Antimicrobial Potential of Nanomaterials Synthesized with Extracts from Annona Plants: A Review
Abstract
1. Introduction
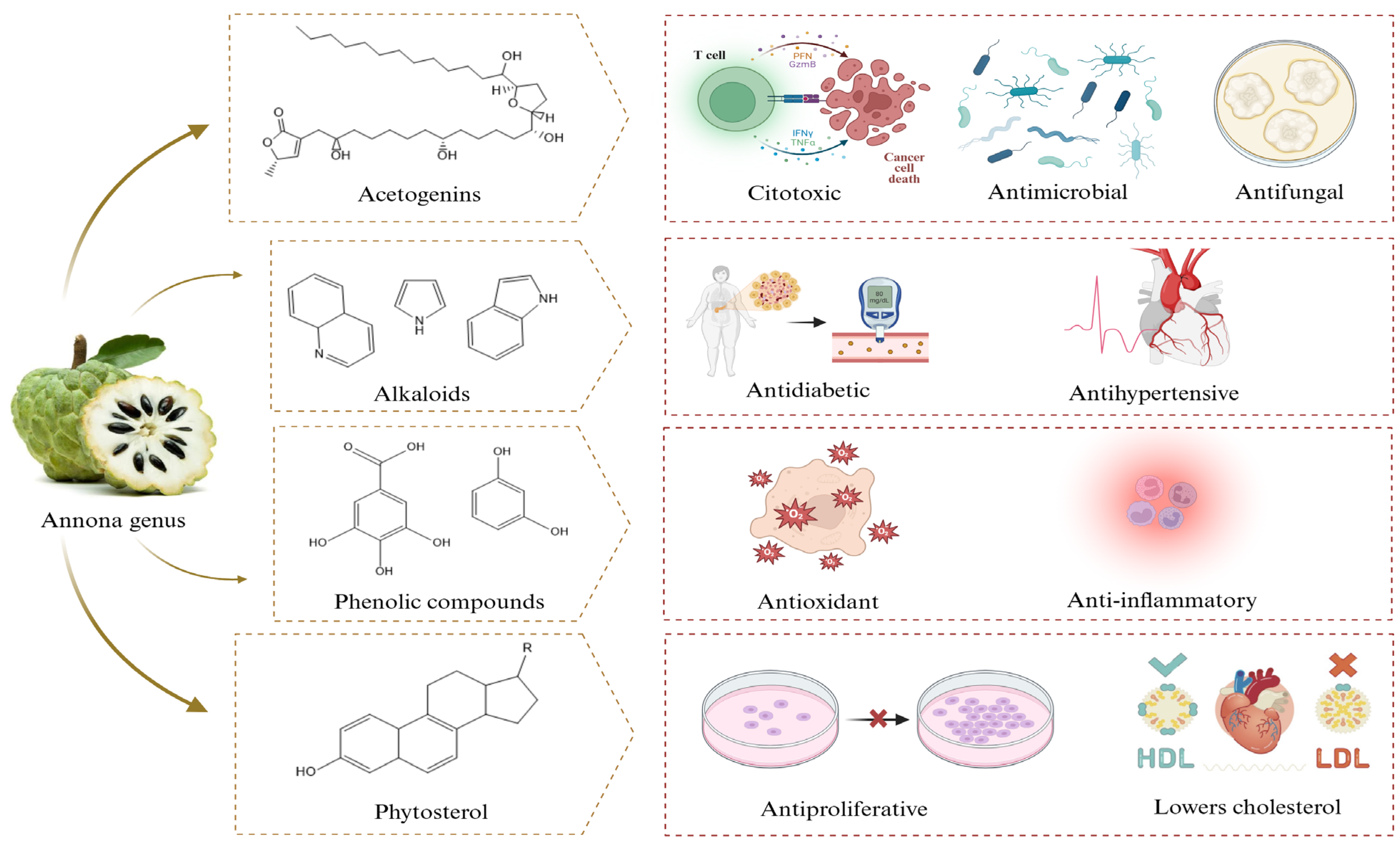
2. Phytochemical Composition of Annona Extracts
2.1. Acetogenins
2.2. Phenolic Compounds
2.3. Alkaloids and Terpenoids
2.4. Other Metabolites
3. Phytosynthesis of Nanomaterials with Annona Extracts
4. Importance of Nanomaterials Synthesized with Plant Extracts of the Annona Genus
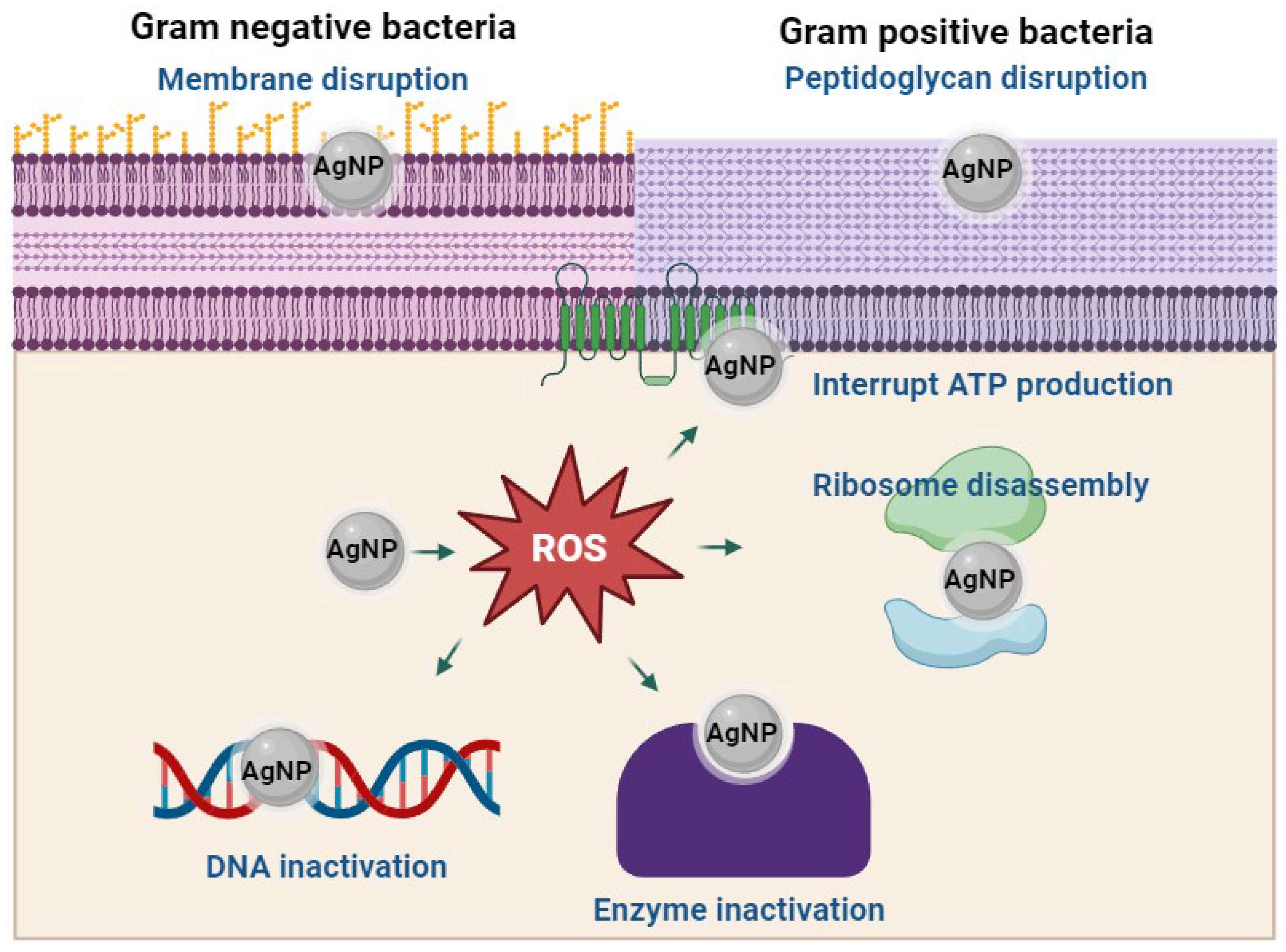
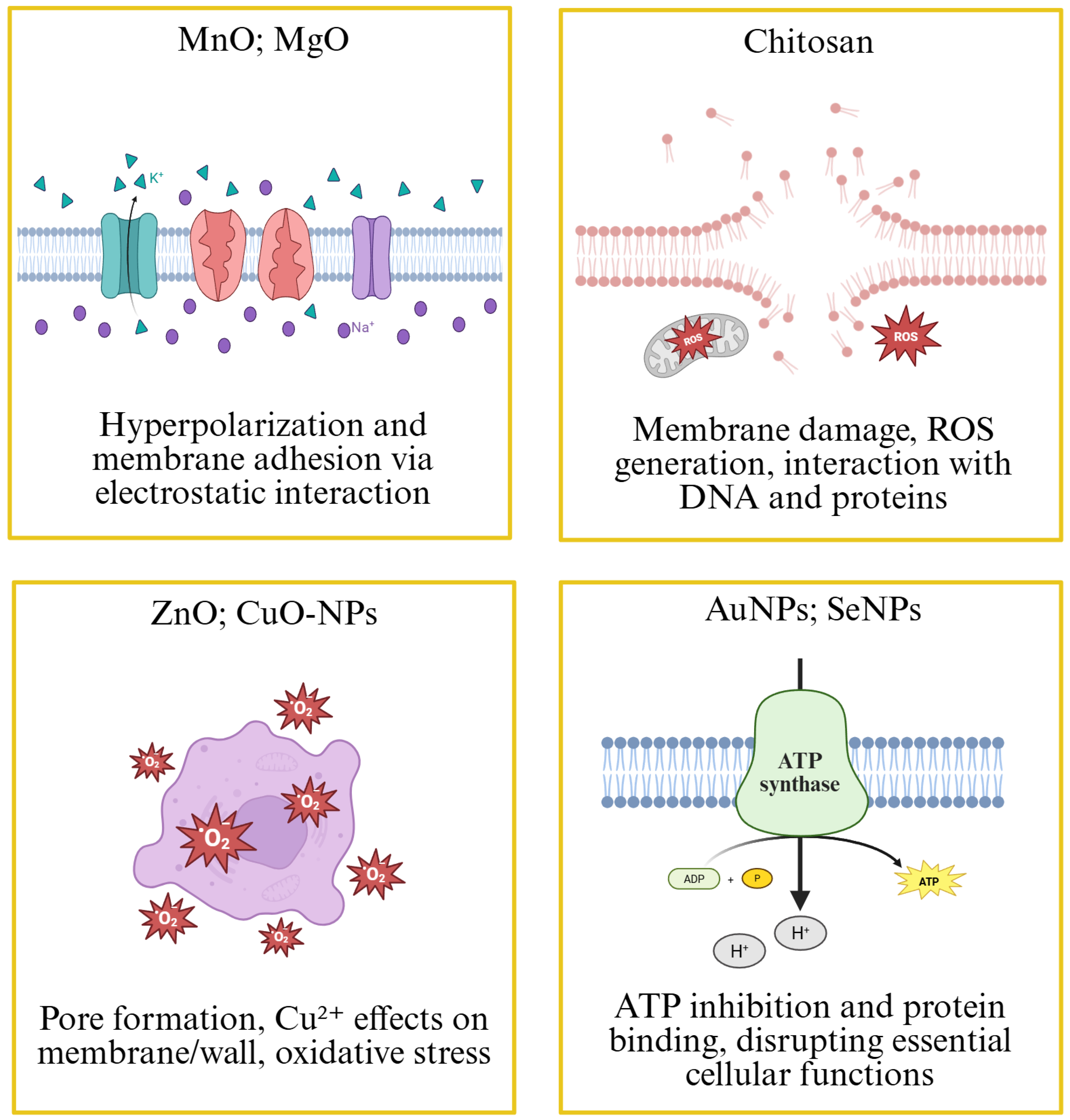
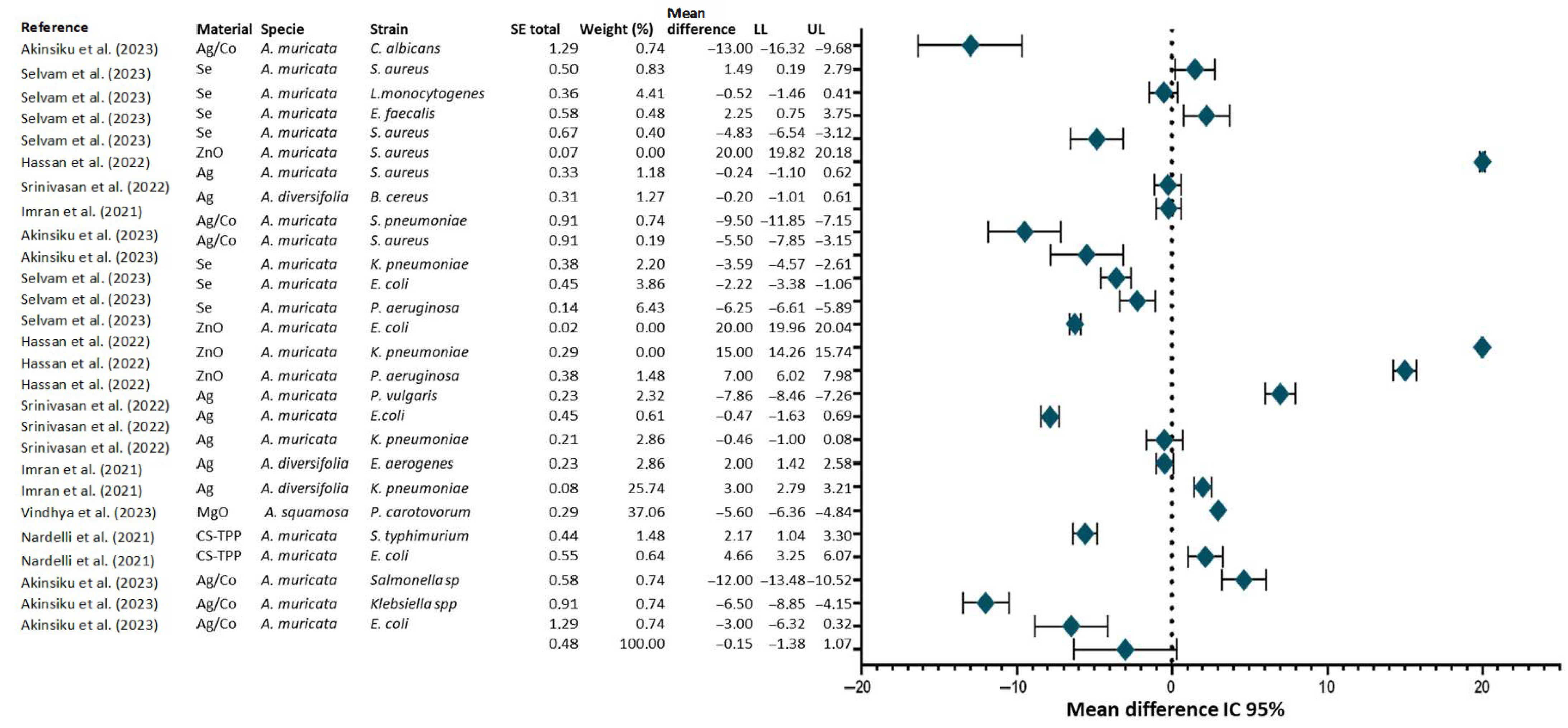
4.1. Antimicrobial Activity of Nanomaterials Synthesized with Annona Extracts
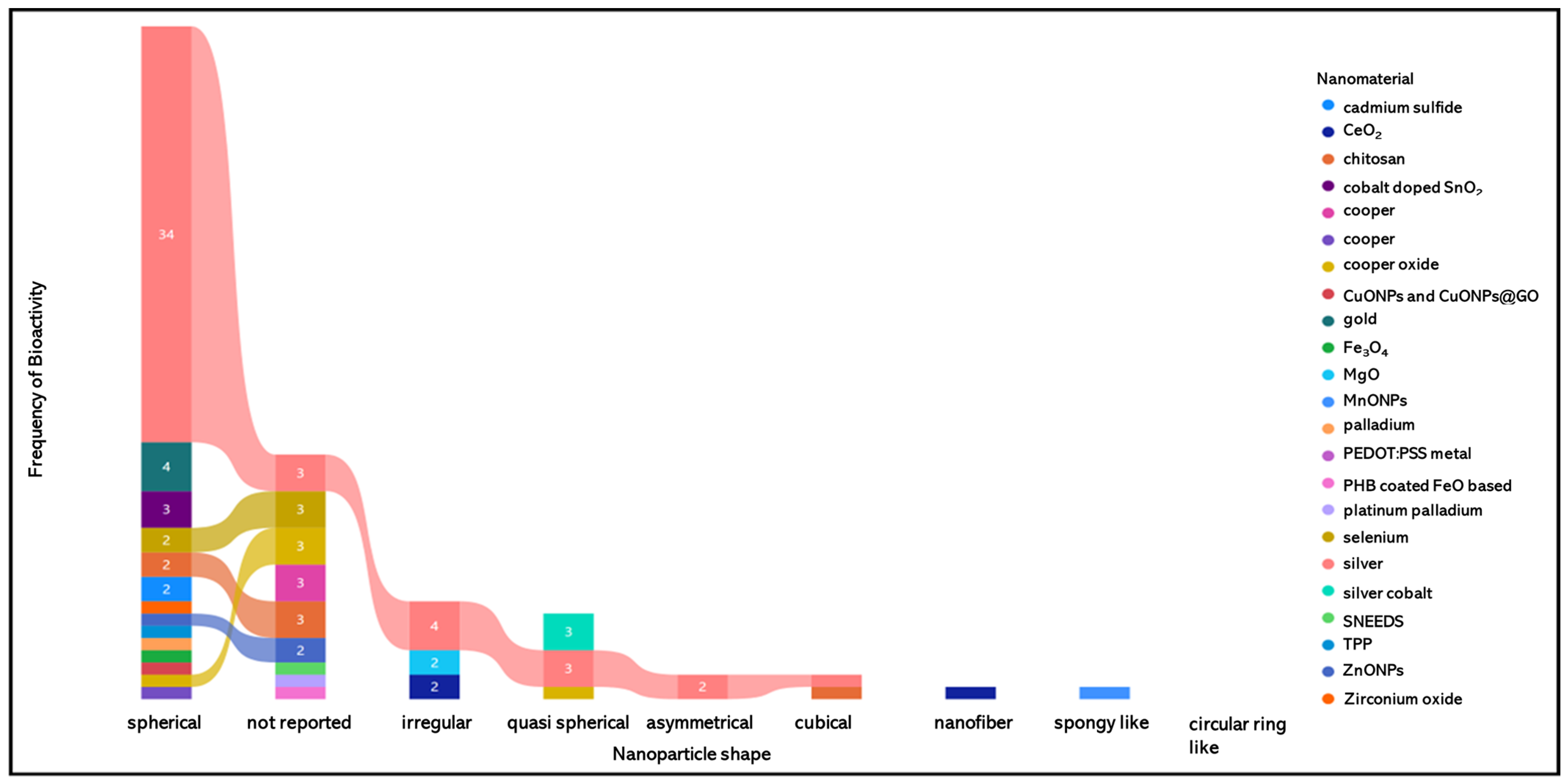
| Specie | Tissue | Extract Solvent | Nanomaterial | Size (nm) | Shape | Bioactivity | Reference |
|---|---|---|---|---|---|---|---|
| A. muricata | Leaves | Methanol | CdS | 3.42 | Spherical | Antimicrobial activity against Staphylococcus aureus and antifungal activity against Aspergillus niger | [67] |
| A. muricata | Fruit | Water | CeO2 | Not reported | Nanofiber | Antimicrobial activity against Staphylococcus aureus and Enterococcus faecalis | [68] |
| A. reticulata | Leaves | Water | CeO2 | 3.7–10.3 | Irregular | Antioxidant and antidiabetic activity | [69] |
| A. muricata | Leaves | Chloroform | Chitosan | 248–317 | Not reported | Anticancer activity against HeLa cells | [62] |
| A. muricata | Leaves | Ethanol | Chitosan | 234 | Spherical | Antibacterial activity against Escherichia coli and Salmonella typhimurium | [63] |
| A. muricata | Leaves | Ethanol | Chitosan | 282.75 | Not reported | Not reported | [70] |
| A. squamosa | Leaves | Ethanol | Chitosan | 535.1 | Spherical | Cytotoxic against HeLa cells by induction of caspase-3 expression | [71] |
| A. squamosa | Leaves | Ethanol | Chitosan | 531.1 | Cubical | Induces caspase-3 expression on HeLa cells | [72] |
| A. squamosa | Leaves | Ethanol | Chitosan | 535.1 | Not reported | Induces caspase-3 expression and apoptosis | [73] |
| A. squamosa | Leaves | Ethanol | Chitosan | 535.1 | Not reported | Induces caspase-3 expression on WiDr cells | [72] |
| A. muricata | Leaves | Water | Cobalt-doped SnO2 | 0.33 | Spherical | Antibacterial activity against Pseudomonas aeruginosa and Staphylococcus aureus, antifungal activity against Candida albicans and Aspergillus niger, antioxidant activity | [73] |
| A. squamosa | Stem barks | Water | Cu | Not reported | Not reported | Antimicrobial against Staphylococcus aureus and Escherichia coli, antifungal against Candida albicans, cytotoxicity against breast cancer MCF-7 | [74] |
| A. squamosa | Seed | Water | Cu | 5.99–24.48 | Spherical | Insecticidal activity of Anopheles stephensi and Tenebrio molitor larvae | [75] |
| A. muricata | Fruit | Water | CuO | Not reported | Not reported | Antiproliferative activity against AMJ-13, MCF-7 breast cancer cell lines, and the human breast epithelial cell line (HBL-100) | [76] |
| A. reticulata | leaves | Water | CuO | Not reported | Not reported | Antioxidant and catalytic activity | [77] |
| A. squamosa | Seed | Water | CuO | 11 | Spherical | Antimicrobial against Xanthomonas oryzae | [78] |
| A. squamosa | Seeds | Ethanol | CuO | 30.27 | Semiglobular | Molluscicidal activity | [79] |
| A. muricata | Leaves | Water | CuONPs and CuONPs@GO | 40 | Spherical | Antibacterial activity towards both Staphylococcus aureus and Salmonella typhi | [80] |
| A. muricata | Leaves | Water | Au | 25.55 | Spherical | Antimicrobial against Staphylococcus aureus, Enterococcus faecalis, Klebsiella pneumoniae, and Clostridium sporogenes, antifungal against Aspergillus flavus, Candida albicans, Fusarium oxysporum, and Penicillium camemeri. | [81] |
| A. muricata | Peel and pulp | Water | Au | 15 | Spherical | Anticancer activity in treated Hep2 liver cancer cell line and non-toxic effect on regular VERO cell line | [82] |
| A. muricata | leaves | Ethanol 80% | Au | 89.34 | Smooth Spherical | Anticancer activity against metastatic melanoma MM-138 and primary melanoma FM-55, as well as breast cancer cell lines | [83] |
| A. muricata | leaves | Water | Fe3O4 | 23 | Spherical | Antidiabetic activity | [84] |
| A. squamosa | Seeds | Water | MgO | 27 to 68 | Irregular | Antibacterial activity against Pectobacterium carotovorum, antioxidant activity and cytotoxicity against HeLa cells | [85] |
| A. muricata | Seed | Water | MnO | Not reported | Spongy-like agglomeration of smooth particles | Antimicrobial activity against Escherichia coli and Staphylococcus aureus | [86] |
| A. squamosa | Seed | Methanol | Pd | Less than 300 | Spherical | Oxidative damage in hepatic tissue | [87] |
| A. muricata | Leaves | Not reported | PHB-coated Fe3O4–based | 30 to 40 | Not reported | Antiproliferative against HeLa and MDA-MB-231 cell lines | [88] |
| A. muricata | Leaves | Water | PtPd | 3.97–10.68 | Not reported | Antibacterial activity against Escherichia coli and Staphylococcus aureus | [89] |
| A. reticulata | Leaves | Water | Poly (3,4-ethylenedioxythiophene) | 23.7 | Circular ring-like | Not reported | [90] |
| Poly (4-styrene sulfonate) Gold | |||||||
| A. muricata | Fruit | Water | Se | Not reported | Not reported | Anticancer against lung cells (A-549) | [91] |
| A. muricata | Fruit | Water | Se | Not reported | Not reported | Antioxidant | [92] |
| A. muricata | Fruits | Water | Se | Not reported | Not reported | Antifungal against Candida albicans | [93] |
| A. muricata | Fruit | Water | Se | 80–120 | Spherical | Antioxidant, antimicrobial activity against Escherichia coli, Pseudomonas aeruginosa, Klebsiella pneumoniae, Staphylococcus aureus, Enterococcus faecalis, and Listeria monocytogenes | [94] |
| A. diversifolia | Leaves | Water | Ag | 45 to 58 | Spherical | Antibacterial activity against Klebsiella pneumoniae and Enterobacter aerogenes | [95] |
| A. glabra | Leaves | Water | Ag | 10–100 | Spherical | Larvicidal against Aedes aegypti and Aedes albopictus mosquito larvae | [60] |
| A. glabra | Fruit | Ethanol | Ag | 7.11 | Spherical | Antibacterial activity against Pseudomonas aeruginosa and Escherichia coli | [96] |
