Evaluating Antimicrobial Susceptibility Testing Methods for Cefiderocol: A Review and Expert Opinion on Current Practices and Future Directions
Abstract
1. Introduction
2. Materials and Methods
- Focused on Enterobacterales, P. aeruginosa, and A. baumannii complex clinical isolates.
- Reported on comparing AST methodologies specifically for FDC versus broth microdilution method (BMD).
2.1. Data Synthesis Approach
2.2. Collection of Expert Opinions
2.3. Terminology Notes
- ○
- Resistance refers to classification based on clinical breakpoints (EUCAST, CLSI, FDA).
- ○
- Reduced susceptibility is used in the absence of species-specific clinical breakpoints, typically in reference to MICs above PK/PD thresholds.
- ○
- Non-susceptibility is retained when referring to prevalence/mechanism data or published surveillance reports that used this term.
3. Non-Susceptibility to FDC: Global Prevalence and General Mechanisms
- Expression of metallo-β-lactamase NDM and/or β-lactamases (e.g., SHV, PER, and VEB) [10,11,12,13,14,15,16,17,18,19]. In A. baumannii, combining FDC with avibactam has been shown to reduce MIC, indicating that β -lactamase activity in this species contributes to reduced susceptibility [20]. In addition, increased copy numbers of the blaNDM-5 gene due to translocation events have enhanced NDM production, leading to decreased activity of FDC. However, the extent to which blaNDM-5 expression contributes to FDC reduced susceptibility remains to be fully elucidated [21].
- Structural alterations in β-lactamases, such as AmpC, for example, the region encoding the R2 loop [22,23,24]. Furthermore, comparable variations in Oxacillinases include specific point mutations in the Ω loop of OXA-2 (Ala149Pro and Asp150Gly) and the OXA-10 subgroup (Trp154Cys and Gly157Asp). Additionally, the OXA-10 subgroup exhibits a duplication of Thr206 and Gly207 in the β5–β6 loop [25]. In vivo and in vitro emergence of NS-FDC due to KPC variants (e.g., KPC-41 and KPC-50) has also been reported [19].
- Mutations in FDC’s PBP3 target (i.e., 4-amino acid insertion at position 333) reduce FDC’s access to the specific transpeptidase pocket [26,27,28]. Mutations like these do not directly confer non-susceptibility but are commonly observed in isolates that produce β-lactamases, such as NDM-type. Such mutations can contribute to reaching a certain level of clinical non-susceptibility when associated with other mechanisms [26,27,28].
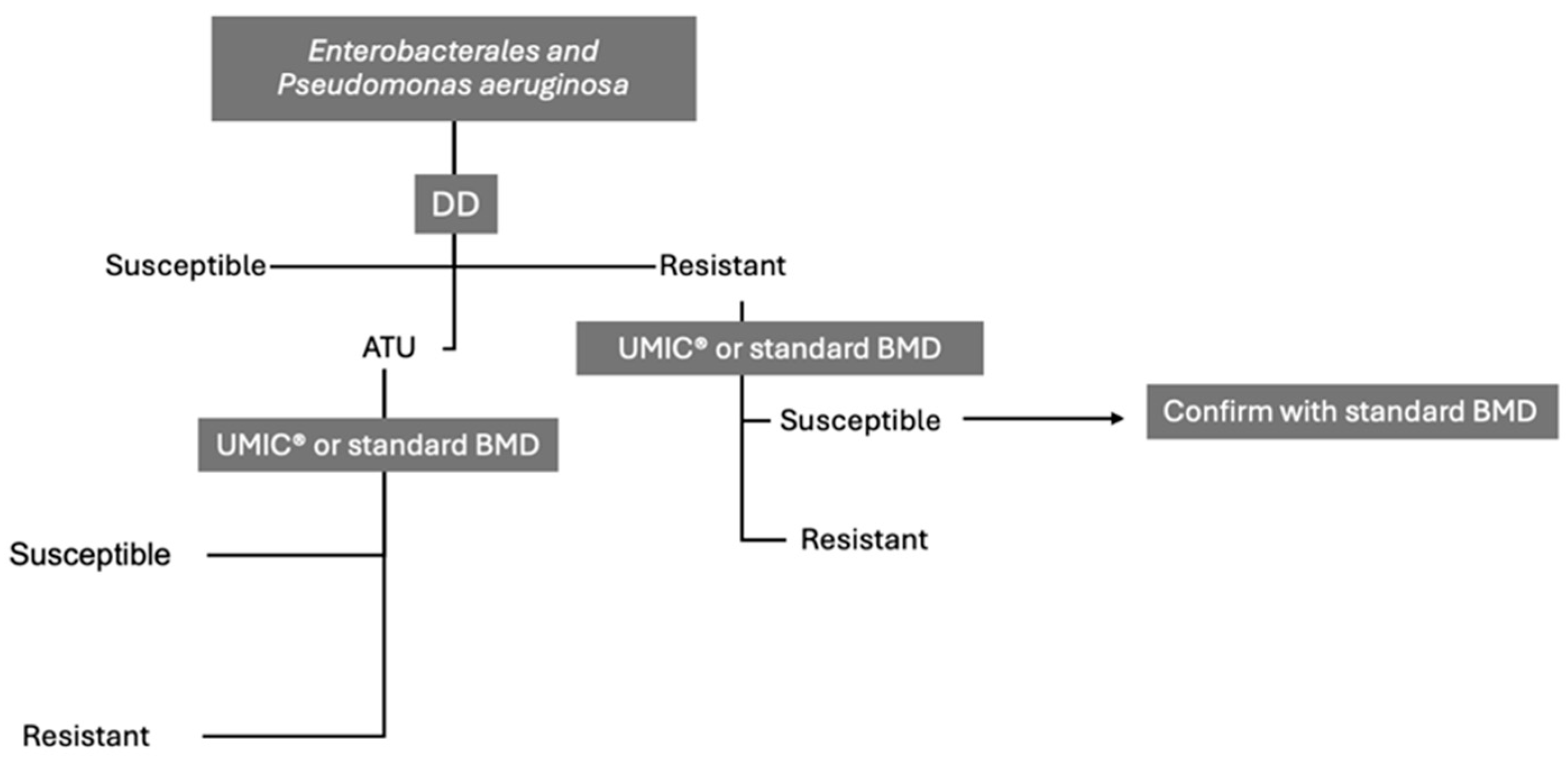
4. Current Standards and AST Methodologies for FDC
5. Variability in Testing Outcomes Across Different Microorganisms
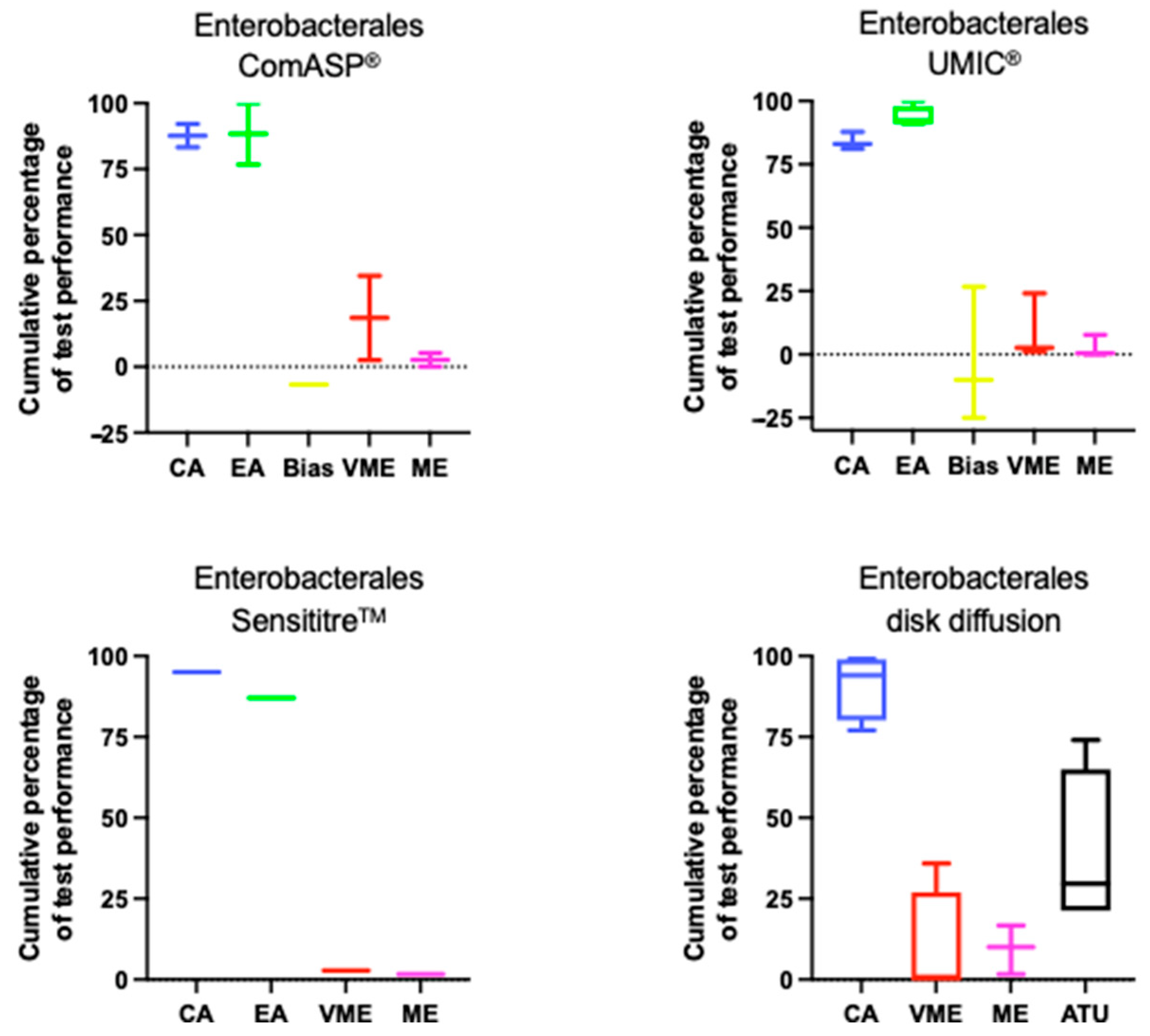
5.1. Enterobacterales
| Dimension | Key points |
| Agreement | Average CA: ComASP® 87.7%; UMIC® 84%; Sensititre™ 95%; DD 91.05% Average EA: ComASP® 88.35%; UMIC® 93.6%; Sensititre™ 87% |
| Errors | Bias range: ComASP® −6.7%; UMIC® −25—+26.7%; Sensititre™ ND Average VME: ComASP® 18.5%; UMIC® 9.3%; Sensititre™ 2.8%; DD 9% Average ME: ComASP® 2.6%; UMIC® 2.7%; Sensititre™ 1.6%; DD 9.4% Average DD ATU: 38.6% |
| Test Conditions | Disk/media combinations highly influence DD performance. Bio-Rad MH agar and Oxoid disks may produce inflated zone diameters. 20–30% of isolates fall within ATU, requiring confirmatory testing. |
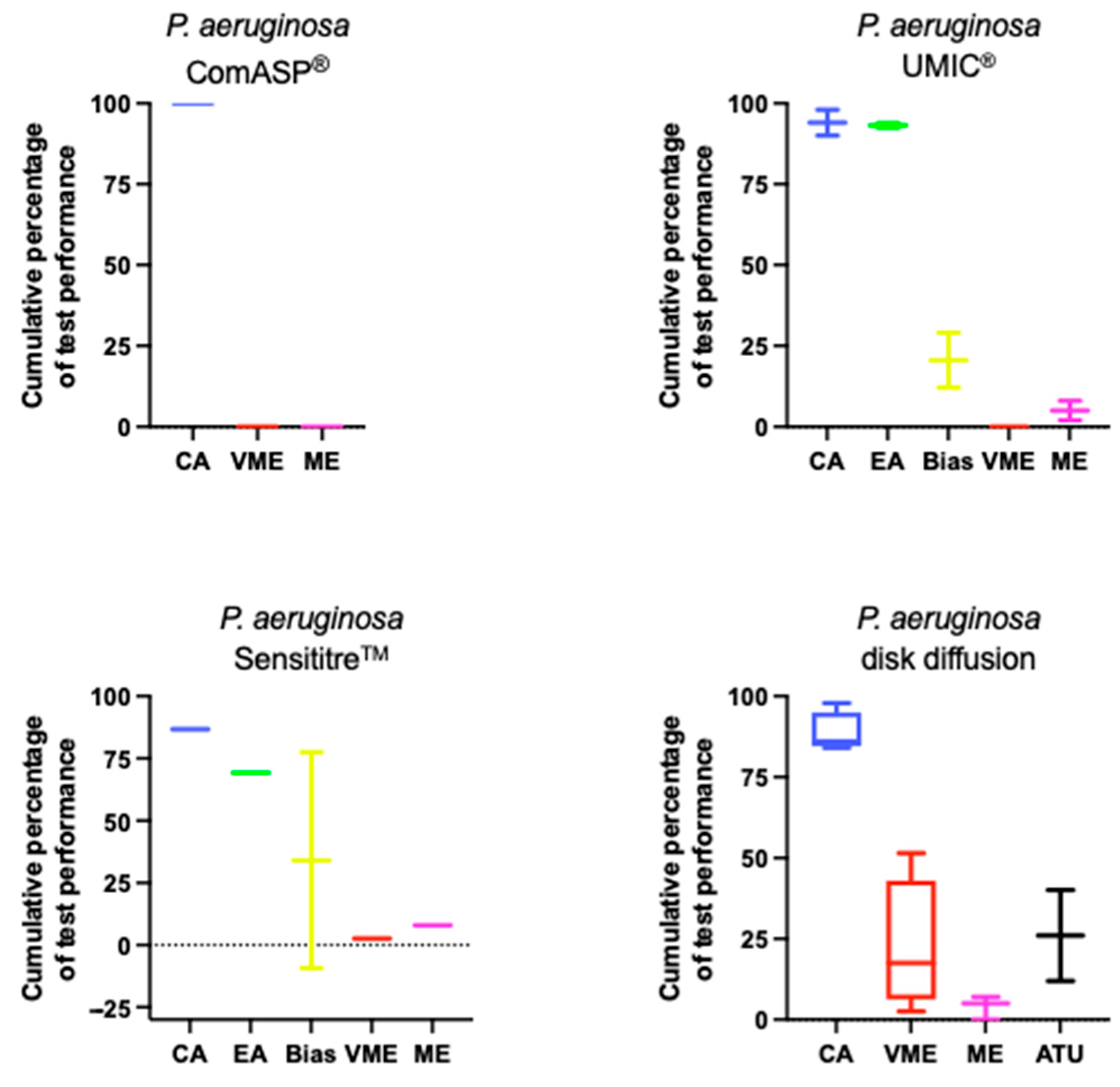
5.2. Pseudomonas aeruginosa
| Dimension | Key Points |
| Agreement | Average CA: ComASP® 100%; UMIC® 94%; Sensititre™ 86.7%; DD 88.5% Average EA: ComASP® ND%; UMIC® 93.2%; Sensititre™ 69.3% |
