High Prevalence of Multidrug-Resistant Bacterial Colonization Among Patients and Healthcare Workers in a Rural Ethiopian Hospital
Abstract
1. Introduction
2. Results
2.1. Patient and Sample Characteristics
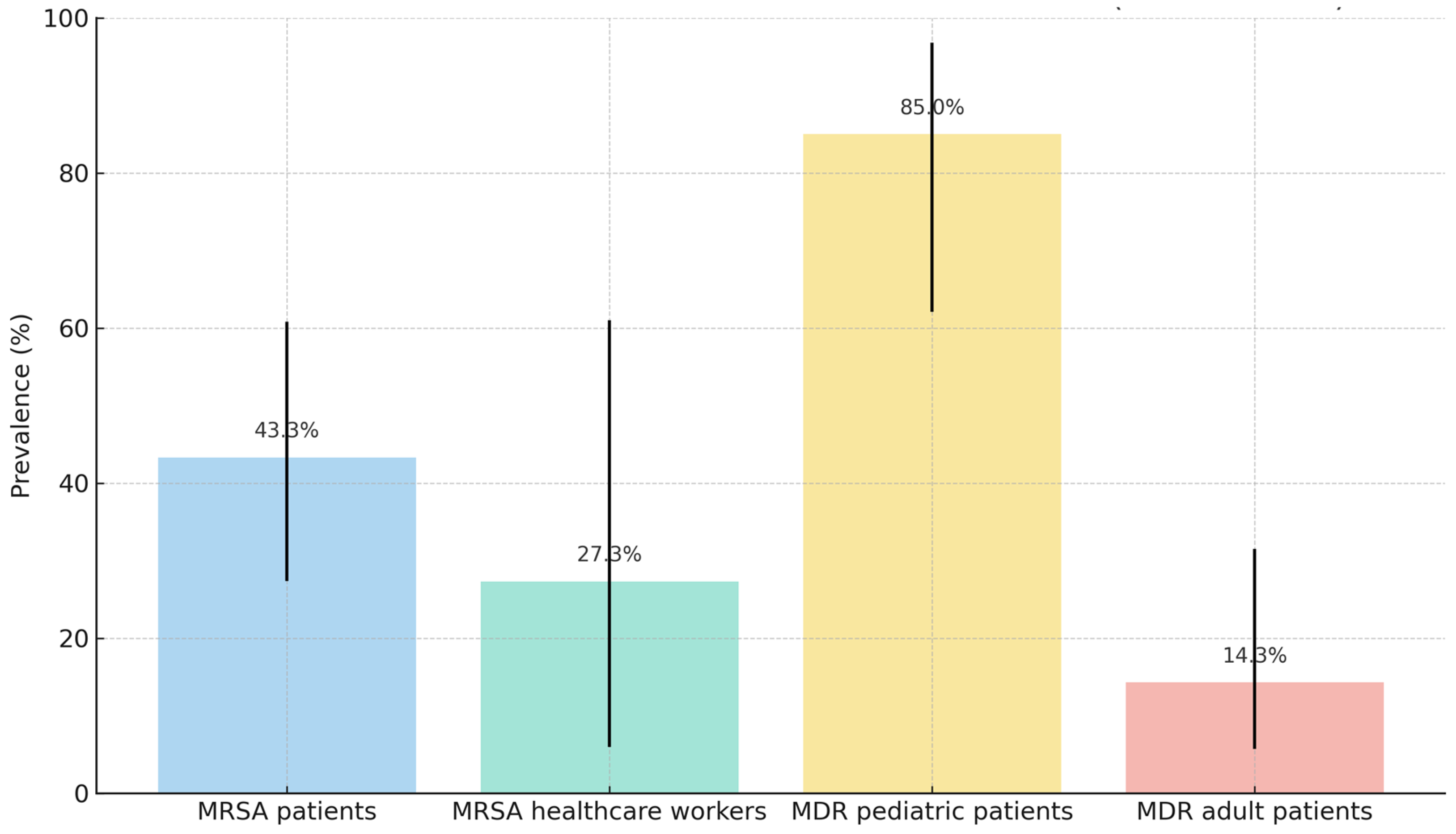
2.2. Nasal Colonization Study
2.3. Rectal Colonization Study
3. Discussion
4. Materials and Methods
Data Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| MDR | Multidrug-resistant |
| MRSA | Methicillin-resistant Staphylococcus aureus |
| VRE | Vancomycin-resistant Enterococcus spp. |
| CPE | Carbapenemase-producing Enterobacteriaceae |
| AMR | Antimicrobial resistance |
| WHO | World Health Organization |
| ESBL | Extended-spectrum beta-lactamase |
| LMICs | Low- and middle-income countries |
| MIC | Minimum inhibitory concentration |
| IQRs | Interquartile ranges |
References
- European Centre for Disease Prevention and Control (ECDC). Antimicrobial Resistance in the EU/EEA (EARS-Net)—Annual Epidemiological Report for 2022; ECDC: Stockholm, Sweden, 2024. Available online: https://www.ecdc.europa.eu/en/publications-data/surveillance-antimicrobial-resistance-europe-2022 (accessed on 23 October 2024).
