Potential Shifts in the Oral Microbiome Induced by Bariatric Surgery—A Scoping Review
Abstract
1. Introduction
2. Methods
3. Results
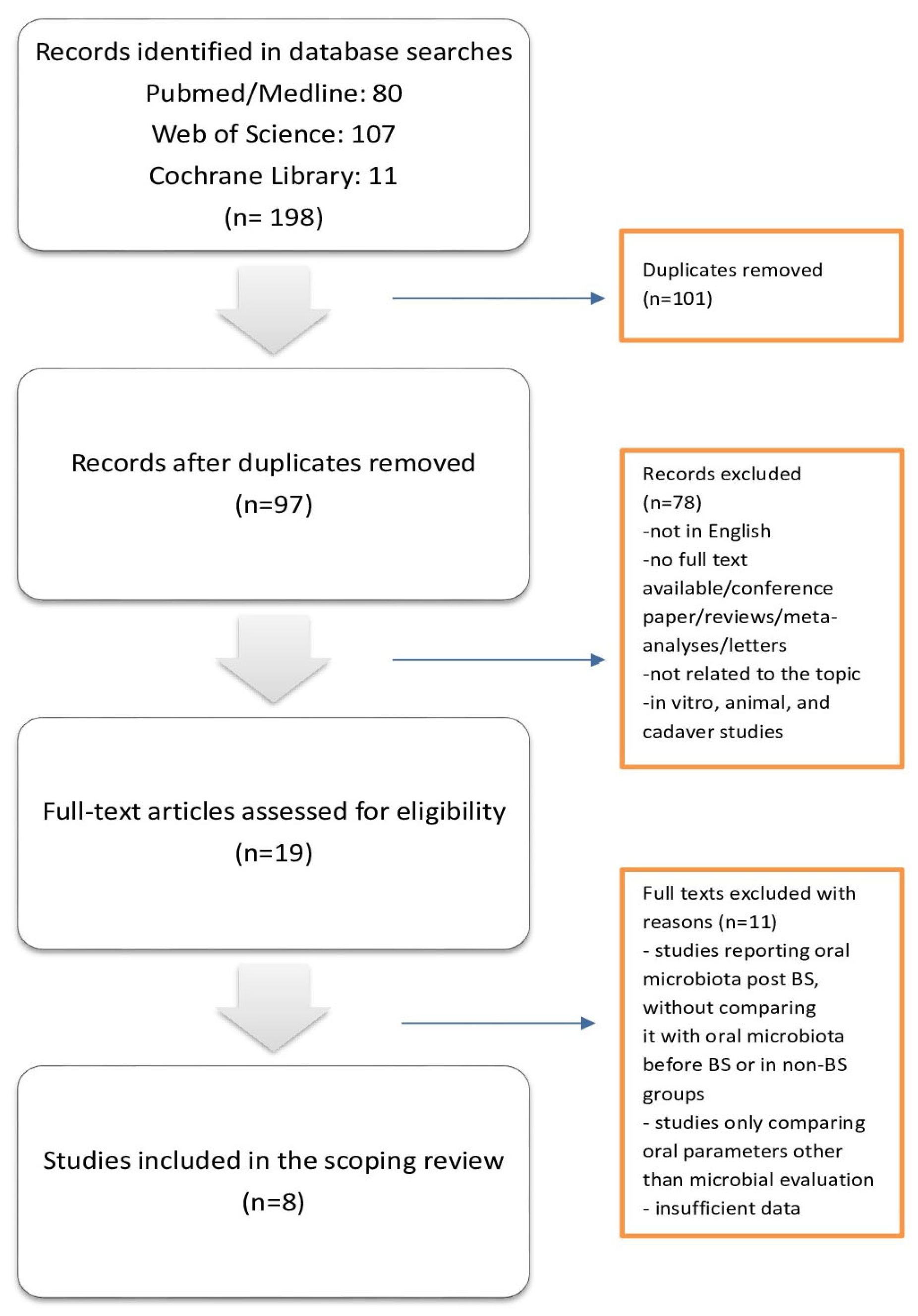
3.1. Sources Identification
3.2. Study Characteristics
3.3. Results of the Studies
4. Discussion
5. Limitations
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- NCD Risk Factor Collaboration (NCD-RisC). Worldwide trends in body-mass index, underweight, overweight, and obesity from 1975 to 2016: A pooled analysis of 2416 population-based measurement studies in 128.9 million children, adolescents, and adults. Lancet 2017, 390, 2627–2642. [Google Scholar] [CrossRef] [PubMed]
- Hruby, A.; Hu, F.B. The Epidemiology of Obesity: A Big Picture. Pharmacoeconomics 2015, 33, 673–689. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Liu, J.; Ni, Y.; Yi, C.; Fang, Y.; Ning, Q.; Shen, B.; Zhang, K.; Liu, Y.; Yang, L.; et al. Global Prevalence of Overweight and Obesity in Children and Adolescents: A Systematic Review and Meta-Analysis. JAMA Pediatr. 2024, 178, 800–813. [Google Scholar] [CrossRef] [PubMed]
- Lingvay, I.; Cohen, R.V.; Roux, C.W.L.; Sumithran, P. Obesity in adults. Lancet 2024, 404, 972–987. [Google Scholar] [CrossRef]
- Chandrasekaran, P.; Weiskirchen, R. The Role of Obesity in Type 2 Diabetes Mellitus-An Overview. Int. J. Mol. Sci. 2024, 25, 1882. [Google Scholar] [CrossRef]
- Džunková, M.; Lipták, R.; Vlková, B.; Gardlík, R.; Čierny, M.; Moya, A.; Celec, P. Salivary microbiome composition changes after bariatric surgery. Sci. Rep. 2020, 10, 20086. [Google Scholar] [CrossRef]
- Zhao, L. The gut microbiota and obesity: From correlation to causality. Nat. Rev. Microbiol. 2013, 11, 639–647. [Google Scholar] [CrossRef]
- Fetissov, S.O. Role of the gut microbiota in host appetite control: Bacterial growth to animal feeding behaviour. Nat. Rev. Endocrinol. 2017, 13, 11–25. [Google Scholar] [CrossRef]
- Muscogiuri, G.; Cantone, E.; Cassarano, S.; Tuccinardi, D.; Barrea, L.; Savastano, S.; Colao, A. Gut microbiota: A new path to treat obesity. Int. J. Obes. Suppl. 2019, 9, 10–19. [Google Scholar] [CrossRef]
- Zeigler, C.C.; Persson, G.R.; Wondimu, B.; Marcus, C.; Sobko, T.; Modéer, T. Microbiota in the oral subgingival bioflm is associated with obesity in adolescence. Obesity 2012, 20, 157–164. [Google Scholar] [CrossRef]
