Exploring the Prevalence of Antimicrobial Resistance in the Environment Through Bonelli’s Eagles (Aquila fasciata) as Sentinels
Abstract
1. Introduction
2. Results
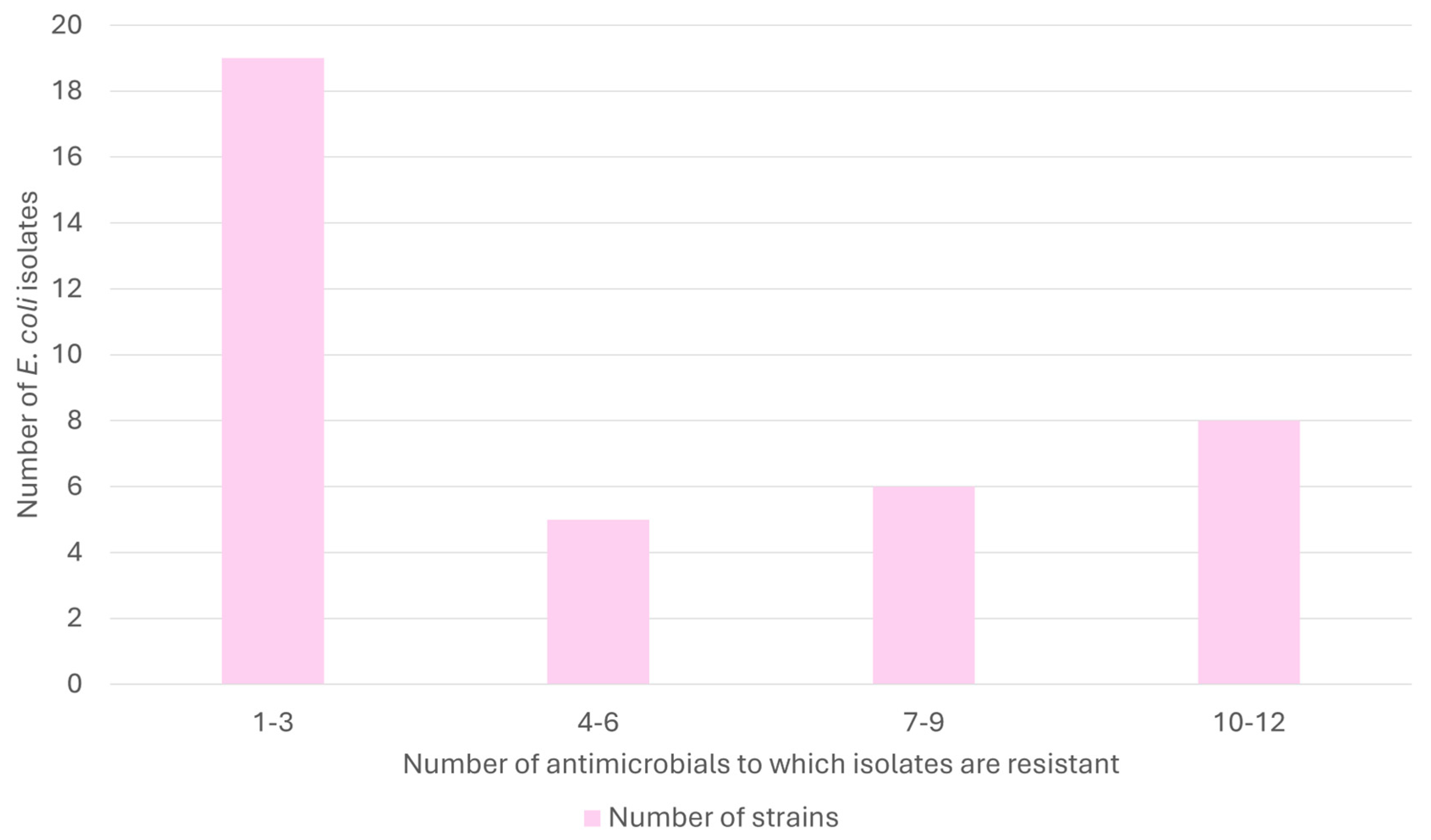
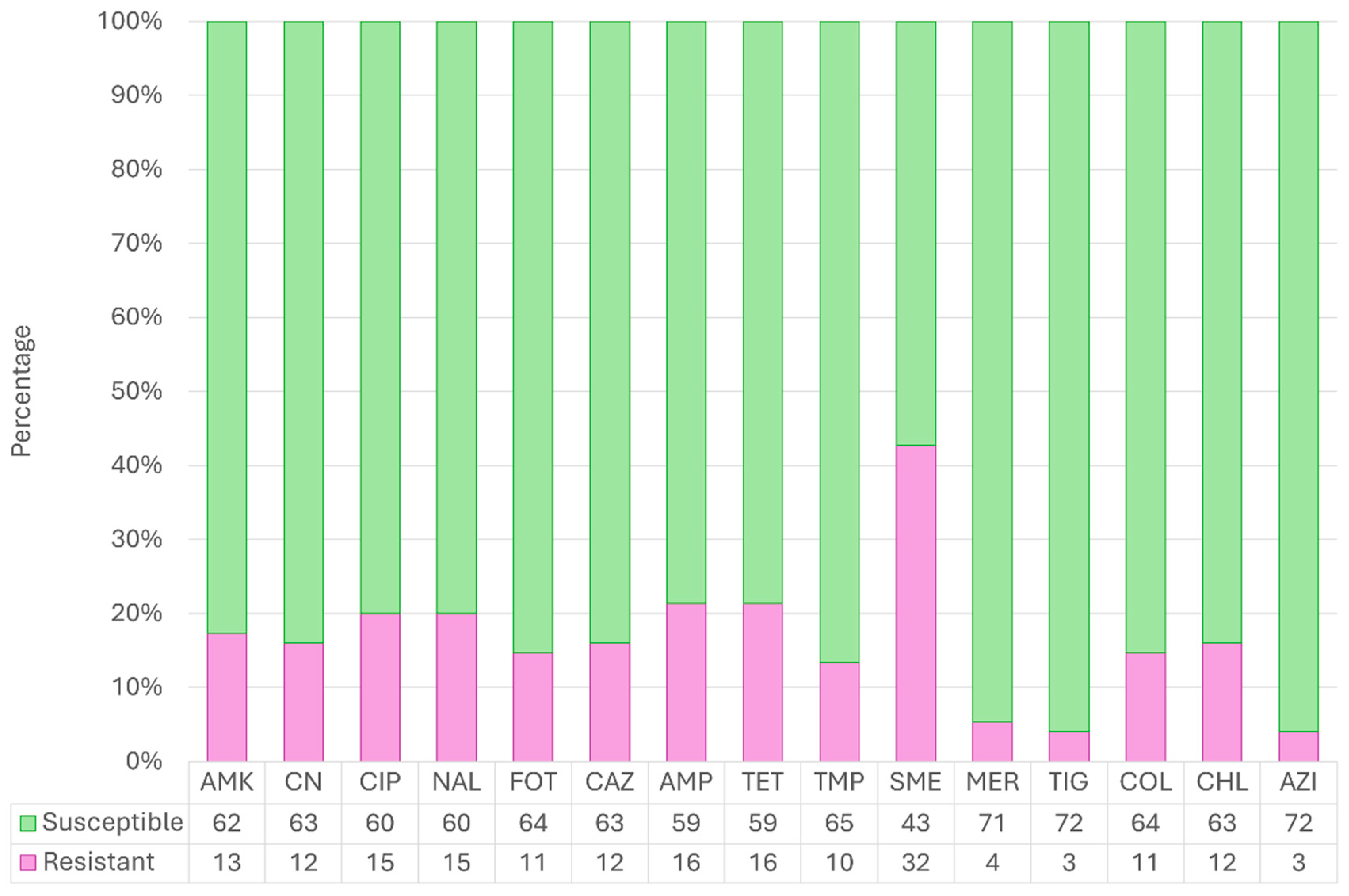
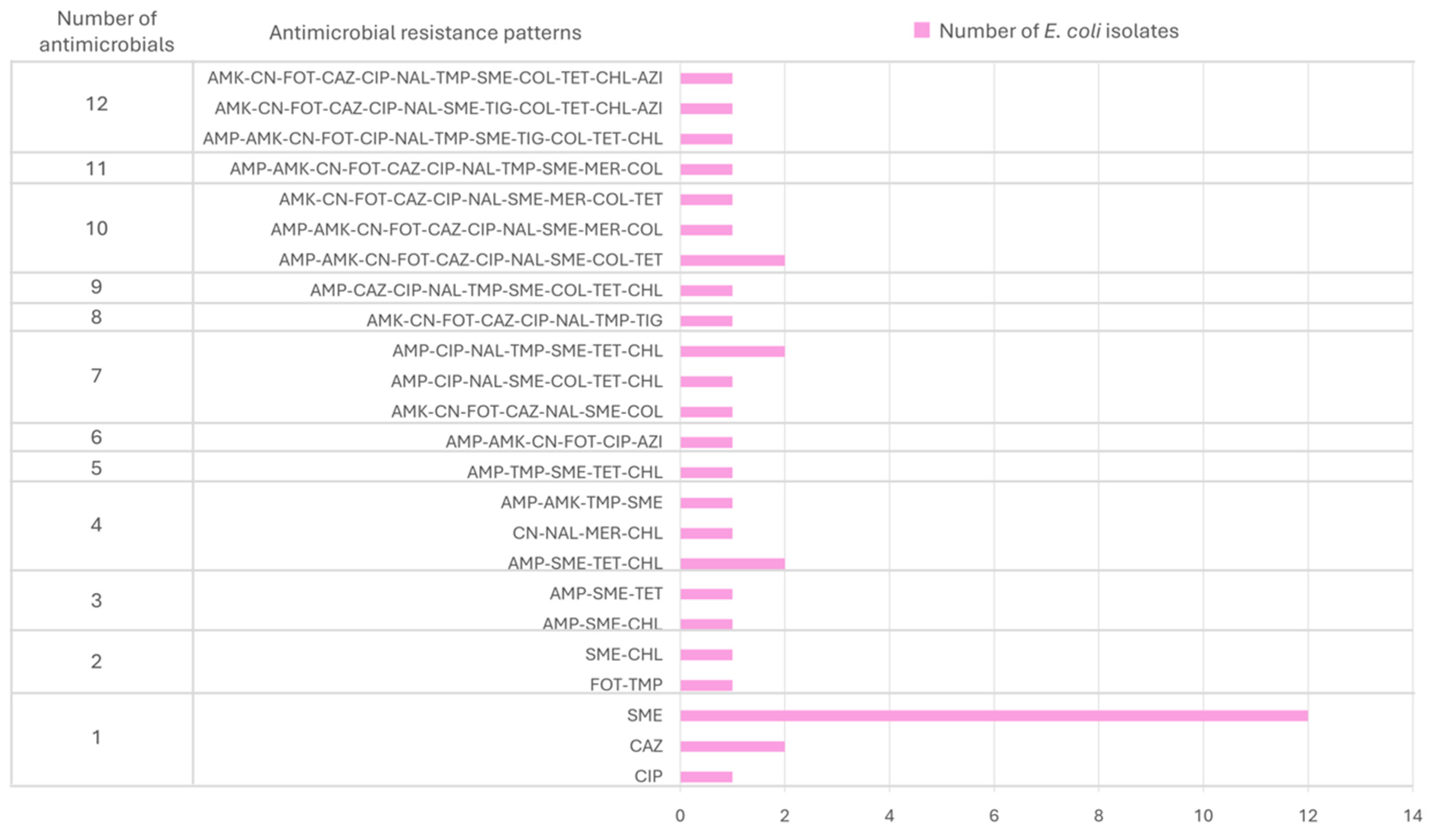
2.1. Global Antimicrobial Resistance Results
2.2. Antimicrobial Resistance Fluctuation Through the Years
3. Discussion
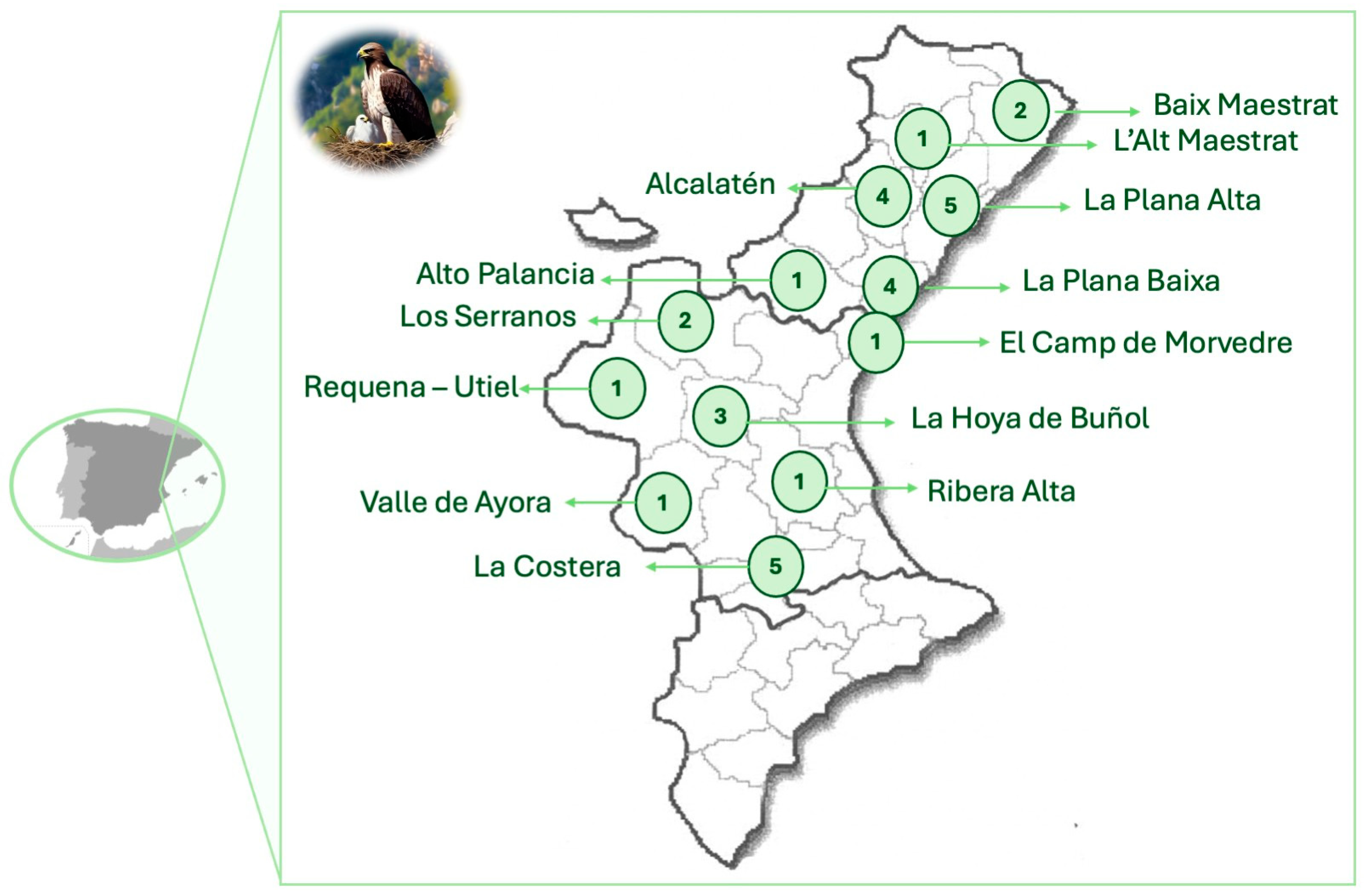
4. Materials and Methods
4.1. Study Population and Sampling Collection
4.2. Escherichia coli Isolation and Characterization
4.3. Antimicrobial Susceptibility Test
4.4. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AMR | Antimicrobial resistance |
| ARB | Antimicrobial-resistant bacteria |
| BPW | Buffered peptone water |
| EUCAST | European Committee on Antimicrobial Susceptibility Testing |
| EPEC | Enteropathogenic E. coli |
| GLM | General Linear Model |
| MDR | Multidrug-resistant |
| MIC | Minimum inhibitory concentration |
| IUCN | International Union for Conservation of Nature |
| STEC | Shiga-toxigenic E. coli |
| TBX | X-glucuronide agar |
References
- O’Neill, J. Antimicrobial Resistance: Tackling a Crisis for the Health and Wealth of Nations; HM Government: London, UK, 2014.
- Chowdhury, G.; Ramamurthy, T. Influence of Antimicrobials on the Gut Microbiota. In Antimicrobial Resistance: Global Challenges and Future Interventions; Thomas, S., Ed.; Springer: Singapore, 2020; pp. 53–79. [Google Scholar] [CrossRef]
- Almansour, A.M.; Alhadlaq, M.A.; Alzahrani, K.O.; Mukhtar, L.E.; Alharbi, A.L.; Alajel, S.M. The silent threat: Antimicrobial-resistant pathogens in food-producing animals and their impact on public health. Microorganisms 2023, 11, 2127. [Google Scholar] [CrossRef] [PubMed]