- World Health Organization (WHO). WHO Bacterial Priority Pathogens List 2024: Bacterial Pathogens of Public Health Importance, to Guide Research, Development, and Strategies to Prevent and Control Antimicrobial Resistance, 1st ed.; WHO: Geneva, Switzerland, 2024; ISBN 978-92-4-009346-1. [Google Scholar]
- Jesudason, T. WHO publishes updated list of bacterial priority pathogens. Lancet Microbe 2024, 5, 100940. [Google Scholar] [CrossRef]
- Nordmann, P. Carbapenemase-producing Enterobacteriaceae: Overview of a major public health challenge. Med. Mal. Infect. 2014, 44, 51–56. [Google Scholar] [CrossRef]
- Ruppé, É.; Woerther, P.-L.; Barbier, F. Mechanisms of antimicrobial resistance in Gram-negative bacilli. Ann. Intensive Care 2015, 5, 61. [Google Scholar] [CrossRef] [PubMed]
- Tadesse, S.; Mulu, W.; Genet, C.; Kibret, M.; Belete, M.A.; Mascellino, M.T. Emergence of High Prevalence of Extended-Spectrum Beta-Lactamase and Carbapenemase-Producing Enterobacteriaceae Species among Patients in Northwestern Ethiopia Region. BioMed Res. Int. 2022, 2022, 5727638. [Google Scholar] [CrossRef] [PubMed]
- Ardanuy, C.; Cercenado, E.; Morosini, M.I.; Torres, C. Procedimientos en Microbiología Clínica. No 39, Detección Fenotípica de Mecanismos de Resistencia en Grampositivos; Sociedad Española de Enfermedades Infecciosas y Microbiología Clínica (SEIMC): Madrid, Spain, 2011; Available online: https://seimc.org/wp-content/uploads/2025/06/seimc-procedimientomicrobiologia39.pdf (accessed on 30 October 2024).
- Arthur, M.; Courvalin, P. Genetics and mechanisms of glycopeptide resistance in enterococci. Antimicrob. Agents Chemother. 1993, 37, 1563–1571. [Google Scholar] [CrossRef]
- Verdú-Expósito, C.; Romanyk, J.; Cuadros-González, J.; TesfaMariam, A.; Copa-Patiño, J.L.; Pérez-Serrano, J.; Soliveri, J.; del Campo, R. Study of susceptibility to antibiotics and molecular characterization of high virulence Staphylococcus aureus strains isolated from a rural hospital in Ethiopia. PLoS ONE 2020, 15, e0230031. [Google Scholar] [CrossRef]
- Siegel, J.D.; Rhinehart, E.; Jackson, M.; Chiarello, L. Management of multidrug-resistant organisms in health care settings, 2006. Am. J. Infect. Control 2007, 35, S165–S193. [Google Scholar] [CrossRef]
- Schwaber, M.J.; Lev, B.; Israeli, A.; Solter, E.; Smollan, G.; Rubinovitch, B.; Shalit, I.; Carmeli, Y.; the Israel Carbapenem-Resistant Enterobacteriaceae Working Group. Containment of a Country-wide Outbreak of Carbapenem-Resistant Klebsiella pneumoniae in Israeli Hospitals via a Nationally Implemented Intervention. Clin. Infect. Dis. 2011, 52, 848–855. [Google Scholar] [CrossRef] [PubMed]
- Swaminathan, M.; Sharma, S.; Blash, S.P.; Patel, G.; Banach, D.B.; Phillips, M.; LaBombardi, V.; Anderson, K.F.; Kitchel, B.; Srinivasan, A.; et al. Prevalence and Risk Factors for Acquisition of Carbapenem-Resistant Enterobacteriaceae in the Setting of Endemicity. Infect. Control Hosp. Epidemiol. 2013, 34, 809–817. [Google Scholar] [CrossRef]
- Lucet, J.-C.; Chevret, S.; Decré, D.; Vanjak, D.; Macrez, A.; Bédos, J.-P.; Wolff, M.; Regnier, B. Outbreak of Multiply Resistant Enterobacteriaceae in an Intensive Care Unit: Epidemiology and Risk Factors for Acquisition. Clin. Infect. Dis. 1996, 22, 430–436. [Google Scholar] [CrossRef][Green Version]
- Peña, C.; Pujol, M.; Ardanuy, C.; Ricart, A.; Pallares, R.; LiñaRes, J.; Ariza, J.; Gudiol, F. Epidemiology and Successful Control of a Large Outbreak Due to Klebsiella pneumoniae Producing ExtendedSpectrum β-Lactamases. Antimicrob. Agents Chemother. 1998, 42, 53–58. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization (WHO). Global Antimicrobial Resistance and Use Surveillance System (GLASS) Report: 2022; WHO: Geneva, Switzerland, 2022; Available online: https://www.who.int/publications/i/item/9789240062702 (accessed on 23 October 2024).