| A. muricata | Fruit Juice | Water | Ag | 31.95 | Spherical | Anticancer activity against HeLa cells, cytotoxicity against AMJ−13 | [97] |
| A. muricata | Leaves | Water | Ag | 10.87 | Asymmetrical | Antimicrobial activity against Escherichia coli, Staphylococcus aureus, and Enterococcus faecalis, cytotoxicity against oral fibroblasts | [98] |
| A. muricata | Pulp | Water | Ag | 51.5 | Spherical | Antimicrobial activity against Staphylococcus aureus, Enterococcus faecalis, Escherichia coli, Klebsiella pneumoniae, Proteus mirabilis, and Pseudomonas aeruginosa, fungistatic action against Candida albicans | [61] |
| A. muricata | Pulp | Water | Ag | 87 | Spherical | Antimicrobial activity against Staphylococcus aureus, Enterococcus faecalis, Escherichia coli, Klebsiella pneumoniae, and Proteus mirabilis | [99] |
| A. muricata | Seeds | Water | Ag | 62 | Spherical | Antimicrobial activity against Staphylococcus aureus, Enterococcus faecalis, Escherichia coli, Klebsiella pneumoniae, and Proteus mirabilis | [99] |
| A. muricata | Seeds | Water | Ag | 194 | Spherical | Antimicrobial activity against Staphylococcus aureus, Enterococcus faecalis, Escherichia coli, Klebsiella pneumoniae, and Proteus mirabilis | [99] |
| A. muricata | Leaves | Water | Ag | 205 | Spherical | Antimicrobial activity against Staphylococcus aureus, Enterococcus faecalis, Escherichia coli, Klebsiella pneumoniae, and Proteus mirabilis | [99] |
| A. muricata | Leaves | Water | Ag | 60 | Spherical | Antimicrobial activity against Pseudomonas aeruginosa and fungistatic action against Candida albicans | [99] |
| A. muricata | Root bark | Water | Ag | 22 | Spherical | Antimicrobial activity against Bacillus subtilis, Staphylococcus aureus, Klebsiella pneumoniae, Escherichia coli, and Pseudomonas aeruginosa | [99] |
| A. muricata | Leaves | Water | Ag | Not reported | Not reported | Ability to cleave DNA into fragments | [100] |
| A. muricata | Leaves | Water | Ag | 35 | Spherical | Antimicrobial activity against Klebsiella pneumoniae, Escherichia coli, Proteus vulgaris, and Staphylococcus aureus | [101] |
| A. muricata | Leaves | Water | Ag | 35 | Spherical | Larvicidal activity against larvae of Aedes aegypti, Anopheles stephensi, and Culex quinquefasciatus | [102] |
| A. muricata | Peels | Water | Ag | 11 to 23 | Spherical | Antiproliferative against THP−1, HBL, and AMJ−13 | [103] |
| A. muricata | Leaves | Water | Ag | 35 | Spherical | Antioxidant, antidiabetic, cytotoxic (HaCaT), and antimicrobial (Staphylococcus aureus, Serratia marcescens, and Pseudomonas aeruginosa) | [67] |
| A. muricata | Leaves | Ethanol | Ag | 60.12 | Spherical | Anticancer activity via CASP9 activation | [104,105] |
| A. muricata | Fruits | Ethanol | Ag | 60.12 | Spherical | Anticancer activity via CASP9 activation | [104,105] |
| A. muricata | Root | Water | Ag | 34 | Spherical | Antioxidant activity, selective cytotoxicity against HCT116, without affecting the growth of normal human lymphocytes and erythrocytes, and an anticancer agent for colon cancer | [106] |
| A. muricata | Leaves | Water | Ag | 30 to 40 | Not reported | Antimicrobial against Staphylococcus aureus, Escherichia coli, Bacillus subtilis, and Pseudomonas aeruginosa, antifungal activity against Candida albicans | [107] |
| A. muricata | Peel | Water | Ag | 19.63 | Quasi-Spherical | Antiproliferative against breast cancer (MCF−7, MDA-MB−468), colon cancer (HCT−116), and melanoma (A−375) | [108] |
| A. reticulata | Leaves | Water and ethanol | Ag | 22 | Not reported | [109] | |
| A. squamosa | Leaves | Water | Ag | 84.9 | Irregular | Antimicrobial activity against Escherichia coli, Bacillus subtilis, Xanthomonas campestris, and Staphylococcus aureus, antifungal against Aspergillus niger | [110] |
| A. squamosa | Leaves | Different fractions | Ag | 100–200 | Cubical | Larvicidal activity against Anopheles stephensi | [111] |
| A. squamosa | Peel | Water | Ag | 18–35 | Spherical | Antioxidant activity examined by DPPH-scavenging assay and amylase inhibition, | [112] |
| A. squamosa | Leaves | Water | Ag | 20–100 | Spherical | Cytotoxic against MCF−7 | [113] |
| A. squamosa | Leaves | Water | Ag | 28.47 | Spherical | Antimicrobial activity against Bacillus cereus, Bacillus subtilis, Staphylococcus aureus, Salmonella typhimurium, Pseudomonas aeruginosa and Proteus vulgaris | [114] |
| A. squamosa | Fruit | Water | Ag | 15–50 | Spherical | Antimicrobial activity against Escherichia coli and Pseudomonas aeruginosa | [115] |
| A. squamosa | Leaves | Water | Ag | 35–90 | Spherical | Antimicrobial activity against Escherichia coli and Pseudomonas aeruginosa | [116] |
| A. squamosa | Fruit | Ethanol | Ag | 6.63 | Spherical | Antibacterial activity against Pseudomonas aeruginosa and Escherichia coli | [96] |
| A. squamosa | Seed | Water | Ag | 73.5 | Irregular | Antimicrobial activity against Escherichia coli, Bacillus subtilis, Xanthomonas campestris, and Staphylococcus aureus, antifungal against Aspergillus niger | [110] |
| A. squamosa | Seeds | Water | Ag | 22 | Spherical | Larvicidal activity against mosquito Anopheles stephensi larvae | [116] |
| A. squamosa | Seeds | Water | Ag | 50–80 | Quasi-Spherical | Antimicrobial Escherichia coli, Streptococcus mutans, and Staphylococcus aureus | [117] |
| A. squamosa | Leaves | Water | Ag | 52 | Spherical | Antimicrobial against Escherichia coli | [96] |
| A. muricata | Leaves | Water | Ag | 16.56 | Quasi-Spherical | Antiproliferative against breast cancer (MCF−7, MDA-MB−468), colon cancer (HCT−116), and melanoma (A−375) | [108] |
| A. muricata | Leaves | Water | Ag-Co | 39.34 | pseudo-Spherically | Toxicity against Drosophila melanogaster, antibacterial against Klebsiella sp., Salmonella sp., Streptococcus pneumoniae, Staphylococcus aureus, and Escherichia coli, antifungal against Candida albicans | [81] |
| A. muricata | Leaves | Chloroform | SNEEDS | 411.4 | Not reported | Antioxidant | [62] |
| A. muricata | Leaves | Ethanol | TPP | 234 | Spherical | Antibacterial activity against Escherichia coli and Salmonella typhimurium | [63] |
| A. muricata | Fruit | Water | ZnO | 29 | Not reported | Antibacterial activity against Escherichia coli, Klebsiella pneumoniae, Pseudomonas aeruginosa, and Staphylococcus aureus, cytotoxicity against HCT116, K562., | [118] |