- Hwengwere, K.; Paramel Nair, H.; Hughes, K.A.; Peck, L.S.; Clark, M.S.; Walker, C.A. Antimicrobial resistance in Antarctica: Is it still a pristine environment? Microbiome 2022, 10, 71. [Google Scholar] [CrossRef] [PubMed]
- Murray, C.J.; Ikuta, K.S.; Sharara, F.; Swetschinski, L.; Aguilar, G.R.; Gray, A.; Han, C.; Bisignano, C.; Rao, P.; Wool, E.; et al. Global burden of bacterial antimicrobial resistance in 2019: A systematic analysis. Lancet 2022, 399, 629–655. [Google Scholar] [CrossRef] [PubMed]
- Paul, R.J.; Varghese, D. AMR in animal health: Issues and One Health solutions for LMICs. In Antimicrobial Resistance: Global Challenges and Future Interventions; Thomas, S., Ed.; Springer: Singapore, 2020; pp. 135–149. [Google Scholar] [CrossRef]
- Dadgostar, P. Antimicrobial resistance: Implications and costs. Infect. Drug Resist. 2019, 12, 3903–3910. [Google Scholar] [CrossRef] [PubMed]
- EFSA (European Food Safety Authority); ECDC (European Centre for Disease Prevention and Control). The European Union summary report on antimicrobial resistance in zoonotic and indicator bacteria from humans, animals and food in 2021–2022. EFSA J. 2024, 22, e8583. [Google Scholar] [CrossRef] [PubMed]
- Tardón, A.; Bataller, E.; Llobat, L.; Jiménez-Trigos, E. Bacteria and antibiotic resistance detection in fractures of wild birds from wildlife rehabilitation centres in Spain. Comp. Immunol. Microbiol. Infect. Dis. 2021, 74, 101575. [Google Scholar] [CrossRef] [PubMed]
- Sacristán, I.; Esperón, F.; Acuña, F.; Aguilar, E.; García, S.; López, M.J.; Cevidanes, A.; Neves, E.; Cabello, J.; Hidalgo-Hermoso, E. Antibiotic resistance genes as landscape anthropization indicators: Using a wild felid as sentinel in Chile. Sci. Total Environ. 2020, 703, 134900. [Google Scholar] [CrossRef] [PubMed]