- Tadesse, B.T.; Ashley, E.A.; Ongarello, S.; Havumaki, J.; Wijegoonewardena, M.; González, I.J.; Dittrich, S. Antimicrobial resistance in Africa: A systematic review. BMC Infect. Dis. 2017, 17, 616. [Google Scholar] [CrossRef] [PubMed]
- Eshetie, S.; Tarekegn, F.; Moges, F.; Amsalu, A.; Birhan, W.; Huruy, K. Methicillin resistant Staphylococcus aureus in Ethiopia: A meta-analysis. BMC Infect. Dis. 2016, 16, 689. [Google Scholar] [CrossRef] [PubMed]
- Reta, A.; Mengist, A.; Tesfahun, A. Nasal colonization of methicillin resistant Staphylococcus aureus in Ethiopia: A systematic review and meta-analysis. Ann. Clin. Microbiol. Antimicrob. 2019, 18, 25. [Google Scholar] [CrossRef]
- Mekuriya, E.; Manilal, A.; Aklilu, A.; Woldemariam, M.; Hailu, T.; Wasihun, B. Methicillin-resistant Staphylococcus aureus colonization among medicine and health science students, Arba Minch University, Ethiopia. Sci. Rep. 2022, 12, 10161. [Google Scholar] [CrossRef]
- Gebremeskel, F.T.; Alemayehu, T.; Ali, M.M. Methicillin-resistant Staphylococcus aureus antibiotic susceptibility profile and associated factors among hospitalized patients at Hawassa University Comprehensive Specialized Hospital, Ethiopia. IJID Reg. 2022, 3, 129–134. [Google Scholar] [CrossRef]
- Rodríguez-Villodres, Á.; Martín-Gandul, C.; Peñalva, G.; Guisado-Gil, A.B.; Crespo-Rivas, J.C.; Pachón-Ibáñez, M.E.; Lepe, J.A.; Cisneros, J.M. Prevalence and Risk Factors for Multidrug-Resistant Organisms Colonization in Long-Term Care Facilities Around the World: A Review. Antibiotics 2021, 10, 680. [Google Scholar] [CrossRef]
- Fernández, M.C.; Rodríguez, R.A.; Arias, Á.; Aguirre-Jaime, A.; Hernández, M.B.C.; Real, M.J.R.; Fernández, Y.P.; Lecuona, M. The Impact of MRSA Colonization on Healthcare-Associated Infections in Long-Term Care Facility Residents: A Whole-Genome Sequencing-Based Study. Microorganisms 2023, 11, 2842. [Google Scholar] [CrossRef]
- Peters, C.; Dulon, M.; Kleinmüller, O.; Nienhaus, A.; Schablon, A.; Melo-Cristino, J. MRSA Prevalence and Risk Factors among Health Personnel and Residents in Nursing Homes in Hamburg, Germany—A Cross-Sectional Study. PLoS ONE 2017, 12, e0169425. [Google Scholar] [CrossRef][Green Version]
- Fulchini, R.; Albrich, W.C.; Kronenberg, A.; Egli, A.; Kahlert, C.R.; Schlegel, M.; Kohler, P. Antibiotic-resistant pathogens in different patient settings and identification of surveillance gaps in Switzerland—A systematic review. Epidemiol. Infect. 2019, 147, e259. [Google Scholar] [CrossRef]
- van Dulm, E.; Klok, S.; Boyd, A.; Joore, I.K.; Prins, M.; van Dam, A.P.; Tramper-Stranders, G.A.; van Duijnhoven, Y.T.H.P. Nasal carriage of methicillin-resistant Staphylococcus aureus (MRSA) among undocumented migrants and uninsured legal residents in Amsterdam, the Netherlands: A cross-sectional study. Antimicrob. Resist. Infect. Control 2020, 9, 118. [Google Scholar] [CrossRef] [PubMed]
- Ita, T.; Luvsansharav, U.-O.; Smith, R.M.; Mugoh, R.; Ayodo, C.; Oduor, B.; Jepleting, M.; Oguta, W.; Ouma, C.; Juma, J.; et al. Prevalence of colonization with multidrug-resistant bacteria in communities and hospitals in Kenya. Sci. Rep. 2022, 12, 22290. [Google Scholar] [CrossRef]
- Schaumburg, F.; Alabi, A.; Peters, G.; Becker, K. New epidemiology of Staphylococcus aureus infection in Africa. Clin. Microbiol. Infect. 2014, 20, 589–596. [Google Scholar] [CrossRef]
- World Health Organization (WHO). Promoting Rational Use of Medicines: Core Components; WHO Policy Perspectives on Medicines No. 005; WHO: Geneva, Switzerland, 2002; Available online: https://iris.who.int/bitstream/handle/10665/67438/WHO_EDM_2002.3.pdf (accessed on 7 November 2024).