| Errors | Bias range: ComASP® ND; UMIC® +12.12—+29%; Sensititre™ −9.3—+77.5 Average VME: ComASP® 0%; UMIC® 0%; Sensititre™ 2.7%; DD 22.25% Average ME: ComASP® 0%; UMIC® 5%; Sensititre™ 8%; DD 4% Average DD ATU: 25.9% Few VMEs reported, mainly in isolates with borderline MICs. MEs increase when MICs are slightly overestimated (e.g., 4 mg/L vs. 2 mg/L). |
| Test Conditions | Zone diameter variability is affected by the manufacturer of agar and the disk. ATU inhibition zones (20–22 mm) may be associated with reduced susceptibility. |
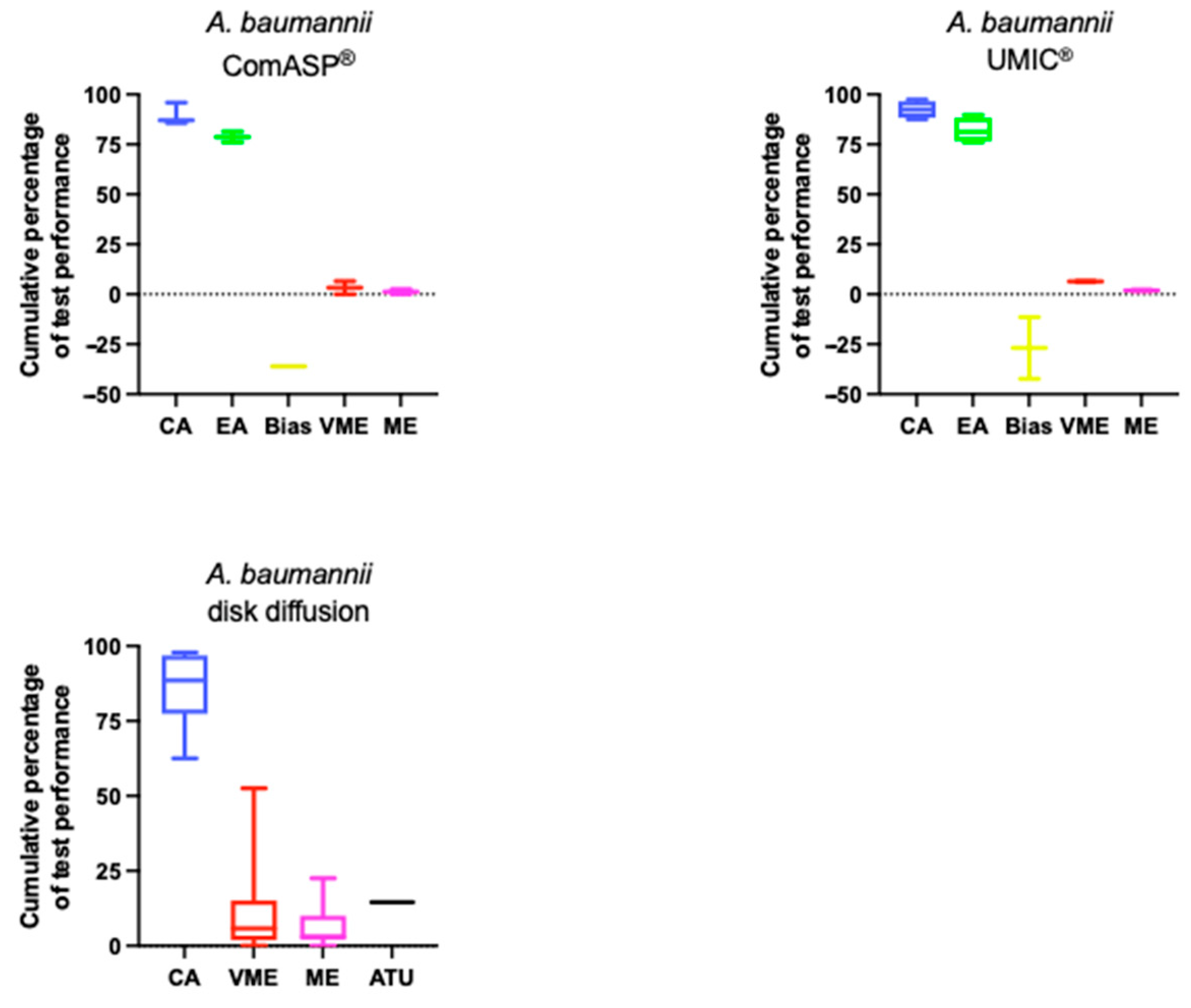
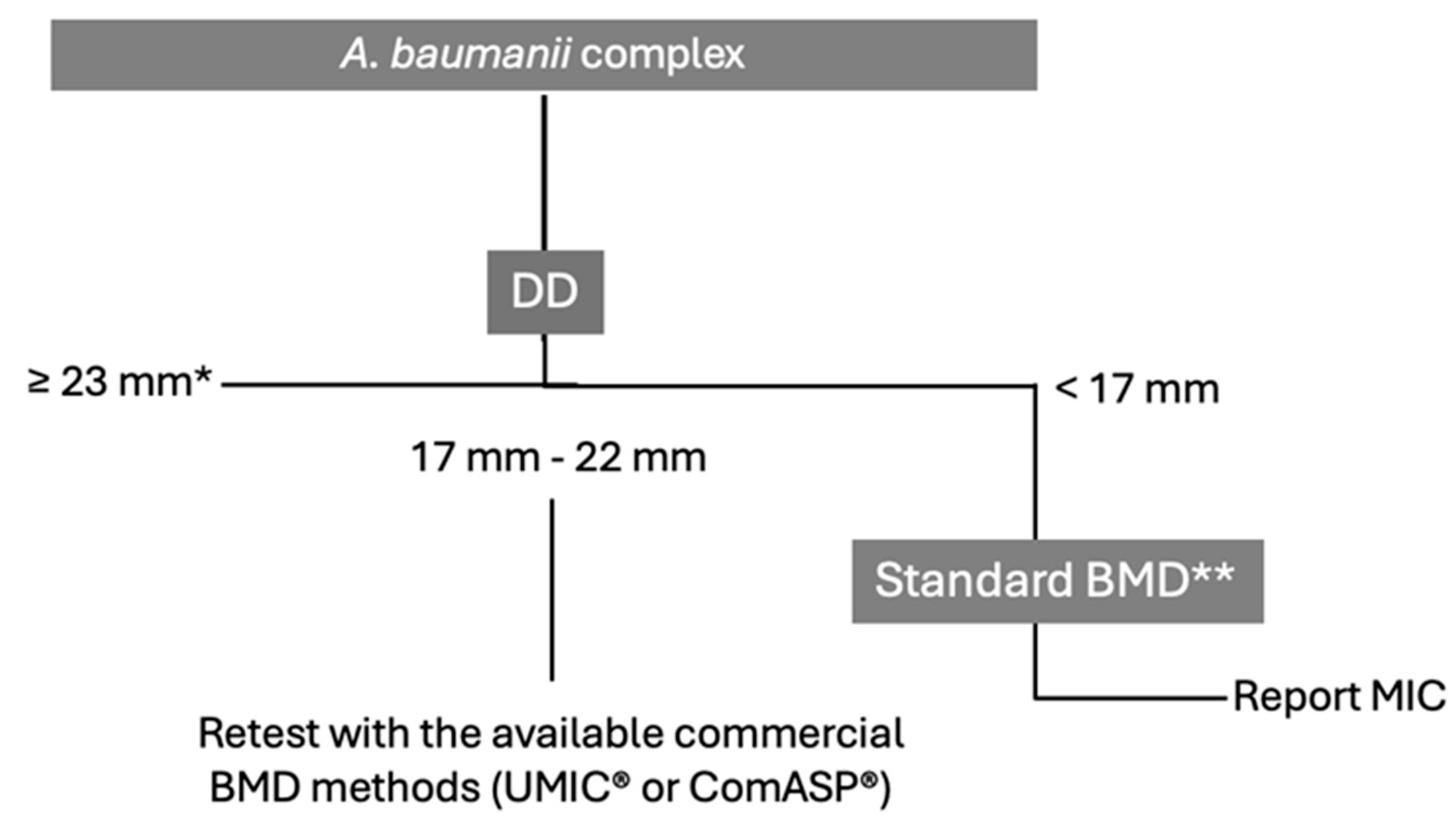
5.3. Acinetobacter baumannii
| Dimension | Key points |
| Agreement | Average CA: ComASP® 89.5%; UMIC® 92.4; DD 85.7% Average EA: ComASP® 78.7%; UMIC® 82% |
| Errors | Bias range: ComASP® −36.1; UMIC® −42.3–−11.4% Average VME: ComASP® 0%; UMIC® 0%; DD 12% Average ME: ComASP® 1.2%; UMIC® 1.9%; DD 6.4% Average DD ATU: 14.5% VMEs are prominent in DD within 17–22 mm. |
| Test conditions | Media and disk combinations strongly influence performance. Confirmatory BMD testing is critical for isolates in the 17–22 mm zone. |
6. Expert Opinion and Discussion
7. Conclusions
| Specific Gaps | Implications | Future Research Suggestions |
| Heterogeneity in Study Design | Diverse methodologies across studies lead to difficulty in comparing and synthesizing results. | Standardize and report frameworks in AST studies to ensure consistency and comparability. |
| Small Sample Sizes | Limited sample sizes reduce the statistical power and generalizability of findings. | Implement multicenter studies to gather larger, more diverse bacterial isolates and enhance the representativeness of results. |
| Use of Standard Reference Strains Only | Reliance solely on ATCC strains might not accurately represent clinical scenarios. | Incorporate clinical isolates with known resistance profiles as controls in testing protocols. |
| Longitudinal Surveillance | Evolving resistance patterns are not adequately monitored over time, affecting the relevance of AST protocols. | Conduct longitudinal surveillance studies to monitor resistance evolution and method efficacy. |
| Variations in ATU | Changes in ATU boundaries may impact susceptibility interpretations, increasing variability in results. | Evaluate the influence of ATU variations on susceptibility testing outcomes and explore strategies to minimize inconsistencies. |
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| FDC | Cefiderocol |
| AST | Antimicrobial susceptibility testing |
| BMD | Broth microdilution |
| ID-CAMHB | Iron-depleted cation-adjusted Mueller-Hinton broth |
| ATUs | Areas of technical uncertainty |
| CRE | Carbapenem-resistant Enterobacterales |
| DD | Disk diffusion |
| S | Susceptible |
| R | Resistant |
| AMR | Antimicrobial resistance |
| GN | Gram-negative |
| ICU | Intensive Care Units |
| CAESAR | Central Asian and European Surveillance of Antimicrobial Resistance |
| EARS-Net | European Antimicrobial Resistance Surveillance Network |
| MBLs | metallo- β -lactamases |
| CR | carbapenemase-resistant |
| CA | Categorical Agreement |
| EA | Essential Agreement |
| VME | Very Major Errors |
| ME | Major Errors |
| mE | minor errors |
| NS-FDC | non-susceptibility to FDC |
| MH | Mueller-Hinton |
| iROMPs | iron-regulated outer membrane proteins |
| ECOFF | epidemiological cut-off |
References