| A. muricata | Leaves | Water | ZnO | 80 | Spherical | Anticancer against A549 and MOLT4 | [20] |
| A. reticulata | Leaves | Water | ZrO2 | 13–20 | Spherical | Antibacterial action against Salmonella enterica (multidrug-resistant) | [119] |
4.2. Antiparasitic Activity
4.3. Other Activities
4.4. Anticancer Activity
5. Conclusions
Funding
Acknowledgments
Conflicts of Interest
References
- Begum, S.L.R.; Jayawardana, N.U. Green Synthesized Metal Nanoparticles as an Ecofriendly Measure for Plant Growth Stimulation and Disease Resistance. Plant Nano Biol. 2023, 3, 100028. [Google Scholar] [CrossRef]
- Aswathi, V.P.; Meera, S.; Maria, C.G.A.; Nidhin, M. Green Synthesis of Nanoparticles from Biodegradable Waste Extracts and Their Applications: A Critical Review. Nanotechnol. Environ. Eng. 2023, 8, 377–397. [Google Scholar] [CrossRef]
- Akintelu, S.A.; Bo, Y.; Folorunso, A.S. A Review on Synthesis, Optimization, Mechanism, Characterization, and Antibacterial Application of Silver Nanoparticles Synthesized from Plants. J. Chem. 2020, 2020, 3189043. [Google Scholar] [CrossRef]
- Gavas, S.; Quazi, S.; Karpiński, T.M. Nanoparticles for Cancer Therapy: Current Progress and Challenges. Nanoscale Res. Lett. 2021, 16, 173. [Google Scholar] [CrossRef]
- Akinsiku, A.A.; Odaudu, R.O.; De Campos, O.C.; Adeyemi, A.O.; Ejilude, O. Synthesis of Low Toxic Silver-Cobalt Nanoparticles Using Annona muricata Leaf Extract: Antimicrobial Evaluation. Inorg. Chem. Commun. 2023, 153, 110837. [Google Scholar] [CrossRef]
- Singh, P.; Jain, S.K. Biosynthesis of Nanomaterials: Growth and Properties. Rev. Adv. Sci. Eng. 2014, 3, 231–238. [Google Scholar] [CrossRef]
- Husen, A.; Iqbal, M. Nanomaterials and Plant Potential: An Overview. In Nanomaterials and Plant Potential; Husen, A., Iqbal, M., Eds.; Springer International Publishing: Cham, Switzerland, 2019; pp. 3–29. ISBN 978-3-030-05569-1. [Google Scholar]
- Kemala, P.; Idroes, R.; Khairan, K.; Tallei, T.E.; Ramli, M.; Efendi, R. Green Synthesis of Silver Nanoparticles Using Calotropis Gigantea and Its Characterization Using UV-Vis Spectroscopy. IOP Conf. Ser. Earth Environ. Sci. 2022, 951, 12090. [Google Scholar] [CrossRef]
- Sidhu, A.K.; Agrawal, S.B.; Verma, N.; Kaushal, P.; Sharma, M. Fungal-Mediated Synthesis of Multimetallic Nanoparticles: Mechanisms, Unique Properties, and Potential Applications. Front. Nanotechnol. 2025, 7, 1549713. [Google Scholar] [CrossRef]
- Adewale, A.S. The Efficacy of Methanol, Dichloromethane and N-Butanol Extracts of Anonna Muricata Leaves on Selected Bacteria And Fungi. N. Y. Sci. J. 2019, 12, 53–56. [Google Scholar] [CrossRef]
- Amala Dev, A.R.; Joseph, S.M. Anticancer Potential of Annona Genus: A Detailed Review. J. Indian Chem. Soc. 2021, 98, 100231. [Google Scholar] [CrossRef]
- Aguilar-Villalva, R.; Molina, G.A.; España-Sánchez, B.L.; Díaz-Peña, L.F.; Elizalde-Mata, A.; Valerio, E.; Azanza-Ricardo, C.; Estevez, M. Antioxidant Capacity and Antibacterial Activity from Annona Cherimola Phytochemicals by Ultrasound-Assisted Extraction and Its Comparison to Conventional Methods. Arab. J. Chem. 2021, 14, 103239. [Google Scholar] [CrossRef]
- Aliyu, N.; Duniya, V.A. Effect of Custard Apple (Annona squamosa L.) Seeds Extracts on In-Vitro Activity of Malassezia Globosa. J. Chem. Eng. Ind. Biotechnol. 2023, 9, 13–19. [Google Scholar]
- Goñi, O.; Sanchez-Ballesta, M.T.; Merodio, C.; Escribano, M.I. Two Cold-Induced Family 19 Glycosyl Hydrolases from Cherimoya (Annona Cherimola) Fruit: An Antifungal Chitinase and a Cold-Adapted Chitinase. Phytochemistry 2013, 95, 94–104. [Google Scholar] [CrossRef] [PubMed]
- Patil, H.V.; Dhankani, M.A.; Dhankani, A.R. A Review on Marvel Fruit: Annona Muricata. Med. Sci. Forum 2023, 21, 26. [Google Scholar] [CrossRef]
- Tundis, R.; Xiao, J.; Loizzo, M.R. Annona Species (Annonaceae): A Rich Source of Potential Antitumor Agents? Ann. N. Y. Acad. Sci. 2017, 1398, 30–36. [Google Scholar] [CrossRef]
- Yahya, M.; Ginting, B. In-Vitro anti-cervical cancer activity of isolate ase 3.3.3 from ethyl acetate extract of Annona squamosa l. leaf. Rasayan J. Chem. 2023, 16, 1503–1508. [Google Scholar] [CrossRef]
- Ferraz, C.R.; Silva, D.B.; da Silva Prado, L.C.; Canabrava, H.A.N.; Bispo-da-Silva, L.B. Antidiarrhoeic Effect and Dereplication of the Aqueous Extract of Annona Crassiflora (Annonaceae). Nat. Prod. Res. 2019, 33, 563–567. [Google Scholar] [CrossRef]
- Onuoha, C.; Nwachi, E.C.; Nwanya, E.U.; Osuagwu, O.L.; Nsofor, W.N.; Nzebude, C.P.; Ujowundu, F.N.; Chukwudoruo, C.S. Comparative Phytochemical Composition and Functional Group Detection of Annona muricata Linn Seeds and Leaves. Trop. J. Phytochem. Pharm. Sci. 2023, 2, 59–64. [Google Scholar] [CrossRef]
- Chabattula, S.C.; Gupta, P.K.; Tripathi, S.K.; Gahtori, R.; Padhi, P.; Mahapatra, S.; Biswal, B.K.; Singh, S.K.; Dua, K.; Ruokolainen, J.; et al. Anticancer Therapeutic Efficacy of Biogenic Am-ZnO Nanoparticles on 2D and 3D Tumor Models. Mater. Today Chem. 2021, 22, 100618. [Google Scholar] [CrossRef]
- Kazman, B.S.M.A.; Harnett, J.E.; Hanrahan, J.R. The Phytochemical Constituents and Pharmacological Activities of Annona Atemoya: A Systematic Review. Pharmaceuticals 2020, 13, 269. [Google Scholar] [CrossRef]
- Kusmardiyani, S.; Suharli, Y.A.; Insanu, M.; Fidrianny, I. Phytochemistry and Pharmacological Activities of Annona Genus: A Review. Curr. Res. Biosci. Biotechnol. 2020, 2, 77–88. [Google Scholar] [CrossRef]
- Quílez, A.M.; Fernández-Arche, M.A.; García-Giménez, M.D.; De la Puerta, R. Potential Therapeutic Applications of the Genus Annona: Local and Traditional Uses and Pharmacology. J. Ethnopharmacol. 2018, 225, 244–270. [Google Scholar] [CrossRef] [PubMed]
- Kumar, M.; Changan, S.; Tomar, M.; Prajapati, U.; Saurabh, V.; Hasan, M.; Sasi, M.; Maheshwari, C.; Singh, S.; Dhumal, S.; et al. Custard Apple (Annona squamosa L.) Leaves: Nutritional Composition, Phytochemical Profile, and Health-Promoting Biological Activities. Biomolecules 2021, 11, 614. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, J.C.L.; Bruginski, E.; Zuccolotto, T.; Santos, A.D.D.C.; Bomfim, L.M.; Rocha, S.L.A.; Barison, A.; Sassaki, G.; Cavalcanti, S.C.D.H.; Costa, E.V.; et al. Chemical Composition, Larvicidal and Cytotoxic Activity of Annona Salzmannii (Annonaceae) Seed Oil. Braz. J. Pharm. Sci. 2021, 57, e18479. [Google Scholar] [CrossRef]