- Hofman, K.L.; Hutchinson, D.S.; Fowler, J.; Smith, D.P.; Ajami, N.J.; Zhao, H.; Scheet, P.; Chow, W.-H.; Petrosino, J.F.; Daniel, C.R. Oral microbiota reveals signs of acculturation in Mexican American women. PLoS ONE 2018, 13, e0194100. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Chi, X.; Zhang, Q.; Chen, F.; Deng, X. Characterization of the salivary microbiome in people with obesity. PeerJ 2018, 6, e4458. [Google Scholar] [CrossRef] [PubMed]
- Duvallet, C.; Gibbons, S.M.; Gurry, T.; Irizarry, R.A.; Alm, E.J. Meta-analysis of gut microbiome studies identifes disease-specifc and shared responses. Nat. Commun. 2017, 8, 1784. [Google Scholar] [CrossRef] [PubMed]
- Aceves-Martins, M.; Godina-Flores, N.L.; Gutierrez-Gómez, Y.Y.; Richards, D.; López-Cruz, L.; García-Botello, M.; Moreno-García, C.F. Obesity and oral health in Mexican children and adolescents: Systematic review and meta-analysis. Nutr. Rev. 2022, 80, 1694–1710. [Google Scholar] [CrossRef]
- Taghat, N.; Lingström, P.; Mossberg, K.; Fändriks, L.; Eliasson, B.; Östberg, A.L. Oral health by obesity classification in young obese women—A cross-sectional study. Acta Odontol. Scand. 2022, 80, 596–604. [Google Scholar] [CrossRef]
- Schmidt, J.; Vogel, M.; Poulain, T.; Kiess, W.; Hirsch, C.; Ziebolz, D.; Haak, R. Association of Oral Health Conditions in Adolescents with Social Factors and Obesity. Int. J. Environ. Res. Public Health 2022, 19, 2905. [Google Scholar] [CrossRef]
- Esposito, K.; Giugliano, G.; Scuderi, N.; Giugliano, D. Role of adipokines in the obesity—Inflammation relationship: The effect of fat removal. Plast. Reconstr. Surg. 2006, 118, 1048–1057. [Google Scholar] [CrossRef]
- Pischon, N.; Heng, N.; Bernimoulin, J.P.; Kleber, B.M.; Willich, S.N.; Pischon, T. Obesity, inflammation, and periodontal disease. J. Dent. Res. 2007, 86, 400–409. [Google Scholar] [CrossRef]
- Adams, T.D.; Davidson, L.E.; Litwin, S.E.; Kim, J.; Kolotkin, R.L.; Nanjee, M.N.; Gutierrez, J.M.; Frogley, S.J.; Ibele, A.R.; Brinton, E.A.; et al. Weight and metabolic outcomes 12 years after gastric bypass. N. Engl. J. Med. 2017, 377, 1143–1155. [Google Scholar] [CrossRef]
- Paganelli, F.L.; Luyer, M.; Hazelbag, C.M.; Uh, H.W.; Rogers, M.R.C.; Adriaans, D.; Berbers, R.-M.; Hendrickx, A.P.A.; Viveen, M.C.; Groot, J.A.; et al. Roux-Y gastric bypass and sleeve gastrectomy directly change gut microbiota composition independent of surgery type. Sci. Rep. 2019, 9, 10979. [Google Scholar] [CrossRef]