- García-García, J.A.; Santos-Morano, J.; Castro, C.; Bayoll-Serradilla, E.; Martín-Ponce, M.L.; Vergara-López, S.; Martín-Rodríguez, L.M.; Mateos-Gómez, A.; de la Cueva, J.; Martín-Mazuelos, E.; et al. Prevalencia y factores asociados a la colonización por Staphylococcus aureus resistente a meticilina en centros de larga estancia en el sur de España. Enfermedades Infecc. Y Microbiol. Clin. (Engl. Ed.) 2011, 29, 405–410. [Google Scholar] [CrossRef] [PubMed]
- von Baum, H.; Schmidt, C.; Svoboda, D.; Bock-Hensley, O.; Wendt, C. Risk Factors for Methicillin-Resistant Staphylococcus Aureus Carriage in Residents of German Nursing Homes. Infect. Control Hosp. Epidemiol. 2002, 23, 511–515. [Google Scholar] [CrossRef] [PubMed]
- Vovko, P.; Retelj, M.; Cretnik, T.Z.; Jutersek, B.; Harlander, T.; Kolman, J.; Gubina, M. Risk Factors for Colonization with Methicillin-Resistant Staphylococcus aureus in a Long-Term-Care Facility in Slovenia. Infect. Control Hosp. Epidemiol. 2005, 26, 191–195. [Google Scholar] [CrossRef]
- Barr, B.; Wilcox, M.H.; Brady, A.; Parnell, P.; Darby, B.; Tompkins, D. Prevalence of Methicillin-Resistant Staphylococcus aureus Colonization Among Older Residents of Care Homes in the United Kingdom. Infect. Control Hosp. Epidemiol. 2007, 28, 853–859. [Google Scholar] [CrossRef]
- Shibabaw, A.; Abebe, T.; Mihret, A. Nasal carriage rate of methicillin resistant Staphylococcus aureus among Dessie Referral Hospital Health Care Workers; Dessie, Northeast Ethiopia. Antimicrob. Resist. Infect. Control 2013, 2, 25. [Google Scholar] [CrossRef][Green Version]
- Desta, K.; Aklillu, E.; Gebrehiwot, Y.; Enquselassie, F.; Cantillon, D.; Al-Hassan, L.; Price, J.R.; Newport, M.J.; Davey, G.; Woldeamanuel, Y. High Levels of Methicillin-Resistant Staphylococcus aureus Carriage Among Healthcare Workers at a Teaching Hospital in Addis Ababa Ethiopia: First Evidence Using mecA Detection. Infect. Drug Resist. 2022, 15, 3135–3147. [Google Scholar] [CrossRef]
- Wolde, W.; Mitiku, H.; Sarkar, R.; Shume, T. Nasal Carriage Rate of Staphylococcus aureus, Its Associated Factors, and Antimicrobial Susceptibility Pattern Among Health Care Workers in Public Hospitals, Harar, Eastern Ethiopia. Infect. Drug Resist. 2023, 16, 3477–3486. [Google Scholar] [CrossRef]
- Amare, A.; Eshetie, S.; Kasew, D.; Moges, F.; Algammal, A.M. High prevalence of fecal carriage of Extended-spectrum beta-lactamase and carbapenemase-producing Enterobacteriaceae among food handlers at the University of Gondar, Northwest Ethiopia. PLoS ONE 2022, 17, e0264818. [Google Scholar] [CrossRef] [PubMed]