- Stevenson, P. Links between industrial livestock production, disease including zoonoses and antimicrobial resistance. Anim. Res. One Health 2023, 1, 137–144. [Google Scholar] [CrossRef]
- Caneschi, A.; Bardhi, A.; Barbarossa, A.; Zaghini, A. The Use of Antibiotics and Antimicrobial Resistance in Veterinary Medicine, a Complex Phenomenon: A Narrative Review. Antibiotics 2023, 12, 487. [Google Scholar] [CrossRef] [PubMed]
- Plaza, P.I.; Lambertucci, S.A. How are garbage dumps impacting vertebrate demography, health, and conservation? Glob. Ecol. Conserv. 2017, 12, 9–20. [Google Scholar] [CrossRef]
- Rossi, F.; Péguilhan, R.; Turgeon, N.; Veillette, M.; Baray, J.L.; Deguillaume, L.; Amato, P.; Duchaine, C. Quantification of antibiotic resistance genes (ARGs) in clouds at a mountain site (puy de Dôme, central France). Sci. Total Environ. 2023, 865, 161264. [Google Scholar] [CrossRef] [PubMed]
- Vargas-Villalobos, S.; Hernández, F.; Fabregat-Safont, D.; Salas-González, D.; Quesada-Alvarado, F.; Botero-Coy, A.M.; Esperón, F.; Martín-Maldonado, B.; Monrós-Gonzalez, J.; Ruepert, C.; et al. A case study on pharmaceutical residues and antimicrobial resistance genes in Costa Rican rivers: A possible route of contamination for feline and other species. Environ. Res. 2024, 242, 117665. [Google Scholar] [CrossRef] [PubMed]
- Guitart-Matas, J.; Espunyes, J.; Illera, L.; Gonzalez-Escalona, N.; Ribas, M.P.; Marco, I.; Migura-Garcia, L. High-risk lineages of extended spectrum cephalosporinase producing Escherichia coli from Eurasian griffon vultures (Gyps fulvus) foraging in landfills in north-eastern Spain. Sci. Total Environ. 2024, 909, 168625. [Google Scholar] [CrossRef] [PubMed]
- Mukerji, S.; Sahibzada, S.; Abraham, R.; Stegger, M.; Jordan, D.; Hampson, D.J.; O’Dea, M.; Lee, T.; Abraham, S. Proximity to human settlement is directly related to carriage of critically important antimicrobial-resistant Escherichia coli and Klebsiella pneumoniae in Silver Gulls. Vet. Microbiol. 2023, 280, 109702. [Google Scholar] [CrossRef] [PubMed]