- Shrivastava, A.; Tripathi, A.D.; Paul, V.; Chandra Rai, D. Optimization of Spray Drying Parameters for Custard Apple (Annona squamosa L.) Pulp Powder Development Using Response Surface Methodology (RSM) with Improved Physicochemical Attributes and Phytonutrients. LWT 2021, 151, 112091. [Google Scholar] [CrossRef]
- Albuquerque, T.G.; Santos, F.; Sanches-Silva, A.; Beatriz Oliveira, M.; Bento, A.C.; Costa, H.S. Nutritional and Phytochemical Composition of Annona Cherimola Mill. Fruits and by-Products: Potential Health Benefits. Food Chem. 2016, 193, 187–195. [Google Scholar] [CrossRef]
- Neske, A.; Ruiz Hidalgo, J.; Cabedo, N.; Cortes, D. Acetogenins from Annonaceae Family. Their Potential Biological Applications. Phytochemistry 2020, 174, 112332. [Google Scholar] [CrossRef]
- Bhardwaj, R.; Pareek, S.; Sagar, N.A.; Vyas, N. Bioactive Compounds of Annona. In Bioactive Compounds in Underutilized Fruits and Nuts; Springer: Berlin/Heidelberg, Germany, 2020; pp. 37–62. [Google Scholar] [CrossRef]
- das Chagas Lima, N.N.; Faustino, D.C.; Allahdadi, K.J.; de Aragão França, L.S.; Pinto, L.C. Acetogenins from Annonaceae Plants: Potent Antitumor and Neurotoxic Compounds. PharmaNutrition 2022, 20, 100295. [Google Scholar] [CrossRef]
- Terezan, A.P.; Junqueira, J.G.M.; Wakui, V.G.; Kato, L.; Oliveira, C.M.A.; Martins, C.H.G.; Santiago, M.B.; Severino, V.G.P. Qualitative Analysis of the Acetogenins from Annona Coriacea (Annonaceae) Leaves by HPLC-Q-Orbitrap and Their Antibacterial Potential against Oral Pathogens. Nat. Prod. Res. 2022, 36, 765–771. [Google Scholar] [CrossRef]
- Aguilar-Hernández, G.; López-Romero, B.A.; Pérez-Larios, A.; Ruvalcaba-Gómez, J.M.; Castellanos-Huerta, I.; Tellez-Isaias, G.; Petrone-García, V.M.; Anaya-Esparza, L.M.; Montalvo-González, E. Antibacterial Activity of Crude Extract and Purified Acetogenins from Annona muricata Seeds. Appl. Sci. 2022, 13, 558. [Google Scholar] [CrossRef]
- Le Dang, Q.; Quoc Nguyen, C.; De Tran, Q.; Kim, J.-C.; Thi Vo, K.A.; Thi Cao, H.; Thi Nguyen, X.; Huy Nguyen, T.; Thi Nguyen, T.T.; Huu Nguyen, T.; et al. Anti-Phytopathogenic and Phytotoxic Effects of Annona glabra Lin. and Annona muricata Lin. Seed Extracts: In Vitro and In Vivo Assessment, Bioactive Compound Quantification, and Mechanism Involved. ChemistrySelect 2024, 9, e202401730. [Google Scholar] [CrossRef]
- Han, J.; Zhou, X.; Fu, J.; Gao, G.; Zuo, C.; Guo, Y.; Wang, X. Annonaceous Acetogenins Nanosuspensions Stabilized by Poloxamer 188: Preparation, Properties and in Vivo Evaluation. J. Drug Deliv. Sci. Technol. 2021, 66, 102676. [Google Scholar] [CrossRef]
- Ao, H.; Song, H.; Li, J.; Wang, X. Enhanced Anti-Glioma Activity of Annonaceous Acetogenins Based on a Novel Liposomal Co-Delivery System with Ginsenoside Rh2. Drug Deliv. 2024, 31, 2324716. [Google Scholar] [CrossRef] [PubMed]
- López-Romero, B.A.; Téllez-Isaías, G.; Luna-Bárcenas, G.; Zamudio-Ojeda, A.; Velázquez-Juárez, G.; de Lourdes Garcia-Magaña, M.; Aguilar-Hernández, G.; Montalvo-González, E. Nanosuspensions as Carriers of Annona muricata Acetogenins: Antibacterial Activity against Enterococcus Faecalis and Listeria Monocytogenes. CyTA-J. Food 2023, 21, 771–780. [Google Scholar] [CrossRef]
- Moazzen, A.; Öztinen, N.; Ak-Sakalli, E.; Koşar, M. Structure-Antiradical Activity Relationships of 25 Natural Antioxidant Phenolic Compounds from Different Classes. Heliyon 2022, 8, e10467. [Google Scholar] [CrossRef]
- Zhang, Y.; Cai, P.; Cheng, G.; Zhang, Y. A Brief Review of Phenolic Compounds Identified from Plants: Their Extraction, Analysis, and Biological Activity. Nat. Prod. Commun. 2022, 17, 1934578x211069721. [Google Scholar] [CrossRef]
- Fahim, M.; Shahzaib, A.; Nishat, N.; Jahan, A.; Bhat, T.A.; Inam, A. Green Synthesis of Silver Nanoparticles: A Comprehensive Review of Methods, Influencing Factors, and Applications. JCIS Open 2024, 16, 100125. [Google Scholar] [CrossRef]
- Amini, S.M.; Akbari, A. Metal Nanoparticles Synthesis through Natural Phenolic Acids. IET Nanobiotechnol. 2019, 13, 771–777. [Google Scholar] [CrossRef]
- Nam, J.-S.; Park, S.-Y.; Jang, H.-L.; Rhee, Y.H. Phenolic Compounds in Different Parts of Young Annona muricata Cultivated in Korea and Their Antioxidant Activity. Appl. Biol. Chem. 2017, 60, 535–543. [Google Scholar] [CrossRef]
- Nolasco-González, Y.; Chacón-López, M.A.; Ortiz-Basurto, R.I.; Aguilera-Aguirre, S.; González-Aguilar, G.A.; Rodríguez-Aguayo, C.; Navarro-Cortez, M.C.; García-Galindo, H.S.; García-Magaña, M.D.L.; Meza-Espinoza, L.; et al. Annona muricata Leaves as a Source of Bioactive Compounds: Extraction and Quantification Using Ultrasound. Horticulturae 2022, 8, 560. [Google Scholar] [CrossRef]
- Ahmed, S.H. The Biological Activity of the Annona muricata L. Plant. South Asian Res. J. Agric. Fish. 2023, 5, 23–27. [Google Scholar] [CrossRef]
- Dey, P.; Kundu, A.; Kumar, A.; Gupta, M.; Lee, B.M.; Bhakta, T.; Dash, S.; Kim, H.S. Analysis of Alkaloids (Indole Alkaloids, Isoquinoline Alkaloids, Tropane Alkaloids). In Recent Advances in Natural Products Analysis; Elsevier: Amsterdam, The Netherlands, 2020; pp. 505–567. [Google Scholar] [CrossRef]
- Letchuman, S.; Madhuranga, H.D.T.; Madhurangi, B.L.N.K.; Premarathna, A.D.; Saravanan, M. Alkaloids Unveiled: A Comprehensive Analysis of Novel Therapeutic Properties, Mechanisms, and Plant-Based Innovations. Intell. Pharm. 2024, in press. [Google Scholar] [CrossRef]
- Edo, G.I.; Mafe, A.N.; Ali, A.B.M.; Akpoghelie, P.O.; Yousif, E.; Isoje, E.F.; Igbuku, U.A.; Zainulabdeen, K.; Owheruo, J.O.; Essaghah, A.E.A.; et al. Eco-Friendly Nanoparticle Phytosynthesis via Plant Extracts: Mechanistic Insights, Recent Advances, and Multifaceted Uses. Nano TransMed 2025, 4, 100080. [Google Scholar] [CrossRef]
- Al Kazman, B.S.M.; Harnett, J.E.; Hanrahan, J.R. Identification of Annonaceous Acetogenins and Alkaloids from the Leaves, Pulp, and Seeds of Annona Atemoya. Int. J. Mol. Sci. 2023, 24, 2294. [Google Scholar] [CrossRef]