- Mekonnen, Y.; Solomon, S.; Gebreyohanns, A.; Teklu, D.S.; Ayenew, Z.; Mihret, A.; Bonger, Z.T. Fecal Carriage of Carbapenem Resistant Enterobacterales and Associated Factors Among Admitted Patients in Saint Paul’s Hospital Millennium Medical College, Addis Ababa, Ethiopia. Infect. Drug Resist. 2023, 16, 6345–6355. [Google Scholar] [CrossRef]
- Boyce, J.M.; Pittet, D. Guideline for Hand Hygiene in Health-Care Settings: Recommendations of the Healthcare Infection Control Practices Advisory Committee and the HICPAC/SHEA/APIC/IDSA Hand Hygiene Task Force. Infect. Control Hosp. Epidemiol. 2002, 23, S3–S40. [Google Scholar] [CrossRef] [PubMed]
- Desta, K.; Woldeamanuel, Y.; Azazh, A.; Mohammod, H.; Desalegn, D.; Shimelis, D.; Gulilat, D.; Lamisso, B.; Makonnen, E.; Worku, A.; et al. High Gastrointestinal Colonization Rate with Extended-Spectrum β-Lactamase-Producing Enterobacteriaceae in Hospitalized Patients: Emergence of Carbapenemase-Producing K. pneumoniae in Ethiopia. PLoS ONE 2016, 11, e0161685. [Google Scholar] [CrossRef]
- Abamecha, A.; Wondafrash, B.; Abdissa, A. Antimicrobial resistance profile of Enterococcus species isolated from intestinal tracts of hospitalized patients in Jimma, Ethiopia. BMC Res. Notes 2015, 8, 213. [Google Scholar] [CrossRef] [PubMed]
- Khairy, R.M.; Mahmoud, M.S.; Esmail, M.A.M.; Gamil, A.N. First detection of vanB phenotype-vanA genotype vancomycin-resistant enterococci in Egypt. J. Infect. Dev. Ctries. 2019, 13, 837–842. [Google Scholar] [CrossRef]
- Siddig, L.A.; Bayoumi, M.; Elhadi, N. Sociodemographic distributions and molecular characterization of colonized Enterococcus faecium isolates from locality hospitals in Khartoum, Sudan. PeerJ 2023, 11, e16169. [Google Scholar] [CrossRef]
- Benamrouche, N.; Guettou, B.; Henniche, F.Z.; Assaous, F.; Laouar, H.; Ziane, H.; Djennane, F.; Tiouit, D.; Bentchouala, C.; Yamouni, F.; et al. Vancomycin-resistant Enterococcus faecium in Algeria: Phenotypic and genotypic characterization of clinical isolates. J. Infect. Dev. Ctries. 2021, 15, 95–101. [Google Scholar] [CrossRef]
- Founou, R.C.; Founou, L.L.; Allam, M.; Ismail, A.; Essack, S.Y. Genome analysis of multidrug resistant Enterococcus faecium and Enterococcus faecalis circulating among hospitalized patients in uMgungundlovu District, KwaZulu-Natal, South Africa. BMC Infect. Dis. 2024, 24, 671. [Google Scholar] [CrossRef]
- European Committee on Antimicrobial Susceptibility Testing (EUCAST). Breakpoint Tables for Interpretation of MICs and Zone Diameters, Version 14.0; EUCAST: Växjö, Sweden, 2024; Available online: https://www.eucast.org/eucast_news/news_singleview?tx_ttnews%5Btt_news%5D=566&cHash=db55f3a8829726044512a1fe74cce41b (accessed on 2 January 2025).