- European Centre for Disease Prevention and Control. Antimicrobial Resistance Surveillance in Europe 2023–2021 Data. Available online: https://www.ecdc.europa.eu/en/publications-data/antimicrobial-resistance-surveillance-europe-2023-2021-data (accessed on 5 June 2025).
- Centers for Disease Control and Prevention (U.S.). Antibiotic Resistance Threats in the United States; CDC: Atlanta, GA, USA, 2019. Available online: https://stacks.cdc.gov/view/cdc/82532 (accessed on 5 June 2025).
- Ong’uti, S.; Czech, M.; Robilotti, E.; Holubar, M. Cefiderocol: A New Cephalosporin Stratagem Against Multidrug-Resistant Gram-Negative Bacteria. Clin. Infect. Dis. 2022, 74, 1303–1312. [Google Scholar] [CrossRef] [PubMed]
- Sato, T.; Yamawaki, K. Cefiderocol: Discovery, Chemistry, and In Vivo Profiles of a Novel Siderophore Cephalosporin. Clin. Infect. Dis. 2019, 69 (Suppl. 7), S538–S543. [Google Scholar] [CrossRef] [PubMed]
- Kaye, K.S.; Naas, T.; Pogue, J.M.; Rossolini, G.M. Cefiderocol, a Siderophore Cephalosporin, as a Treatment Option for Infections Caused by Carbapenem-Resistant Enterobacterales. Infect. Dis. Ther. 2023, 12, 777–806. [Google Scholar] [CrossRef] [PubMed]
- Marino, A.; Stracquadanio, S.; Campanella, E.; Munafò, A.; Gussio, M.; Ceccarelli, M.; Bernardini, R.; Nunnari, G.; Cacopardo, B. Intravenous Fosfomycin: A Potential Good Partner for Cefiderocol. Clinical Experience and Considerations. Antibiotics 2022, 12, 49. [Google Scholar] [CrossRef]
- Brauncajs, M.; Bielec, F.; Macieja, A.; Pastuszak-Lewandoska, D. Cefiderocol—An effective antimicrobial for MDR infections but a challenge for routine antimicrobial susceptibility testing. Adv. Med. Sci. 2024, 69, 256–263. [Google Scholar] [CrossRef]
- Simner, P.J.; Patel, R. Cefiderocol Antimicrobial Susceptibility Testing Considerations: The Achilles’ Heel of the Trojan Horse? J. Clin. Microbiol. 2020, 59, e00951-20. [Google Scholar] [CrossRef]
- Karakonstantis, S.; Rousaki, M.; Vassilopoulou, L.; Kritsotakis, E.I. Global prevalence of cefiderocol non-susceptibility in Enterobacterales, Pseudomonas aeruginosa, Acinetobacter baumannii, and Stenotrophomonas maltophilia: A systematic review and meta-analysis. Clin. Microbiol. Infect. 2024, 30, 178–188. [Google Scholar] [CrossRef]
- Yamano, Y.; Ishibashi, N.; Kuroiwa, M.; Takemura, M.; Sheng, W.H.; Hsueh, P.R. Characterisation of cefiderocol-non-susceptible Acinetobacter baumannii isolates from Taiwan. J. Glob. Antimicrob. Resist. 2022, 28, 120–124. [Google Scholar] [CrossRef]
- Poirel, L.; Sadek, M.; Nordmann, P. Contribution of PER-Type and NDM-Type β-Lactamases to Cefiderocol Resistance in Acinetobacter baumannii. Antimicrob. Agents Chemother. 2021, 65, e00877-21. [Google Scholar] [CrossRef]
- Poirel, L.; Ortiz de la Rosa, J.M.; Sakaoglu, Z.; Kusaksizoglu, A.; Sadek, M.; Nordmann, P. NDM-35-Producing ST167 Escherichia coli Highly Resistant to β-Lactams Including Cefiderocol. Antimicrob. Agents Chemother. 2022, 66, e00311-22. [Google Scholar] [CrossRef]
- Nurjadi, D.; Kocer, K.; Chanthalangsy, Q.; Klein, S.; Heeg, K.; Boutin, S. New Delhi Metallo-Beta-Lactamase Facilitates the Emergence of Cefiderocol Resistance in Enterobacter cloacae. Antimicrob. Agents Chemother. 2022, 66, e02011-21. [Google Scholar] [CrossRef]
- Ito, A.; Sato, T.; Ota, M.; Takemura, M.; Nishikawa, T.; Toba, S.; Kohira, N.; Miyagawa, S.; Ishibashi, N.; Matsumoto, S.; et al. In Vitro Antibacterial Properties of Cefiderocol, a Novel Siderophore Cephalosporin, Against Gram-Negative Bacteria. Antimicrob. Agents Chemother. 2017, 62, e01454-17. [Google Scholar] [CrossRef] [PubMed]