- Martín-Maldonado, B.; Vega, S.; Mencía-Gutiérrez, A.; Lorenzo-Rebenaque, L.; De Frutos, C.; González, F.; Revuelta, L.; Marin, C. Urban birds: An important source of antimicrobial resistant Salmonella strains in Central Spain. Comp. Immunol. Microbiol. Infect. Dis. 2020, 72, 101519. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Pal, D. Antibiotic resistance and wastewater: Correlation, impact and critical human health challenges. J. Environ. Chem. Eng. 2018, 6, 52–58. [Google Scholar] [CrossRef]
- Lagerstrom, K.M.; Hadly, E.A. The under-investigated wild side of Escherichia coli: Genetic diversity, pathogenicity and antimicrobial resistance in wild animals. Proc. R. Soc. B 2021, 288, 20210399. [Google Scholar] [CrossRef] [PubMed]
- Tîrziu, E.; Bulucea, A.V.; Imre, K.; Nichita, I.; Muselin, F.; Dumitrescu, E.; Tîrziu, A.; Mederle, N.G.; Moza, A.; Bucur, I.M.; et al. The Behavior of Some Bacterial Strains Isolated from Fallow Deer Compared to Antimicrobial Substances in Western Romania. Antibiotics 2023, 12, 743. [Google Scholar] [CrossRef] [PubMed]
- Badry, A.; Palma, L.; Beja, P.; Ciesielski, T.M.; Dias, A.; Lierhagen, S.; Jenssen, B.M.; Sturaro, N.; Eulaers, I.; Jaspers, V.L. Using an apex predator for large-scale monitoring of trace element contamination: Associations with environmental, anthropogenic, and dietary proxies. Sci. Total Environ. 2019, 676, 746–755. [Google Scholar] [CrossRef] [PubMed]
- Conde de Dios, M. Establecimiento y Dinámica del Territorio del Águila de Bonelli (Aquila fasciata) en la Isla de Mallorca. Master’s Thesis, Facultad de Ciencias Biológicas, Universidad Complutense de Madrid, Madrid, Spain, 2018. [Google Scholar]
