Galactose Inhibits the Translation of Erg1 that Enhances the Antifungal Activities of Azoles Against Candida albicans
Abstract
1. Introduction
2. Results
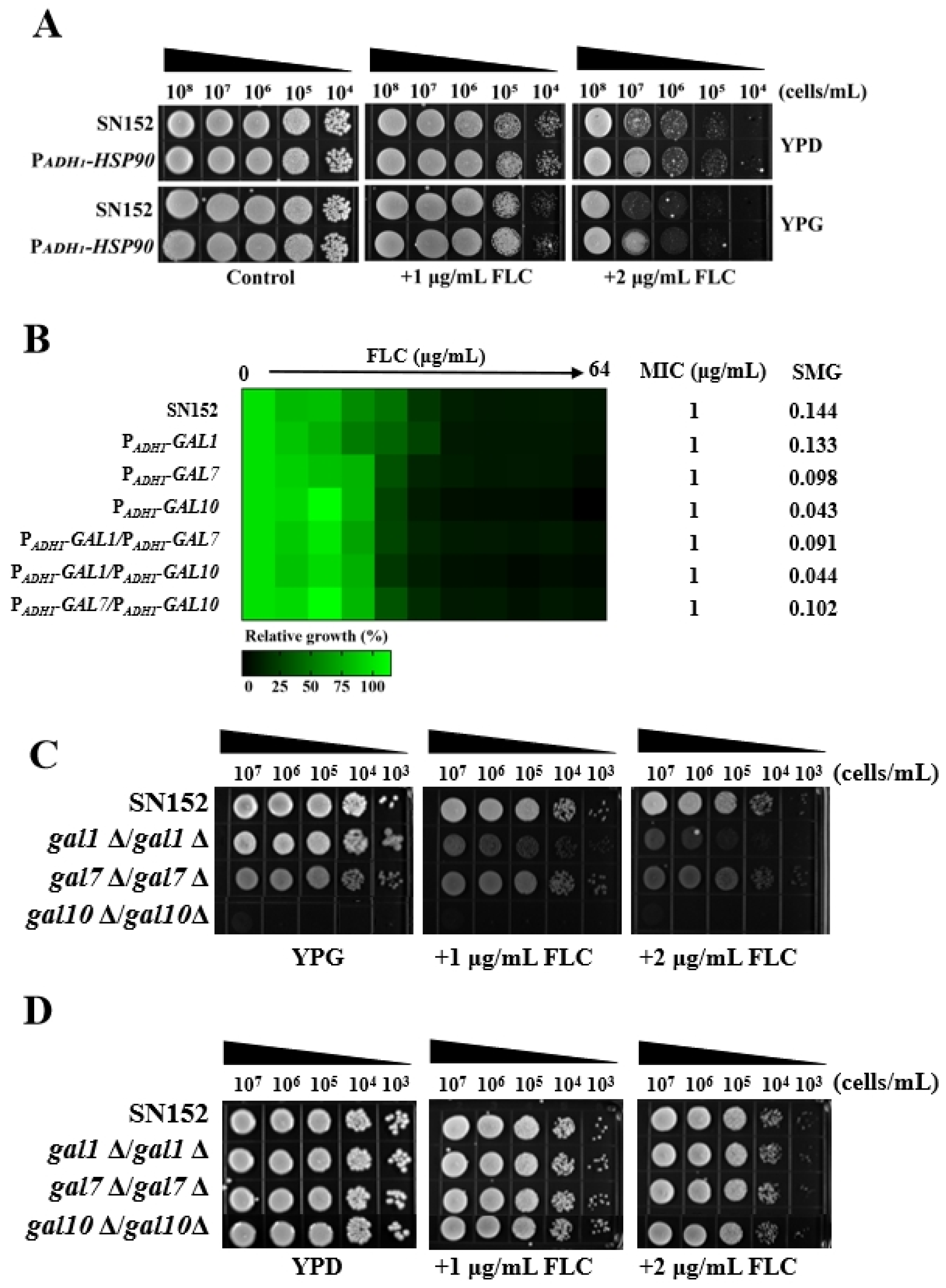
2.1. Galactose Enhanced the Antifungal Activities of Azoles Against C. albicans
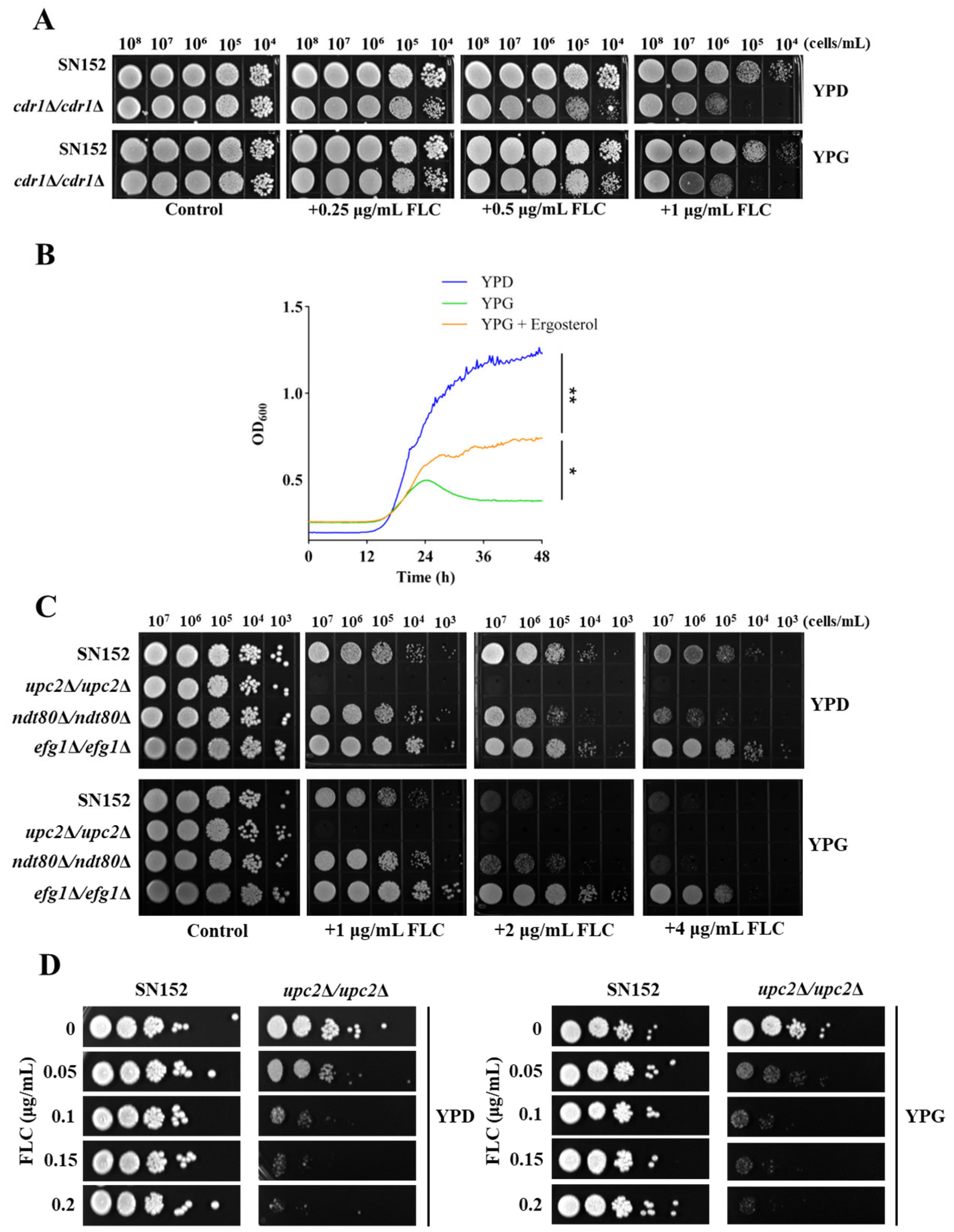
2.2. Galactose Enhanced the Antifungal Activity of Azoles Independent of the Activated Leloir Pathway
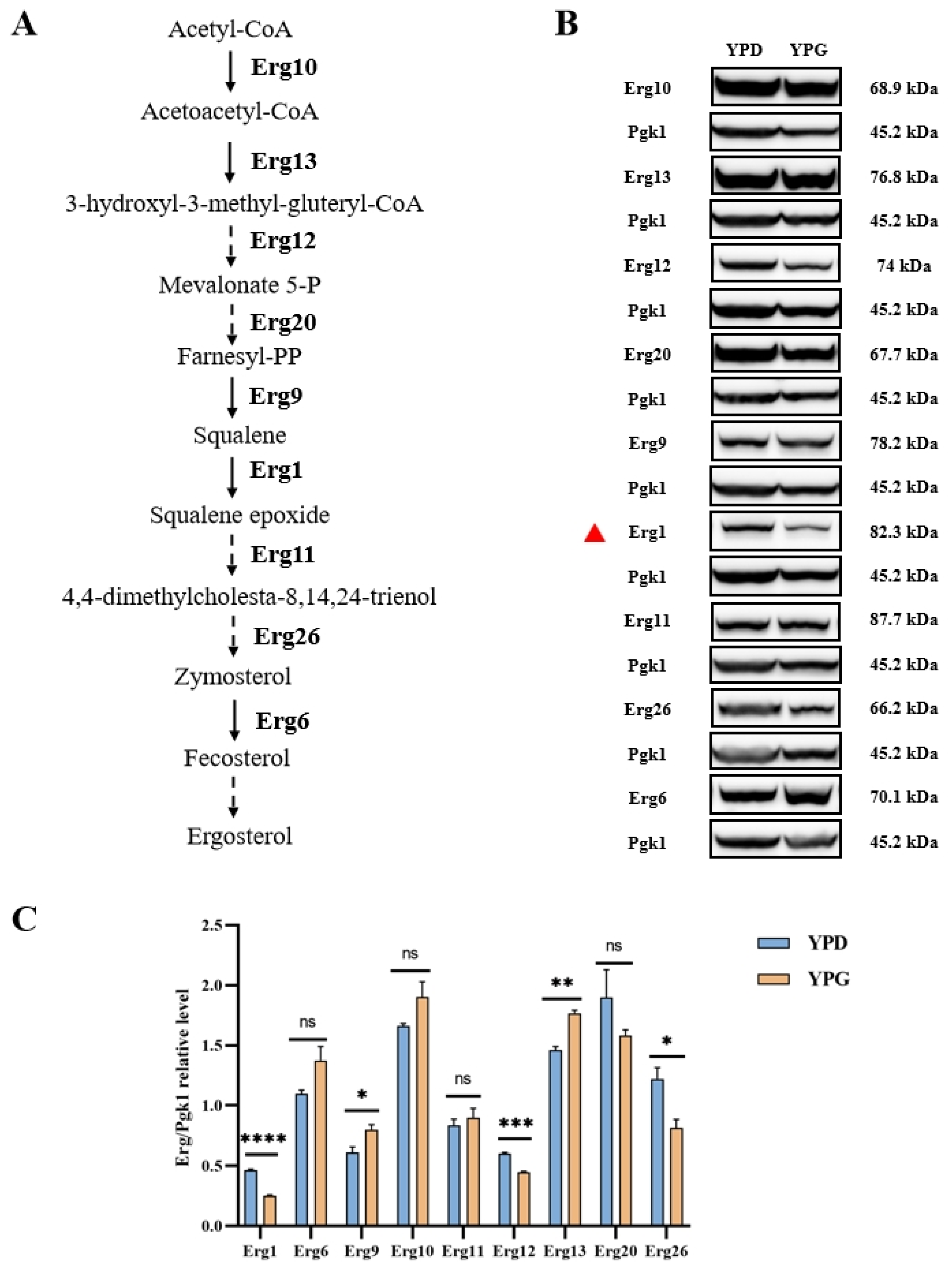
2.3. Galactose Inhibits Ergosterol Biosynthesis in C. albicans
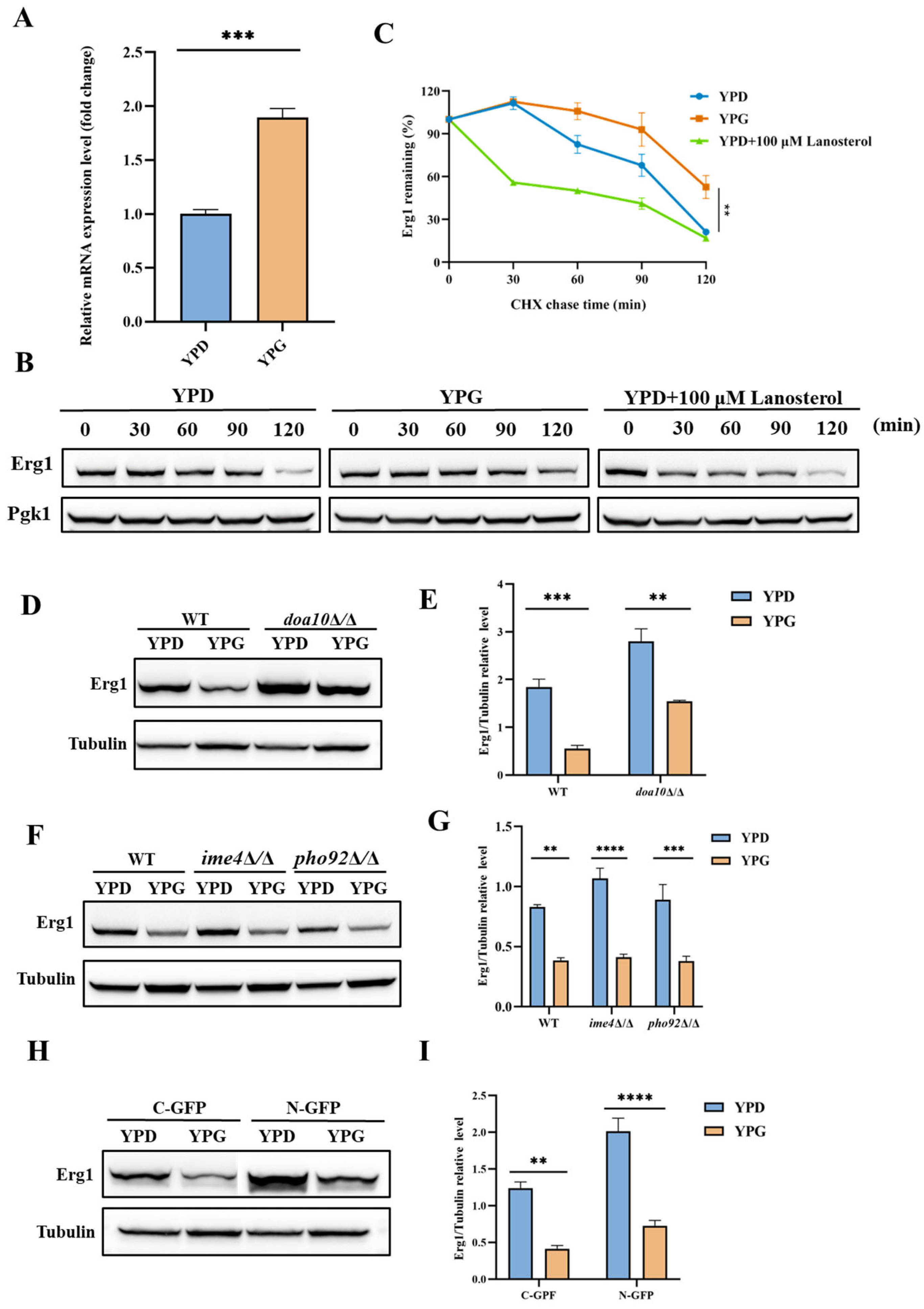
2.4. Galactose Suppresses the Expression of Erg1 in C. albicans
2.5. Galactose Inhibits the Translation of Erg1 in C. albicans
3. Discussion
4. Materials and Methods
4.1. Strains, Primers, Agents, and Cultural Conditions
4.2. MIC and SMG Assay
4.3. Disk Diffusion Assay
4.4. Spotting Assay
4.5. Growth Inhibition Curve Assay
4.6. Disruption of Target Genes
4.7. Ectopic Overexpression of Target Genes
4.8. C-Terminal or N-Terminal of Proteins Tagging GFP
4.9. RNA Extractions and Quantitative RT-PCR
4.10. Western Blotting
4.11. CHX Chase Analysis
4.12. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| FLC | Fluconazole |
| ER | Endoplasmic reticulum |
| FAD | Flavin adenosine dinucleotide |
| NADPH | Nicotinamide adenine dinucleotide phosphate |
| SRE | Sterol regulatory elements |
| ERAD | Endoplasmic reticulum-associated degradation |
| MIC | Minimum inhibitory concentration |
| SMG | Super-MIC growth |
| GFP | Green fluorescent protein |
| CHX | Cycloheximide |
| m6A | N6-methyladenosine |
| AREs | AU-rich elements |
| UTR | Untranslated region |
| DMSO | Dimethyl sulfoxide |
| PBS | Phosphate-buffered saline |
| OD600 | Optical density measurements at 600 nm |
| EDTA | Ethylene diamine tetraacetic acid |
| PMSF | Phenylmethylsulfonyl fluoride |
| SDS | Sodium dodecyl sulfate |
| PAGE | Polyacrylamide gel electrophoresis |
| PVDF | Polyvinylidene difluoride |
| SEM | Standard error of the mean |
| ANOVA | Analysis of variance |
References
- Lu, H.; Hong, T.; Jiang, Y.; Whiteway, M.; Zhang, S. Candidiasis: From cutaneous to systemic, new perspectives of potential targets and therapeutic strategies. Adv. Drug Deliv. Rev. 2023, 199, 114960. [Google Scholar] [CrossRef]
- Denning, D.W. Global incidence and mortality of severe fungal disease. Lancet Infect. Dis. 2024, 24, e428–e438. [Google Scholar] [CrossRef] [PubMed]
- Sokolov, S.S.; Trushina, N.I.; Severin, F.F.; Knorre, D.A. Ergosterol Turnover in Yeast: An Interplay between Biosynthesis and Transport. Biochemistry 2019, 84, 346–357. [Google Scholar] [CrossRef] [PubMed]
- Shafiei, M.; Peyton, L.; Hashemzadeh, M.; Foroumadi, A. History of the development of antifungal azoles: A review on structures, SAR, and mechanism of action. Bioorg. Chem. 2020, 104, 104240. [Google Scholar] [CrossRef]