- Vitro S.A. MDR Direct Flow Chip Kit: Detección de Patógenos Multirresistentes Mediante PCR Múltiple e Hibridación Reversa; Inserto Técnico; Vitro S.A.: Sevilla, España, 2022; p. 36. [Google Scholar]
- Rodríguez-Villodres, Á.; Galiana-Cabrera, A.; Fink, I.T.; Jiménez, R.D.; Cisneros, J.M.; Lepe, J.A. Evaluation of the MDR Direct Flow Chip Kit for the Detection of Multiple Antimicrobial Resistance Determinants. Microb. Drug Resist. 2023, 29, 381–385. [Google Scholar] [CrossRef]

| Study | Group | n | Median Age (IQR) | Sex |
|---|---|---|---|---|
| Nasal Colonization | Patients | 30 | 25.5 (19.25–40) | 10 males; 20 females |
| Healthcare workers | 11 | 28 (25–35) | 7 males; 4 females | |
| Rectal Colonization | Pediatric patients | 20 | 1.2 (0.75–4) | 20 males; 8 females |
| Adult patients | 28 | 25 (14–40) | 1 males; 27 females |
| Antimicrobial Susceptibility Testing (MIC g/dL) * | Genotypic Analysis | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Order | OX | CFXS | VNC | TEC | LIN | DAP | ERY | CMN | CIP | LVX | GMN | TMN | AKN | mecA | acc(6′)-lb | msrA |
| 1 | - ** | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
| 2 | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
| 3 | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
| 4 | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
| 5 | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
| 6 | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
| 7 | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
| 8 | - | - | - | - | - | - | - | - | - | - | - | - | - | |||
| 9 | >2 | >4 | 1 | ≤1 | 2 | ≤1 | >4 | ≤0.25 | 2 | 2 | 4 | 8 | ≤8 | + *** | + | − |
| 10 | >2 | >4 | 1 | ≤1 | 2 | ≤1 | >4 | ≤0.25 | >2 | 4 | >8 | >8 | ≤8 | + | + | − |
| 11 | >2 | >4 | 1 | ≤1 | ≤1 | ≤1 | >4 | ≤0.25 | ≤1 | ≤1 | ≤1 | ≤1 | ≤8 | + | + | − |
| 12 | >2 | >4 | 1 | 1 | 2 | ≤1 | >4 | ≤0.25 | >2 | 4 | >8 | 8 | ≤8 | + | + | − |
| 13 | >2 | >4 | 1 | ≤1 | ≤1 | ≤1 | >4 | ≤0.25 | >2 | 4 | >8 | >8 | ≤8 | + | + | − |
| 14 | >2 | >4 | 1 | ≤1 | ≤1 | ≤1 | >4 | ≤0.25 | ≤1 | ≤1 | ≤1 | ≤1 | ≤8 | + | + | − |
| 15 | >2 | >4 | 1 | ≤1 | ≤1 | ≤1 | >4 | ≤0.25 | >2 | >4 | ≤1 | ≤1 | ≤8 | + | + | + |
| 16 | >2 | >4 | 1 | ≤1 | 2 | ≤1 | >4 | ≤0.25 | ≤1 | ≤1 | ≤1 | ≤1 | ≤8 | + | − | − |
| Order | Patient/ Healthcare Workers | Age (Years) | Gender | Residential Area | Hospital Department (Admission/Work) | History of Empirical Cloxacillin Treatment for Ulcers in the Previous 3 Months | Genotypic Analysis | Antimicrobial Susceptibility Testing |
|---|---|---|---|---|---|---|---|---|
| 1 | Healthcare worker | 30 | M | Kore | Maternity Ward | No | No | No |
| 2 | Patient | 35 | F | Negele | OPD * | Yes | No | No |
| 3 | Patient | 48 | M | Kore | Leprosy Ward | Yes | No | No |
| 4 | Patient | 28 | F | Kore | Leprosy Ward | Yes | No | No |
| 5 | Healthcare worker | 25 | M | Kore | OPD | No | No | No |
| 6 | Healthcare worker | 60 | M | Kore | OPD | No | No | No |
| 7 | Patient | 40 | F | Kore | OPD | Yes | No | No |
| 8 | Patient | 51 | F | Lepis | Emergency Department | No | No | No |
| 9 | Patient | 72 | M | Kokosa | Leprosy Ward | Yes | Yes | Yes |
| 10 | Patient | 24 | F | Kabira | Leprosy Ward | Yes | Yes | Yes |
| 11 | Patient | 46 | F | Kore | Leprosy Ward | Yes | Yes | Yes |
| 12 | Patient | 81 | M | Rigelu | Leprosy Ward | Yes | Yes | Yes |
| 13 | Patient | 63 | F | Hogiso | Leprosy Ward | Yes | Yes | Yes |
| 14 | Patient | 55 | M | Kore | Leprosy Ward | Yes | Yes | Yes |
