Multifaceted Biological Properties of Verbascoside/Acteoside: Antimicrobial, Cytotoxic, Anti-Inflammatory, and Immunomodulatory Effects
Abstract
1. Introduction
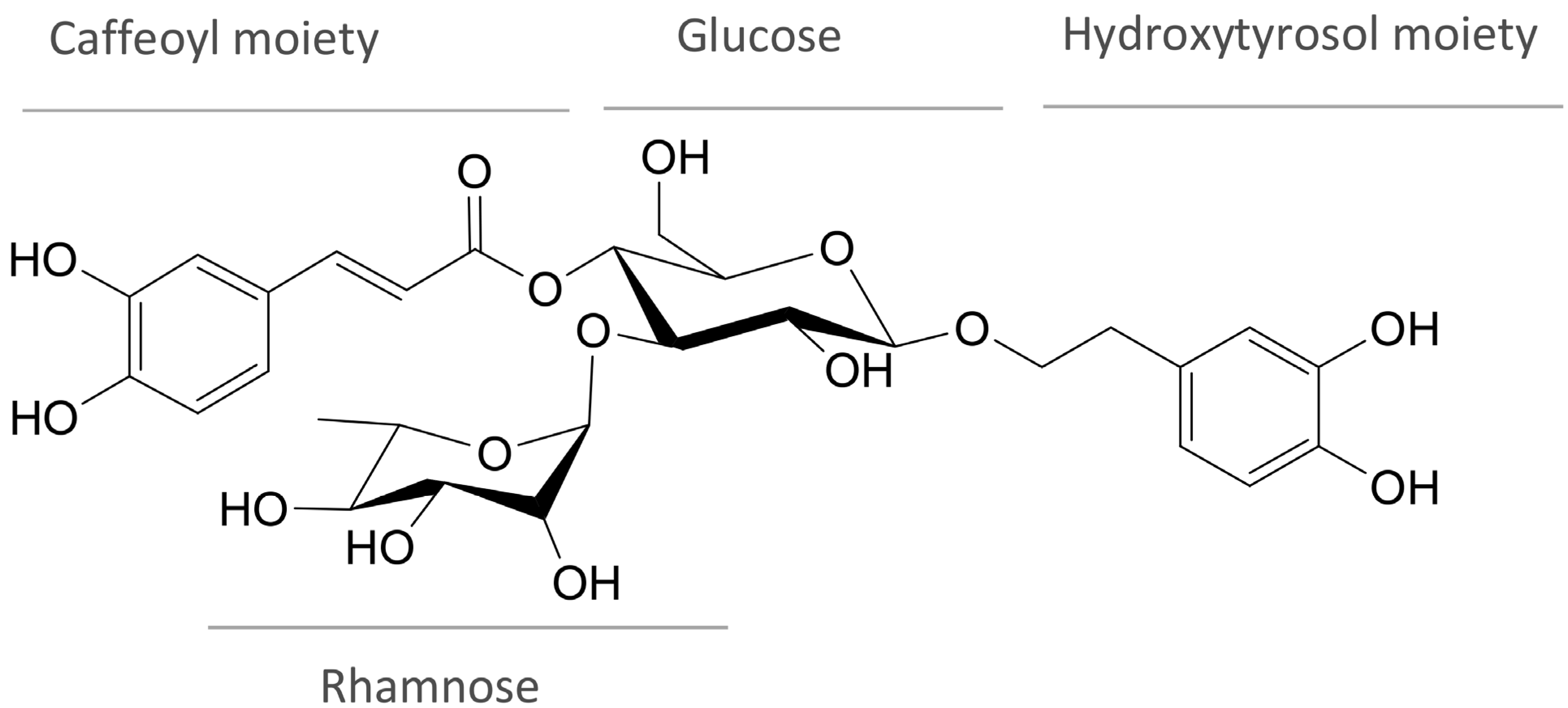
2. Chemistry and Occurrence of Verbascoside
3. Biosynthesis of Verbacoside
4. Cytotoxicity of Verbacoside
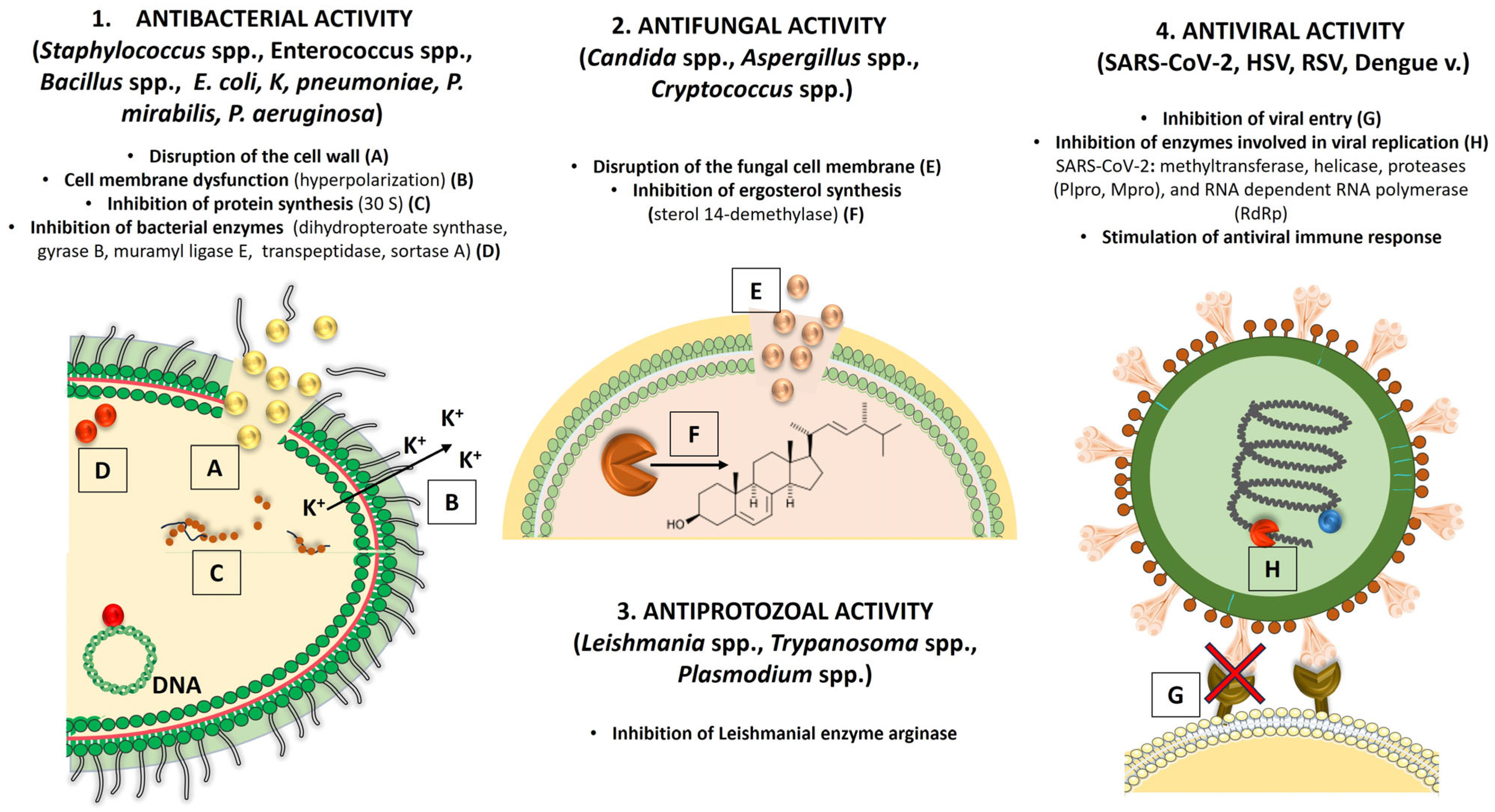
5. Antimicrobial Activity of Verbacoside
5.1. Antibacterial Activity
5.2. Antifungal and Antiprotozoal Activity
5.2.1. Antifungal Activity
5.2.2. Antiprotozoal Activity
5.3. Antiviral Activity
6. Anti-Inflammatory and Immunomodulatory Effects of Verbacoside
7. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Alipieva, K.; Korkina, L.; Orhan, I.E.; Georgiev, M.I. Verbascoside—A review of its occurrence, (bio)synthesis and pharmacological significance. Biotechnol. Adv. 2014, 32, 1065–1076. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Y.; Ren, Q.; Wu, L. The pharmacokinetic property and pharmacological activity of acteoside: A review. Biomed. Pharmacother. 2022, 153, 113296. [Google Scholar] [CrossRef]
- Saha, R.; Majie, A.; Baidya, R.; Sarkar, B. Verbascoside: Comprehensive review of a phenylethanoid macromolecule and its journey from nature to bench. Inflammopharmacology 2024, 32, 2729–2751. [Google Scholar] [CrossRef] [PubMed]
- Jan, F.; Jan, B.; Akbar Dar, M.; Sofi, F.A.; Alsuwayni, B.M.; Afzal, S.; Fawzi, M. A Review on Traditional Uses, Phytochemistry, and Pharmacological Activities of Verbascum thapsus. In Edible Plants in Health and Diseases: Volume II: Phytochemical and Pharmacological Properties; Masoodi, M.H., Rehman, M.U., Eds.; Springer Nature: Singapore, 2022; pp. 483–500. [Google Scholar] [CrossRef]
- Pereira, A.M.; Hernandes, C.; Pereira, S.I.; Bertoni, B.W.; França, S.C.; Pereira, P.S.; Taleb-Contini, S.H. Evaluation of anticandidal and antioxidant activities of phenolic compounds from Pyrostegia venusta (Ker Gawl.) Miers. Chem. Biol. Interact. 2014, 224, 136–141. [Google Scholar] [CrossRef]
- Maquiaveli, C.C.; Lucon-Júnior, J.F.; Brogi, S.; Campiani, G.; Gemma, S.; Vieira, P.C.; Silva, E.R. Verbascoside Inhibits Promastigote Growth and Arginase Activity of Leishmania amazonensis. J. Nat. Prod. 2016, 79, 1459–1463. [Google Scholar] [CrossRef] [PubMed]
- Cheimonidi, C.; Samara, P.; Polychronopoulos, P.; Tsakiri, E.N.; Nikou, T.; Myrianthopoulos, V.; Sakellaropoulos, T.; Zoumpourlis, V.; Mikros, E.; Papassideri, I.; et al. Selective cytotoxicity of the herbal substance acteoside against tumor cells and its mechanistic insights. Redox. Biol. 2018, 16, 169–178. [Google Scholar] [CrossRef] [PubMed]
- Andary, C.; Wylde, R.; Laffite, C.; Privat, G.; Winternitz, F. Structures of verbascoside and orobanchoside, caffeic acid sugar esters from Orobanche rapum-genistae. Phytochemistry 1982, 21, 1123–1127. [Google Scholar] [CrossRef]
- Scarpati, M.L.; Delle Monache, F. Isolation from Verbascum sinuatum of Two New Glucosides, Verbascoside and Isoverbascoside. Ann. Chim. 1963, 53, 356–367. [Google Scholar]
- Birkofer, L.; Kaiser, C.; Thomas, U. Acteosid und neoacteosid: Zukerester aus Syringa vulgaris. Z. Naturforsch. B 1968, 23, 1051–1058. [Google Scholar] [CrossRef]
- Sakurai, A.; Kato, T. A New Glycoside, Kusaginin Isolated from Clerodendron trichotomum. Bull. Chem. Soc. Jpn. 1983, 56, 1573–1574. [Google Scholar] [CrossRef]
- Pereira, A.M.S.; Guimarães, C.C.; Pereira, S.I.V.; Crevelin, E.J.; Pinto, G.H.T.; Morel, L.J.F.; Bertoni, B.W.; França, S.C.; Taleb-Contini, S.H. Isolation and Identification of Phenylethanoid Glycosides from Aloysia polystachya and Its Activity as Inhibitors of Monoamine Oxidase-A. Planta Med. Int. Open 2019, 6, e1–e6. [Google Scholar] [CrossRef]
- Shahat, A.A.; Nazif, N.M.; Abousetta, L.M.; Ibrahim, N.A.; Cos, P.; Van Miert, S.; Pieters, L.; Vlietinck, A.J. Phytochemical investigation and antioxidant activity of Duranta repens. Phytother. Res. 2005, 19, 1071–1073. [Google Scholar] [CrossRef] [PubMed]
- Herbert, J.M.; Maffrand, J.P.; Taoubi, K.; Augereau, J.M.; Fouraste, I.; Gleye, J. Verbascoside isolated from Lantana camara, an inhibitor of protein kinase C. J. Nat. Prod. 1991, 54, 1595–1600. [Google Scholar] [CrossRef] [PubMed]
- Pereira, A.C.; Carvalho, H.W.P.; Silva, G.H.; Oliveira, D.F.; Figueiredo, H.C.P.; Cavalheiro, A.J.; Carvalho, D.A. Purification of an antibacterial compound from Lantana lilacina. Rev. Bras. Farmacogn. 2008, 18, 204–208. [Google Scholar] [CrossRef]
- Imbenzi, P.S.; He, Y.-Z.; Yan, Z.-X.; Osoro, E.K.; Cheplogoi, P.K. Chemical Constituents in Extracts from Leaves of Lantana trifolia and Their In Vitro Anti-oxidative Activity. Chin. Herb. Med. 2014, 6, 242–246. [Google Scholar] [CrossRef]
- Leitão, G.G.; Pinto, S.C.; Oliveira, D.R.; Timoteo, P.; Guimarães, M.G.; Cordova, W.H.P.; Leitão, S.G. Gradient x Isocratic Elution CCC on the Isolation of Verbascoside and Other Phenylethanoids: Influence of the Complexity of the Matrix. Planta Med. 2015, 81, 1609–1613. [Google Scholar] [CrossRef]
- Funes, L.; Laporta, O.; Cerdán-Calero, M.; Micol, V. Effects of verbascoside, a phenylpropanoid glycoside from lemon verbena, on phospholipid model membranes. Chem. Phys. Lipids 2010, 163, 190–199. [Google Scholar] [CrossRef]
- Kaneda, N.; Lee, I.S.; Gupta, M.P.; Soejarto, D.D.; Kinghorn, D. (+)-4β-Hydroxy-hernandulcin, a new sweet sesquiterpene from the leaves and flowers of Lippia dulcis. J. Nat. Prod. 1992, 55, 1136–1141. [Google Scholar] [CrossRef]
- Pham, H.C.; Koffi, Y.; Pham, H.C. Comparative effects on TXA2 biosynthesis of products extracted from Lippia multiflora Moldenke leaves. Prostaglandins leukot. 1988, 34, 83–88. [Google Scholar] [CrossRef]
- Cheng, L.-C.; Murugaiyah, V.; Chan, K.-L. In Vitro Xanthine Oxidase Inhibitory Studies of Lippia nodiflora and Isolated Flavonoids and Phenylethanoid Glycosides as Potential Uric Acid-Lowering Agents. Nat. Prod. Commun. 2015, 10, 945–948. [Google Scholar] [CrossRef]
- Nakamura, T.; Okuyama, E.; Tsukada, A.; Yamazaki, M.; Satake, M.; Nishibe, S.; Deyama, T.; Moriya, A.; Maruno, M.; Nishimura, H. Acteoside as the analgesic principle of Cedron (Lippia triphylla), a Peruvian medicinal plant. Chem. Pharm. Bull. 1997, 45, 499–504. [Google Scholar] [CrossRef]
- Singh, N.; Shukla, N.; Singh, P.; Sharma, R.; Rajendran, S.M.; Maurya, R.; Palit, G. Verbascoside isolated from Tectona grandis mediates gastric protection in rats via inhibiting proton pump activity. Fitoterapia 2010, 81, 755–761. [Google Scholar] [CrossRef] [PubMed]
- Delazar, A.; Delnavazi, M.-R.; Yassa, N.; Parkhideh, S.; Delazar, N.; Nahar, L.; Sarker, S.D. Essential oil composition and isolation of freeradical-scavenging phenolic glycosides from the aerial parts of Ajuga chamaepitys growing in Iran. Rev. Bras. Farmacogn. 2012, 22, 299–305. [Google Scholar] [CrossRef]
- Shimomura, H.; Sashida, Y.; Ogawa, K. Iridoid glucosides and phenylpropanoid glycosides in Ajuga species of Japan, Lamiaceae. Phytochemistry 1987, 26, 1981–1983. [Google Scholar] [CrossRef]
- Niu, C.; Li, Q.; Yang, L.-P.; Zhang, Z.-Z.; Zhang, W.-K.; Liu, Z.-Q.; Wang, J.-H.; Wang, Z.-H.; Wang, H. Phenylethanoid glycosides from Callicarpa macrophylla Vahl. Phytochem. Lett. 2020, 38, 65–69. [Google Scholar] [CrossRef]
- Wu, A.-Z.; Zhai, Y.-J.; Zhao, Z.-X.; Zhang, C.-X.; Lin, C.-Z.; Zhu, C.-C. Phenylethanoid glycosides from the stems of Callicarpa peii (hemostatic drug). Fitoterapia 2013, 84, 237–241. [Google Scholar] [CrossRef]
- Zhu, J.; Li, G.; Zhou, J.; Xu, Z.; Xu, J. Cytoprotective effects and antioxidant activities of acteoside and various extracts of Clerodendrum cyrtophyllum Turcz leaves against t-BHP induced oxidative damage. Sci. Rep. 2022, 12, 12630. [Google Scholar] [CrossRef]
- Uddin, M.J.; Çiçek, S.S.; Willer, J.; Shulha, O.; Abdalla, M.A.; Sönnichsen, F.; Girreser, U.; Zidorn, C. Phenylpropanoid and flavonoid glycosides from the leaves of Clerodendrum infortunatum (Lamiaceae). Biochem. Syst. Ecol. 2020, 92, 104131. [Google Scholar] [CrossRef]
- Fauvel, M.T.; Gleye, J.; Andary, C. Verbascoside: A Constituent of Clerodendrum inerme. Planta Med. 1989, 55, 57. [Google Scholar] [CrossRef]
- Viswanatha, G.L.; Shylaja, H.; Kishore, D.V.; Venkataranganna, M.V.; Prasad, N.B.L. Acteoside Isolated from Colebrookea oppositifolia Smith Attenuates Epilepsy in Mice Via Modulation of Gamma-Aminobutyric Acid Pathways. Neurotox. Res. 2020, 38, 1010–1023. [Google Scholar] [CrossRef]
- Lv, Y.; Wang, Z.; Wu, Q.; Fang, Y.; Wang, Q.; Li, G.; Dang, J. Preparation and Antioxidant Activities of Phenylethanoids from Dracocephalum heterophyllum. Separations 2022, 9, 111. [Google Scholar] [CrossRef]