- Poirel, L.; Sadek, M.; Kusaksizoglu, A.; Nordmann, P. co-resistance to ceftazidime-avibactam and cefiderocol in clinical isolates producing KPC Variants. Eur. J. Clin. Microbiol. Infect. Dis. 2022, 41, 677–680. [Google Scholar] [CrossRef] [PubMed]
- Poirel, L.; Ortiz de la Rosa, J.M.; Sadek, M.; Nordmann, P. Impact of Acquired Broad-Spectrum β-Lactamases on Susceptibility to Cefiderocol and Newly Developed β-Lactam/β-Lactamase Inhibitor Combinations in Escherichia coli and Pseudomonas aeruginosa. Antimicrob. Agents Chemother. 2022, 66, e00039-22. [Google Scholar] [CrossRef] [PubMed]
- Coppi, M.; Antonelli, A.; Niccolai, C.; Bartolini, A.; Bartolini, L.; Grazzini, M.; Mantengoli, E.; Farese, A.; Pieralli, F.; Mechi, M.T.; et al. Nosocomial outbreak by NDM-1-producing Klebsiella pneumoniae highly resistant to cefiderocol, Florence, Italy, August 2021 to June 2022. Euro Surveill. 2022, 27, 2200795. [Google Scholar] [CrossRef]
- Liu, X.; Lei, T.; Yang, Y.; Zhang, L.; Liu, H.; Leptihn, S.; Yu, Y.; Hua, X. Structural Basis of PER-1-Mediated Cefiderocol Resistance and Synergistic Inhibition of PER-1 by Cefiderocol in Combination with Avibactam or Durlobactam in Acinetobacter baumannii. Antimicrob. Agents Chemother. 2022, 66, e00828-22. [Google Scholar] [CrossRef]
- Karakonstantis, S.; Rousaki, M.; Kritsotakis, E.I. Cefiderocol: Systematic Review of Mechanisms of Resistance, Heteroresistance and In Vivo Emergence of Resistance. Antibiotics 2022, 11, 723. [Google Scholar] [CrossRef]
- Abdul-Mutakabbir, J.C.; Nguyen, L.; Maassen, P.T.; Stamper, K.C.; Kebriaei, R.; Kaye, K.S.; Castanheira, M.; Rybak, M.J. In Vitro Antibacterial Activity of Cefiderocol Against Multidrug-Resistant Acinetobacter baumannii. Antimicrob. Agents Chemother. 2021, 65, e02646-20. [Google Scholar] [CrossRef]
- Simner, P.J.; Mostafa, H.H.; Bergman, Y.; Ante, M.; Tekle, T.; Adebayo, A.; Beisken, S.; Dzintars, K.; Tamma, P.D. Progressive Development of Cefiderocol Resistance in Escherichia coli During Therapy Is Associated with an Increase in blaNDM-5 Copy Number and Gene Expression. Clin. Infect. Dis. 2022, 75, 47–54. [Google Scholar] [CrossRef]
- Hobson, C.A.; Cointe, A.; Jacquier, H.; Choudhury, A.; Magnan, M.; Courroux, C.; Tenaillon, O.; Bonacorsi, S.; Birgy, A. Cross-resistance to cefiderocol and ceftazidime-avibactam in KPC β-Lactamase mutants and the inoculum effect. Clin. Microbiol. Infect. 2021, 27, 1172.e7–1172.e10. [Google Scholar] [CrossRef]
- Shields, R.K.; Iovleva, A.; Kline, E.G.; Kawai, A.; McElheny, C.L.; Doi, Y. Clinical Evolution of AmpC-Mediated Ceftazidime-Avibactam and Cefiderocol Resistance in Enterobacter cloacae Complex Following Exposure to Cefepime. Clin. Infect. Dis. 2020, 71, 2713–2716. [Google Scholar] [CrossRef] [PubMed]
- Kawai, A.; McElheny, C.L.; Iovleva, A.; Kline, E.G.; Sluis-Cremer, N.; Shields, R.K.; Doi, Y. Structural Basis of Reduced Susceptibility to Ceftazidime-Avibactam and Cefiderocol in Enterobacter cloacae Due to AmpC R2 Loop Deletion. Antimicrob. Agents Chemother. 2020, 64, e00198-20. [Google Scholar] [CrossRef] [PubMed]
- Vuillemin, X.; Da Silva, M.; Bour, M.; Landon, C.; Plésiat, P.; Jeannot, K. Cefiderocol activity is compromised by acquired extended-spectrum oxacillinases in Pseudomonas aeruginosa. Int. J. Antimicrob. Agents 2023, 62, 106917. [Google Scholar] [CrossRef] [PubMed]