- Pinto, N.C.C.; Silva, J.B.; Menegati, L.M.; Guedes, M.C.M.R.; Marques, L.B.; Silva, T.P.D.; Melo, R.C.N.D.; Souza-Fagundes, E.M.D.; Salvador, M.J.; Scio, E.; et al. Cytotoxicity and Bacterial Membrane Destabilization Induced by Annona squamosa L. Extracts. An. Acad. Bras. Ciênc. 2017, 89, 2053–2073. [Google Scholar] [CrossRef]
- De-la-Cruz-Chacón, I.; Riley-Saldaña, C.A.; Arrollo-Gómez, S.; Sancristóbal-Domínguez, T.J.; Castro-Moreno, M.; González-Esquinca, A.R. Spatio-Temporal Variation of Alkaloids in Annona Purpurea and the Associated Influence on Their Antifungal Activity. Chem. Biodivers. 2019, 16, e1800284. [Google Scholar] [CrossRef] [PubMed]
- Masyita, A.; Mustika Sari, R.; Dwi Astuti, A.; Yasir, B.; Rahma Rumata, N.; Emran, T.B.; Nainu, F.; Simal-Gandara, J. Terpenes and Terpenoids as Main Bioactive Compounds of Essential Oils, Their Roles in Human Health and Potential Application as Natural Food Preservatives. Food Chem. X 2022, 13, 100217. [Google Scholar] [CrossRef] [PubMed]
- Thang, T.D.; Dai, D.N.; Hoi, T.M.; Ogunwande, I.A. Study on the Volatile Oil Contents of Annona glabra L., Annona squamosa L., Annona muricata L. and Annona reticulata L., from Vietnam. Nat. Prod. Res. 2013, 27, 1232–1236. [Google Scholar] [CrossRef]
- Okoye, T.C.; Akah, P.A.; Okoli, C.O.; Ezike, A.C.; Omeje, E.O.; Odoh, U.E. Antimicrobial Effects of a Lipophilic Fraction and Kaurenoic Acid Isolated from the Root Bark Extracts of Annona Senegalensis. Evid.-Based Complement. Altern. Med. ECAM 2012, 2012, 831327. [Google Scholar] [CrossRef]
- De Souza Araújo, M.; Araújo Da Silva, F.M.; Ferreira Koolen, H.H.; Costa, E.V. Isoquinoline-Derived Alkaloids from the Bark of Guatteria Olivacea (Annonaceae). Biochem. Syst. Ecol. 2020, 92, 104105. [Google Scholar] [CrossRef]
- Nardelli, V.B.; Silva De Souza, C.A.; Da Silva Chaar, J.; Ferreira Koolen, H.H.; Araújo Da Silva, F.M.; Costa, E.V. Isoquinoline-derived alkaloids and one terpene lactone from the leaves of Duguetia pycnastera (Annonaceae). Biochem. Syst. Ecol. 2021, 94, 104206. [Google Scholar] [CrossRef]
- Haykal, T.; Nasr, P.; Hodroj, M.H.; Taleb, R.I.; Sarkis, R.; Moujabber, M.N.E.; Rizk, S. Annona Cherimola Seed Extract Activates Extrinsic and Intrinsic Apoptotic Pathways in Leukemic Cells. Toxins 2019, 11, 506. [Google Scholar] [CrossRef]
- Khan, M.F.; Khan, M.A. Plant-Derived Metal Nanoparticles (PDMNPs): Synthesis, Characterization, and Oxidative Stress-Mediated Therapeutic Actions. Future Pharmacol. 2023, 3, 252–295. [Google Scholar] [CrossRef]
- Garg, D.; Sarkar, A.; Chand, P.; Bansal, P.; Gola, D.; Sharma, S.; Khantwal, S.; Surabhi; Mehrotra, R.; Chauhan, N.; et al. Synthesis of Silver Nanoparticles Utilizing Various Biological Systems: Mechanisms and Applications—A Review. Prog. Biomater. 2020, 9, 81–95. [Google Scholar] [CrossRef] [PubMed]
- da Silva Avelino Oliveira Rocha, G.N.; Dutra, L.M.; Paz, W.H.P.; da Silva, F.M.A.; Costa, E.V.; da Silva Almeida, J.R.G. Chemical Constituents from the Leaves and Branches of Annona Coriacea Mart. (Annonaceae). Biochem. Syst. Ecol. 2021, 97, 104297. [Google Scholar] [CrossRef]
- Santos, T.C.B.; De Oliveira, R.C.; De Vasconcelos, L.G.; Sousa, P.T.D.; Silva, V.C.P.; De Carvalho, M.G.; Ribeiro, T.A.N. Chemical Constituents from Roots of Duguetia furfuracea (A. St.-Hil.) Saff. (Annonaceae). Biochem. Syst. Ecol. 2019, 87, 103951. [Google Scholar] [CrossRef]
- Amarasinghe, L.D.; Wickramarachchi, P.A.S.R.; Aberathna, A.A.A.U.; Sithara, W.S.; De Silva, C.R. Comparative Study on Larvicidal Activity of Green Synthesized Silver Nanoparticles and Annona glabra (Annonaceae) Aqueous Extract to Control Aedes Aegypti and Aedes Albopictus (Diptera: Culicidae). Heliyon 2020, 6, e04322. [Google Scholar] [CrossRef]
- Assunção, D.P.D.S.F.D.; Justus, B.; Toledo, A.C.O.; Paula, J.D.F.P.D. Development, Characterization and Microbiological Evaluation of Silver Nanoparticles, Obtained by Green Synthesis, from the Extract of Annona muricata L. Braz. Arch. Biol. Technol. 2021, 64, e21200469. [Google Scholar] [CrossRef]
- Astirin, O.P.; Prayitno, A.; Artanti, A.N.; Herawati, E.; Aini Saad, A.N.; Firstlia, A.D. Single-Dose and Combined-Dose of Nanoparticles from Soursop Leaves (Annona muricata L.) and Sappan Wood (Caesalpinia sappan L.) Induced Apoptosis and Necrosis in HeLA Cells. Pharmacogn. J. 2021, 13, 1134–1142. [Google Scholar] [CrossRef]
- Durga, B.; Raziya, S.; Rajamahanti, S.G.; Govindh, B.; Raju, K.V.; Annapurna, N. Synthesis and Characterization of Cadmium Sulphide Nanoparticles Using Annona muricata Leaf Extract as Reducing/Capping Agent. Chem. Sci. Trans. 2016, 5, 1035–1041. [Google Scholar] [CrossRef]
- Fajriyah, N.N.; Rosyid, F.N.; Mugiyanto, E. Formulation and Evaluation of Nano-Zinc and Annona muricata Extract Particles Loaded Topical Gel. Indones. J. Pharm. 2024, 34, 291–301. [Google Scholar][Green Version]
- Soleman, D.M.; Eldahshan, O.A.; Ibrahim, M.H.; Ogaly, H.A.; Galal, H.M.; Batiha, G.E.-S.; Elkousy, R.H. GC/MS Analysis, Cytotoxicity, and Antiviral Activities of Annona glabra Hexane Extract Supported by In Silico Study. Molecules 2023, 28, 1628. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Hu, C.; Shao, L. The Antimicrobial Activity of Nanoparticles: Present Situation and Prospects for the Future. Int. J. Nanomed. 2017, 12, 1227–1249. [Google Scholar] [CrossRef] [PubMed]
- Badmus, J.A.; Oyemomi, S.A.; Adedosu, O.T.; Yekeen, T.A.; Azeez, M.A.; Adebayo, E.A.; Lateef, A.; Badeggi, U.M.; Botha, S.; Hussein, A.A.; et al. Photo-Assisted Bio-Fabrication of Silver Nanoparticles Using Annona muricata Leaf Extract: Exploring the Antioxidant, Anti-Diabetic, Antimicrobial, and Cytotoxic Activities. Heliyon 2020, 6, e05413. [Google Scholar] [CrossRef]
- Sebastiammal, S.; Mariappan, A.; Neyvasagam, K.; Fathima, A.L. Annona Muricata Inspired Synthesis of CeO2 Nanoparticles and Their Antimicrobial Activity. Mater. Today Proc. 2019, 9, 627–632. [Google Scholar] [CrossRef]
- Rajan, A.R.; Vilas, V.; Rajan, A.; John, A.; Philip, D. Synthesis of CeO2 Nanostructures with Its Exceptional Biological and Chemocatalytic Activities: A Comparative Study. Bull. Mater. Sci. 2021, 44, 16. [Google Scholar] [CrossRef]