- Yang, L.; Zeng, H.; Xia, X.; Wang, H.; Zhao, B.; He, J. Natural phenylethanoid glycosides diuretics derived from Lagopsis supina: Biological activity, mechanism, molecular docking, and structure-activity relationship. Bioorg Chem. 2022, 129, 106165. [Google Scholar] [CrossRef] [PubMed]
- Caliş, I.; Ersöz, T.; Taşdemir, D.; Rüedi, P. Two phenylpropanoid glycosides from Leonurus glaucescens. Phytochemistry 1992, 31, 357–359. [Google Scholar] [CrossRef]
- Martins, F.O.; Esteves, P.F.; Mendes, G.S.; Barbi, N.S.; Menezes, F.S.; Romanos, M.T.V. Verbascoside isolated from Lepechinia speciosa has inhibitory Activity against HSV-1 and HSV-2 in vitro. Nat. Prod. Commun. 2009, 4, 1693–1696. [Google Scholar] [CrossRef] [PubMed]
- Miyase, T.; Koizumi, A.; Ueno, A.; Noro, T.; Kuroyanagi, M.; Fukushima, S.; Akiyama, Y.; Takemoto, T. Studies on the acyl glycosides from Leucoseptrum japonicum (Miq.) Kitamura et Murata. Chem. Pharm. Bull. 1982, 30, 2732–2737. [Google Scholar] [CrossRef]
- Caliş, I.; Hosny, M.; Khalifa, T.; Rüedi, P. Phenylpropanoid glycosides from Marrubium alysson. Phytochemistry 1992, 31, 3624–3626. [Google Scholar] [CrossRef]
- Zaabat, N.; Hay, A.-E.; Michalet, S.; Darbour, N.; Bayet, C.; Skandrani, I.; Chekir-Ghedira, L.; Akkal, S.; Dijoux-Franca, M.-G. Antioxidant and antigenotoxic properties of compounds isolated from Marrubium deserti de Noé. Food Chem. Toxicol. 2011, 49, 3328–3335. [Google Scholar] [CrossRef]
- Argyropoulou, A.; Samara, P.; Tsitsilonis, O.; Skaltsa, H. Polar Constituents of Marrubium thessalum Boiss. & Heldr. (Lamiaceae) and their Cytotoxic/Cytostatic Activity. Phytother. Res. 2012, 26, 1800–1806. [Google Scholar] [CrossRef]
- Sahpaz, S.; Garbacki, N.; Tits, M.; Bailleul, F. Isolation and pharmacological activity of phenylpropanoid esters from Marrubium vulgare. J. Ethnopharmacol. 2002, 79, 389–392. [Google Scholar] [CrossRef]
- Akbay, P.; Calis, I.; Ündeger, Ü.; Basaran, N.; Basaran, A.A. In vitro Immunomodulatory Activity of Verbascoside from Nepeta ucrainica L. Phytother. Res. 2002, 16, 593–595. [Google Scholar] [CrossRef]
- Ersöz, T.; Schühly, W.; Popov, S.; Handjieva, N.; Sticher, O.; Çalýţ, Ý. Iridoid and phenylethanoid glycosides from Phlomis longifolia var. longifolia. Nat. Prod. Lett. 2001, 15, 345–351. [Google Scholar] [CrossRef] [PubMed]
- Yalçın, F.N.; Ersöz, T.; Bedir, E.; Dönmez, A.A.; Stavri, M.Z.; Gibbons, S.; Çalış, İ. Amanicadol, a pimarane type diterpene from Phlomis amanica Vierch. Z. Naturforsch. B 2006, 61b, 1433–1436. [Google Scholar] [CrossRef]
- Saracoglu, I.; Inoue, M.; Calis, I.; Ogihara, Y. Studies on constituents with cytotoxic and cytostatic activity of two Turkish medicinal plants Phlomis armeniaca and Scutellaria salviifolia. Biol. Pharm. Bull. 1995, 18, 1396–1400. [Google Scholar] [CrossRef]
- Ersöz, T.; Saracođlu, Ý.; Harput, U.S.; Çalýţ, Ý.; Dönmez, A.A. Iridoid and phenylpropanoid glycosides from Phlomis grandiflora var. fimbrilligera and Phlomis fruticosa. Turk. J. Chem. 2002, 26, 171–178. [Google Scholar]
- Saracoğlu, İ.; Varel, M.; Çalış, İ.; Dönmez, A.A. Neolignan, Flavonoid, Phenylethanoid and iridoid glycosides from Phlomis integrifolia. Turk. J. Chem. 2003, 27, 739–747. [Google Scholar]
- Caliş, I.; Kirmizibekmez, H. Glycosides from Phlomis lunariifolia. Phytochemistry 2004, 65, 2619–2625. [Google Scholar] [CrossRef]
- Ersöz, T.; Ivancheva, S.; Akbay, P.; Sticher, O.; Caliş, I. Iridoid and phenylethanoid glycosides from Phlomis tuberosa L. Z. Naturforsch. C J. Biosci. 2001, 56, 695–698. [Google Scholar] [CrossRef]
- Yalçın, F.N.; Ersöz, T.; Akbay, P.; Çalış, İ.; Dönmez, A.A.; Sticher, O. Iridoid and phenylpropanoid glycosides from Phlomis samia, P. monocephala and P. carica. Turk. J. Chem. 2003, 27, 295–305. [Google Scholar]
- Ersöz, T.; Alipieva, K.I.; Yalçın, F.N.; Akbay, P.; Handjieva, N.; Dönmez, A.A.; Popov, S.; Çalış, İ. Physocalycoside, a new phenylethanoid glycoside from Phlomis physocalyx Hub. Mor. Z. Naturforsch. C 2003, 58c, 471–476. [Google Scholar] [CrossRef]
- Çalış, İ.; Kırmızıbekmez, H.; Beutler, J.A.; Dönmez, A.A.; Yalçın, F.N.; Kılıç, E.; Özalp, M.; Taşdemir, D. Secondary metabolites from Phlomis viscosa and their biological activities. Turk. J. Chem. 2005, 29, 71–81. [Google Scholar]
- Rungsimakan, S.; Rowan, M.G. Terpenoids, flavonoids and caffeic acid derivatives from Salvia viridis L. cvar. Blue Jeans. Phytochemistry 2014, 108, 177–188. [Google Scholar] [CrossRef]
- To, D.C.; Nguyen, P.-H.; Hoang, L.M.; Nguyen, H.T.; Hoa, T.T.V.; Nhung, T.T.T.; Nguyen Nguyen, P.D.; Nhan, N.T.; Pham, H.K.T.; Truong, P.C.H. Nitric oxide production inhibitors from Vietnamese Scutellaria indica: An in vitro and in silico study. J. Chem. Res. 2024, 48, 17475198241272457. [Google Scholar] [CrossRef]
- Kuroda, M.; Iwabuchi, K.; Mimaki, Y. Chemical Constituents of the Aerial Parts of Scutellaria lateriflora and their α-Glucosidase Inhibitory Activities. Nat. Prod. Commun. 2012, 7, 471–474. [Google Scholar] [CrossRef]
- Bhat, G.; Lone, S.H.; Rather, M.A.; Shawl, A.S. Isolation, bioevaluation and RP-HPLC method development for the chemical constituents of aerial parts of Scutellaria prostrata Jacq. ex Benth. S. Afr. J. Bot. 2022, 148, 720–726. [Google Scholar] [CrossRef]
- Krystalia, L.; Ekaterina-Michaela, T.; Antonios, C.; Chryssoula, D.; Helen, S.; Nikolaos, T. Traditionally Used Sideritis cypria Post.: Phytochemistry, Nutritional Content, Bioactive Compounds of Cultivated Populations. Front. Pharmacol. 2020, 11, 650. [Google Scholar] [CrossRef]
- Takeda, Y.; Zhang, H.; Masuda, T.; Honda, G.; Otsuka, H.; Sezik, E.; Yesilada, E.; Sun, H. Megastigmane glucosides from Stachys byzantina. Phytochemistry 1997, 44, 1335–1337. [Google Scholar] [CrossRef]
- Venditti, A.; Serrilli, A.M.; Di Cecco, M.; Ciaschetti, G.; Andrisano, T.; Bianco, A. Phytochemical composition of polar fraction of Stachys germanica L. subsp. salviifolia (Ten.) Gams, a typical plant of Majella National Park. Nat. Prod. Res. 2013, 27, 190–193. [Google Scholar] [CrossRef]
- Pritsas, A.; Tomou, E.M.; Tsitsigianni, E.; Papaemmanouil, C.D.; Diamantis, D.A.; Chatzopoulou, P.; Tzakos, A.G.; Skaltsa, H. Valorisation of stachysetin from cultivated Stachys iva Griseb. as anti-diabetic agent: A multi-spectroscopic and molecular docking approach. J. Biomol. Struct. Dyn. 2021, 39, 6452–6466. [Google Scholar] [CrossRef]
- Murata, T.; Endo, Y.; Miyase, T.; Yoshizaki, F. Iridoid Glycoside Constituents of Stachys lanata. J. Nat. Prod. 2008, 71, 1768–1770. [Google Scholar] [CrossRef]
- Tundis, R.; Bonesi, M.; Pugliese, A.; Nadjafi, F.; Menichini, F.; Loizzo, M.R. Tyrosinase, Acetyl- and Butyryl-Cholinesterase Inhibitory Activity of Stachys lavandulifolia Vahl (Lamiaceae) and Its Major Constituents. Rec. Nat. Prod. 2015, 9, 81–93. [Google Scholar]
- Çalis, İ.; Başaran, A.A.; Saracoglu, İ.; Sticher, O. Iridoid and phenylpropanoid glycosides from Stachys macrantha. Phytochemistry 1992, 31, 167–169. [Google Scholar] [CrossRef]
- Miyase, T.; Yamamoto, R.; Ueno, A. Phenylethanoid glycosides from Stachys officinalis. Phytochemistry 1996, 43, 475–479. [Google Scholar] [CrossRef]
- Nishimura, H.; Sasaki, H.; Inagaki, N.; Chin, M.; Mitsuhashi, H. Nine phenethyl alcohol glycosides from Stachys sieboldii. Phytochemistry 1991, 30, 965–969. [Google Scholar] [CrossRef] [PubMed]
- Nazemiyeh, H.; Shoeb, M.; Movahhedin, N.; Kumarasamy, Y.; Talebpour, A.-H.; Delazar, A.; Nahar, L.; Sarker, S.D. Phenolic compounds and their glycosides from Stachys schtschegleevii (Lamiaceae). Biochem. Syst. Ecol. 2006, 34, 721–723. [Google Scholar] [CrossRef]
- Afouxenidi, A.; Milošević-Ifantis, T.; Skaltsa, H. Secondary metabolites from Stachys tetragona Boiss. & Heldr. ex Boiss. and their chemotaxonomic significance. Biochem. Syst. Ecol. 2018, 81, 83–85. [Google Scholar] [CrossRef]
- Ghasemi, S.; Evazalipour, M.; Peyghanbari, N.; Zamani, E.; Bellstedt, P.; Molaee, M.; Eghbali Koohi, D.; Yousefbeyk, F. Isolation and structure elucidation of the compounds from Teucrium hyrcanicum L. and the investigation of cytotoxicity, antioxidant activity, and protective effect on hydrogen peroxide-induced oxidative stress. BMC Complement. Med. Ther. 2023, 23, 447. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.-D.; Kim, J.H.; Pang, Q.Q.; Jung, P.-M.; Cho, E.J.; Lee, S. Antioxidant Activity and Acteoside Analysis of Abeliophyllum distichum. Antioxidants 2020, 9, 1148. [Google Scholar] [CrossRef]
- Zürn, M.; Tóth, G.; Kraszni, M.; Sólyomváry, A.; Mucsi, Z.; Deme, R.; Rózsa, B.; Fodor, B.; Molnár-Perl, I.; Horváti, K.; et al. Galls of European Fraxinus trees as new and abundant sources of valuable phenylethanoid and coumarin glycosides. Ind. Crops Prod. 2019, 139, 111517. [Google Scholar] [CrossRef]