- Monk, B.C.; Sagatova, A.A.; Hosseini, P.; Ruma, Y.N.; Wilson, R.K.; Keniya, M.V. Fungal Lanosterol 14alpha-demethylase: A target for next-generation antifungal design. Biochim. Biophys. Acta Proteins Proteom. 2020, 1868, 140206. [Google Scholar] [CrossRef]
- Feng, Y.; Lu, H.; Whiteway, M.; Jiang, Y. Understanding fluconazole tolerance in Candida albicans: Implications for effective treatment of candidiasis and combating invasive fungal infections. J. Glob. Antimicrob. Resist. 2023, 35, 314–321. [Google Scholar] [CrossRef]
- Lee, Y.; Puumala, E.; Robbins, N.; Cowen, L.E. Antifungal Drug Resistance: Molecular Mechanisms in Candida albicans and Beyond. Chem. Rev. 2021, 121, 3390–3411. [Google Scholar] [CrossRef]
- Xiong, J.; Lu, H.; Jiang, Y. Mechanisms of Azole Potentiation: Insights from Drug Repurposing Approaches. ACS Infect. Dis. 2025, 11, 305–322. [Google Scholar] [CrossRef]
- Lok, B.; Adam, M.A.A.; Kamal, L.Z.M.; Chukwudi, N.A.; Sandai, R.; Sandai, D. The assimilation of different carbon sources in Candida albicans: Fitness and pathogenicity. Med. Mycol. 2021, 59, 115–125. [Google Scholar] [CrossRef]
- Van Ende, M.; Wijnants, S.; Van Dijck, P. Sugar Sensing and Signaling in Candida albicans and Candida glabrata. Front. Microbiol. 2019, 10, 99. [Google Scholar] [CrossRef]
- Segal, E.S.; Gritsenko, V.; Levitan, A.; Yadav, B.; Dror, N.; Steenwyk, J.L.; Silberberg, Y.; Mielich, K.; Rokas, A.; Gow, N.A.R.; et al. Gene Essentiality Analyzed by In Vivo Transposon Mutagenesis and Machine Learning in a Stable Haploid Isolate of Candida albicans. mBio 2018, 9. [Google Scholar] [CrossRef]
- Li, L.; Lu, H.; Zhang, X.; Whiteway, M.; Wu, H.; Tan, S.; Zang, J.; Tian, S.; Zhen, C.; Meng, X.; et al. Baicalein Acts against Candida albicans by Targeting Eno1 and Inhibiting Glycolysis. Microbiol. Spectr. 2022, 10, e0208522. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.L.; Chen, H.F.; Kuo, T.J.; Lin, C.Y. Mutations on CaENO1 in Candida albicans inhibit cell growth in the presence of glucose. J. Biomed. Sci. 2006, 13, 313–321. [Google Scholar] [CrossRef] [PubMed]
- Ko, H.C.; Hsiao, T.Y.; Chen, C.T.; Yang, Y.L. Candida albicans ENO1 null mutants exhibit altered drug susceptibility, hyphal formation, and virulence. J. Microbiol. 2013, 51, 345–351. [Google Scholar] [CrossRef] [PubMed]
- Wijnants, S.; Riedelberger, M.; Penninger, P.; Kuchler, K.; Van Dijck, P. Sugar Phosphorylation Controls Carbon Source Utilization and Virulence of Candida albicans. Front. Microbiol. 2020, 11, 1274. [Google Scholar] [CrossRef]
- Rodaki, A.; Bohovych, I.M.; Enjalbert, B.; Young, T.; Odds, F.C.; Gow, N.A.; Brown, A.J. Glucose promotes stress resistance in the fungal pathogen Candida albicans. Mol. Biol. Cell 2009, 20, 4845–4855. [Google Scholar] [CrossRef]
