–e77. [Google Scholar] [CrossRef]
- Velasco-Arnaiz, E.; Simó-Nebot, S.; Ríos-Barnés, M.; López Ramos, M.G.; Monsonís, M.; Urrea-Ayala, M.; Jordan, I.; Mas-Comas, A.; Casadevall-Llandrich, R.; Ormazábal-Kirchner, D.; et al. Benefits of a Pediatric Antimicrobial Stewardship Program in Antimicrobial Use and Quality of Prescriptions in a Referral Children’s Hospital. J. Pediatr. 2020, 225, 222–230.e1. [Google Scholar] [CrossRef]
- Molina, J.; Noguer, M.; Lepe, J.A.; Pérez-Moreno, M.A.; Aguilar-Guisado, M.; Lasso de la Vega, R.; Peñalva, G.; Crespo-Rivas, J.C.; Gil-Navarro, M.V.; Salvador, J.; et al. Clinical impact of an educational antimicrobial stewardship program associated with infectious diseases consultation targeting patients with cancer: Results of a 9-year quasi-experimental study with an interrupted time-series analysis. J. Infect. 2019, 79, 206–211. [Google Scholar] [CrossRef] [PubMed]
- Pillinger, K.E.; Bouchard, J.; Withers, S.T.; Mediwala, K.; McGee, E.U.; Gibson, G.M.; Bland, C.M.; Bookstaver, P.B. Inpatient Antibiotic Stewardship Interventions in the Adult Oncology and Hematopoietic Stem Cell Transplant Population: A Review of the Literature. Ann. Pharmacother. 2020, 54, 594–610. [Google Scholar] [CrossRef] [PubMed]
- Muratore, E.; Baccelli, F.; Leardini, D.; Campoli, C.; Belotti, T.; Viale, P.; Prete, A.; Pession, A.; Masetti, R.; Zama, D. Antimicrobial Stewardship Interventions in Pediatric Oncology: A Systematic Review. J. Clin. Med. 2022, 11, 4545. [Google Scholar] [CrossRef]
- Liberati, C.; Barbieri, E.; Cavagnero, F.; Petris, M.G.; Brigadoi, G.; Reggiani, G.; De Pieri, M.; Pierobon, M.; Marzollo, A.; Gabelli, M.; et al. Impact of a two step antimicrobial stewardship program in a paediatric haematology and oncology unit. Sci. Rep. 2024, 14, 29296. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lehrnbecher, T.; Groll, A.H. Infectious complications in the paediatric immunocompromised host: A narrative review. Clin. Microbiol. Infect. 2025, 31, 37–42. [Google Scholar] [CrossRef] [PubMed]
- Sung, L.; Phillips, R.; Lehrnbecher, T. Time for paediatric febrile neutropenia guidelines - children are not little adults. Eur. J. Cancer 2011, 47, 811–813. [Google Scholar] [CrossRef] [PubMed]
- Berkley, J.A.; Walson, J.L.; Gray, G.; Russell, F.; Bhutta, Z.; Ashorn, P.; Norris, S.A.; Adejuyigbe, E.A.; Grais, R.; Ogutu, B.; et al. Strengthening the paediatric clinical trial ecosystem to better inform policy and programmes. Lancet Glob. Health 2025, 13, e732–e739. [Google Scholar] [CrossRef] [PubMed]
- Upton, D.A. Management of infections in theimmunocompromised child: General principles. LymphoSign J. 2016, 3, 87–98. [Google Scholar] [CrossRef]
- Lamy, B. Blood culture time-to-positivity: Making use of the hidden information. Clin. Microbiol. Infect. 2019, 25, 268–271. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, M.D.; Chao, T.; Pettengill, M.A. Modern Blood Culture: Management Decisions and Method Options. Clin. Lab. Med. 2020, 40, 379–392. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Puerta-Alcalde, P.; Cardozo, C.; Suárez-Lledó, M.; Rodríguez-Núñez, O.; Morata, L.; Fehér, C.; Marco, F.; Del Río, A.; Martínez, J.A.; Mensa, J.; et al. Current time-to-positivity of blood cultures in febrile neutropenia: A tool to be used in stewardship de-escalation strategies. Clin. Microbiol. Infect. 2019, 25, 447–453. [Google Scholar] [CrossRef]
- Lambregts, M.M.C.; Warreman, E.B.; Bernards, A.T.; Veelken, H.; von dem Borne, P.A.; Dekkers, O.M.; Visser, L.G.; de Boer, M.G. Distribution and clinical determinants of time-to-positivity of blood cultures in patients with neutropenia. Eur. J. Haematol. 2018, 100, 206–214. [Google Scholar] [CrossRef] [PubMed]