- Kołtun-Jasion, M.; Sawulska, P.; Patyra, A.; Woźniak, M.; Dudek, M.K.; Filipek, A.; Kiss, A.K. Bio-Guided Isolation of Compounds from Fraxinus excelsior Leaves with Anti-Inflammatory Activity. Int. J. Mol. Sci. 2023, 24, 3750. [Google Scholar] [CrossRef]
- Kitagawa, S.; Hisada, S.; Nishibe, S. Phenolic compounds from Forsythia leaves. Phytochemistry 1984, 23, 1635–1636. [Google Scholar] [CrossRef]
- Gao, L.; Liu, X.; Li, C.; Wang, Z. Bioactivity-Guided Fractionation of Antioxidative Constituents of Ligustrum lucidum. Chem. Nat. Compd. 2017, 53, 553–554. [Google Scholar] [CrossRef]
- Mučaji, P.; Nagy, M.; Záhradníková, A.; Záhradníková, A.; Holková, I.; Bezáková, L.; Švajdlenka, E.; Liptaj, T.; Prónayová, N. Polar constituents of Ligustrum vulgare L. and their effect on lipoxygenase activity. Chem. Pap. 2011, 65, 367–372. [Google Scholar] [CrossRef]
- Pettit, G.R.; Numata, A.; Takemura, T.; Ode, R.H.; Narula, A.S.; Schmidt, J.M.; Cragg, G.M.; Pase, C.P. Antineoplastic agents, 107. Isolation of acteoside and isoacteoside from Castilleja linariaefolia. J. Nat. Prod. 1990, 53, 456–458. [Google Scholar] [CrossRef] [PubMed]
- Carrillo-Ocampo, D.; Bazaldúa-Gómez, S.; Bonilla-Barbosa, J.R.; Aburto-Amar, R.; Rodríguez-López, V. Anti-Inflammatory Activity of Iridoids and Verbascoside Isolated from Castilleja tenuiflora. Molecules 2013, 18, 12109–12118. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Tsao, R.; Yang, R.; Liu, C.; Young, J.C.; Zhu, H. Isolation and purification of phenylethanoid glycosides from Cistanche deserticola by high-speed counter-current chromatography. Food Chem. 2008, 108, 702–710. [Google Scholar] [CrossRef]
- Cui, Q.; Pan, Y.; Zhang, W.; Zhang, Y.; Ren, S.; Wang, D.; Wang, Z.; Liu, X.; Xiao, W. Metabolites of Dietary Acteoside: Profiles, Isolation, Identification, and Hepatoprotective Capacities. J. Agric. Food Chem. 2018, 66, 2660–2668. [Google Scholar] [CrossRef]
- Lei, L.; Yang, F.Q.; Zhang, T.Y.; Tu, P.F.; Wu, L.J.; Ito, Y. Preparative isolation and purification of acteoside and 2′-acetyl acteoside from Cistanches salsa (C.A. Mey) G. Beck by countercurrent chromatography. J. Chromatogr. A 2001, 912, 181–185. [Google Scholar] [CrossRef]
- Daňková, I.; Žemlička, M.; Švajdlenka, E.; Bartl, T.; Šmejkal, K. The chemotaxonomic significance of phenylethanoid glycosides of Lathraea squamaria L. (Orobanchaceae). Biochem. Syst. Ecol. 2016, 64, 53–56. [Google Scholar] [CrossRef]
- Qu, Z.-Y.; Zhang, Y.-W.; Yao, C.-L.; Jin, Y.-P.; Zheng, P.-H.; Sun, C.-H.; Liu, J.-X.; Wang, Y.-S.; Wang, Y.-P. Chemical constituents from Orobanche cernua Loefling. Biochem. Syst. Ecol. 2015, 60, 199–203. [Google Scholar] [CrossRef]
- Murai, Y.; Iwashina, T. Phenolic Compounds in the Leaves of Pedicularis chamissonis in Japan. Bull. Natl. Mus. Nat. Sci. Ser. B 2015, 41, 131–136. [Google Scholar]
- Akdemir, Z.; Çali, I.; Junior, P. Iridoid and phenylpropanoid glycosides from Pedicularis condensata. Phytochemistry 1991, 30, 2401–2402. [Google Scholar] [CrossRef]
- Tian, M.; Li, C.; Ahmad, N.; Luo, Z.; Zhang, Y.; Cheng, J.; Zhao, C. Alternative strategy for purification of acteoside with hypoglycemic activity from Rehmannia glutinosa Libosch. leaves: Preparation of ZIF-8 @D110 resin and its application. Ind. Crops Prod. 2023, 193, 116193. [Google Scholar] [CrossRef]
- Harput, U.S.; Arihan, O.; Iskit, A.B.; Nagatsu, A.; Saracoglu, I. Antinociceptive, free radical-scavenging, and cytotoxic activities of Acanthus hirsutus Boiss. J. Med. Food 2011, 14, 767–774. [Google Scholar] [CrossRef]
- Zhang, Y.; Shen, J.; Ma, X.; Yao, M.; Zhang, Y.; Cao, D. Anti-infammatory and antioxidant activities of acteoside isolated from Acanthus ilicifolius var. xiamenensis. Appl. Biol. Chem. 2022, 65, 29. [Google Scholar] [CrossRef]
- Burgos, C.; Muñoz-Mingarro, D.; Navarro, I.; Martín-Cordero, C.; Acero, N. Neuroprotective Potential of Verbascoside Isolated from Acanthus mollis L. Leaves through Its Enzymatic Inhibition and Free Radical Scavenging Ability. Antioxidants 2020, 9, 1207. [Google Scholar] [CrossRef] [PubMed]
- El-Shanawany, M.A.; Sayed, H.M.; Ibrahim, S.R.M.; Fayed, M.A.A.; Radwan, M.M.; Ross, S.A. A new isoflavone from Blepharis ciliaris of an Egyptian origin. Med. Chem. Res. 2013, 22, 2346–2350. [Google Scholar] [CrossRef]
- Ashour, M.A.-G. Isolation, HPLC/UV characterization and antioxidant activity of phenylethanoids from Blepharis edulis (Forssk.) Pers. growing in Egypt. Bull. Fac. Pharm. Cairo Univ. 2012, 50, 67–72. [Google Scholar] [CrossRef]
- Refaey, M.S.; Hassanein, A.M.M.; Mostafa, M.A.H.; Wanas, A.S.; Ali, A.A. Two new iridoid glycosides from Odontonema cuspidatum and their bioactivities. Phytochem. Lett. 2017, 22, 27–32. [Google Scholar] [CrossRef]
- Gu, W.; Zhang, Y.; Hao, X.J.; Yang, F.M.; Sun, Q.Y.; Morris-Natschke, S.L.; Lee, K.H.; Wang, Y.H.; Long, C.L. Indole Alkaloid Glycosides from the Aerial Parts of Strobilanthes cusia. J. Nat. Prod. 2014, 77, 2590–2594. [Google Scholar] [CrossRef]
- Utlu, M.; Ercil, D. Phytochemical Content, In Vitro Antioxidant, and Cholinesterase Inhibitory Activities Determination of Endemic Linaria corifolia Desf. Nat. Prod. Commun. 2024, 19, 1934578X241272734. [Google Scholar] [CrossRef]
- Xie, J.; Tan, F.; Zhu, J.; Yue, C.; Li, Q. Separation, purification and quantification of verbascoside from Penstemon barbatus (Cav.) Roth. Food Chem. 2012, 135, 2536–2541. [Google Scholar] [CrossRef]
- Gering, B.; Wichtl, M. Phytochemical Investigations on Penstemon hirsutus. J. Nat. Prod. 1987, 50, 1048–1054. [Google Scholar] [CrossRef]
- Ravn, H.; Nishibe, S.; Sasahara, M.; Li, X. Phenolic compounds from Plantago asiatica. Phytochemistry 1990, 29, 3627–3631. [Google Scholar] [CrossRef]
- Olennikov, D.N.; Tankhaeva, L.M.; Stolbikova, A.V.; Petrov, E.V. Phenylpropanoids and polysaccharides from Plantago depressa and P. media growing in Buryatia. Chem. Nat. Compd. 2011, 47, 165–169. [Google Scholar] [CrossRef]
- Guragac Dereli, F.T.; Genc, Y.; Saracoglu, I.; Akkol, E.K. Enzyme inhibitory assessment of the isolated constituents from Plantago holosteum Scop. Z. Naturforsch. C 2020, 75, 121–128. [Google Scholar] [CrossRef]
- Budzianowska, A.; Totoń, E.; Romaniuk-Drapała, A.; Kikowska, M.; Budzianowski, J. Cytotoxic Effect of Phenylethanoid Glycosides Isolated from Plantago lanceolata L. Life 2023, 13, 556. [Google Scholar] [CrossRef] [PubMed]
- Eldesoky, A.H.; Abdel-Rahman, R.F.; Ahmed, O.K.; Soliman, G.A.; Saeedan, A.S.; Elzorba, H.Y.; Elansary, A.A.; Hattori, M. Antioxidant and hepatoprotective potential of Plantago major growing in Egypt and its major phenylethanoid glycoside, acteoside. J. Food Biochem. 2018, 42, e12567. [Google Scholar] [CrossRef]
- Li, L.; Tsao, R.; Liu, Z.Q.; Liu, S.Y.; Yang, R.; Young, J.C.; Zhu, H.; Deng, Z.; Xie, M.; Fu, Z. Isolation and purification of acteoside and isoacteoside from Plantago psyllium L. by high-speed counter-current chromatography. J. Chromatogr. A 2005, 1063, 161–169. [Google Scholar] [CrossRef]
- Yu, S.Y.; Lee, I.-S.; Jung, S.-H.; Lee, Y.M.; Lee, Y.-R.; Kim, J.-H.; Sun, H.; Kim, J.S. Caffeoylated Phenylpropanoid Glycosides from Brandisia hancei Inhibit Advanced Glycation End Product Formation and Aldose Reductase in Vitro and Vessel Dilation in Larval Zebrafish in Vivo. Planta Med. 2013, 79, 1705–1709. [Google Scholar] [CrossRef]
- Filho, A.G.; Morel, A.F.; Adolpho, L.; Ilha, V.; Giralt, E.; Tarragó, T.; Dalcol, I.I. Inhibitory Effect of Verbascoside Isolated from Buddleja brasiliensis Jacq. ex Spreng on Prolyl Oligopeptidase Activity. Phytother. Res. 2012, 26, 1472–1475. [Google Scholar] [CrossRef]
- Fan, P.; Hay, A.E.; Marston, A.; Hostettmann, K. Acetylcholinesterase-Inhibitory Activity of Linarin from Buddleja davidii, Structure-Activity Relationships of Related Flavonoids, and Chemical Investigation of Buddleja nitida. Pharm. Biol. 2008, 46, 596–601. [Google Scholar] [CrossRef]
- Hien, T.T.T.; Quang, T.H.; Nhiem, N.X.; Phi, V.P.; Van Dong, L.; Dung, H.V.; Trung, D.M.; Tram, L.H.; Van Kiem, P. Secondary metabolites from the aerial parts of Buddleja macrostachya Benth. Vietnam, J. Chem. 2018, 56, 139–145. [Google Scholar] [CrossRef]
- Xie, G.; Yang, J.; Wei, X.; Xu, Q.; Qin, M. Separation of acteoside and linarin from Buddlejae Flos by high-speed countercurrent chromatography and their anti-inflammatory activities. J. Sep. Sci. 2020, 43, 1450–1457. [Google Scholar] [CrossRef] [PubMed]
- Pendota, S.C.; Aderogba, M.A.; Ndhlala, A.R.; Van Staden, J. Antimicrobial and acetylcholinesterase inhibitory activities of Buddleja salviifolia (L.) Lam. leaf extracts and isolated compounds. J. Ethnopharmacol. 2013, 148, 515–520. [Google Scholar] [CrossRef]