- Tremaroli, V.; Karlsson, F.; Werling, M.; Ståhlman, M.; Kovatcheva-Datchary, P.; Olbers, T.; Fändriks, L.; le Roux, C.W.; Nielsen, J.; Bäckhed, F. Roux-en-Y gastric bypass and vertical banded gastroplasty induce long-term changes on the human gut microbiome contributing to fat mass regulation. Cell Metab. 2015, 22, 228–238. [Google Scholar] [CrossRef] [PubMed]
- Shillitoe, E.; Weinstock, R.; Kim, T.; Simon, H.; Planer, J.; Noonan, S.; Cooney, R. The oral microflora in obesity and type-2 diabetes. J. Oral Microbiol. 2012, 4, 19013. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.S.; Na, H.S.; Oh, T.J.; Han, H.; Kim, J.; Hong, J.S.; Lee, H.J.; Park, Y.S.; Chung, J. Oral microbiome changes in subjects with obesity following bariatric surgery compared to lean counterparts. Front. Microbiol. 2025, 16, 1553404. [Google Scholar] [CrossRef] [PubMed]
- Hashizume, L.N.; Bastos, L.F.; Cardozo, D.D.; Hilgert, J.B.; Hugo, F.N.; Stein, A.T.; Souto, K.E.; Meinhardt, N.G. Impact of Bariatric Surgery on the Saliva of Patients with Morbid Obesity. Obes. Surg. 2015, 25, 1550–1555. [Google Scholar] [CrossRef]
- Sales-Peres, S.H.; de Moura-Grec, P.G.; Yamashita, J.M.; Torres, E.A.; Dionísio, T.J.; Leite, C.V.; Sales-Peres, A.; Ceneviva, R. Periodontal status and pathogenic bacteria after gastric bypass: A cohort study. J. Clin. Periodontol. 2015, 42, 530–536. [Google Scholar] [CrossRef]
- Ribeiro, A.S.P.; Marquezin, M.C.S.; Pacheco, E.R.P.; Rasera, I., Jr.; Klein, M.I.; de Vasconcellos, S.P.; Landgraf, R.G.; Okamoto, D.; Calixto, L.A.; Castelo, P.M. Bypass gastroplasty impacts oral health, salivary inflammatory biomarkers, and microbiota: A controlled study. Clin. Oral Investig. 2023, 27, 4735–4746. [Google Scholar] [CrossRef] [PubMed]
- Stefura, T.; Zapała, B.; Gosiewski, T.; Skomarovska, O.; Pędziwiatr, M.; Major, P. Changes in the Composition of Oral and Intestinal Microbiota After Sleeve Gastrectomy and Roux-En-Y Gastric Bypass and Their Impact on Outcomes of Bariatric Surgery. Obes. Surg. 2022, 32, 1439–1450. [Google Scholar] [CrossRef]
- Balogh, B.; Somodi, S.; Tanyi, M.; Miszti, C.; Márton, I.; Kelentey, B. Follow-up Study of Microflora Changes in Crevicular Gingival Fluid in Obese Subjects After Bariatric Surgery. Obes. Surg. 2020, 30, 5157–5161. [Google Scholar] [CrossRef]
- Damms-Machado, A.; Mitra, S.; Schollenberger, A.E.; Kramer, K.M.; Meile, T.; Königsrainer, A.; Huson, D.H.; Bischoff, S.C. Effects of surgical and dietary weight loss therapy for obesity on gut microbiota composition and nutrient absorption. Biomed Res. Int. 2015, 806248. [Google Scholar] [CrossRef]