| 15 | Patient | 66 | M | Hogiso | Leprosy Ward | Yes | Yes | Yes |
| 16 | Patient | 51 | M | Kore | Leprosy Ward | Yes | Yes | Yes |
| Patient | Age | Gender | Residential Area | Admission Department | History of Empirical Antibiotic in the Previous 3 Months | Sample | Isolate |
|---|---|---|---|---|---|---|---|
| 1 | 1 months | M | Basaku Ilala | Pediatric Ward | Ceftriaxone, Cloxacillin | Stool | E. faecium |
| 2 | 3 years old | M | Ashoka | Pediatric Ward | Ceftriaxone, Cloxacillin | Stool | E. coli |
| 3 | 11 months | M | Kore | Pediatric Ward | Ceftriaxone, Ampicillin, Gentamicin | Stool | E. coli K. pneumoniae E. faecium |
| 4 | 14 months | F | Kore | Pediatric Ward | Ceftriaxone | Stool | E. coli E. faecium |
| 5 | 5 months | M | Lepis | Pediatric Ward | Ampicillin, Gentamicin, Ceftriaxone, Cloxacillin | Stool | E. coli E. faecium |
| 6 | 6 months | M | Kore | Pediatric Ward | Ceftriaxone, Cloxacillin, Gentamicin | Stool | E. coli E. faecium |
| 7 | 2 years old 7 months | M | Basaku Ilala | Pediatric Ward | Ceftriaxone, Gentamicin | Stool | E. coli K. pneumoniae |
| 8 | 13 years old | M | Lepis | Pediatric Ward | Ceftriaxone | Stool | E. coli |
| 9 | 35 years old | F | Negele | OPD * | Cloxacillin | Rectal exudate | E. coli |
| 10 | 28 years old | F | Kore | Leprosy Ward | Cloxacillin | Rectal exudate | E. coli E. coli |
| 11 | 4 years old | M | Basaku Ilala | Pediatric Ward | Ceftriaxone, Gentamicin, Ampicillin | Stool | E. coli K. pneumoniae E. faecium |
| 12 | 4 years old | M | Basaku Ilala | Pediatric Ward | Ampicillin, Gentamicin | Stool | K. pneumoniae |
| 13 | 9 months | F | Lepis | Pediatric Ward | Ceftriaxone, Vancomycin | Stool | E. coli |
| 14 | 11 months | M | Aga Nia | Pediatric Ward | Ceftriaxone, Gentamicin, Ampicillin | Stool | E. coli K. pneumoniae E. faecium |
| 15 | 18 months | F | Kore | Pediatric Ward | Ceftriaxone | Stool | E. coli E. faecium |
| 16 | 11 years old | F | Kore | Pediatric Ward | Ceftriaxone | Stool | E. coli |
| 17 | 8 months | F | Kore | Pediatric Ward | Ceftriaxone | Stool | E. coli E. faecium |
| 18 | 3 years old | F | Kore | Pediatric Ward | Ceftriaxone | Stool | E. coli E. faecium |
| 19 | 11 months | M | Lepis | Pediatric Ward | Ceftriaxone, Amoxicillin | Stool | E. coli E. faecium |
| 20 | 40 years old | F | Kore | OPD | Cloxacillin, Amoxicillin, Ceftriaxone, Ciprofloxacin | Rectal exudate | E. coli |
| 21 | 18 years old | F | Aga Nia | Antenatal Department | None | Vagino-rectal exudate | E. faecium |
| Patient | Isolate | blaCTX-M | blaCMY | blaNDM | blaoxa48 like | sul1 | sul2 | qnrS | ermB | aac (6′)-Ib | catB3 | vanA |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | E. faecium | − | − | − | − | − | − | − | + | − | − | + |
| 2 | E. coli | + | − | + | − | + | − | − | + | + | − | − |
| 3 | E. coli | + | − | − | − | − | − | − | − | − | − | − |
| K. pneumoniae | + | − | − | − | − | − | − | − | − | − | − | |
| E. faecium | − | − | − | − | − | − | − | − | − | − | + | |
| 4 | E. coli | + | + | + | − | + | − | − | + | − | − | − |
| E. faecium | − | − | − | − | − | − | − | + | − | − | + | |
| 5 | E. coli | + | + | − | − | − | − | − | + | + | − | − |
| E. faecium | − | − | − | − | − | − | − | + | − | − | + | |
| 6 | E. coli | + | − | + | − | + | − | − | + | + | − | − |
| E. faecium | − | − | − | − | − | − | − | + | − | − | + | |
| 7 | E. coli | + | − | − | − | − | − | − | − | − | − | − |
| K. pneumoniae | + | − | − | − | − | − | − | − | − | − | − | |