- Blazics, B.; Alberti, Á.; Kursinszki, L.; Kéry, Á.; Béni, S.; Tölgyesi, L. Identification and LC-MS-MS Determination of Acteoside, the Main Antioxidant Compound of Euphrasia Rostkoviana, Using the Isolated Target Analyte as External Standard. J. Chromatogr. Sci. 2011, 49, 203–208. [Google Scholar] [CrossRef]
- Jaramillo-Morales, O.A.; Díaz-Cervantes, E.; Via, L.D.; Garcia-Argaez, A.N.; Espinosa-Juárez, J.V.; Ovando-Zambrano, J.C.; Muñoz-Pérez, V.M.; Valadez-Vega, C.; Bautista, M. Hepatoprotective Activity, In Silico Analysis, and Molecular Docking Study of Verbascoside from Leucophyllum frutescens in Rats with Post-Necrotic Liver Damage. Sci. Pharm. 2023, 91, 40. [Google Scholar] [CrossRef]
- Zhang, Z.; Dai, L.; Wang, H.; Chang, X.; Ren, S.; Lai, H.; Liu, L. Phytochemical profiles and antioxidant, anticholinergic, and antidiabetic activities of Odontites serotina (Lam.) Dum. Eur. J. Integr. Med. 2021, 44, 101340. [Google Scholar] [CrossRef]
- Si, C.-L.; Lu, Y.-Y.; Qin, P.-P.; Sun, R.-C.; Ni, Y.-H. Phenolic extractives from Paulownia. BioResources 2011, 6, 5086–5098. [Google Scholar] [CrossRef]
- Huang, C.G.; Shang, Y.J.; Zhang, J.R.; Li, W.J.; Jiao, B.H. Hypouricemic effects of phenylpropanoid glycosides acteoside of Scrophularia ningpoensis on serum uric acid levels in potassium oxonate-pretreated mice. Am. J. Chin. Med. 2008, 36, 149–157. [Google Scholar] [CrossRef]
- Monsef-Esfahani, H.R.; Hajiaghaee, R.; Shahverdi, A.R.; Khorramizadeh, M.R.; Amini, M. Flavonoids, cinnamic acid and phenyl propanoid from aerial parts of Scrophularia striata. Pharm. Biol. 2010, 48, 333–336. [Google Scholar] [CrossRef]
- Kahraman, Ç.; Tatli, İ.İ.; Kart, D.; Ekizoğlu, M.; Akdemir, Z.Ş. Structure Elucidation and Antimicrobial Activities of Secondary Metabolites from the Flowery Parts of Verbascum mucronatum Lam. Turk. J. Pharm. Sci. 2018, 15, 231–237. [Google Scholar] [CrossRef] [PubMed]
- Luca, S.V.; Czerwińska, M.E.; Miron, A.; Aprotosoaie, A.C.; Marcourt, L.; Wolfender, J.-L.; Granica, S.; Skalicka-Woźniak, K. High-performance countercurrent chromatographic isolation of acylated iridoid diglycosides from Verbascum ovalifolium Donn ex Sims and evaluation of their inhibitory potential on IL-8 and TNF-α production. J. Pharm. Biomed. Anal. 2019, 166, 295–303. [Google Scholar] [CrossRef]
- Demirci, S.; Alp, C.; Akşit, H.; Ulutaş, Y.; Altay, A.; Yeniçeri, E.; Köksal, E.; Yaylı, N. Isolation, characterization and anticancer activity of secondary metabolites from Verbascum speciosum. Chem. Biol. Drug Des. 2023, 101, 1273–1282. [Google Scholar] [CrossRef] [PubMed]
- Marcoccia, D.; Georgiev, M.I.; Alipieva, K.I.; Lorenzetti, S. Inhibition of the DHT-induced PSA secretion by Verbascum xanthophoeniceum and Serenoa repens extracts in human LNCaP prostate epithelial cells. J. Ethnopharmacol. 2014, 155, 616–625. [Google Scholar] [CrossRef]
- Nonaka, G.; Nishioka, I. Bitter phenylpropanoid glycosides from Conandron ramoidioides. Phytochemistry 1977, 16, 1265–1267. [Google Scholar] [CrossRef]
- Takizawa, R.; Minamizono, T.; Tsuji, D.; Yan, X.Y.; Lu, F.L.; Yang, X.R.; Li, D.P.; Akagi, R.; Kashiwada, Y.; Tanaka, N. Methoxyflavone glucosides and caffeoyl phenylethanoid glycoside from Lysionotus pauciflorus: Their structures and anti-ferroptosis activity. J. Nat. Med. 2024, 79, 196–203. [Google Scholar] [CrossRef]
- Gutiérrez-Rebolledo, G.A.; Garduño-Siciliano, L.; García-Rodríguez, R.V.; Pérez-González, M.Z.; Chávez, M.I.; Bah, M.; Siordia-Reyes, G.A.; Chamorro-Cevallos, G.A.; Jiménez-Arellanes, M.A. Anti-inflammatory and toxicological evaluation of Moussonia deppeana (Schldl. & Cham) Hanst and Verbascoside as a main active metabolite. J. Ethnopharmacol. 2016, 187, 269–280. [Google Scholar] [CrossRef] [PubMed]
- Gu, W.; Xie, R.-X.; Chen, J.-L.; Yuan, C.-M.; Huang, L.-J.; Yi, P.; Hao, X.-J. Two new secondary metabolites from Oreocharis auricula and their chemotaxonomic significance. Biochem. Syst. Ecol. 2022, 104, 104477. [Google Scholar] [CrossRef]
- Schmeda-Hirschmann, G.; Burgos-Edwards, A.; Jiménez-Aspee, F.; Mieres-Castro, D.; Theoduloz, C.; Pormetter, L.; Fogel, R.; Céspedes, C.; Soria, N.; Valdez, S. Iridoids and Amino Acid Derivatives from the Paraguayan Crude Drug Adenocalymma marginatum (ysypó hû). Molecules 2020, 25, 180. [Google Scholar] [CrossRef]
- Samy, M.N.; Attia, E.Z.; Shoman, M.E.; Khalil, H.E.; Sugimoto, S.; Matsunami, K.; Fahim, J.R. Phytochemical investigation of Amphilophium paniculatum; an underexplored Bignoniaceae species as a source of SARS-CoV-2 Mpro inhibitory metabolites: Isolation, identification, and molecular docking study. S. Afr. J. Bot. 2021, 141, 421–430. [Google Scholar] [CrossRef]
- Lima, C.S.A.; Amorim, E.L.C.; Sena, K.X.F.R.; Chiappeta, A.A.; Nunes, X.P.; Agra, M.F.; da-Cunha, E.V.L.; Silva, M.S.; Barbosa-Filho, J.M. Antimicrobial activity of a mixture of two isomeric phenylpropanoid glycosides from Arrabidaea harleyi A.H. Gentry (Bignoniaceae). Rev. Bras. Cienc. Farm. 2003, 39, 77–81. [Google Scholar] [CrossRef]
- Brandão, G.C.; Kroon, E.G.; Souza, D.E.R.; Filho, J.D.S.; Oliveira, A.B. Chemistry and Antiviral Activity of Arrabidaea pulchra (Bignoniaceae). Molecules 2013, 18, 9919–9932. [Google Scholar] [CrossRef] [PubMed]
- Samy, M.N.; Hamed, A.N.E.; Attia, E.Z.; Abdelmohsen, U.R.; Fawzy, M.A.; Kamel, M.S. LC-MS-based identification of bioactive compounds and hepatoprotective and nephroprotective activities of Bignonia binata leaves against carbon tetrachloride-induced injury in rats. Nat. Prod. Res. 2021, 36, 1375–1379. [Google Scholar] [CrossRef] [PubMed]
- Han, X.H.; Oh, J.-H.; Hong, S.S.; Lee, C.; Park, J.I.; Lee, M.K.; Hwang, B.Y.; Lee, M.-S. Novel Iridoids from the Flowers of Campsis grandiflora. Arch. Pharm. Res. 2012, 35, 327–332. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.Q.; Wang, Y.M.; Dang, J.; Wang, Q.L.; Shao, Y.; Juan Mei, L.J.; Tao, J.D. Chemical Constituents of Incarvillea compacta. Chem. Nat. Compd. 2017, 53, 548–550. [Google Scholar] [CrossRef]
- Pan, W.; Jiang, S.; Luo, P.; Wu, J.; Gao, P. Isolation, purification and structure identification of antioxidant compound from the roots of Incarvillea younghusbandii Sprague and its life span prolonging effect in Drosophila melanogaster. Nat. Prod. Res. 2008, 22, 719–725. [Google Scholar] [CrossRef]
- Martin, F.; Hay, A.E.; Quinteros Condoretty, V.R.; Cressend, D.; Reist, M.; Gupta, M.P.; Carrupt, P.A.; Hostettmann, K. Antioxidant phenylethanoid glycosides and a neolignan from Jacaranda caucana. J. Nat. Prod. 2009, 72, 852–856. [Google Scholar] [CrossRef]
- Arruda, A.L.; Vieira, C.J.; Sousa, D.G.; Oliveira, R.F.; Castilho, R.O. Jacaranda cuspidifolia Mart. (Bignoniaceae) as an antibacterial agent. J. Med. Food 2011, 14, 1604–1608. [Google Scholar] [CrossRef]
- El-Marasy, S.A.; El-Shenawy, S.M.; Moharram, F.A.; El-Sherbeeny, N.A. Antidiabetic and Antioxidant Effects of Acteoside from Jacaranda mimosifolia Family Biognoniaceae in Streptozotocin–Nicotinamide Induced Diabetes in Rats. Open Access Maced. J. Med. Sci. 2020, 8, 125–133. [Google Scholar] [CrossRef]
- Liu, S.; Zheng, Z.; Zheng, G.; Chen, L.; Zhonh, T.; Ming, Y. A New Flavonoside from the Invasive Plant Macfadyena unguis-cati. Chem. Nat. Compd. 2015, 51, 844–846. [Google Scholar] [CrossRef]
- Sofidiya, M.O.; Agunbiade, F.O.; Koorbanally, N.A.; Sowemimo, A.; Soesan, D.; Familusi, T. Antiulcer activity of the ethanolic extract and ethyl acetate fraction of the leaves of Markhamia tomentosa in rats. J. Ethnopharmacol. 2014, 157, 1–6. [Google Scholar] [CrossRef]
- Kernan, M.R.; Amarquaye, A.; Chen, J.L.; Chan, J.; Sesin, D.F.; Parkinson, N.; Ye, Z.; Barrett, M.; Bales, C.; Stoddart, C.A.; et al. Antiviral phenylpropanoid glycosides from the medicinal plant Markhamia lutea. J. Nat. Prod. 1998, 61, 564–570. [Google Scholar] [CrossRef] [PubMed]
- Mahmoud, B.K.; Hamed, A.N.E.; Samy, M.N.; Mostafa, E.M.; Wanas, A.S.; Radwan, M.M.; Elsohly, M.A.; Kamel, M.S. Phytochemical composition and antimicrobial properties of Markhamia platycalyx (Baker) Sprague leaf. Trop. J. Pharm. Res. 2019, 18, 2623–2631. [Google Scholar] [CrossRef]
- Vien, L.T.; Hanh, T.T.H.; Quang, T.H.; Cuong, N.T.; Cuong, N.X.; Oh, H.; Van Minh, C. Phenolic glycosides from Oroxylum indicum. Nat. Prod. Res. 2020, 36, 2336–2340. [Google Scholar] [CrossRef]
- Martin, F.; Hay, A.E.; Corno, L.; Gupta, M.P.; Hostettmann, K. Iridoid glycosides from the stems of Pithecoctenium crucigerum (Bignoniaceae). Phytochemistry 2007, 68, 1307–1311. [Google Scholar] [CrossRef] [PubMed]