- Mandal, S.M.; Mahata, D.; Migliolo, L.; Parekh, A.; Addy, P.S.; Mandal, M.; Basak, A. Glucose directly promotes antifungal resistance in the fungal pathogen, Candida spp. J. Biol. Chem. 2014, 289, 25468–25473. [Google Scholar] [CrossRef]
- Alves, R.; Mota, S.; Silva, S.; Rodrigues, C.F.; Brown, A.J.P.; Henriques, M.; Casal, M.; Paiva, S. The carboxylic acid transporters Jen1 and Jen2 affect the architecture and fluconazole susceptibility of Candida albicans biofilm in the presence of lactate. Biofouling 2017, 33, 943–954. [Google Scholar] [CrossRef]
- Ene, I.V.; Adya, A.K.; Wehmeier, S.; Brand, A.C.; MacCallum, D.M.; Gow, N.A.; Brown, A.J. Host carbon sources modulate cell wall architecture, drug resistance and virulence in a fungal pathogen. Cell. Microbiol. 2012, 14, 1319–1335. [Google Scholar] [CrossRef]
- Suchodolski, J.; Muraszko, J.; Bernat, P.; Krasowska, A. Lactate Like Fluconazole Reduces Ergosterol Content in the Plasma Membrane and Synergistically Kills Candida albicans. Int. J. Mol. Sci. 2021, 22, 5219. [Google Scholar] [CrossRef]
- Suchodolski, J.; Krasowska, A. Fructose Induces Fluconazole Resistance in Candida albicans through Activation of Mdr1 and Cdr1 Transporters. Int. J. Mol. Sci. 2021, 22, 2127. [Google Scholar] [CrossRef]
- Lao, M.; Li, C.; Li, J.; Chen, D.; Ding, M.; Gong, Y. Opportunistic invasive fungal disease in patients with type 2 diabetes mellitus from Southern China: Clinical features and associated factors. J. Diabetes Investig. 2020, 11, 731–744. [Google Scholar] [CrossRef] [PubMed]
- de Leon, E.M.; Jacober, S.J.; Sobel, J.D.; Foxman, B. Prevalence and risk factors for vaginal Candida colonization in women with type 1 and type 2 diabetes. BMC Infect. Dis. 2002, 2, 1. [Google Scholar] [CrossRef] [PubMed]
- Nazir, M.A.; AlGhamdi, L.; AlKadi, M.; AlBeajan, N.; AlRashoudi, L.; AlHussan, M. The burden of Diabetes, Its Oral Complications and Their Prevention and Management. Open Access Maced. J. Med. Sci. 2018, 6, 1545–1553. [Google Scholar] [CrossRef]
- Martinez, R.F.; Jaimes-Aveldanez, A.; Hernandez-Perez, F.; Arenas, R.; Miguel, G.F. Oral Candida spp carriers: Its prevalence in patients with type 2 diabetes mellitus. Bras. Dermatol. 2013, 88, 222–225. [Google Scholar] [CrossRef]
- Xiong, J.; Lu, H.; Jiang, Y. A Causal Relationship between Type 2 Diabetes and Candidiasis through Two-Sample Mendelian Randomization Analysis. Microorganisms 2024, 12, 1984. [Google Scholar] [CrossRef]
- Huang, L.J.; Chen, R.H. Lipid saturation induces degradation of squalene epoxidase for sterol homeostasis and cell survival. Life Sci. Alliance 2023, 6, e202201612. [Google Scholar] [CrossRef]
- Mascotti, M.L.; Juri Ayub, M.; Furnham, N.; Thornton, J.M.; Laskowski, R.A. Chopping and Changing: The Evolution of the Flavin-dependent Monooxygenases. J. Mol. Biol. 2016, 428, 3131–3146. [Google Scholar] [CrossRef]