- Ontiveros, D. Águila perdicera Hieraaetus fasciatus. In Enciclopedia Virtual de los Vertebrados Españoles; Carrascal, L.M., Salvador, A., Eds.; Museo Nacional de Ciencias Naturales: Madrid, Spain, 2016. [Google Scholar]
- BirdLife International. Aquila fasciata (Amended Version of 2016 Assessment); The IUCN Red List of Threatened Species: Cambridge, UK, 2019. [Google Scholar] [CrossRef]
- Ahmed, N.A.; Gulhan, T. Determination of antibiotic resistance patterns and genotypes of Escherichia coli isolated from wild birds. Microbiome 2024, 12, 8. [Google Scholar] [CrossRef] [PubMed]
- Martín-Maldonado, B.; Rodríguez-Alcázar, P.; Fernández-Novo, A.; González, F.; Pastor, N.; López, I.; Suárez, L.; Moraleda, V.; Aranaz, A. Urban birds as antimicrobial resistance sentinels: White storks showed higher multidrug-resistant Escherichia coli levels than seagulls in Central Spain. Animals 2022, 12, 2714. [Google Scholar] [CrossRef] [PubMed]
- Chung, D.M.; Ferree, E.; Simon, D.M.; Yeh, P.J. Patterns of bird–bacteria associations. Ecohealth 2018, 15, 627–641. [Google Scholar] [CrossRef] [PubMed]
- Giacopello, C.; Foti, M.; Mascetti, A.; Grosso, F.; Ricciardi, D.; Fisichella, V.; Lo Piccolo, F. Antimicrobial resistance patterns of Enterobacteriaceae in European wild bird species admitted in a wildlife rescue centre. Vet. Ital. 2016, 52, 139–144. [Google Scholar] [CrossRef] [PubMed]
- Russo, T.P.; Minichino, A.; Gargiulo, A.; Varriale, L.; Borrelli, L.; Pace, A.; Santaniello, A.; Pompameo, M.; Fioretti, A.; Dipineto, L. Prevalence and phenotypic antimicrobial resistance among ESKAPE bacteria and Enterobacterales strains in wild birds. Antibiotics 2022, 11, 1825. [Google Scholar] [CrossRef] [PubMed]