- Hakim, H.; Flynn, P.M.; Knapp, K.M.; Srivastava, D.K.; Gaur, A.H. Etiology and clinical course of febrile neutropenia in children with cancer. J. Pediatr. Hematol. Oncol. 2009, 31, 623–629. [Google Scholar] [CrossRef]
- MacBrayne, C.E.; Williams, M.C.; Prinzi, A.; Pearce, K.; Lamb, D.; Parker, S.K. Time to Blood Culture Positivity by Pathogen and Primary Service. Hosp. Pediatr. 2021, 11, 953–961. [Google Scholar] [CrossRef]
- Dierig, A.; Berger, C.; Agyeman, P.K.A.; Bernhard-Stirnemann, S.; Giannoni, E.; Stocker, M.; Posfay-Barbe, K.M.; Niederer-Loher, A.; Kahlert, C.R.; Donas, A.; et al. Swiss Pediatric Sepsis Study. Time-to-Positivity of Blood Cultures in Children With Sepsis. Front. Pediatr. 2018, 6, 222. [Google Scholar] [CrossRef]
- Defrance, G.; Birgand, G.; Ruppé, E.; Billard, M.; Ruimy, R.; Bonnal, C.; Andremont, A.; Armand-Lefèvre, L. Time-to-positivity-based discrimination between Enterobacteriaceae, Pseudomonas aeruginosa and strictly anaerobic Gram-negative bacilli in aerobic and anaerobic blood culture vials. J. Microbiol. Methods 2013, 93, 77–79. [Google Scholar] [CrossRef] [PubMed]
- Hamilton, F.; Evans, R.; Ghazal, P.; MacGowan, A. Time to positivity in bloodstream infection is not a prognostic marker for mortality: Analysis of a prospective multicentre randomized control trial. Clin. Microbiol. Infect. 2022, 28, 136.e7–136.e13. [Google Scholar] [CrossRef] [PubMed]
- WHO. Bacterial Priority Pathogens List. In Bacterial Pathogens of Public Health Importance to Guide Research, Development and Strategies to Prevent and Control Antimicrobial Resistance; World Health Organization: Geneva, Switzerland, 2024. [Google Scholar]
- Palmer, H.R.; Palavecino, E.L.; Johnson, J.W.; Ohl, C.A.; Williamson, J.C. Clinical and microbiological implications of time-to-positivity of blood cultures in patients with Gram-negative bacilli bacteremia. Eur. J. Clin. Microbiol. Infect. Dis. 2013, 32, 955–959. [Google Scholar] [CrossRef]
- Xu, H.; Cheng, J.; Yu, Q.; Li, Q.; Yi, Q.; Luo, S.; Li, Y.; Zhang, G.; Tian, X.; Cheng, D.; et al. Prognostic role of time to positivity of blood culture in children with Pseudomonas aeruginosa bacteremia. BMC Infect. Dis. 2020, 20, 665. [Google Scholar] [CrossRef] [PubMed]
- Cheng, J.; Zhang, G.; Li, Q.; Xu, H.; Yu, Q.; Yi, Q.; Luo, S.; Li, Y.; Tian, X.; Chen, D.; et al. Time to positivity of Klebsiella pneumoniae in blood culture as prognostic indicator for pediatric bloodstream infections. Eur. J. Pediatr. 2020, 179, 1689–1698, Erratum in Eur. J. Pediatr. 2020, 179, 1699. [Google Scholar] [CrossRef] [PubMed]
- Haimi-Cohen, Y.; Vellozzi, E.M.; Rubin, L.G. Initial concentration of Staphylococcus epidermidis in simulated pediatric blood cultures correlates with time to positive results with the automated, continuously monitored BACTEC blood culture system. J. Clin. Microbiol. 2002, 40, 898–901. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
| TTP (h) | Sex/Age | Microorganism | Underlying Diseases | Infectious Focus | Antibiotic Pretreatment | ANC (/mm3) |
|---|---|---|---|---|---|---|
| 25.7 | Male/10 y | Acinetobacter spp. | HR-ALL, C/I | Unknown | No | 0 |
| 27.1 | Male/17 y | Klebsiella pneumoniae | Astrocytoma | UTI | Yes | 7800 |
| 29.3 | Female/8 y | Achromobacter xylosoxidans | HR-ALL, relapse | CRBSI | No | 2100 |
| 37.4 | Female/7 y | Pseudomonas putida | HR-ALL, relapse | CRBSI | Yes | 0 |
| 41.4 | Male/6 y | Campylobacter jejuni | HR-ALL, C/I | Unknown | Yes | 1500 |
| 57.0 | Male/5 y | Yersinia enterocolitica | HR-ALL, induction | CRBSI | No | 100 |