- Pasrija, R.; Prasad, T.; Prasad, R. Membrane raft lipid constituents affect drug susceptibilities of Candida albicans. Biochem. Soc. Trans. 2005, 33, 1219–1223. [Google Scholar] [CrossRef]
- Liu, M.; Healy, M.D.; Dougherty, B.A.; Esposito, K.M.; Maurice, T.C.; Mazzucco, C.E.; Bruccoleri, R.E.; Davison, D.B.; Frosco, M.; Barrett, J.F.; et al. Conserved fungal genes as potential targets for broad-spectrum antifungal drug discovery. Eukaryot. Cell 2006, 5, 638–649. [Google Scholar] [CrossRef]
- Xu, D.; Jiang, B.; Ketela, T.; Lemieux, S.; Veillette, K.; Martel, N.; Davison, J.; Sillaots, S.; Trosok, S.; Bachewich, C.; et al. Genome-wide fitness test and mechanism-of-action studies of inhibitory compounds in Candida albicans. PLoS Pathog. 2007, 3, e92. [Google Scholar] [CrossRef] [PubMed]
- Tsai, H.F.; Bard, M.; Izumikawa, K.; Krol, A.A.; Sturm, A.M.; Culbertson, N.T.; Pierson, C.A.; Bennett, J.E. Candida glabrata erg1 mutant with increased sensitivity to azoles and to low oxygen tension. Antimicrob. Agents Chemother. 2004, 48, 2483–2489. [Google Scholar] [CrossRef] [PubMed]
- Tan, L.; Chen, L.; Yang, H.; Jin, B.; Kim, G.; Im, Y.J. Structural basis for activation of fungal sterol receptor Upc2 and azole resistance. Nat. Chem. Biol. 2022, 18, 1253–1262. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Tong, J.; Lee, C.W.; Ha, S.; Eom, S.H.; Im, Y.J. Structural mechanism of ergosterol regulation by fungal sterol transcription factor Upc2. Nat. Commun. 2015, 6, 6129. [Google Scholar] [CrossRef]
- Vasicek, E.M.; Berkow, E.L.; Flowers, S.A.; Barker, K.S.; Rogers, P.D. UPC2 is universally essential for azole antifungal resistance in Candida albicans. Eukaryot. Cell 2014, 13, 933–946. [Google Scholar] [CrossRef]
- MacPherson, S.; Akache, B.; Weber, S.; De Deken, X.; Raymond, M.; Turcotte, B. Candida albicans zinc cluster protein Upc2p confers resistance to antifungal drugs and is an activator of ergosterol biosynthetic genes. Antimicrob. Agents Chemother. 2005, 49, 1745–1752. [Google Scholar] [CrossRef]
- Lu, H.; Li, W.; Whiteway, M.; Wang, H.; Zhu, S.; Ji, Z.; Feng, Y.; Yan, L.; Fang, T.; Li, L.; et al. A Small Molecule Inhibitor of Erg251 Makes Fluconazole Fungicidal by Inhibiting the Synthesis of the 14alpha-Methylsterols. mBio 2023, 14, e0263922. [Google Scholar] [CrossRef]
- Zhen, C.; Wang, L.; Feng, Y.; Whiteway, M.; Hang, S.; Yu, J.; Lu, H.; Jiang, Y. Otilonium Bromide Exhibits Potent Antifungal Effects by Blocking Ergosterol Plasma Membrane Localization and Triggering Cytotoxic Autophagy in Candida Albicans. Adv. Sci. 2024, 11, e2406473. [Google Scholar] [CrossRef]