- Martín-Maldonado, B.; Montoro-Dasi, L.; Pérez-Gracia, M.T.; Jordá, J.; Vega, S.; Marco-Jiménez, F.; Marin, C. Wild Bonelli’s eagles (Aquila fasciata) as carrier of antimicrobial resistant Salmonella and Campylobacter in Eastern Spain. Comp. Immunol. Microbiol. Infect. Dis. 2019, 67, 101372. [Google Scholar] [CrossRef] [PubMed]
- Mencía-Gutiérrez, A.; Martín-Maldonado, B.; Pastor-Tiburón, N.; Moraleda, V.; González, F.; García-Peña, F.J.; Pérez-Cobo, I.; Revuelta, L.; Marín, M. Prevalence and antimicrobial resistance of Campylobacter from wild birds of prey in Spain. Comp. Immunol. Microbiol. Infect. Dis. 2021, 79, 101712. [Google Scholar] [CrossRef] [PubMed]
- Fearon, M.L.; Wood, C.L.; Tibbetts, E.A. Habitat quality influences pollinator pathogen prevalence through both habitat–disease and biodiversity–disease pathways. Ecology 2023, 104, e3933. [Google Scholar] [CrossRef] [PubMed]
- Timmis, K.; Verstraete, W.; Regina, V.R.; Hallsworth, J.E. The Pareto principle: To what extent does it apply to resource acquisition in stable microbial communities and thereby steer their geno−/ecotype compositions and interactions between their members? Environ. Microbiol. 2023, 25, 1221–1231. [Google Scholar] [CrossRef] [PubMed]
- Rehman, M.U.; Zhang, H.; Iqbal, M.K.; Mehmood, K.; Huang, S.; Nabi, F.; Luo, H.; Lan, Y.; Li, J. Antibiotic resistance, serogroups, virulence genes, and phylogenetic groups of Escherichia coli isolated from yaks with diarrhea in Qinghai Plateau, China. Gut Pathog. 2017, 9, 24. [Google Scholar] [CrossRef] [PubMed]
- WHO. WHO List of Medically Important Antimicrobials: A Risk Management Tool for Mitigating Antimicrobial Resistance Due to Non-Human Use; WHO: Geneva, Switzerland, 2024; ISBN 978-92-4-008462-9. Available online: https://www.who.int/news/item/08-02-2024-who-medically-important-antimicrobial-list-2024 (accessed on 13 February 2025).