- Maesaroh, U.; Martien, R.; Dono, N.D.; Zuprizal. Antibacterial Activity and Characterization of Annona muricata Linn Leaf Extract-Nanoparticles against Escherichia Coli FNCC-0091 and Salmonella Typhimurium FNCC-0050. IOP Conf. Ser. Earth Environ. Sci. 2019, 387, 012055. [Google Scholar] [CrossRef]
- Fadholly, A.; Ansori, A.N.M.; Proboningrat, A.; Nugraha, A.P.; Iskandar, R.P.D.; Rantam, F.A.; Sudjarwo, S.A. Apoptosis of HeLa Cells via Caspase-3 Expression Induced by Chitosan-Based Nanoparticles of Annona squamosa Leaf Extract: In Vitro Study. Indian J. Pharm. Educ. Res. 2020, 54, 416–421. [Google Scholar] [CrossRef]
- Sudjarwo, S.; Fadholly, A.; Proboningrat, A.; Dewi Iskandar, R.; Rantam, F. In Vitro Anticancer Activity Annona squamosa Extract Nanoparticle on WiDr Cells. J. Adv. Pharm. Technol. Res. 2019, 10, 149. [Google Scholar] [CrossRef]
- Vindhya, P.S.; Suresh, S.; Kunjikannan, R.; Kavitha, V.T. Antimicrobial, Antioxidant, Cytotoxicity and Photocatalytic Performance of Co Doped ZnO Nanoparticles Biosynthesized Using Annona muricata Leaf Extract. J. Environ. Health Sci. Eng. 2023, 21, 167–185. [Google Scholar] [CrossRef]
- Maulana, I.; Ginting, B.; Azizah, K. Green Synthesis of Copper Nanoparticles Employing Annona squamosa L. Extract as Antimicrobial and Anticancer Agents. S. Afr. J. Chem. Eng. 2023, 46, 65–71. [Google Scholar] [CrossRef]
- Vivekanandhan, P.; Swathy, K.; Thomas, A.; Krutmuang, P.; Kweka, E.J. Green Copper Nano-Pesticide Synthesized by Using Annona squamosa L., Seed and Their Efficacy on Insect Pest as Well as Non-Target Species. Int. J. Plant Anim. Environ. Sci. 2021, 11, 456–473. [Google Scholar] [CrossRef]
- Mahmood, R.I.; Kadhim, A.A.; Ibraheem, S.; Albukhaty, S.; Mohammed-Salih, H.S.; Abbas, R.H.; Jabir, M.S.; Mohammed, M.K.A.; Nayef, U.M.; AlMalki, F.A.; et al. Biosynthesis of Copper Oxide Nanoparticles Mediated Annona muricata as Cytotoxic and Apoptosis Inducer Factor in Breast Cancer Cell Lines. Sci. Rep. 2022, 12, 16165. [Google Scholar] [CrossRef]
- Chauhan, N.; Singh, A.; Singhal, D.; Johri, S. Characterisation of Copper Oxide Nano Particles Using Annona reticulata Extract and Their Antioxidant and Photocatalytic Activities. Int. J. Chem. Pharm. Sci 2019, 10, 14–19. [Google Scholar]
- Singh, P.; Singh, K.R.; Singh, J.; Das, S.N.; Singh, R.P. Tunable Electrochemistry and Efficient Antibacterial Activity of Plant-Mediated Copper Oxide Nanoparticles Synthesized by Annona squamosa Seed Extract for Agricultural Utility. RSC Adv. 2021, 11, 18050–18060. [Google Scholar] [CrossRef] [PubMed]
- Hussein, A.A.A.; Abd El-latif, M.B.; Saad El-Din, M.I.; El-Shenawy, N.S.; Hammam, O.; Ibrahim, A.M. The Molluscicidal Activity of Green Synthesized Copper Oxide–Based Annona squamosa Seed Extract Nanoparticles on the Feeding Behavior, Biochemical, Molecular, and Immunohistochemical Alterations of Biomphalaria Alexandrina Snails. Biol. Trace Elem. Res. 2024, 202, 2327–2337. [Google Scholar] [CrossRef] [PubMed]
- Kayalvizhi, S.; Selvam, K.; Sudhakar, C.; Thangaswamy, S.; Al-Ansari, M.; Al-Humaid, L.; Selvakumar, V. Biofabrication of Copper Oxide Nanoparticles@graphene Oxide Nanocomposite Using Annona muricata Leaf Extract and Its Antibacterial and Photocatalytic activity. Appl. Nanosci. 2021, 13, 1601–1609. [Google Scholar] [CrossRef]
- Folorunso, A.; Akintelu, S.; Oyebamiji, A.K.; Ajayi, S.; Abiola, B.; Abdusalam, I.; Morakinyo, A. Biosynthesis, Characterization and Antimicrobial Activity of Gold Nanoparticles from Leaf Extracts of Annona Muricata. J. Nanostruct. Chem. 2019, 9, 111–117. [Google Scholar] [CrossRef]
- Priya, M.R.K.; Iyer, P.R. Antiproliferative Effects on Tumor Cells of the Synthesized Gold Nanoparticles against Hep2 Liver Cancer Cell Line. Egypt. Liver J. 2020, 10, 15. [Google Scholar] [CrossRef]
- Imran, M.; Husseini, G.; Awad, N.; Paul, V.; M El-Haj, B.; Saad Ali, H. An Effective Anticancer Nano-Approach for Melanoma and Breast Cancers Using Annona Muricate Gold Nanoparticles. Acta Sci. Pharm. Sci. 2021, 5, 46–54. [Google Scholar] [CrossRef]
- Athithan, A.S.S.; Jeyasundari, J.; Renuga, D.; Jacob, Y.B.A. Annona muricata fruit mediated biosynthesis, physicochemical characterization of magnetite (fe3o4) nanoparticles and assessment of its in vitro antidiabetic activity. Rasayan J. Chem. 2020, 13, 1759–1766. [Google Scholar] [CrossRef]
- Sharma, S.K.; Khan, A.U.; Khan, M.; Gupta, M.; Gehlot, A.; Park, S.; Alam, M. Biosynthesis of MgO Nanoparticles Using Annona squamosa Seeds and Its Catalytic Activity and Antibacterial Screening. Micro Nano Lett. 2020, 15, 30–34. [Google Scholar] [CrossRef]
- Mamuru, S.A.; Okoye, C.; Eseyin, A.E.; Dalen, M.B. Antimicrobial evaluation of Annona muricata seed mediated synthesized manganese oxide nanoparticles. FUW Trends Sci. Technol. J. 2020, 5, 952–955. [Google Scholar]
- Anila, P.A.; Keerthiga, B.; Ramesh, M.; Muralisankar, T. Synthesis and Characterization of Palladium Nanoparticles by Chemical and Green Methods: A Comparative Study on Hepatic Toxicity Using Zebrafish as an Animal Model. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2021, 244, 108979. [Google Scholar] [CrossRef] [PubMed]
- Köksal, R.; Yalcin, S. The Cytotoxic Effect of Annona Muricata-Loaded PHB-Coated Magnetic Nanoparticles on Cancer Cell Lines and Molecular Docking Analyses. Curr. Pharmacol. Rep. 2020, 6, 121–130. [Google Scholar] [CrossRef]
- Velidandi, A.; Sarvepalli, M.; Pabbathi, N.P.P.; Baadhe, R.R. Biogenic Synthesis of Novel Platinum-Palladium Bimetallic Nanoparticles from Aqueous Annona muricata Leaf Extract for Catalytic Activity. 3 Biotech 2021, 11, 385. [Google Scholar] [CrossRef]
- Sarkar, S.; Bhowal, A.C.; Kandimalla, R.; Kundu, S. Structural and Electrical Behaviours of PEDOT:PSS Thin Films in Presence of Negatively Charged Gold and Silver Nanoparticles: A Green Synthesis Approach. Synth. Met. 2021, 279, 116848. [Google Scholar] [CrossRef]
- Srinivasan, A.; Ramani, P.; Kumar, R.; Ramalingam, K. Antimicrobial Activity of Zinc Oxide Nanoparticles Synthesized Using Annona muricata Leaves Extract-An Invitro Study. J. Pharm. Negat. Results 2022, 13. [Google Scholar] [CrossRef]