- Foresti, O.; Ruggiano, A.; Hannibal-Bach, H.K.; Ejsing, C.S.; Carvalho, P. Sterol homeostasis requires regulated degradation of squalene monooxygenase by the ubiquitin ligase Doa10/Teb4. eLife 2013, 2, e00953. [Google Scholar] [CrossRef]
- Sharma, P.; Bhalla, M.; Thami, G.P.; Chander, J. Evaluation of efficacy and safety of oral terbinafine and itraconazole combination therapy in the management of dermatophytosis. J. Dermatol. Treat. 2020, 31, 749–753. [Google Scholar] [CrossRef]
- Hassaan, Z.; Mohamed, H.A.K.; Eldahshan, R.M.; Elsaie, M.L. Comparison between the efficacy of terbinafine and itraconazole orally vs. the combination of the two drugs in treating recalcitrant dermatophytosis. Sci. Rep. 2023, 13, 19037. [Google Scholar] [CrossRef]
- Rosenberg, A.; Ene, I.V.; Bibi, M.; Zakin, S.; Segal, E.S.; Ziv, N.; Dahan, A.M.; Colombo, A.L.; Bennett, R.J.; Berman, J. Antifungal tolerance is a subpopulation effect distinct from resistance and is associated with persistent candidemia. Nat. Commun. 2018, 9, 2470. [Google Scholar] [CrossRef] [PubMed]
- Akinosoglou, K.; Rigopoulos, E.A.; Papageorgiou, D.; Schinas, G.; Polyzou, E.; Dimopoulou, E.; Gogos, C.; Dimopoulos, G. Amphotericin B in the Era of New Antifungals: Where Will It Stand? J. Fungi 2024, 10, 278. [Google Scholar] [CrossRef] [PubMed]
- Szymanski, M.; Chmielewska, S.; Czyzewska, U.; Malinowska, M.; Tylicki, A. Echinocandins—Structure, mechanism of action and use in antifungal therapy. J. Enzym. Inhib. Med. Chem. 2022, 37, 876–894. [Google Scholar] [CrossRef] [PubMed]
- Sigera, L.S.M.; Denning, D.W. Flucytosine and its clinical usage. Ther. Adv. Infect. Dis. 2023, 10, 20499361231161387. [Google Scholar] [CrossRef]
- Kim, I.S.; Sohn, H.Y.; Jin, I. Adaptive stress response to menadione-induced oxidative stress in Saccharomyces cerevisiae KNU5377. J. Microbiol. 2011, 49, 816–823. [Google Scholar] [CrossRef]
- O’Meara, T.R.; O’Meara, M.J.; Polvi, E.J.; Pourhaghighi, M.R.; Liston, S.D.; Lin, Z.Y.; Veri, A.O.; Emili, A.; Gingras, A.C.; Cowen, L.E. Global proteomic analyses define an environmentally contingent Hsp90 interactome and reveal chaperone-dependent regulation of stress granule proteins and the R2TP complex in a fungal pathogen. PLoS Biol. 2019, 17, e3000358. [Google Scholar] [CrossRef]
- Gopinath, R.K.; Leu, J.Y. Hsp90 mediates the crosstalk between galactose metabolism and cell morphology pathways in yeast. Curr. Genet. 2017, 63, 23–27. [Google Scholar] [CrossRef]
- Sangster, T.A.; Lindquist, S.; Queitsch, C. Under cover: Causes, effects and implications of Hsp90-mediated genetic capacitance. Bioessays 2004, 26, 348–362. [Google Scholar] [CrossRef]
- Choudhury, B.I.; Whiteway, M. Evolutionary Transition of GAL Regulatory Circuit from Generalist to Specialist Function in Ascomycetes. Trends Microbiol. 2018, 26, 692–702. [Google Scholar] [CrossRef]