- Sato, T.; Ito, A.; Ishioka, Y.; Matsumoto, S.; Rokushima, M.; Kazmierczak, K.M.; Hackel, M.; Sahm, D.F.; Yamano, Y. Escherichia coli strains possessing a four amino acid yrin insertion in PBP3 identified as part of the SIDERO-WT-2014 surveillance study. JAC Antimicrob. Resist. 2020, 2, dlaa081. [Google Scholar] [CrossRef]
- Nordmann, P.; Shields, R.K.; Doi, Y.; Takemura, M.; Echols, R.; Matsunaga, Y.; Yamano, Y. Mechanisms of Reduced Susceptibility to Cefiderocol Among Isolates from the CREDIBLE-CR and APEKS-NP Clinical Trials. Microb. Drug Resist. 2022, 28, 398–407. [Google Scholar] [CrossRef]
- Takemura, M.; Yamano, Y.; Matsunaga, Y.; Ariyasu, M.; Echols, R.; Den Nagata, T. Characterization of Shifts in Minimum Inhibitory Concentrations During Treatment with Cefiderocol or Comparators in the Phase 3 CREDIBLE-CR and APEKS-NP Studies. Open Forum Infect. Dis. 2020, 7 (Suppl. 1), S649–S650. [Google Scholar] [CrossRef]
- Kocer, K.; Boudour-Halil, D.; Chanthalangsy, Q.; Sähr, A.; Heeg, K.; Boutin, S.; Nurjadi, D. Genomic Modification of TonB and Emergence of Small-Colony Phenotype in VIM- and NDM-Producing Escherichia coli Following Cefiderocol Exposure In Vitro. Antimicrob. Agents Chemother. 2023, 67, e00118-23. [Google Scholar] [CrossRef]
- Klein, S.; Boutin, S.; Kocer, K.; Fiedler, M.O.; Störzinger, D.; Weigand, M.A.; Tan, B.; Richter, D.; Rupp, C.; Mieth, M.; et al. Rapid Development of Cefiderocol Resistance in Carbapenem-Resistant Enterobacter cloacae During Therapy Is Associated with Heterogeneous Mutations in the Catecholate Siderophore Receptor cirA. Clin. Infect. Dis. 2022, 74, 905–908. [Google Scholar] [CrossRef]
- Price, T.K.; Davar, K.; Contreras, D.; Ward, K.W.; Garner, O.B.; Simner, P.J.; Yang, S.; Chandrasekaran, S. Case Report and Genomic Analysis of Cefiderocol-Resistant Escherichia coli Clinical Isolates. Am. J. Clin. Pathol. 2022, 157, 257–265. [Google Scholar] [CrossRef]
- Hall, C.M.; Somprasong, N.; Hagen, J.P.; Nottingham, R.; Sahl, J.W.; Webb, J.R.; Mayo, M.; Currie, B.J.; Podin, Y.; Wagner, D.M.; et al. Exploring Cefiderocol Resistance Mechanisms in Burkholderia pseudomallei. Antimicrob. Agents Chemother. 2023, 67, e00171-23. [Google Scholar] [CrossRef]
- McElheny, C.L.; Fowler, E.L.; Iovleva, A.; Shields, R.K.; Doi, Y. In Vitro Evolution of Cefiderocol Resistance in an NDM-Producing Klebsiella pneumoniae Due to Functional Loss of CirA. Microbiol. Spectr. 2021, 9, e01779-21. [Google Scholar] [CrossRef]
- Gupta, A.; Landman, D.; Quale, J. Relationship of TonB-Dependent Receptors with Susceptibility to Cefiderocol in Clinical Isolates of Pseudomonas aeruginosa. J. Antimicrob. Chemother. 2022, 77, 1282–1285. [Google Scholar] [CrossRef] [PubMed]
- Daoud, L.; Al-Marzooq, F.; Moubareck, C.A.; Ghazawi, A.; Collyns, T. Elucidating the Effect of Iron Acquisition Systems in Klebsiella pneumoniae on Susceptibility to the Novel Siderophore-Cephalosporin Cefiderocol. PLoS ONE 2022, 17, e0277946. [Google Scholar] [CrossRef] [PubMed]
- Stracquadanio, S.; Nicolosi, A.; Privitera, G.F.; Massimino, M.; Marino, A.; Bongiorno, D.; Stefani, S. Role of Transcriptomic and Genomic Analyses in Improving the Comprehension of Cefiderocol Activity in Acinetobacter baumannii. mSphere 2024, 9, e00617-23. [Google Scholar] [CrossRef] [PubMed]
- Galdino, A.C.M.; Vaillancourt, M.; Celedonio, D.; Huse, K.; Doi, Y.; Lee, J.S.; Jorth, P. Siderophores Promote Cooperative Interspecies and Intraspecies Cross-Protection Against Antibiotics In Vitro. Nat. Microbiol. 2024, 9, 631–646. [Google Scholar] [CrossRef]