| N (%) | Median TTP (p25–75) | p-Value | |
|---|---|---|---|
| Total | 128 (100) | 11.4 (9.8–14.1) | |
| ANC (/mm3) at diagnosis | 0.906 | ||
| 99 (77.3) | 11.4 (10.1–13.4) | |
| 29 (22.7) | 8.7 (7.7–14.4) | |
| Previous antibiotic treatment | 0.257 | ||
| 20 (15.6) | 11.5 (9.6–14.8) | |
| 108 (84.4) | 11.3 (9.1–13.4) | |
| Disease-related risk factor | 0.071 | ||
| 38 (29.7) | 11.4 (9.6–17.0) | |
| 17 (13.3) | 11.3 (8.9–12.0) | |
| 5 (3.9) | 13.6 (11.4–18.0) | |
| 40 (31.3) | 10.9 (8.8–12.3) | |
| 20 (15.6) | 11.8 (8.8–14.3) | |
| 8 (6.2) | 10.9 (8.4–13.6) | |
| Infectious focus | 0.868 | ||
| 84 (65.6) | 11.3 (10.0–13.0) | |
| 24 (18.8) | 8.8 (7.5–13.9) | |
| 13 (10.2) | 12.7 (11.3–15.0) | |
| 7 (5.4) | 9.4 (8.1–17.6) | |
| Microorganism | <0.001 | ||
| 41 (32.0) | 10.5 (9.3–11.3) | |
| 22 (17.2) | 11.5 (8.6–12.0) | |
| 25 (19.5) | 16.0 (13.9–16.8) | |
| 12 (9.4) | 10.2 (9.0–11.2) | |
| 28 (21.9) | 12.6 (8.0–19.5) | |
| Admission to the PICU | 0.175 | ||
| 15 (11.7) | 10.5 (7.5–12.0) | |
| 113 (88.3) | 11.3 (9.6–14.3) | |
| 30-day mortality | 0.236 | ||
| 7 (5.5) | 10.6 (7.9–11.3) | |
| 121 (94.5) | 11.4 (9.4–14.3) |
| TTP < 24 h | TTP > 24 h | ||
|---|---|---|---|
| N (%) | N (%) | p-Value | |
| Total | 122 (95.3) | 6 (4.7) | |
| Severe neutropenia (ANC < 500/mm3) | 93 (76.2) | 3 (50.0) | 0.129 |
| Previous antibiotic treatment | 17 (13.9) | 3 (50.0) | 0.048 |
| Disease-related risk factor | 0.239 | ||
| 33 (27.0) | 5 (83.3) | |
| 17 (13.9) | 0 (0.0) | |
| 5 (4.1) | 0 (0.0) | |
| 39 (32.0) | 1 (16.7) | |
| 20 (16.4) | 0 (0.0) | |
| 8 (6.5) | 0 (0.0) | |
| Infectious focus | 0.080 | ||
| 82 (62.7) | 2 (33.3) | |
| 21 (17.2) | 3 (50.0) | |
| 13 (10.7) | 0 (0.0) | |
| 6 (4.9) | 1 (16.7) | |
| Microorganism | 0.005 | ||
| 41 (33.6) | 0 (0.0) | |
| 21 (17.2) | 1 (16.7) | |
| 25 (20.5) | 0 (0.0) | |
| 12 (9.8) | 0 (0.0) | |
| 23 (18.9) | 5 (83.3) | |
| Admission to PICU | 15 (12.3) | 0 (0.0) | 1.000 |
| 30-day mortality | 7 (5.7) | 0 (0.0) | 1.000 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gotzens, J.; Colom-Balañà, A.; Monsonís, M.; Alsina, L.; Ruiz-Cobo, M.A.; Ríos-Barnés, M.; Gamell, A.; Velasco-Arnaiz, E.; Martínez-de-Albéniz, I.; Fumadó, V.; et al. Blood Cultures Time-to-Positivity as an Antibiotic Stewardship Tool in Immunocompromised Children with Gram-Negative Bacteraemia. Antibiotics 2025, 14, 847. https://doi.org/10.3390/antibiotics14080847
Gotzens J, Colom-Balañà A, Monsonís M, Alsina L, Ruiz-Cobo MA, Ríos-Barnés M, Gamell A, Velasco-Arnaiz E, Martínez-de-Albéniz I, Fumadó V, et al. Blood Cultures Time-to-Positivity as an Antibiotic Stewardship Tool in Immunocompromised Children with Gram-Negative Bacteraemia. Antibiotics. 2025; 14(8):847. https://doi.org/10.3390/antibiotics14080847
Chicago/Turabian StyleGotzens, Julià, Aina Colom-Balañà, Manuel Monsonís, Laia Alsina, María Antonia Ruiz-Cobo, María Ríos-Barnés, Anna Gamell, Eneritz Velasco-Arnaiz, Irene Martínez-de-Albéniz, Victoria Fumadó, and et al. 2025. "Blood Cultures Time-to-Positivity as an Antibiotic Stewardship Tool in Immunocompromised Children with Gram-Negative Bacteraemia" Antibiotics 14, no. 8: 847. https://doi.org/10.3390/antibiotics14080847
APA StyleGotzens, J., Colom-Balañà, A., Monsonís, M., Alsina, L., Ruiz-Cobo, M. A., Ríos-Barnés, M., Gamell, A., Velasco-Arnaiz, E., Martínez-de-Albéniz, I., Fumadó, V., Fortuny, C., Noguera-Julian, A., & Simó-Nebot, S. (2025). Blood Cultures Time-to-Positivity as an Antibiotic Stewardship Tool in Immunocompromised Children with Gram-Negative Bacteraemia. Antibiotics, 14(8), 847. https://doi.org/10.3390/antibiotics14080847