- Xiong, J.; Wang, L.; Feng, Y.; Zhen, C.; Hang, S.; Yu, J.; Lu, H.; Jiang, Y. Geldanamycin confers fungicidal properties to azole by triggering the activation of succinate dehydrogenase. Life Sci. 2024, 348, 122699. [Google Scholar] [CrossRef]
- Cowen, L.E. Hsp90 orchestrates stress response signaling governing fungal drug resistance. PLoS Pathog. 2009, 5, e1000471. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.D.; Robbins, N.; Zaas, A.K.; Schell, W.A.; Perfect, J.R.; Cowen, L.E. Hsp90 governs echinocandin resistance in the pathogenic yeast Candida albicans via calcineurin. PLoS Pathog. 2009, 5, e1000532. [Google Scholar] [CrossRef] [PubMed]
- LaFayette, S.L.; Collins, C.; Zaas, A.K.; Schell, W.A.; Betancourt-Quiroz, M.; Gunatilaka, A.A.; Perfect, J.R.; Cowen, L.E. PKC signaling regulates drug resistance of the fungal pathogen Candida albicans via circuitry comprised of Mkc1, calcineurin, and Hsp90. PLoS Pathog. 2010, 6, e1001069. [Google Scholar] [CrossRef] [PubMed]
- Girstmair, H.; Tippel, F.; Lopez, A.; Tych, K.; Stein, F.; Haberkant, P.; Schmid, P.W.N.; Helm, D.; Rief, M.; Sattler, M.; et al. The Hsp90 isoforms from S. cerevisiae differ in structure, function and client range. Nat. Commun. 2019, 10, 3626. [Google Scholar] [CrossRef]
- Robbins, N.; Uppuluri, P.; Nett, J.; Rajendran, R.; Ramage, G.; Lopez-Ribot, J.L.; Andes, D.; Cowen, L.E. Hsp90 governs dispersion and drug resistance of fungal biofilms. PLoS Pathog. 2011, 7, e1002257. [Google Scholar] [CrossRef]
- Wang, H.; Ji, Z.; Feng, Y.; Yan, T.; Cao, Y.; Lu, H.; Jiang, Y. Myriocin enhances the antifungal activity of fluconazole by blocking the membrane localization of the efflux pump Cdr1. Front. Pharmacol. 2022, 13, 1101553. [Google Scholar] [CrossRef]
- Lo, H.J.; Wang, J.S.; Lin, C.Y.; Chen, C.G.; Hsiao, T.Y.; Hsu, C.T.; Su, C.L.; Fann, M.J.; Ching, Y.T.; Yang, Y.L. Efg1 involved in drug resistance by regulating the expression of ERG3 in Candida albicans. Antimicrob. Agents Chemother. 2005, 49, 1213–1215. [Google Scholar] [CrossRef]
- Sellam, A.; Tebbji, F.; Nantel, A. Role of Ndt80p in sterol metabolism regulation and azole resistance in Candida albicans. Eukaryot. Cell 2009, 8, 1174–1183. [Google Scholar] [CrossRef]
- Oerum, S.; Meynier, V.; Catala, M.; Tisne, C. A comprehensive review of m6A/m6Am RNA methyltransferase structures. Nucleic Acids Res. 2021, 49, 7239–7255. [Google Scholar] [CrossRef]
- Zhou, Y.; Zeng, P.; Li, Y.H.; Zhang, Z.; Cui, Q. SRAMP: Prediction of mammalian N6-methyladenosine (m6A) sites based on sequence-derived features. Nucleic Acids Res. 2016, 44, e91. [Google Scholar] [CrossRef]
- Puig, S.; Askeland, E.; Thiele, D.J. Coordinated remodeling of cellular metabolism during iron deficiency through targeted mRNA degradation. Cell 2005, 120, 99–110. [Google Scholar] [CrossRef]