- Teran, N.; Egge, S.L.; Phe, K.; Baptista, R.P.; Tam, V.H.; Miller, W.R. The Emergence of Cefiderocol Resistance in Pseudomonas aeruginosa from a Heteroresistant Isolate During Prolonged Therapy. Antimicrob. Agents Chemother. 2024, 68, e01009-23. [Google Scholar] [CrossRef]
- Smoke, S.M.; Brophy, A.; Reveron, S.; Iovleva, A.; Kline, E.G.; Marano, M.; Miller, L.P.; Shields, R.K. Evolution and Transmission of Cefiderocol-Resistant Acinetobacter baumannii During an Outbreak in the Burn Intensive Care Unit. Clin. Infect. Dis. 2023, 76, e1261–e1265. [Google Scholar] [CrossRef]
- Tascini, C.; Coppi, M.; Antonelli, A.; Niccolai, C.; Bartolini, A.; Pecori, D.; Sartor, A.; Giani, T.; Rossolini, G.M. In Vivo Evolution to High-Level Cefiderocol Resistance of NDM-1-Producing Klebsiella pneumoniae, Followed by Intra-Hospital Cross-Transmission. Clin. Microbiol. Infect. 2024, 30, 398–400. [Google Scholar] [CrossRef]
- Sadek, M.; Le Guern, R.; Kipnis, E.; Gosset, P.; Poirel, L.; Dessein, R.; Nordmann, P. Progressive In Vivo Development of Resistance to Cefiderocol in Pseudomonas aeruginosa. Eur. J. Clin. Microbiol. Infect. Dis. 2023, 42, 61–66. [Google Scholar] [CrossRef]
- He, Y.; Wang, Y.; Ma, X.; Zhao, L.; Guan, J.; Zhao, J.; Yu, W.; Li, Y.; Ni, W.; Gao, Z. Resistance to Cefiderocol Involved Expression of PER-1 β-Lactamase and Downregulation of Iron Transporter System in Carbapenem-Resistant Acinetobacter baumannii. Infect. Drug Resist. 2022, 15, 7177–7187. [Google Scholar] [CrossRef]
- Simner, P.J.; Beisken, S.; Bergman, Y.; Posch, A.E.; Cosgrove, S.E.; Tamma, P.D. Cefiderocol Activity Against Clinical Pseudomonas aeruginosa Isolates Exhibiting Ceftolozane-Tazobactam Resistance. Open Forum Infect. Dis. 2021, 8, ofab311. [Google Scholar] [CrossRef]
- Simner, P.J.; Bergman, Y.; Conzemius, R.; Jacobs, E.; Tekle, T.; Beisken, S.; Tamma, P.D. An NDM-Producing Escherichia coli Clinical Isolate Exhibiting Resistance to Cefiderocol and the Combination of Ceftazidime-Avibactam and Aztreonam: Another Step Toward Pan-β-Lactam Resistance. Open Forum Infect. Dis. 2023, 10, ofad276. [Google Scholar] [CrossRef]
- Domingues, S.; Lima, T.; Saavedra, M.J.; Da Silva, G.J. An Overview of Cefiderocol’s Therapeutic Potential and Underlying Resistance Mechanisms. Life 2023, 13, 1427. [Google Scholar] [CrossRef]
- Yamano, Y. In Vitro Activity of Cefiderocol Against a Broad Range of Clinically Important Gram-Negative Bacteria. Clin. Infect. Dis. 2019, 69 (Suppl. 7), S544–S551. [Google Scholar] [CrossRef]
- Simner, P.J.; Palavecino, E.L.; Satlin, M.J.; Mathers, A.J.; Weinstein, M.P.; Lewis, J.S., 2nd; Humphries, R. Potential of Inaccurate Cefiderocol Susceptibility Results: A CLSI AST Subcommittee Advisory. J. Clin. Microbiol. 2023, 61, e01600-22. [Google Scholar] [CrossRef]
- Stracquadanio, S.; Nicolosi, A.; Marino, A.; Calvo, M.; Stefani, S. Issues with Cefiderocol Testing: Comparing Commercial Methods to Broth Microdilution in Iron-Depleted Medium—Analyses of the Performances, ATU, and Trailing Effect According to EUCAST Initial and Revised Interpretation Criteria. Diagnostics 2024, 14, 2318. [Google Scholar] [CrossRef] [PubMed]
- Uskudar-Guclu, A.; Danyildiz, S.; Mirza, H.C.; Akcil Ok, M.; Basustaoglu, A. In Vitro Activity of Cefiderocol Against Carbapenem-Resistant Acinetobacter baumannii Carrying Various β-Lactamase-Encoding Genes. Eur. J. Clin. Microbiol. Infect. Dis. 2024, 43, 1171–1179. [Google Scholar] [CrossRef] [PubMed]
- CLSI M100; Performance Standards for Antimicrobial Susceptibility Testing—Thirty-Fourth Edition. CLSI: Berwyn, PA, USA, 2024.