- Ramirez, G.; Zamilpa, A.; Zavala, M.; Perez, J.; Morales, D.; Tortoriello, J. Chrysoeriol and other polyphenols from Tecoma stans with lipase inhibitory activity. J. Ethnopharmacol. 2016, 185, 1–8. [Google Scholar] [CrossRef]
- Burger, J.F.W.; Brandt, E.V.; Ferreira, D. Iridoid and phenolic glycosides from Harpagophytum procumbens. Phytochemistry 1987, 25, 1453–1457. [Google Scholar] [CrossRef]
- Fuji, Y.; Ohtsuki, T.; Matsufuji, H. Accumulation and Subcellular Localization of acteoside in sesame plants (Sesamum indicum L.). ACS Omega 2018, 3, 17287–17294. [Google Scholar] [CrossRef]
- Rossi, R.; Mainardi, E.; Vizzarri, F.; Corino, C. Verbascoside-Rich Plant Extracts in Animal Nutrition. Antioxidants 2024, 13, 39. [Google Scholar] [CrossRef]
- Kostyuk, V.; Potapovich, A.; Suhan, T.; De Luca, C.; Pressi, G.; Dal Toso, R.; Korkina, L. Plant polyphenols against UV-C-induced cellular death. Planta Med. 2008, 74, 509–514. [Google Scholar] [CrossRef]
- Hänsel, R.; Sticher, O. Pharmakognosie—Phytopharmazie, 10th ed.; Wissenschaftliche Verlagsgesellschaft: Stuttgart, Germany, 2015. [Google Scholar]
- Daels-Rakotoarison, D.A.; Seidel, V.; Gressier, B.; Brunet, C.; Tillequin, F.; Bailleul, F.; Luyckx, M.; Dine, T.; Cazin, M.; Cazin, J.-C. Neurosedative and Antioxidant Activities of Phenylpropanoids from Ballota nigra. Arzneimittelforschung 2000, 50, 16–23. [Google Scholar] [CrossRef]
- Taskova, R.M.; Gotfredsen, C.H.; Jensen, S.R. Chemotaxonomic markers in Digitalideae (Plantaginaceae). Phytochemistry 2005, 66, 1440–1447. [Google Scholar] [CrossRef]
- Schlauer, J.; Budzianowski, J.; Kukułczanka, K.; Ratajczak, L. Acteoside and related phenylethanoid glycosides in Byblis liniflora Salisb. plants propagated in vitro and its systematic significance. Acta Soc. Bot. Pol. 2011, 73, 9–15. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhu, J.; Shao, L.; Guo, M. Current advances in acteoside biosynthesis pathway elucidation and biosynthesis. Fitoterapia 2020, 142, 104495. [Google Scholar] [CrossRef] [PubMed]
- Fuji, Y.; Uchida, K.; Akashi, T.; Ohtsuki, T.; Matsufuji, H.; Hirai, M.Y. Molecular Identification of UDP-Sugar-Dependent Glycosyltransferase and Acyltransferase Involved in the Phenylethanoid Glycoside Biosynthesis Induced by Methyl Jasmonate in Sesamum indicum L. Plant Cell Physiol. 2023, 64, 716–728. [Google Scholar] [CrossRef]
- Yang, Y.; Xi, D.; Wu, Y.; Liu, T. Complete biosynthesis of the phenylethanoid glycoside verbascoside. Plant Commun. 2023, 4, 100592. [Google Scholar] [CrossRef] [PubMed]
- Herrmann, K.M.; Weaver, L.M. The shikimate pathway. Annu. Rev. Plant Biol. 1999, 50, 473–503. [Google Scholar] [CrossRef]
- Shende, V.V.; Bauman, K.D.; Moore, B.S. The shikimate pathway: Gateway to metabolic diversity. Nat. Prod. Rep. 2024, 41, 604–648. [Google Scholar] [CrossRef]
- Fuji, Y.; Matsufuji, H.; Hirai, M.Y. Distribution, biosynthesis, and synthetic biology of phenylethanoid glycosides in the order Lamiales. Plant Biotechnol. 2024, 41, 231–241. [Google Scholar] [CrossRef]
- Chen, W.; Yao, J.; Meng, J.; Han, W.; Tao, Y.; Chen, Y.; Guo, Y.; Shi, G.; He, Y.; Jin, J.-M.; et al. Promiscuous enzymatic activity-aided multiple pathway network design for metabolic flux rearrangement in hydroxytyrosol biosynthesis. Nat. Commun. 2019, 10, 960. [Google Scholar] [CrossRef]
- Şenol, H.; Tulay, P.; Ergören, M.Ç.; Hanoğlu, A.; Çalış, İ.; Mocan, G. Cytotoxic Effects of Verbascoside on MCF-7 and MDA-MB-231. Turk. J. Pharm. Sci. 2021, 18, 637–644. [Google Scholar] [CrossRef] [PubMed]
- Shi, C.; Ma, Y.; Tian, L.; Li, J.; Qiao, G.; Liu, C.; Cao, W.; Liang, C. Verbascoside: An Efficient and Safe Natural Antibacterial Adjuvant for Preventing Bacterial Contamination of Fresh Meat. Molecules 2022, 27, 4943. [Google Scholar] [CrossRef] [PubMed]
- Etemad, L.; Zafari, R.; Vahdati-Mashhadian, N.; Adel Moallem, S.A.; Shirvan, Z.O.; Hosseinzadeh, H. Acute, Sub-Acute and Cell Toxicity of Verbascoside. Res. J. Med. Plant. 2015, 9, 354–360. [Google Scholar] [CrossRef]
- Song, X.; He, J.; Xu, H.; Hu, X.P.; Wu, X.L.; Wu, H.Q.; Liu, L.Z.; Liao, C.H.; Zeng, Y.; Li, Y.; et al. The antiviral effects of acteoside and the underlying IFN-γ-inducing action. Food. Funct. 2016, 7, 3017–3030. [Google Scholar] [CrossRef] [PubMed]
- Khan, R.A.; Hossain, R.; Roy, P.; Jain, D.; Mohammad Saikat, A.S.; Roy Shuvo, A.P.; Akram, M.; Elbossaty, W.F.; Khan, I.N.; Painuli, S.; et al. Anticancer effects of acteoside: Mechanistic insights and therapeutic status. Eur. J. Pharmacol. 2022, 916, 174699. [Google Scholar] [CrossRef]
- Khazir, J.; Ali, I.; Khan, I.A.; Kumar, H.M. Enzyme mediated transesterification of verbascoside and evaluation of antifungal activity of synthesised compounds. Nat. Prod. Res. 2015, 29, 727–734. [Google Scholar] [CrossRef]
- Sharmila Devi, N.; Mythili, R.; Cherian, T.; Dineshkumar, R.; Sivaraman, G.K.; Jayakumar, R.; Prathaban, M.; Duraimurugan, M.; Chandrasekar, V.; Peijnenburg, W.J.G.M. Overview of antimicrobial resistance and mechanisms: The relative status of the past and current. Microbe 2024, 3, 100083. [Google Scholar] [CrossRef]
- Cheesman, M.J.; Ilanko, A.; Blonk, B.; Cock, I.E. Developing New Antimicrobial Therapies: Are Synergistic Combinations of Plant Extracts/Compounds with Conventional Antibiotics the Solution? Pharmacogn. Rev. 2017, 11, 57–72. [Google Scholar] [CrossRef]
- De Souza, P.A.; Silva, C.G.; Machado, B.R.P.; De Lucas, N.C.; Leitao, G.G.; Eleutherio, E.C.A.; Ortiz, G.M.D.; Benchetrit, L.C. Evaluation of antimicrobial, antioxidant and phototoxic activities of extracts and isolated compounds from Stachytarpheta cayennensis (Rich.) Vahl. Verbenaceae. Rev. Bras. Farmacogn. 2010, 20, 922–928. [Google Scholar] [CrossRef]
- Pardo, F.; Perich, F.; Villarroel, L.; Torres, R. Isolation of verbascoside, an antimicrobial constituent of Buddleja globosa leaves. J. Ethnopharmacol. 1993, 39, 221–222. [Google Scholar] [CrossRef]
- Guillermo Avila, J.; de Liverant, J.G.; Martínez, A.; Martínez, G.; Muñoz, J.L.; Arciniegas, A.; Romo de Vivar, A. Mode of action of Buddleja cordata verbascoside against Staphylococcus aureus. J. Ethnopharmacol. 1999, 66, 75–78. [Google Scholar] [CrossRef]
- Didry, N.; Seidel, V.; Dubreuil, L.; Tillequin, F.; Bailleul, F. Isolation and antibacterial activity of phenylpropanoid derivatives from Ballota nigra. J. Ethnopharmacol. 1999, 67, 197–202. [Google Scholar] [CrossRef]
- Fazly Bazzaz, B.S.; Khameneh, B.; Zahedian Ostad, M.R.; Hosseinzadeh, H. In vitro evaluation of antibacterial activity of verbascoside, lemon verbena extract and caffeine in combination with gentamicin against drug-resistant Staphylococcus aureus and Escherichia coli clinical isolates. Avicenna J. Phytomed. 2018, 8, 246–253. [Google Scholar]
- Sermukhamedova, O.; Wojtanowski, K.K.; Widelski, J.; Korona-Głowniak, I.; Elansary, H.O.; Sakipova, Z.; Malm, A.; Głowniak, K.; Skalicka-Woźniak, K. Metabolic profile of and antimicrobial activity in the aerial part of Leonurus turkestanicus V.I. Krecz. et Kuprian. from Kazakhstan. J. AOAC Int. 2017, 100, 1700–1705. [Google Scholar] [CrossRef] [PubMed]
- Šimat, V.; Skroza, D.; Tabanelli, G.; Čagalj, M.; Pasini, F.; Gómez-Caravaca, A.M.; Fernández-Fernández, C.; Sterniša, M.; Smole Možina, S.; Ozogul, Y.; et al. Antioxidant and Antimicrobial Activity of Hydroethanolic Leaf Extracts from Six Mediterranean Olive Cultivars. Antioxidants 2022, 11, 1656. [Google Scholar] [CrossRef]
- Lara-Issasi, G.; Salgado, C.; Pedraza-Chaverri, J.; Medina-Campos, O.N.; Morales, A.; Águila, M.A.; Avilés, M.; Rivero-Cruz, B.E.; Navarro, V.; Ríos-Gómez, R.; et al. Antimicrobial, Antioxidant Activities, and HPLC Determination of the Major Components of Verbena carolina (Verbenaceae). Molecules 2019, 24, 1970. [Google Scholar] [CrossRef] [PubMed]
- Rúa, J.; López-Rodríguez, I.; Sanz, J.; Del Valle Fernández, P.; Garcia, M.D.C.; Garcia Armesto, M.R. Antimicrobial efficacy of Lippia citriodora natural extract against Escherichia coli and Enterococcus faecalis in “Piel de Sapo” melon juice. Food Sci. Nutr. 2019, 7, 3986–3992. [Google Scholar] [CrossRef]
- Saqallah, F.G.; Hamed, W.M.; Talib, W.H.; Dianita, R.; Wahab, H.A. Antimicrobial activity and molecular docking screening of bioactive components of Antirrhinum majus (snapdragon) aerial parts. Heliyon 2022, 8, e10391. [Google Scholar] [CrossRef] [PubMed]
- Reddy, G.K.K.; Padmavathi, A.R.; Nancharaiah, Y.V. Fungal infections: Pathogenesis, antifungals and alternate treatment approaches. Curr. Res. Microb. Sci. 2022, 3, 100137. [Google Scholar] [CrossRef]
- Fernandes, V.S.; da Rosa, R.; Zimmermann, L.A.; Rogério, K.R.; Kümmerle, A.E.; Bernardes, L.S.C.; Graebin, C.S. Antiprotozoal agents: How have they changed over a decade? Arch. Pharm. 2022, 355, e2100338. [Google Scholar] [CrossRef]