- Puig, S.; Vergara, S.V.; Thiele, D.J. Cooperation of two mRNA-binding proteins drives metabolic adaptation to iron deficiency. Cell Metab. 2008, 7, 555–564. [Google Scholar] [CrossRef]
- Jorda, T.; Martinez-Martin, A.; Martinez-Pastor, M.T.; Puig, S. Modulation of yeast Erg1 expression and terbinafine susceptibility by iron bioavailability. Microb. Biotechnol. 2022, 15, 2705–2716. [Google Scholar] [CrossRef]
- Li, W.; Feng, Y.; Feng, Z.; Wang, L.; Whiteway, M.; Lu, H.; Jiang, Y. Pitavastatin Calcium Confers Fungicidal Properties to Fluconazole by Inhibiting Ubiquinone Biosynthesis and Generating Reactive Oxygen Species. Antioxidants 2024, 13, 667. [Google Scholar] [CrossRef] [PubMed]
- Thompson, P.D.; Panza, G.; Zaleski, A.; Taylor, B. Statin-Associated Side Effects. J. Am. Coll. Cardiol. 2016, 67, 2395–2410. [Google Scholar] [CrossRef] [PubMed]
- Kane, A.; Campbell, L.; Ky, D.; Hibbs, D.; Carter, D. The Antifungal and Synergistic Effect of Bisphosphonates in Cryptococcus. Antimicrob. Agents Chemother. 2021, 65, e01753-20. [Google Scholar] [CrossRef] [PubMed]
- Drake, M.T.; Clarke, B.L.; Khosla, S. Bisphosphonates: Mechanism of action and role in clinical practice. Mayo Clin. Proc. 2008, 83, 1032–1045. [Google Scholar] [CrossRef]
- Khodavandi, A.; Alizadeh, F.; Vanda, N.A.; Karimi, G.; Chong, P.P. Possible mechanisms of the antifungal activity of fluconazole in combination with terbinafine against Candida albicans. Pharm. Biol. 2014, 52, 1505–1509. [Google Scholar] [CrossRef]
- Bidaud, A.L.; Schwarz, P.; Chowdhary, A.; Dannaoui, E. In Vitro Antifungal Combination of Terbinafine with Itraconazole against Isolates of Trichophyton Species. Antimicrob. Agents Chemother. 2022, 66, e0144921. [Google Scholar] [CrossRef]
- Canete-Gibas, C.F.; Mele, J.; Patterson, H.P.; Sanders, C.J.; Ferrer, D.; Garcia, V.; Fan, H.; David, M.; Wiederhold, N.P. Terbinafine-Resistant Dermatophytes and the Presence of Trichophyton indotineae in North America. J. Clin. Microbiol. 2023, 61, e0056223. [Google Scholar] [CrossRef]
- Alves, R.; Kastora, S.L.; Gomes-Goncalves, A.; Azevedo, N.; Rodrigues, C.F.; Silva, S.; Demuyser, L.; Van Dijck, P.; Casal, M.; Brown, A.J.P.; et al. Transcriptional responses of Candida glabrata biofilm cells to fluconazole are modulated by the carbon source. NPJ Biofilms Microbiomes 2020, 6, 4. [Google Scholar] [CrossRef]
- Fang, T.; Xiong, J.; Wang, L.; Feng, Z.; Hang, S.; Yu, J.; Li, W.; Feng, Y.; Lu, H.; Jiang, Y. Unexpected Inhibitory Effect of Octenidine Dihydrochloride on Candida albicans Filamentation by Impairing Ergosterol Biosynthesis and Disrupting Cell Membrane Integrity. Antibiotics 2023, 12, 1675. [Google Scholar] [CrossRef]
- Noble, S.M.; Johnson, A.D. Strains and strategies for large-scale gene deletion studies of the diploid human fungal pathogen Candida albicans. Eukaryot. Cell 2005, 4, 298–309. [Google Scholar] [CrossRef] [PubMed]