- Stracquadanio, S.; Bonomo, C.; Marino, A.; Bongiorno, D.; Privitera, G.F.; Bivona, D.A.; Mirabile, A.; Bonacci, P.G.; Stefani, S. Acinetobacter baumannii and Cefiderocol, Between Cidality and Adaptability. Microbiol. Spectr. 2022, 10, e02347-22. [Google Scholar] [CrossRef] [PubMed]
- Leonildi, A. In Vitro Susceptibility Test to Cefiderocol of NDM-Producing K. pneumoniae from Bloodstream Isolates: A Comparison of Commercial Methods and the Reference Method. In Proceedings of the Congress of the European Society of Clinical Microbiology and Infectious Diseases Global, Barcelona, Spain, 27–30 April 2024. [Google Scholar]
- EUCAST. EUCAST Warnings Concerning Antimicrobial Susceptibility Testing Products or Procedures. 2024. Available online: https://www.eucast.org/ast-of-bacteria/warnings (accessed on 5 June 2025).
- Critchley, I.A.; Basker, M.J. Conventional Laboratory Agar Media Provide an Iron-Limited Environment for Bacterial Growth. FEMS Microbiol. Lett. 1988, 50, 35–39. [Google Scholar] [CrossRef]
- EUCAST. EUCAST Breakpoint Table 14.0 (2024) Available for Consultation (5–19 December 2023). 2023. Available online: https://www.eucast.org/eucast_news/news_singleview?tx_ttnews%5Btt_news%5D=566&cHash=db55f3a8829726044512a1fe74cce41b (accessed on 5 June 2025).
- Nordmann, P.; Bouvier, M.; Poirel, L.; Sadek, M. Rapid Cefiderocol NP Test for Detection of Cefiderocol Susceptibility/Resistance in Enterobacterales. J. Antimicrob. Chemother. 2022, 77, 3456–3461. [Google Scholar] [CrossRef]
- Raro, O.H.F.; Bouvier, M.; Kerbol, A.; Decousser, J.W.; Poirel, L.; Nordmann, P. Rapid Detection of Cefiderocol Susceptibility/Resistance in Acinetobacter baumannii. Eur. J. Clin. Microbiol. Infect. Dis. 2023, 42, 1511–1518. [Google Scholar] [CrossRef]
- Jeannot, K.; Gaillot, S.; Triponney, P.; Portets, S.; Pourchet, V.; Fournier, D.; Potron, A. Performance of the Disc Diffusion Method, MTS Gradient Tests and Two Commercially Available Microdilution Tests for the Determination of Cefiderocol Susceptibility in Acinetobacter spp. Microorganisms 2023, 11, 1971. [Google Scholar] [CrossRef] [PubMed]
- Kolesnik-Goldmann, N.; Seth-Smith, H.M.B.; Haldimann, K.; Imkamp, F.; Roloff, T.; Zbinden, R.; Hobbie, S.N.; Egli, A.; Mancini, S. Comparison of Disk Diffusion, E-Test, and Broth Microdilution Methods for Testing In Vitro Activity of Cefiderocol in Acinetobacter baumannii. Antibiotics 2023, 12, 1212. [Google Scholar] [CrossRef]
- Bonnin, R.A.; Emeraud, C.; Jousset, A.B.; Naas, T.; Dortet, L. Comparison of Disk Diffusion, MIC Test Strip and Broth Microdilution Methods for Cefiderocol Susceptibility Testing on Carbapenem-Resistant Enterobacterales. Clin. Microbiol. Infect. 2022, 28, 1156.e1–1156.e5. [Google Scholar] [CrossRef] [PubMed]
- Albano, M.; Karau, M.J.; Schuetz, A.N.; Patel, R. Comparison of Agar Dilution to Broth Microdilution for Testing In Vitro Activity of Cefiderocol Against Gram-Negative Bacilli. J. Clin. Microbiol. 2020, 59, e00966-20. [Google Scholar] [CrossRef]
- Bassetti, M.; Echols, R.; Matsunaga, Y.; Ariyasu, M.; Doi, Y.; Ferrer, R.; Lodise, T.P.; Naas, T.; Niki, Y.; Paterson, D.L.; et al. Efficacy and safety of cefiderocol or best available therapy for the treatment of serious infections caused by carbapenem-resistant Gram-Negative Bacteria (CREDIBLE-CR): A randomised, open-label, multicentre, pathogen-focused, descriptive, phase 3 trial. Lancet Infect. Dis. 2021, 21, 226–240. [Google Scholar] [CrossRef]
- Ibrahim, A.; Bouvier, M.; Sadek, M.; Decousser, J.W.; Poirel, L.; Nordmann, P. A Selective Culture Medium for Screening Cefiderocol Resistance in Enterobacterales, Pseudomonas aeruginosa, and Acinetobacter baumannii. J. Clin. Microbiol. 2023, 61, e01883-22. [Google Scholar] [CrossRef]
- McCreary, E.K.; Heil, E.L.; Tamma, P.D. New Perspectives on Antimicrobial Agents: Cefiderocol. Antimicrob. Agents Chemother. 2021, 65, e02171-20. [Google Scholar] [CrossRef]
- Jena, J.; Behera, B.; Nayak, G.; Mohanty, S.; Mahapatra, A.; Purushotham, P.; Radhakrishnan, A.; Tripathy, M. In Vitro Susceptibility of Burkholderia pseudomallei Isolates to Cefiderocol and Ceftazidime/Avibactam from Odisha, India. J. Lab. Physicians 2023, 15, 573–577. [Google Scholar] [CrossRef]
- Matuschek, E.; Longshaw, C.; Takemura, M.; Yamano, Y.; Kahlmeter, G. Cefiderocol: EUCAST Criteria for Disc Diffusion and Broth Microdilution for Antimicrobial Susceptibility Testing. J. Antimicrob. Chemother. 2022, 77, 1662–1669. [Google Scholar] [CrossRef]
- Morris, C.P.; Bergman, Y.; Tekle, T.; Fissel, J.A.; Tamma, P.D.; Simner, P.J. Cefiderocol Antimicrobial Susceptibility Testing Against Multidrug-Resistant Gram-Negative Bacilli: A Comparison of Disk Diffusion to Broth Microdilution. J. Clin. Microbiol. 2020, 59, e01649-20. [Google Scholar] [CrossRef]
- Emeraud, C.; Gonzalez, C.; Dortet, L. Comparison of ComASP® and UMIC® Methods with the Reference Method for Cefiderocol Susceptibility Testing on Carbapenem-Resistant Enterobacterales. J. Antimicrob. Chemother. 2023, 78, 1800–1801. [Google Scholar] [CrossRef]