| 8 | E. coli | + | − | + | − | + | − | − | + | + | − | − |
| 9 | E. coli | + | − | + | − | + | − | − | + | + | − | − |
| 10 | E. coli | + | − | + | − | + | − | − | + | + | − | − |
| E. coli | − | − | − | + | − | − | − | − | − | − | − | |
| 11 | E. coli | + | − | + | − | + | − | − | + | + | − | − |
| K. pneumoniae | + | − | − | − | − | − | − | − | − | − | − | |
| E. faecium | − | − | − | − | − | − | − | + | − | − | + | |
| 12 | K. pneumoniae | + | − | − | − | − | − | − | − | − | − | − |
| 13 | E. coli | + | − | + | − | + | − | − | + | + | − | − |
| 14 | E. coli | + | − | + | − | + | − | − | + | + | + | − |
| K. pneumoniae | + | − | − | − | − | − | − | − | − | − | − | |
| E. faecium | − | − | − | − | − | − | − | + | − | − | + | |
| 15 | E. coli | + | − | + | − | + | − | − | + | + | + | − |
| E. faecium | − | − | − | − | − | − | − | + | − | − | + | |
| 16 | E. coli | + | − | − | − | − | − | − | − | − | − | − |
| 17 | E. coli | + | − | + | − | + | − | − | + | + | + | − |
| E. faecium | − | − | − | − | − | − | − | + | − | − | + | |
| 18 | E. coli | + | + | − | − | − | − | − | + | + | − | − |
| E. faecium | − | − | − | − | − | − | − | + | − | − | + | |
| 19 | E. coli | + | − | + | − | + | − | − | + | + | − | − |
| E. faecium | − | − | − | − | − | − | − | + | − | − | + | |
| 20 | E. coli | + | − | − | − | − | − | − | − | − | − | − |
| 21 | E. faecium | − | − | − | − | − | − | − | + | − | − | + |
| Organism | n | Main Resistance Genes Detected | Most Frequent Phenotypic Resistances |
|---|---|---|---|
| MRSA (S. aureus) | 8 | mecA, msrA, acc(6′)-Ib | Oxacillin, cefoxitin, erythromycin, clindamycin |
| MDR E. coli | 19 | blaNDM, blaCTX-M, blaCMY, blaOXA-48, ermB | Third-gen cephalosporins, carbapenems, TMP-SMX |
| MDR K. pneumoniae | 5 | blaCTX-M | Third-gen cephalosporins, gentamicin, tobramycin |
| VRE (E. faecium) | 12 | vanA, ermB | Vancomycin, teicoplanin |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hidalgo, E.; Alvaredo-Carrillo, T.; Gil-Belda, J.-M.; Portela-Pino, C.; Bares-Moreno, C.; Jareño-Moreno, S.; de la Fuente, P.; Platero, L.; Pérez-Tanoira, R. High Prevalence of Multidrug-Resistant Bacterial Colonization Among Patients and Healthcare Workers in a Rural Ethiopian Hospital. Antibiotics 2025, 14, 717. https://doi.org/10.3390/antibiotics14070717
Hidalgo E, Alvaredo-Carrillo T, Gil-Belda J-M, Portela-Pino C, Bares-Moreno C, Jareño-Moreno S, de la Fuente P, Platero L, Pérez-Tanoira R. High Prevalence of Multidrug-Resistant Bacterial Colonization Among Patients and Healthcare Workers in a Rural Ethiopian Hospital. Antibiotics. 2025; 14(7):717. https://doi.org/10.3390/antibiotics14070717
Chicago/Turabian StyleHidalgo, Elena, Teresa Alvaredo-Carrillo, Josefina-Marina Gil-Belda, Clara Portela-Pino, Clara Bares-Moreno, Sara Jareño-Moreno, Paula de la Fuente, Lucía Platero, and Ramón Pérez-Tanoira. 2025. "High Prevalence of Multidrug-Resistant Bacterial Colonization Among Patients and Healthcare Workers in a Rural Ethiopian Hospital" Antibiotics 14, no. 7: 717. https://doi.org/10.3390/antibiotics14070717
APA StyleHidalgo, E., Alvaredo-Carrillo, T., Gil-Belda, J.-M., Portela-Pino, C., Bares-Moreno, C., Jareño-Moreno, S., de la Fuente, P., Platero, L., & Pérez-Tanoira, R. (2025). High Prevalence of Multidrug-Resistant Bacterial Colonization Among Patients and Healthcare Workers in a Rural Ethiopian Hospital. Antibiotics, 14(7), 717. https://doi.org/10.3390/antibiotics14070717