- Arthi, S.; Rajeshkumar; Ramani, P. Green Synthesis of Selenium Nanoparticles Using Annona muricata Fruit Extract and Its Free Radical Scavenging Activities. J. Popul. Ther. Clin. Pharmacol. 2023, 30, 260–265. [Google Scholar] [CrossRef]
- Arthi, S.; Ramani, P.; Rajeshkumar, S. Green Synthesis of Annona muricata Mediated Selenium Nanoparticles and Its Antifungal Activity against Candida Albicans. J. Popul. Ther. Clin. Pharmacol. 2023, 30, 282–287. [Google Scholar] [CrossRef]
- Rao, T.C.K.; Rosaiah, G.; Mangamuri, U.K.; Sikharam, A.S.; Devaraj, K.; Kalagatur, N.K.; Kadirvelu, K. Biosynthesis of Selenium Nanoparticles from Annona muricata Fruit Aqueous Extract and Investigation of Their Antioxidant and Antimicrobial Potentials. Curr. Trends Biotechnol. Pharm. 2022, 16, 101–107. [Google Scholar] [CrossRef]
- Solorzano-Toala, R.; Gonzalez-Mendoza, D.; Valdez-Salas, B.; Mendez-Trujillo, V.; Gutierrez-Miceli, F.; Beltran-Partida, E.; Tzintzun-Camacho, O. Green Synthesis of Silver Nanoparticles Using Annona Diversifolia Leaf Extract and Their Antimicrobial Application. J. Renew. Mater. 2020, 8, 1129–1137. [Google Scholar] [CrossRef]
- Mokhtar, F.A.; Selim, N.M.; Elhawary, S.S.; Abd El Hadi, S.R.; Hetta, M.H.; Albalawi, M.A.; Shati, A.A.; Alfaifi, M.Y.; Elbehairi, S.E.I.; Fahmy, L.I.; et al. Green Biosynthesis of Silver Nanoparticles Using Annona glabra and Annona squamosa Extracts with Antimicrobial, Anticancer, Apoptosis Potentials, Assisted by In Silico Modeling, and Metabolic Profiling. Pharmaceuticals 2022, 15, 1354. [Google Scholar] [CrossRef] [PubMed]
- Hamid, M.K.; Ghafoor, A.; Allawi, S.; Obaid, S.A. Characteristic and anticancer activity of silver nanoparticles of graviola (Annona) fruit juice as a reducing agent. Biochem. Cell. Arch. 2021, 21, 1003–1010. [Google Scholar]
- Sánchez-Navarro, M.D.C.; Ruiz-Torres, C.A.; Niño-Martínez, N.; Sánchez-Sánchez, R.; Martínez-Castañón, G.A.; DeAlba-Montero, I.; Ruiz, F. Cytotoxic and Bactericidal Effect of Silver Nanoparticles Obtained by Green Synthesis Method Using Annona muricata Aqueous Extract and Functionalized with 5-Fluorouracil. Bioinorg. Chem. Appl. 2018, 2018, 6506381. [Google Scholar] [CrossRef] [PubMed]
- Ezealisiji, K.M.; Noundou, X.S.; Ukwueze, S.E. Green Synthesis and Characterization of Monodispersed Silver Nanoparticles Using Root Bark Aqueous Extract of Annona muricata Linn and Their Antimicrobial Activity. Appl. Nanosci. 2017, 7, 905–911. [Google Scholar] [CrossRef]
- Reeba, C.V.; Helen, D.S.M. DNA Cleavage and Molecular Docking Studies Using Green Synthesized Annona muricata Silver Nanoparticles. Int. J. Aquat. Sci. 2021, 12, 2254–2259. [Google Scholar]
- Santhosh, S.B.; Chandrasekar, M.J.N.; Kaviarasan, L.; Deepak, P.; Silambarasan, T.; Gayathri, B.; Natarajan, D. Chemical Composition, Antibacterial, Anti-Oxidant and Cytotoxic Properties of Green Synthesized Silver Nanoparticles from Annona muricata L. (Annonaceae). Res. J. Pharm. Technol. 2020, 13, 33. [Google Scholar] [CrossRef]
- Santhosh, S.B.; Ragavendran, C.; Natarajan, D. Spectral and HRTEM Analyses of Annona muricata Leaf Extract Mediated Silver Nanoparticles and Its Larvicidal Efficacy against Three Mosquito Vectors Anopheles Stephensi, Culex Quinquefasciatus, and Aedes Aegypti. J. Photochem. Photobiol. B 2015, 153, 184–190. [Google Scholar] [CrossRef]
- Jabir, M.S.; Saleh, Y.M.; Sulaiman, G.M.; Yaseen, N.Y.; Sahib, U.I.; Dewir, Y.H.; Alwahibi, M.S.; Soliman, D.A. Green Synthesis of Silver Nanoparticles Using Annona muricata Extract as an Inducer of Apoptosis in Cancer Cells and Inhibitor for NLRP3 Inflammasome via Enhanced Autophagy. Nanomaterials 2021, 11, 384. [Google Scholar] [CrossRef]
- Gavamukulya, Y.; Maina, E.N.; Meroka, A.M.; Madivoli, E.S.; El-Shemy, H.A.; Wamunyokoli, F.; Magoma, G. Green Synthesis and Characterization of Highly Stable Silver Nanoparticles from Ethanolic Extracts of Fruits of Annona Muricata. J. Inorg. Organomet. Polym. Mater. 2020, 30, 1231–1242. [Google Scholar] [CrossRef]
- Gavamukulya, Y.; Maina, E.N.; El-Shemy, H.A.; Meroka, A.M.; Kangogo, G.K.; Magoma, G.; Wamunyokoli, F. Annona muricata Silver Nanoparticles Exhibit Strong Anticancer Activities against Cervical and Prostate Adenocarcinomas through Regulation of CASP9 and the CXCL1/CXCR2 Genes Axis. Tumor Biol. 2021, 43, 37–55. [Google Scholar] [CrossRef] [PubMed]
- Shaniba, V.S.; Aziz, A.A.; Joseph, J.; Jayasree, P.R.; Manish Kumar, P.R. Synthesis, Characterization and Evaluation of Antioxidant and Cytotoxic Potential of Annona muricata Root Extract-Derived Biogenic Silver Nanoparticles. J. Clust. Sci. 2022, 33, 467–483. [Google Scholar] [CrossRef]
- Ezeamalu, S.A.; Aleke, P.C.; Uzor, P.F. Antimicrobial Evaluation and Characterization of Green Synthesized Silver Nanoparticles Using Annona muricata L. Leaf Extract. South Asian Res. J. Nat. Prod. 2023, 6, 64–73. [Google Scholar]
- González-Pedroza, M.; Argueta-Figueroa, L.; García-Contreras, R.; Jiménez-Martínez, Y.; Martínez-Martínez, E.; Navarro-Marchal, S.; Marchal, J.; Morales-Luckie, R.; Boulaiz, H. Silver Nanoparticles from Annona muricata Peel and Leaf Extracts as a Potential Potent, Biocompatible and Low-Cost Antitumor Tool. Nanomaterials 2021, 11, 1273. [Google Scholar] [CrossRef]
- Samrot, A.V.; Silky, V.I.C.; Raji, P.; SaiPriya, C.; Selvarani, J.A. Bioactivity Studies of Datura Metel, Aegle Marmelos, Annona reticulata and Saraca Indica and Their Green Synthesized Silver Nanoparticle. J. Pure Appl. Microbiol. 2019, 13, 329–338. [Google Scholar] [CrossRef]
- Kolekar, Y.; Tamboli, F.; Gaikwad, D.; Memon, S.; Gulavani, S.; Alaskar, K.; Mali, D.; Pawar, V. Biosynthesis of Silver Nanoparticles Using Annona squamosa L. Seed and Leaves Extract: Evaluation of the Anti-Inflammatory, Antifungal, and Antibacterial Potency. Int. J. Pharm. Qual. Assur. 2023, 14, 377–387. [Google Scholar] [CrossRef]
- Khader, S.Z.A.; Syed Zameer Ahmed, S.; Sathyan, J.; Mahboob, M.R.; Venkatesh, K.P.; Ramesh, K. A Comparative Study on Larvicidal Potential of Selected Medicinal Plants over Green Synthesized Silver Nano Particles. Egypt. J. Basic Appl. Sci. 2018, 5, 54–62. [Google Scholar] [CrossRef]