- Schmiedel, Y.; Zimmerli, S. Common invasive fungal diseases: An overview of invasive candidiasis, aspergillosis, cryptococcosis, and Pneumocystis pneumonia. Swiss. Med. Wkly. 2016, 146, w14281. [Google Scholar] [CrossRef] [PubMed]
- Funari, C.S.; Gullo, F.P.; Napolitano, A.; Carneiro, R.L.; Mendes-Giannini, M.J.; Fusco-Almeida, A.M.; Piacente, S.; Pizza, C.; Silva, D.H. Chemical and antifungal investigations of six Lippia species (Verbenaceae) from Brazil. Food Chem. 2012, 135, 2086–2094. [Google Scholar] [CrossRef]
- Ali, I.; Sharma, P.; Suri, K.A.; Satti, N.K.; Dutt, P.; Afrin, F.; Khan, I.A. In vitro antifungal activities of amphotericin B in combination with acteoside, a phenylethanoid glycoside from Colebrookea oppositifolia. J. Med. Microbiol. 2011, 60 Pt 9, 1326–1336. [Google Scholar] [CrossRef] [PubMed]
- Mahlke, J.D.; Boligon, A.A.; Machado, M.M.; Borba Spader, T.; Alves, S.H.; do Canto-Dorow, T.; Linde Athayde, M. In vitro antimicrobial and antioxidant activities of a crude extract and fractions from Buddleja thyrsoides Lam Leaves. Quim. Nova 2009, 32, 277–281. [Google Scholar] [CrossRef]
- Oyourou, J.N.; Combrinck, S.; Regnier, T.; Marston, A. Purification, stability and antifungal activity of verbascoside from Lippia javanica and Lantana camara leaf extracts. Ind. Crops. Prod. 2013, 43, 820–826. [Google Scholar] [CrossRef]
- Mann, S.; Frasca, K.; Scherrer, S.; Henao-Martínez, A.F.; Newman, S.; Ramanan, P.; Suarez, J.A. A Review of Leishmaniasis: Current Knowledge and Future Directions. Curr. Trop. Med. Rep. 2021, 8, 121–132. [Google Scholar] [CrossRef]
- Desquesnes, M.; Gonzatti, M.; Sazmand, A.; Thévenon, S.; Bossard, G.; Boulangé, A.; Gimonneau, G.; Truc, P.; Herder, S.; Ravel, S.; et al. A review on the diagnosis of animal trypanosomoses. Parasites Vectors 2022, 15, 64. [Google Scholar] [CrossRef]
- Rocha, L.G.; Almeida, J.R.G.S.; Macedo, R.O.; Barbosa-Filho, J.B. A review of natural products with antileishmanial activity. Phytomedicine 2005, 12, 514–535. [Google Scholar] [CrossRef]
- Maquiaveli, C.D.C.; Rochetti, A.L.; Fukumasu, H.; Vieira, P.C.; da Silva, E.R. Antileishmanial activity of verbascoside: Selective arginase inhibition of intracellular amastigotes of Leishmania (Leishmania) amazonensis with resistance induced by LPS plus IFN-γ. Biochem. Pharmacol. 2017, 127, 28–33. [Google Scholar] [CrossRef]
- Aoki, J.I.; Laranjeira-Silva, M.F.; Muxel, S.M.; Floeter-Winter, L.M. The impact of arginase activity on virulence factors of Leishmania amazonensis. Curr. Opin. Microbiol. 2019, 52, 110–115. [Google Scholar] [CrossRef]
- Kirmizibekmez, H.; Çalıs, I.; Perozzo, R.; Brunn, R.; Donmez, A.A.; Linden, A.; Rüedi, P.; Tasdemir, D. Inhibiting activities of the secondary metabolites of Phlomis brunneogaleata against parasitic protozoa and plasmodial Enoyl-ACP reductase, a crucial enzyme in fatty acid biosynthesis. Planta Med. 2004, 70, 711–717. [Google Scholar] [CrossRef] [PubMed]
- Atay, I.; Kirmizibekmez, H.; Kaiser, M.; Akaydin, G.; Yesilada, E.; Tasdemir, D. Evaluation of in vitro antiprotozoal activity of Ajuga laxmannii and its secondary metabolites. Pharm. Biol. 2016, 54, 1808–1814. [Google Scholar] [CrossRef] [PubMed]
- Froelich, S.; Gupta, M.P.; Siems, K.; Jenett-Siems, K. Phenylethanoid glycosides from Stachytarpheta cayennensis (Rich.) Vahl, Verbenaceae, a traditional antimalarial medicinal plant. Rev. Bras. Farmacogn. 2008, 18, 517–520. [Google Scholar] [CrossRef]
- Abouzid, S.F.; Wahba, H.M.; Elshamy, A.; Cos, P.; Maes, L.; Apers, S.; Pieters, L.; Shahat, A.A. Antimicrobial activity of some Clerodendrum species from Egypt. Nat. Prod. Res. 2013, 27, 1032–1036. [Google Scholar] [CrossRef] [PubMed]
- Sharma, R.; Bhattu, M.; Tripathi, A.; Verma, M.; Acevedo, R.; Kumar, P.; Rajput, D.V.; Singh, J. Potential medicinal plants to combat viral infections: A way forward to environmental biotechnology. Environ. Res. 2023, 227, 115725. [Google Scholar] [CrossRef]
- Pal, D.; Bareth, K. Respiratory Viral Infections and the Role of Medicinal Plants in Prevention and Treatment. In Anti-Viral Metabolites from Medicinal Plants; Pal, D., Ed.; Reference Series in Phytochemistry; Springer Nature: Berlin/Heidelberg, Germany, 2024; pp. 397–427. [Google Scholar] [CrossRef]
- Strasfeld, L.; Chou, S. Antiviral drug resistance: Mechanisms and clinical implications. Infect. Dis. Clin. N. Am. 2010, 24, 809–833, Erratum in Infect. Dis. Clin. N. Am. 2010, 24, xi. [Google Scholar] [CrossRef]
- Molnár, J.; Gunics, G.; Mucsi, I.; Koltai, M.; Petri, I.; Shoyama, Y.; Matsumoto, M.; Nishioka, I. Antimicrobial and immunomodulating effects of some phenolic glycosides. Acta Microbiol. Hung. 1989, 36, 425–432. [Google Scholar]
- Majrashi, T.A.; El Hassab, M.A.; Mahmoud, S.H.; Mostafa, A.; Wahsh, E.A.; Elkaeed, E.B.; Hassan, F.E.; Eldehna, W.M.; Abdelgawad, S.M. In vitro biological evaluation and in silico insights into the antiviral activity of standardized olive leaves extract against SARS-CoV-2. PLoS ONE 2024, 19, e0301086. [Google Scholar] [CrossRef] [PubMed]
- Okasha, Y.M.; Fathy, F.I.; Soliman, F.M.; Fayek, N.M. The untargeted phytochemical profile of Verbascum thapsus L. with potent antiviral, antibacterial and anticancer activities. S. Afr. J. Bot. 2023, 156, 334–341. [Google Scholar] [CrossRef]
- Narayanan, S.A.; Jamison, D.A.; Guarnieri, J.W.; Zaksas, V.; Topper, M.; Koutnik, A.P.; Park, J.; Clark, K.B.; Enguita, F.J.; Leitão, A.L.; et al. A comprehensive SARS-CoV-2 and COVID-19 review, Part 2: Host extracellular to systemic effects of SARS-CoV-2 infection. Eur. J. Hum. Genet. 2024, 32, 10–20. [Google Scholar] [CrossRef]
- Shawky, E.; Nada, A.A.; Ibrahim, R.S. Potential role of medicinal plants and their constituents in the mitigation of SARS-CoV-2: Identifying related therapeutic targets using network pharmacology and molecular docking analyses. RSC Adv. 2020, 10, 27961–27983. [Google Scholar] [CrossRef]
- Bernardi, M.; Ghaani, M.R.; Bayazeid, O. Phenylethanoid glycosides as a possible COVID-19 protease inhibitor: A virtual screening approach. J. Mol. Model. 2021, 27, 341. [Google Scholar] [CrossRef]
- Johnston, C. Diagnosis and Management of Genital Herpes: Key Questions and Review of the Evidence for the 2021 Centers for Disease Control and Prevention Sexually Transmitted Infections Treatment Guidelines. Clin. Infect. Dis. 2022, 74 (Suppl. 2), S134–S143. [Google Scholar] [CrossRef] [PubMed]
- Kaler, J.; Hussain, A.; Patel, K.; Hernandez, T.; Ray, S. Respiratory Syncytial Virus: A Comprehensive Review of Transmission, Pathophysiology, and Manifestation. Cureus 2023, 15, e36342. [Google Scholar] [CrossRef]
- Chathuranga, K.; Kim, M.S.; Lee, H.C.; Kim, T.H.; Kim, J.H.; Gayan Chathuranga, W.A.; Ekanayaka, P.; Wijerathne, H.M.S.M.; Cho, W.K.; Kim, H.I.; et al. Anti-Respiratory Syncytial Virus Activity of Plantago asiatica and Clerodendrum trichotomum Extracts In Vitro and In Vivo. Viruses 2019, 11, 604. [Google Scholar] [CrossRef] [PubMed]
- Loaiza-Cano, V.; Monsalve-Escudero, L.M.; Filho, C.D.S.M.B.; Martinez-Gutierrez, M.; Sousa, D.P. Antiviral Role of Phenolic Compounds against Dengue Virus: A Review. Biomolecules 2020, 11, 11. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Lee, J.Y.; Kang, H.S.; Jeong, C.H.; Moon, H.; Whang, W.K.; Kim, C.J.; Sim, S.S. The effect of acteoside on histamine release and arachidonic acid release in RBL-2H3 mast cells. Arch. Pharm. Res. 2006, 29, 508–513. [Google Scholar] [CrossRef]
- Song, H.S.; Choi, M.Y.; Ko, M.S.; Jeong, J.M.; Kim, Y.H.; Jang, B.H.; Sung, J.H.; Kim, M.G.; Whang, W.K.; Sim, S.S. Competitive inhibition of cytosolic Ca2+-dependent phospholipase A2 by acteoside in RBL-2H3 cells. Arch. Pharm. Res. 2012, 35, 905–910. [Google Scholar] [CrossRef]
- Pesce, M.; Franceschelli, S.; Ferrone, A.; De Lutiis, M.A.; Patruno, A.; Grilli, A.; Felaco, M.; Speranza, L. Verbascoside down-regulates some pro-inflammatory signal transduction pathways by increasing the activity of tyrosine phosphatase SHP-1 in the U937 cell line. J. Cell. Mol. Med. 2015, 19, 1548–1556. [Google Scholar] [CrossRef]
- Seo, E.S.; Oh, B.K.; Pak, J.H.; Yim, S.-H.; Gurunathan, S.; Kim, Y.-P.; Lee, K.J. Acteoside Improves Survival in Cecal Ligation and Puncture-Induced Septic Mice via Blocking of High Mobility Group Box 1 Release. Mol. Cells 2013, 35, 348–354. [Google Scholar] [CrossRef]
- Pongkitwitoon, B.; Kitisripanya, T.; Putalun, W.; Triwitayakorn, K.; Kanchanapoom, T.; Boonsnongcheep, P. Anti-inflammatory activity of verbascoside-and isoverbascoside-rich Lamiales medicinal plants. Heliyon 2024, 10, e23644. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.Y.; Woo, E.-R.; Kang, K.W. Inhibition of lipopolysaccharide-inducible nitric oxide synthase expression by acteoside through blocking of AP-1 activation. J. Ethnopharmacol. 2005, 97, 561–566. [Google Scholar] [CrossRef]