- Chang, P.; Wang, W.; Igarashi, Y.; Luo, F.; Chen, J. Efficient vector systems for economical and rapid epitope-tagging and overexpression in Candida albicans. J. Microbiol. Methods 2018, 149, 14–19. [Google Scholar] [CrossRef] [PubMed]
- Paul, S.; Singh, S.; Chakrabarti, A.; Rudramurthy, S.M.; Ghosh, A.K. Selection and evaluation of appropriate reference genes for RT-qPCR based expression analysis in Candida tropicalis following azole treatment. Sci. Rep. 2020, 10, 1972. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Buchanan, B.W.; Lloyd, M.E.; Engle, S.M.; Rubenstein, E.M. Cycloheximide Chase Analysis of Protein Degradation in Saccharomyces cerevisiae. J. Vis. Exp. 2016, 110, 53975. [Google Scholar] [CrossRef]

| Compounds | Structures | MIC in YPD Medium | MIC in YPG Medium |
|---|---|---|---|
| Fluconazole |  | 1 μg/mL | 0.5 μg/mL |
| Miconazole | 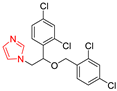 | 0.063 μg/mL | 0.031 μg/mL |
| Ketoconazole |  | 0.031 μg/mL | 0.016 μg/mL |
| Voriconazole | 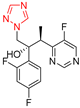 | 0.016 μg/mL | 0.008 μg/mL |
| Itraconazole | 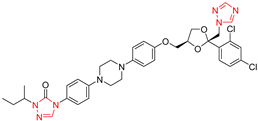 | 0.008 μg/mL | 0.004 μg/mL |
| Terbinafine | 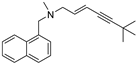 | 2 μg/mL | 0.5 μg/mL |
| Amphotericin B | 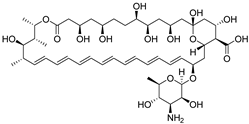 | 0.5 μg/mL | 0.5 μg/mL |
| Caspofungin |  | 40 ng/mL | 40 ng/mL |
| 5-Fluorocytosine | 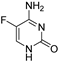 | 2 μg/mL | 2 μg/mL |
| Menadione | 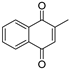 | 8 μg/mL | 8 μg/mL |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hang, S.; Wang, L.; Ji, Z.; Shen, X.; Fang, X.; Li, W.; Jiang, Y.; Lu, H. Galactose Inhibits the Translation of Erg1 that Enhances the Antifungal Activities of Azoles Against Candida albicans. Antibiotics 2025, 14, 799. https://doi.org/10.3390/antibiotics14080799
Hang S, Wang L, Ji Z, Shen X, Fang X, Li W, Jiang Y, Lu H. Galactose Inhibits the Translation of Erg1 that Enhances the Antifungal Activities of Azoles Against Candida albicans. Antibiotics. 2025; 14(8):799. https://doi.org/10.3390/antibiotics14080799
Chicago/Turabian StyleHang, Sijin, Li Wang, Zhe Ji, Xuqing Shen, Xinyu Fang, Wanqian Li, Yuanying Jiang, and Hui Lu. 2025. "Galactose Inhibits the Translation of Erg1 that Enhances the Antifungal Activities of Azoles Against Candida albicans" Antibiotics 14, no. 8: 799. https://doi.org/10.3390/antibiotics14080799
APA StyleHang, S., Wang, L., Ji, Z., Shen, X., Fang, X., Li, W., Jiang, Y., & Lu, H. (2025). Galactose Inhibits the Translation of Erg1 that Enhances the Antifungal Activities of Azoles Against Candida albicans. Antibiotics, 14(8), 799. https://doi.org/10.3390/antibiotics14080799