- Hassan, A.-M.S.; Mahmoud, A.S.; Ramadan, M.F.; Eissa, M.A. Microwave-Assisted Green Synthesis of Silver Nanoparticles Using Annona squamosa Peels Extract: Characterization, Antioxidant, and Amylase Inhibition Activities. Rendiconti Lincei Sci. Fis. E Nat. 2022, 33, 83–91. [Google Scholar] [CrossRef]
- Vivek, R.; Thangam, R.; Muthuchelian, K.; Gunasekaran, P.; Kaveri, K.; Kannan, S. Green Biosynthesis of Silver Nanoparticles from Annona squamosa Leaf Extract and Its In Vitro Cytotoxic Effect on MCF-7 Cells. Process Biochem. 2012, 47, 2405–2410. [Google Scholar] [CrossRef]
- Jagtap, U.B.; Bapat, V.A. Biosynthesis, Characterization and Antibacterial Activity of Silver Nanoparticles by Aqueous Annona squamosa L. Leaf Extract at Room Temperature. J. Plant Biochem. Biotechnol. 2013, 22, 434–440. [Google Scholar] [CrossRef]
- Malik, M.; Iqbal, M.A.; Malik, M.; Raza, M.A.; Shahid, W.; Choi, J.R.; Pham, P.V. Biosynthesis and Characterizations of Silver Nanoparticles from Annona squamosa Leaf and Fruit Extracts for Size-Dependent Biomedical Applications. Nanomaterials 2022, 12, 616. [Google Scholar] [CrossRef]
- Jose, V.; Raphel, L.; Aiswariya, K.S.; Mathew, P. Green Synthesis of Silver Nanoparticles Using Annona squamosa L. Seed Extract: Characterization, Photocatalytic and Biological Activity Assay. Bioprocess Biosyst. Eng. 2021, 44, 1819–1829. [Google Scholar] [CrossRef]
- Va, M.; Ramesh, S.; Muthukrishnan, L. Facile Fabrication of Annona squamosa L. Seed Extract Mediated Silver Nanoparticles Challenged against Biofilm Forming Oral Pathogens. Plant Nano Biol. 2023, 3, 100023. [Google Scholar] [CrossRef]
- Aziz, A.A.; Shaniba, V.S.; Jayasree, P.R.; Manish Kumar, P.R. Physico-Chemical, Photocatalytic and Cytotoxicity Evaluation of Annona muricata L. Fruit Extract Derived Zinc Oxide Nanoparticles in Comparison to the Commercial Chemical Version. Curr. Sci. 2019, 117, 1492–1504. [Google Scholar] [CrossRef]
- Selvam, K.; Sudhakar, C.; Selvankumar, T.; Senthilkumar, B.; Kim, W.; Al-Ansari, M.M.; Al-Humaid, L. Photocatalytic Degradation of Malachite Green and Antibacterial Potential of Biomimetic-Synthesized Zirconium Oxide Nanoparticles Using Annona reticulata Leaf Extract. Appl. Nanosci. 2023, 13, 2837–2843. [Google Scholar] [CrossRef]
- Gomes, J.V.D.; Borges, A.S.; Athaydes, B.R.; Gomes-Copeland, K.K.P.; Silveira, D.; Magalhães, P.O.; Gonçalves, R.D.C.R.; Kitagawa, R.R.; Fonseca-Bazzo, Y.M.; Jamal, C.M. Anti-Helicobacter Pylori Potential, Antioxidant Capacity, and Anti-Inflammatory Activity of Xylopia sericea A. St.-Hil. (Annonaceae) Leaves. Phytomed. Plus 2022, 2, 100214. [Google Scholar] [CrossRef]
- Baran, M.F.; Keskin, C.; Baran, A.; Kurt, K.; İpek, P.; Eftekhari, A.; Khalilov, R.; Fridunbayov, I.; Cho, W.C. Green Synthesis and Characterization of Selenium Nanoparticles (Se NPs) from the Skin (Testa) of Pistacia vera L. (Siirt Pistachio) and Investigation of Antimicrobial and Anticancer Potentials. Biomass Convers. Biorefinery 2024, 14, 23623–23633. [Google Scholar] [CrossRef]
- Sawant, S.S.; Gabhe, S.Y.; Singh, K.K. In Vitro Effect on Plasmodium Falciparum and In Vivo Effect on Plasmodium Berghei of Annomaal, an Oily Fraction Obtained from the Seeds of Annona squamosa. Molecules 2023, 28, 5472. [Google Scholar] [CrossRef]
- Brígido, H.P.C.; Correa-Barbosa, J.; Da Silva-Silva, J.V.; Costa, E.V.S.; Percário, S.; Dolabela, M.F. Antileishmanial Activity of Annona Species (Annonaceae). SN Appl. Sci. 2020, 2, 1524. [Google Scholar] [CrossRef]
- Brito, I.A.; Thevenard, F.; Costa-Silva, T.A.; Oliveira, S.S.; Cunha, R.L.O.R.; De Oliveira, E.A.; Sartorelli, P.; Guadagnin, R.C.; Romanelli, M.M.; Tempone, A.G.; et al. Antileishmanial Effects of Acetylene Acetogenins from Seeds of Porcelia macrocarpa (Warm.) R.E. Fries (Annonaceae) and Semisynthetic Derivatives. Molecules 2022, 27, 893. [Google Scholar] [CrossRef] [PubMed]
- Avan, E.D.; Ngozi, P.O.; Ojowu, J.O.; Faluyi, E. Modulation of Mitochondrial Membrane Permeability Transition Pore by Annonaceous Acetogenins (ACGs)-Loaded Transferrin Conjugated Nanoparticles. J. Biosci. Biotechnol. Discov. 2023, 8, 81–91. [Google Scholar] [CrossRef]
- Siegel, R.L.; Miller, K.D.; Wagle, N.S.; Jemal, A. Cancer statistics, 2023. CA A Cancer J. Clin. 2023, 73, 17–48. [Google Scholar] [CrossRef]
- Anjum, S.; Hashim, M.; Imran, M.; Babur, S.; Adnan, S.; Hano, C.; Ibrahim, W.N. Selenium Nanoparticles in Cancer Therapy: Unveiling Cytotoxic Mechanisms and Therapeutic Potential. Cancer Rep. 2025, 8, e70210. [Google Scholar] [CrossRef]
- Spyridopoulou, K.; Aindelis, G.; Pappa, A.; Chlichlia, K. Anticancer Activity of Biogenic Selenium Nanoparticles: Apoptotic and Immunogenic Cell Death Markers in Colon Cancer Cells. Cancers 2021, 13, 5335. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gutiérrez-Pinzón, Y.; Martínez-Preciado, A.H.; Velázquez-López, J.M.; Pech-Jiménez, C.; Zúñiga-Mayo, V.M.; Guevara-Martínez, S.J.; Velázquez-Juárez, G. Antimicrobial Potential of Nanomaterials Synthesized with Extracts from Annona Plants: A Review. Antibiotics 2025, 14, 748. https://doi.org/10.3390/antibiotics14080748
Gutiérrez-Pinzón Y, Martínez-Preciado AH, Velázquez-López JM, Pech-Jiménez C, Zúñiga-Mayo VM, Guevara-Martínez SJ, Velázquez-Juárez G. Antimicrobial Potential of Nanomaterials Synthesized with Extracts from Annona Plants: A Review. Antibiotics. 2025; 14(8):748. https://doi.org/10.3390/antibiotics14080748
Chicago/Turabian StyleGutiérrez-Pinzón, Yared, Alma Hortensia Martínez-Preciado, José Miguel Velázquez-López, Cristina Pech-Jiménez, Víctor Manuel Zúñiga-Mayo, Santiago José Guevara-Martínez, and Gilberto Velázquez-Juárez. 2025. "Antimicrobial Potential of Nanomaterials Synthesized with Extracts from Annona Plants: A Review" Antibiotics 14, no. 8: 748. https://doi.org/10.3390/antibiotics14080748
APA StyleGutiérrez-Pinzón, Y., Martínez-Preciado, A. H., Velázquez-López, J. M., Pech-Jiménez, C., Zúñiga-Mayo, V. M., Guevara-Martínez, S. J., & Velázquez-Juárez, G. (2025). Antimicrobial Potential of Nanomaterials Synthesized with Extracts from Annona Plants: A Review. Antibiotics, 14(8), 748. https://doi.org/10.3390/antibiotics14080748