- Magalhães, R.; Tavares, L.; Oliveira, M. Antimicrobial Resistance and Virulence Potential of Bacterial Species from Captive Birds of Prey—Consequences of Falconry for Public Health. Animals 2024, 14, 856. [Google Scholar] [CrossRef] [PubMed]
- Musa, L.; Stefanetti, V.; Casagrande Proietti, P.; Grilli, G.; Gobbi, M.; Toppi, V.; Brustenga, L.; Magistrali, C.F.; Franciosini, M.P. Antimicrobial Susceptibility of Commensal E. coli Isolated from Wild Birds in Umbria (Central Italy). Animals 2023, 13, 1776. [Google Scholar] [CrossRef] [PubMed]
- Bosch, R.; Real, J.; Tinto, A.; Zozaya, E.L.; Castell, C. Home-ranges and patterns of spatial use in territorial Bonelli’s Eagles Aquila fasciata. Ibis 2010, 152, 105–117. [Google Scholar] [CrossRef]
- Athanasakopoulou, Z.; Diezel, C.; Braun, S.D.; Sofia, M.; Giannakopoulos, A.; Monecke, S.; Gary, D.; Krähmer, D.; Chatzopoulos, D.C.; Touloudi, A.; et al. Occurrence and characteristics of ESBL-and carbapenemase-producing Escherichia coli from wild and feral birds in Greece. Microorganisms 2022, 10, 1217. [Google Scholar] [CrossRef] [PubMed]
- Suárez-Pérez, A.; Corbera, J.A.; González-Martín, M.; Tejedor-Junco, M.T. Antimicrobial resistance patterns of bacteria isolated from chicks of Canarian Egyptian vultures (Neophron percnopterus majorensis): A “one health” problem? Comp. Immunol. Microbiol. Infect. Dis. 2023, 92, 101925. [Google Scholar] [CrossRef] [PubMed]
- PRAN (Plan Nacional contra la Resistencia a Antibóticos). Mapas de Consumo de Antibióticos. Available online: https://www.resistenciaantibioticos.es/es/lineas-de-accion/vigilancia/mapas-de-consumo (accessed on 12 February 2025).
- Palma, L.; Beja, P.; Pais, M.; Cancela Da Fonseca, L. Why do raptors take domestic prey? The case of Bonelli’s eagles and pigeons. J. Appl. Ecol. 2006, 43, 1075–1086. [Google Scholar] [CrossRef]
- Skarżyńska, M.; Zając, M.; Bomba, A.; Bocian, Ł.; Kozdruń, W.; Polak, M.; Wiącek, J.; Wasyl, D. Antimicrobial resistance glides in the sky—Free-living birds as a reservoir of resistant Escherichia coli with zoonotic potential. Front. Microbiol. 2021, 12, 656223. [Google Scholar] [CrossRef] [PubMed]
- Ahlstrom, C.A.; Woksepp, H.; Sandegren, L.; Mohsin, M.; Hasan, B.; Muzyka, D.; Hernandez, J.; Aguirre, F.; Tok, A.; Söderman, J.; et al. Genomically diverse carbapenem resistant Enterobacteriaceae from wild birds provide insight into global patterns of spatiotemporal dissemination. Sci. Total Environ. 2022, 824, 153632. [Google Scholar] [CrossRef] [PubMed]
- Sevilla, E.; Marín, C.; Delgado-Blas, J.F.; González-Zorn, B.; Vega, S.; Kuijper, E.; Bolea, R.; Mainar-Jaime, R.C. Wild griffon vultures (Gyps fulvus) fed at supplementary feeding stations: Potential carriers of pig pathogens and pig-derived antimicrobial resistance? Transbound. Emerg. Dis. 2020, 67, 1295–1305. [Google Scholar] [CrossRef] [PubMed]
- Tarabai, H.; Krejci, S.; Karyakin, I.; Bitar, I.; Literak, I.; Dolejska, M. Clinically relevant antibiotic resistance in Escherichia coli from black kites in southwestern Siberia: A genetic and phenotypic investigation. mSphere 2023, 8, e00099-23. [Google Scholar] [CrossRef] [PubMed]
- García-Meniño, I.; García, V.; Mora, A.; Díaz-Jiménez, D.; Flament-Simon, S.C.; Alonso, M.P.; Blanco, J.E.; Blanco, M.; Blanco, J. Swine enteric colibacillosis in Spain: Pathogenic potential of mcr-1 ST10 and ST131 E. coli isolates. Front. Microbiol. 2018, 9, 2659. [Google Scholar] [CrossRef] [PubMed]
- European Commission. Directive 2010/63/EU of the European Parliament and of the Council of 22 September 2010 on the Protection of Animals Used for Scientific Purposes. OJ 2010, L 276, 33–79. Available online: http://data.europa.eu/eli/dir/2010/63/oj (accessed on 11 March 2025).