- Marzocco, S.; Piccinelli, A.L.; Rastrelli, L.; Mazzon, E.; Cuzzocrea, S.; Autore, G. Inhibition of inducible nitric oxide synthase in vitro and in vivo by a water-soluble extract of Wendita calysina leaves. Naunyn Schmiedebergs Arch. Pharmacol. 2007, 375, 349–358. [Google Scholar] [CrossRef] [PubMed]
- Picerno, P.; Autore, G.; Marzocco, S.; Meloni, M.; Sanogo, R.; Aquino, R.P. Anti-inflammatory Activity of Verminoside from Kigelia africana and Evaluation of Cutaneous Irritation in Cell Cultures and Reconstituted Human Epidermis. J. Nat. Prod. 2005, 68, 1610–1614. [Google Scholar] [CrossRef] [PubMed]
- Dıaz, A.M.; Abad, M.J.; Fernàndez, L.; Silvàn, A.M.; De Santos, J.; Bermejo, P. Phenylpropanoid glycosides from Scrophularia scorodonia: In vitro anti-inflammatory activity. Life Sci. 2004, 74, 2515–2526. [Google Scholar] [CrossRef]
- Rao, Y.K.; Fang, S.-H.; Hsieh, S.-C.; Yeh, T.-H.; Tzeng, Y.-M. The constituents of Anisomeles indica and their anti-inflammatory activities. J. Ethnopharmacol. 2009, 121, 292–296. [Google Scholar] [CrossRef]
- Nam, S.-Y.; Kim, H.-M.; Jeong, H.-J. Attenuation of IL-32-induced caspase-1 and nuclear factor-κB activations by acteoside. Int. Immunopharmacol. 2015, 29, 574–582. [Google Scholar] [CrossRef]
- Esposito, E.; Dal Toso, R.; Pressi, G.; Bramanti, P.; Meli, R.; Cuzzocrea, S. Protective effect of verbascoside in activated C6 glioma cells: Possible molecular mechanisms. Naunyn Schmiedebergs Arch. Pharmacol. 2010, 381, 93–105. [Google Scholar] [CrossRef]
- Speranza, L.; Franceschelli, S.; Pesce, M.; Reale, M.; Menghini, L.; Vinciguerra, I.; De Lutiis, M.A.; Felaco, M.; Grilli, A. Antiinflammatory effects in THP-1 cells treated with verbascoside. Phytother. Res. 2010, 24, 1398–1404. [Google Scholar] [CrossRef]
- Qiao, Z.; Tang, J.; Wu, W.; Tang, J.; Liu, M. Acteoside inhibits inflammatory response via JAK/STAT signaling pathway in osteoarthritic rats. BMC Complement. Altern. Med. 2019, 19, 264. [Google Scholar] [CrossRef]
- Chang, J.-H.; Chuang, H.-C.; Hsiao, G.; Hou, T.-Y.; Wang, C.-C.; Huang, S.-C.; Li, B.-Y.; Lee, Y.-L. Acteoside exerts immunomodulatory effects on dendritic cells via aryl hydrocarbon receptor activation and ameliorates Th2-mediated allergic asthma by inducing Foxp3(+) regulatory T cells. Int. Immunopharmacol. 2022, 106, 108603. [Google Scholar] [CrossRef]
- Pastore, S.; Potapovich, A.; Kostyuk, V.; Mariani, V.; Lulli, D.; De Luca, C.; Korkina, L. Plant Polyphenols Effectively Protect HaCaT Cells from Ultraviolet C–Triggered Necrosis and Suppress Inflammatory Chemokine Expression. Ann. N.Y. Acad. Sci. 2009, 1171, 305–313. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.N.; Zhuo, J.Y.; Nie, J.; Liu, Y.L.; Chen, B.Y.; Wu, A.Z.; Li, Y.C. Phenylethanoid glycosides from callicarpa Kwangtungensis Chun attenuate TNF- alpha-induced cell damage by inhibiting NF-kappa B pathway and enhancing Nrf2 pathway in A549 cells. Front. Pharm. 2021, 12, 693983. [Google Scholar] [CrossRef]
- Jing, W.; Chunhua, M.; Shumin, W. Effects of acteoside on lipopolysaccharide-in duced inflammation in acute lung injury via regulation of NF-kappaB pathway in vivo and in vitro. Toxicol. Appl. Pharmacol. 2015, 285, 128–135. [Google Scholar] [CrossRef] [PubMed]
- Maltby, S.; Khazaie, K.; McNagny, K.M. Mast cells in tumor growth: Angiogenesis, tissue remodelling and immune-modulation. Biochim. Et Biophys. Acta (BBA)-Rev. Cancer 2009, 1796, 19–26. [Google Scholar] [CrossRef]
- Yoou, M.-S.; Kim, H.-M.; Jeong, H.-J. Acteoside attenuates TSLP-induced mast cell proliferation via down-regulating MDM2. Int. Immunopharmacol. 2015, 26, 23–29. [Google Scholar] [CrossRef] [PubMed]
- Yamada, P.; Iijima, R.; Han, J.; Shigemori, H.; Yokota, S.; Isoda, H. Inhibitory effect of acteoside isolated from Cistanche tubulosa on chemical mediator release and inflammatory cytokine production by RBL-2H3 and KU812 cells. Planta Med. 2010, 76, 1512–1518. [Google Scholar] [CrossRef]
- Motojima, H.; Villareal, M.O.; Iijima, R.; Han, J.; Isoda, H. Acteoside inhibits type Ι allergy through the down-regulation of Ca/NFAT and JNK MAPK signaling pathways in basophilic cells. J. Nat. Med. 2013, 67, 790–798. [Google Scholar] [CrossRef]
- Kim, S. Interleukin-32 in Inflammatory Autoimmune Diseases. Immune. Netw. 2014, 14, 123–127. [Google Scholar] [CrossRef]
- de Albuquerque, R.; Komsi, E.; Starskaia, I.; Ullah, U.; Lahesmaa, R. The role of Interleukin-32 in autoimmunity. Scand. J. Immunol. 2021, 93, e13012. [Google Scholar] [CrossRef]
- Jeong, H.J.; Shin, S.Y.; Oh, H.A.; Kim, M.H.; Cho, J.S.; Kim, H.M. IL-32 up-regulation is as sociated with inflammatory cytokine production in allergic rhinitis. J. Pathol. 2011, 224, 553–563. [Google Scholar] [CrossRef]
- Li, Y.; Yu, H.; Jin, Y.; Li, M.; Qu, C. Verbascoside Alleviates Atopic Dermatitis-Like Symptoms in Mice via Its Potent Anti-Inflammatory Effect. Int. Arch. Allergy. Immunol. 2018, 175, 220–230. [Google Scholar] [CrossRef]
- Wu, M.; Yu, S.; Chen, Y.; Meng, W.; Chen, H.; He, J.; Shen, J.; Lin, X. Acteoside promotes B cell-derived IL-10 production and ameliorates autoimmunity. J. Leukoc. Biol. 2022, 112, 875–885. [Google Scholar] [CrossRef]
- Schapoval, E.E.; Vargas, M.R.; Chaves, C.G.; Bridi, R.; Zuanazzi, J.A.; Henriques, A.T. Antiinflammatory and antinociceptive activities of extracts and isolated compounds from Stachytarpheta cayennensis. J. Ethnopharmacol. 1998, 60, 53–59. [Google Scholar] [CrossRef]
- Drechsler, S.; Osuchowski, M. Cecal Ligation and Puncture. Methods Mol. Biol. 2021, 2321, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Mazzon, E.; Esposito, E.; Di Paola, R.; Riccardi, L.; Caminiti, R.; Dal Toso, R.; Pressi, G.; Cuzzocrea, S. Effects of verbascoside biotechnologically produced by Syringa vulgaris plant cell cultures in a rodent model of colitis. Naunyn Schmiedebergs Arch. Pharmacol. 2009, 380, 79–94. [Google Scholar] [CrossRef] [PubMed]
- Esposito, E.; Mazzon, E.; Paterniti, I.; Dal Toso, R.; Pressi, G.; Caminiti, R.; Cuzzocrea, S. PPAR-alpha Contributes to the Anti-Inflammatory Activity of Verbascoside in a Model of Inflammatory Bowel Disease in Mice. PPAR Res. 2010, 2010, 917312. [Google Scholar] [CrossRef] [PubMed]
- Guo, W.; Wang, X.; Liu, F.; Chen, S.; Wang, S.; Zhang, Q.; Yuan, L.; Du, S. Acteoside alleviates dextran sulphate sodium-induced ulcerative colitis via regulation of the HO-1/HMGB1 signaling pathway. Mol. Med. Rep. 2022, 26, 360. [Google Scholar] [CrossRef]
- Li, W.; Deng, R.; Jing, X.; Chen, J.; Yang, D.; Shen, J. Acteoside ameliorates experimental autoimmune encephalomyelitis through inhibiting peroxynitrite-mediated mitophagy activation. Free Radic. Biol. Med. 2020, 146, 79–91. [Google Scholar] [CrossRef]



| The Source of Verbascoside (Plant Extract/Compound) | Tested Concentrations | Biological Effect | Cell Line/Cells | Refs. |
|---|---|---|---|---|
| Clerodendron trichotomum | 1–10 µM | Inhibition of release of AA and histamine, and PGE2 production; competitive inhibition of cytosolic Ca2+-dependent PLA2 | RBL-2H3 | [200,201] |
| Verbascoside | 10 μg/mL | Inhibition of NO production | RAW 264.7 | [204] |
| Wendita calysina | 0.05 mg/mL, 0.1 mg/mL and 0.5 mg/mL | Inhibition of NO production | J774.A1 | [206] |
| Buddleja officinalis | 10 µM, 30 µM, 100 µM | Inhibition of iNOS gene expression; suppression of NF-kB and AP-1 activation | RAW 264.7 | [205] |
| Verbascum thapsus | 50 µM | Downregulation of expression and activity of COX2 and iNOS; downregulation of TAK-1, JNK, AP-1 activation via activation SHP-1 | U937 | [202] |
| Verbascoside | 1 µM, 10 µM, 100 µM | Inhibition of NF-KB pathway | A549 | [217] |
| Scrophularia scorodonia | 25 µM, 50 µM, 100 µM | Inhibition of NO, PGE2, and TNF-α production | Mouse peritoneal macrophages | [208] |
| Buddleja officinalis | 1 µmol/L, 10 µmol/L, 100 µmol/L | Inhibition of NO, TNF-α, and IL-1β production | HUVEC | [104] |
| Verbascum thapsus | 100 µM | Downregulation of expression and activity of iNOS, O2− formation, and SOD, CAT and GPx activity; diminished expression of NF-kB | THP-1 | [212] |
| Anisomeles indica | 40 µM | Inhibition of NO, IL-12, and TNF-α production | Mouse peritoneal macrophages | [209] |
| Kigelia africana | 0.1–1 mM | Inhibition of NO production | J774.A1 | [207] |
| Verbascoside | 10 µM, 50 µM, 100 µM | Inhibition of HMBG1 release, expression of iNOS and production of NO; increase of HO-1 expression; induction of p38 MAPK/Nrf2 signal pathways | RAW 264.7 | [203] |