- Bianco, G.; Boattini, M.; Comini, S.; Banche, G.; Cavallo, R.; Costa, C. Disc Diffusion and ComASP® Cefiderocol Microdilution Panel to Overcome the Challenge of Cefiderocol Susceptibility Testing in Clinical Laboratory Routine. Antibiotics 2023, 12, 604. [Google Scholar] [CrossRef] [PubMed]
- Bianco, G.; Boattini, M.; Comini, S.; Gaibani, P.; Cavallo, R.; Costa, C. Performance Evaluation of Bruker UMIC® Microdilution Panel and Disc Diffusion to Determine Cefiderocol Susceptibility in Enterobacterales, Acinetobacter baumannii, Pseudomonas aeruginosa, Stenotrophomonas maltophilia, Achromobacter xylosoxidans and Burkholderia Species. Eur. J. Clin. Microbiol. Infect. Dis. 2024, 43, 559–566. [Google Scholar] [PubMed]
- Bovo, F.; Lazzarotto, T.; Ambretti, S.; Gaibani, P. Comparison of Broth Microdilution, Disk Diffusion and Strip Test Methods for Cefiderocol Antimicrobial Susceptibility Testing on KPC-Producing Klebsiella pneumoniae. Antibiotics 2023, 12, 614. [Google Scholar] [CrossRef] [PubMed]
- Dortet, L.; Niccolai, C.; Pfennigwerth, N.; Frisch, S.; Gonzalez, C.; Antonelli, A.; Giani, T.; Hoenings, R.; Gatermann, S.; Rossolini, G.M.; et al. Performance Evaluation of the UMIC® Cefiderocol to Determine MIC in Gram-Negative Bacteria. J. Antimicrob. Chemother. 2023, 78, 1672–1676. [Google Scholar] [CrossRef]
- Devoos, L.; Biguenet, A.; Rousselot, J.; Bour, M.; Plésiat, P.; Fournier, D.; Jeannot, K. Performance of discs, Sensititre EUMDROXF microplates and mts gradient strips for the determination of the susceptibility of multidrug-resistant Pseudomonas aeruginosa to Cefiderocol. Clin. Microbiol. Infect. 2023, 29, 652.e1–652.e8. [Google Scholar] [CrossRef]
- EUCAST. Clinical Breakpoints—Breakpoints and Guidance. Breakpoint Tables for Interpretation of MICs and Zone Diameters. Version 15.0. EUCAST 2025. Available online: https://www.eucast.org/clinical_breakpoints (accessed on 5 June 2025).
- Liu, Y.; Ding, L.; Han, R.; Zeng, L.; Li, J.; Guo, Y.; Hu, F. Assessment of Cefiderocol Disk Diffusion Versus Broth Microdilution Results When Tested Against Acinetobacter baumannii Complex Clinical Isolates. Microbiol. Spectr. 2023, 11, e05355-22. [Google Scholar] [CrossRef]
- Russo, C.; Humphries, R. Approaches to Testing Novel β-Lactam and β-Lactam Combination Agents in the Clinical Laboratory. Antibiotics 2023, 12, 1700. [Google Scholar] [CrossRef]
- Koeth, L.M.; DiFranco-Fisher, J.M.; Palavecino, E.; Kilic, A.; Hardy, D.; Vicino, D.; Stracquadanio, S.; Stefani, S. A Multicenter Performance Evaluation of Cefiderocol MIC Results: ComASP in Comparison to CLSI Broth Microdilution. J. Clin. Microbiol. 2024, 63, e00926-24. [Google Scholar] [CrossRef]
| MIC Breakpoint (mg/L) | Disk Zone Diameter Breakpoint (mm) | ||||||
|---|---|---|---|---|---|---|---|
| Organism | CLSI | EUCAST | FDA | CLSI | EUCAST | ATU (EUCAST) | FDA |
| Enterobacterales | ≤4 (S), 8 (I), ≥16 (R) | ≤2 (S), >2 (R) | M-100 standard is recognized | ≥16 (S), 9–15 (I), ≤8 (R) | ≥23 (S), <23 (R) | 21–23 | M-100 standard is recognized § |
| Pseudomonas aeruginosa | ≤4 (S), 8 (I), ≥16 (R) | ≤2 (S), >2 (R) | ≤1 (S), 2 (I), ≥4 (R) | ≥18 (S), 13–17 (I), ≤12 (R) | ≥22 (S), <22 (R) | 20–21 | ≥22 (S), 13–21 (I), ≤12 (R) |
| Acinetobacter baumannii complex | ≤4 (S), 8 (I), ≥16 (R) | IE | ≤1 (S), 2 (I), ≥4 (R) | ≥15 (S) ** | Note * | ≥19 (S), 12–18 (I), ≤11 (R) | |
| Stenotrophomonas maltophilia | ≤4 (S), 8 (I), ≥16 (R) | IE | Not specified | ≥15 (S) | Note ° | ≥17 (S) | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stefani, S.; Arena, F.; Principe, L.; Stracquadanio, S.; Vismara, C.; Rossolini, G.M. Evaluating Antimicrobial Susceptibility Testing Methods for Cefiderocol: A Review and Expert Opinion on Current Practices and Future Directions. Antibiotics 2025, 14, 760. https://doi.org/10.3390/antibiotics14080760
Stefani S, Arena F, Principe L, Stracquadanio S, Vismara C, Rossolini GM. Evaluating Antimicrobial Susceptibility Testing Methods for Cefiderocol: A Review and Expert Opinion on Current Practices and Future Directions. Antibiotics. 2025; 14(8):760. https://doi.org/10.3390/antibiotics14080760
Chicago/Turabian StyleStefani, Stefania, Fabio Arena, Luigi Principe, Stefano Stracquadanio, Chiara Vismara, and Gian Maria Rossolini. 2025. "Evaluating Antimicrobial Susceptibility Testing Methods for Cefiderocol: A Review and Expert Opinion on Current Practices and Future Directions" Antibiotics 14, no. 8: 760. https://doi.org/10.3390/antibiotics14080760
APA StyleStefani, S., Arena, F., Principe, L., Stracquadanio, S., Vismara, C., & Rossolini, G. M. (2025). Evaluating Antimicrobial Susceptibility Testing Methods for Cefiderocol: A Review and Expert Opinion on Current Practices and Future Directions. Antibiotics, 14(8), 760. https://doi.org/10.3390/antibiotics14080760