- Dashti, A.A.; Jadaon, M.M.; Abdulsamad, A.M.; Dashti, H.M. Heat treatment of bacteria: A simple method of DNA extraction for molecular techniques. Kuwait Med. J. 2009, 41, 117–122. [Google Scholar]
- Hardegen, C.; Messler, S.; Henrich, B.; Pfeffer, K.; Würthner, J.; MacKenzie, C.R. A set of novel multiplex Taqman real-time PCRs for the detection of diarrhoeagenic Escherichia coli and its use in determining the prevalence of EPEC and EAEC in a university hospital. Ann. Clin. Microbiol. Antimicrob. 2010, 9, 5. [Google Scholar] [CrossRef] [PubMed]
- EUCAST. European Committee on Antimicrobial Susceptibility Testing (EUCAST), Breakpoint Tables for Interpretation of MICs and Zone Diameters, Version 14.0. 2024. Available online: http://www.eucast.org (accessed on 15 November 2024).
- CLSI. Performance Standards for Antimicrobial Susceptibility Testing, Part B. CLSI vs. FDA Breakpoints, 34th ed.; CLSI Supplement M100; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2024; Available online: https://clsi.org/resources/breakpoint-implementation-toolkit/ (accessed on 8 July 2024).

| Gene | Primers/Probe |
|---|---|
| eaeA | EAE-S For 5′-ACTGGACTTCTTATTRCCGTTCTATG EAE-B1 5′-CTAAGCGGGTATTGTTACCAGA-3′ EAE- 5′-6-Fam-AATCCTGATCAATGAAGACGTTATAGCCCA-BHQ-1-3′ |
| stx-1 | SLT1-1 5′-CTTCCATCTGCCGGACACATA-3′ SLT1-2 5′-ATTAATACTGAATTGTCATCATCATGC-3′ SLT1-S 5′-6-Fam-AAGGAAACTCATCAGATGCCATTCTGGCA-BHQ-1-3′ |
| stx-2 | SLT2-1 5′-GACGTGGACCTCACTCTGAACTG-3′ SLT2-2 5′-TCCCCACTCTGACACCATCC-3′ SLT2-S 5′-6-Fam-TACTCCGGAAGCACATTGCTGATTCGC-BHQ-1-3′ |
| Antimicrobial Class | Antimicrobial Molecule | Abbreviation | EMA Category * | Cut-off Values (μg/mL) ** |
|---|---|---|---|---|
| Aminoglycosides | Amikacin | AMK | C | >8 |
| Gentamicin | CN | C | >2 | |
| Quinolones | Ciprofloxacin | CIP | B | >0.5 |
| Nalidixic acid | NAL | B | >16 | |
| Cephalosporines | Cefotaxime | FOT | B | >4 |
| Ceftazidime | CAZ | B | >2 | |
| Penicillin | Ampicillin | AMP | D | >8 |
| Tetracycline | Tetracycline | TET | D | >8 |
| Pyrimidine | Trimethoprim | TMP | D | >4 |
| Sulfonamide | Sulfamethoxazole | SME | D | >64 |
| Carbapenem | Meropenem | MER | A | >8 |
| Glycylglycine | Tigecycline | TIG | A | >0.5 |
| Polymyxin | Colistin | COL | B | >2 |
| Amphenicol | Chloramphenicol | CHL | C | >16 |
| Macrolide | Azithromycin | AZI | C | >16 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martin-Maldonado, B.; Marco-Fuertes, A.; Montoro-Dasi, L.; Lorenzo-Rebenaque, L.; Sansano-Maestre, J.; Jordá, J.; Martín Solance, D.; Esperón, F.; Marin, C. Exploring the Prevalence of Antimicrobial Resistance in the Environment Through Bonelli’s Eagles (Aquila fasciata) as Sentinels. Antibiotics 2025, 14, 734. https://doi.org/10.3390/antibiotics14080734
Martin-Maldonado B, Marco-Fuertes A, Montoro-Dasi L, Lorenzo-Rebenaque L, Sansano-Maestre J, Jordá J, Martín Solance D, Esperón F, Marin C. Exploring the Prevalence of Antimicrobial Resistance in the Environment Through Bonelli’s Eagles (Aquila fasciata) as Sentinels. Antibiotics. 2025; 14(8):734. https://doi.org/10.3390/antibiotics14080734
Chicago/Turabian StyleMartin-Maldonado, Barbara, Ana Marco-Fuertes, Laura Montoro-Dasi, Laura Lorenzo-Rebenaque, Jose Sansano-Maestre, Jaume Jordá, Daniel Martín Solance, Fernando Esperón, and Clara Marin. 2025. "Exploring the Prevalence of Antimicrobial Resistance in the Environment Through Bonelli’s Eagles (Aquila fasciata) as Sentinels" Antibiotics 14, no. 8: 734. https://doi.org/10.3390/antibiotics14080734
APA StyleMartin-Maldonado, B., Marco-Fuertes, A., Montoro-Dasi, L., Lorenzo-Rebenaque, L., Sansano-Maestre, J., Jordá, J., Martín Solance, D., Esperón, F., & Marin, C. (2025). Exploring the Prevalence of Antimicrobial Resistance in the Environment Through Bonelli’s Eagles (Aquila fasciata) as Sentinels. Antibiotics, 14(8), 734. https://doi.org/10.3390/antibiotics14080734