| Verbascoside | 10 µM, 50 µM, 100 µM | Inhibition of IL-6, IL-12, TNF-α, and IFN-γ production; enhanced viability; inhibition of JAK/STAT signaling pathway; enhanced Bcl2 expression and dampened Bax/cleaved-caspase 3 expression | IL-1beta stimulated primary rat chondrocytes | [213] |
| Syringa vulgaris vegetal cells | 0.1 µM, 1 µM, 10 µM, 50 µM | Inhibition of CXCl10/ IP-10 and CXCL8/IL-8 production; impaired NF-κB and AP-1 DNA binding activity | HaCaT | [215] |
| Syringa vulgaris IRBSV25/B cells | 10 μg/mL, 50 μg/mL, 100 μg/ml | Inhibition of iNOS expression and NO production, inhibition of COX2 expression; inhibition of the activation of NF-kB and ERK MAPK signaling pathway | C6 | [211] |
| Callicarpa kwangtungensis | 10 μM, 20 μM, 40 μM | Inhibited apoptosis; decreased expression of IL-1β, IL-8, IL-6; decreased levels of caspase -3, -8, -9; upregulated HO-1, GCLC, and NQO1; upregulated expression of Keap1, enhanced activation of Nrf2, decreased expression of p-IκBα and nuclear p65 | TNF-α stimulated A549 | [216] |
| Radix Rehmanniae | 25 μM, 50 μM | Promoted production of IL-10; enhanced PI3K/Akt signaling | LPS-stimulated human and murine B cells | [226] |
| Cistanche deserticola | 1 μM, 10 μM, 50 μM | Increased production of IL-10; decreased production of IL-12, and TNF-α; enhanced expansion of Foxp3 Tregs; AhR activation | LPS-stimulated mouse BMDC | [214] |
| Cistanche tubulosa | 0.1 μg/mL, 1.0 μg/mL, 10.0 μg/mL | Inhibited β-hexosaminidase release and decreased intracellular Ca2+ level in RBL-2H3 cells; inhibited histamine release and TNF-α and IL-4 production in KU812 cells | A23187 plus PMA- or 48/80-stimulated KU812 and IgE-sensitized RBL-2H3 cells | [220] |
| Cistanche tubulosa | 0.1 μg/mL, 1.0 μg/mL | Downregulated expression of CCL1-4, FCER1A, and NFATC1 genes; decreased JNK phosphorylation and inhibition of the MAPK pathway | A23187 plus PMA- PMA-stimulated KU812 | [221] |
| Verbascoside | 0.1 μg/mL, 1.0 μg/mL | Inhibited expression of TSLP, IL-1β, TNF-α, and IL-8, and expression of NO and iNOS, and decreased caspase-1 activation in THP-1 cells; inhibited production of TNF-α in peritoneal macrophages; suppressed nuclear translocation and binding activities of NF-kB and reduced phosphorylation of IƘB-α | IL-32- or LPS-stimulated THP-1 cells and mouse peritoneal macrophages | [210] |
| Verbascoside | 0.1 μg/mL, 1.0 μg/mL | Downregulation of MDM2 and upregulation of p53; reduced production of IL-13, IL-6, TNF-α, and IL-1β; induced activation of caspase-3, the cleavage of poly-ADP-ribose polymerase, and reduction of the procaspase-3 and Bcl2; inhibited expression of TSLP receptor and IL-7 R; increased phosphorylation of STAT 5 and 6 | TSLP-stimulated HMC-1 cells | [219] |
| Verbascoside | 5 µM, 10 µM | Suppressed expression of CD86 and CD54; reduced production of TNF-α and IL-6; downregulated activation (phosphorylation) of p65 and IκBα | DNCB-stimulated THP-1 | [225] |
| The Source of Verbascoside (Plant Extract/Compound) | Tested Doses | Biological Effect | Animal Model | Ref |
|---|---|---|---|---|
| Verbascoside | 30 mg/kg, 60 mg/kg; i.p. | Decreased lung wet-to-dry weight ratio and lung MPO activity; ameliorated histopathological changes; increased SOD level; inhibited MDA content, total cell and neutrophil infiltrations, and levels of pro-inflammatory cytokines (TNF-α, IL-1β, IL-6) in BALF | LPS-induced acute lung injury in BALB/c mice | [217] |
| Stachytarpheta cayennensis | 100 mg/kg, 150 mg/kg; p.o. | Inhibition of edema formation | Carrageenan-induced rat paw edema | [227] |
| Verbascoside | 100 mg/kg; i.p. | Increased survival; decreased levels of HMBG1 in serum and lung | CLP-induced sepsis in BALB/c mice | [203] |
| Verbascoside | 100 mg/kg; i.p. | Reduced production of IL-1β, IL-6, IL-12, TNF-α and IFN-γ in synovial fluid; enhanced Bcl2 expression and decreased Bax and cleaved caspase 3 expression; inhibition of JAK/STAT signaling | OA surgery model in SD rats | [213] |
| Syringa vulgaris IRBSV25/B cell cultures | 0.2 mg/kg, 2 mg/kg; p.o. | Reduced macroscopic damage score, reduced weight loss, MPO activity, thiobarbituric acid-reactant substances, expression of TNF-α, Il-1β, ICAM-1, P-selectin, iNOS, poly(ADP ribose), NF-κB p65 nuclear levels, and activity of pro-active form of MMP 2 and MMP-9 activity | DNBS-induced colitis in SD rats | [229] |
| Syringa vulgaris IRBSV25/B cell cultures | 2 mg/kg; p.o. | Reduces the microscopic and macroscopic signs; inhibited neutrophil infiltration, intestinal permeability, and colon injury more significantly in PPAR-αWT mice | DNBS-induced colitis in PPAR-αKO mice | [230] |
| Acanthus ilicifolius var. xiamenensis | 100 mg/kg, 200 mg/kg; p.o. | Reduced weight loss and DAI score, suppressed colon shortening, alleviated colon pathological injury; up-regulated IL-10, down-regulated IL-1β and TNF-α; decreased MDA and NO, and increased GSH, SOD, and Nrf2 and HO-1 protein in colon; activated Nrf2, and inhibited protein expression of JAK2/STAT3, NF-κB p65, IKKα/β, and IKB in colon | DSS-induced colitis in C57BL/6 mice | [85] |
| Verbascoside | 30 mg/kg, 60 mg/kg; i.p. | Reversal in body weight loss, colon shortening, DAI score, inflammation, oxidative stress, and colonic barrier dysfunction; inhibited apoptosis in the colon; down-regulated protein expression of HMGB1, and up-regulated HO-1 in colon | DSS-induced colitis in C57BL/6 mice data | [231] |
| Radix Rehmanniae | 10 mk/kg; p.o. | Improved salivary flow rate; reduced serum levels of anti- SSA and anti- M3 muscarinic receptor IgG antibodies; reduced IL-17 and increased IL-10 in serum; increased effector T cells (Th1, Th17, Tfh) in LNs, promoted production of IL-10 from B regulatory cells and TLR4+CXCR4+ plasma cells in spleen | Experimental Sjogren’s syndrome in C57BL/6 N | [226] |
| Cistanche deserticola | 25 mg/kg, 50 mg/kg; p.o. | Reduced levels of IL-4, IL-5, IL-13; increased IL-10 and TGF-β; promoted CD4+CD25+Foxp3+ Treg differentiation; suppressed specific T cell proliferation; reduced OVA-specific IgE production; attenuated accumulation of inflammatory cells in lungs and development of airway hyperresponsiveness | OVA-induced allergic asthma in mice data | [214] |
| Verbascoside | 1%, topical application | Relieved AD-like symptoms (scratching and skin lesion severity), reduced the levels of total IgE and DNCB-specific IgE, and IL-4 and IL-13 in serum; decreased TNF-α, IL-6, and IL-4 in skin lesions | DNCB-induced AD model in BALB/c mice | [225] |
| Radix Rehmanniae | prevention: 30 mg/kg treatment: 5 mg/kg, 10 mg/kg, 30 mg/kg; p.o. | Attenuated disease severity and progress as prophylactic or therapeutic; in SC: reduced inflammatory infiltration and demyelination; in spleen: reduced percentages of Ly6+ cells, CD11b+ cells, and CD4+ cells, and reduced levels of IL-6, TLR4, INF-γ, iNOS, IL1-β, CCL-20, CXCL-1-2, CXCL-11 12; in CNS: reduced percentages of Ly6+ cells, CD11b+ cells, and CD4+ cells, and IL-1ra, IL-1α, IL-5, IL-7, IL-12, IL-15, IL-27, IL-28, TNF-α, G-CSF, osteopontin, VCAM-1, ICAM-1, pentraxin 3, CCL-5, CXCL-5, CXCL-10, CXCL-11, CXCL-13; inhibited oxidative stress and suppressed mitochondria damages in SC | EAE in C57BL/6N mice | [232] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marčetić, M.; Bufan, B.; Drobac, M.; Antić Stanković, J.; Arsenović Ranin, N.; Milenković, M.T.; Božić, D.D. Multifaceted Biological Properties of Verbascoside/Acteoside: Antimicrobial, Cytotoxic, Anti-Inflammatory, and Immunomodulatory Effects. Antibiotics 2025, 14, 697. https://doi.org/10.3390/antibiotics14070697
Marčetić M, Bufan B, Drobac M, Antić Stanković J, Arsenović Ranin N, Milenković MT, Božić DD. Multifaceted Biological Properties of Verbascoside/Acteoside: Antimicrobial, Cytotoxic, Anti-Inflammatory, and Immunomodulatory Effects. Antibiotics. 2025; 14(7):697. https://doi.org/10.3390/antibiotics14070697
Chicago/Turabian StyleMarčetić, Mirjana, Biljana Bufan, Milica Drobac, Jelena Antić Stanković, Nevena Arsenović Ranin, Marina T. Milenković, and Dragana D. Božić. 2025. "Multifaceted Biological Properties of Verbascoside/Acteoside: Antimicrobial, Cytotoxic, Anti-Inflammatory, and Immunomodulatory Effects" Antibiotics 14, no. 7: 697. https://doi.org/10.3390/antibiotics14070697
APA StyleMarčetić, M., Bufan, B., Drobac, M., Antić Stanković, J., Arsenović Ranin, N., Milenković, M. T., & Božić, D. D. (2025). Multifaceted Biological Properties of Verbascoside/Acteoside: Antimicrobial, Cytotoxic, Anti-Inflammatory, and Immunomodulatory Effects. Antibiotics, 14(7), 697. https://doi.org/10.3390/antibiotics14070697