- Guo, Y.; Huang, Z.-P.; Liu, C.-Q.; Qi, L.; Sheng, Y.; Zou, D.-J. Modulation of the gut microbiome: A systematic review of the effect of bariatric surgery. Eur. J. Endocrinol. 2018, 178, 43–56. [Google Scholar] [CrossRef]
- Hague, A.L.; Baechle, M. Advanced caries in a patient with a history of bariatric surgery. J. Dent. Hyg. 2008, 82, 22. [Google Scholar] [PubMed]
- Schwenger, K.J.P.; Alghamdi, M.M.; Ghorbani, Y.; Jackson, T.D.; Okrainec, A.; Allard, J.P. Hyposalivation is prevalent in bariatric patients but improves after surgery. Surg. Obes. Relat. Dis. 2020, 16, 1407–1413. [Google Scholar] [CrossRef] [PubMed]
- Kogawa, E.M.; Melo, F.F.; Pires, R.G.; Caetano, P.C.C.; Rodrigues, J.d.L.; Benito, L.A.O.; da Silva, I.C.R.; Cantuária, A.P.d.C.; Sales-Peres, S.H.d.C. The changes on salivary flow rates, buffering capacity and chromogranin A levels in adults after bariatric surgery. Clin. Oral Investig. 2024, 28, 159. [Google Scholar] [CrossRef] [PubMed]
- Marquezin, M.C.S.; Scudine, K.G.O.; Lamy, E.; Finassi, C.M.; Carreira, L.; Segura, W.D.; Rasera, I.; Pessotti, E.R.; Castelo, P.M. Impact of gastroplasty on salivary characteristics, dental health status and oral sensory aspects: A controlled clinical study. J. Oral Rehabil. 2022, 49, 1002–1011. [Google Scholar] [CrossRef]
- de Moura-Grec, P.G.; Marsicano, J.A.; Rodrigues, L.M.; de Carvalho Sales-Peres, S.H. Alveolar bone loss and periodontal status in a bariatric patient: A brief review and case report. Eur. J. Gastroenterol. Hepatol. 2012, 24, 84–89. [Google Scholar] [CrossRef]
- Marsicano, J.A.; Grec, P.G.; Belarmino, L.B.; Ceneviva, R.; Peres, S.H. Interfaces between bariatric surgery and oral health: A longitudinal survey. Acta Cir. Bras. 2011, 26 (Suppl. 2), 79–83. [Google Scholar] [CrossRef]
- Haslam, D.W.; James, W.P. Obesity. Lancet 2005, 366, 1197–1209. [Google Scholar] [CrossRef]
- Conway, B.; Rene, A. Obesity as a disease: No lightweight matter. Obes. Rev. 2004, 5, 145–151. [Google Scholar] [CrossRef]
- Dalile, B.; Van Oudenhove, L.; Vervliet, B.; Verbeke, K. The role of short-chain fatty acids in microbiota–gut–brain communication. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 461–478. [Google Scholar] [CrossRef]
- Jagannathachary, S.; Kamaraj, D. Obesity and periodontal disease. J. Indian. Soc. Periodontol. 2010, 14, 96–100. [Google Scholar] [CrossRef]
- Nascimento, G.G.; Peres, M.A.; Mittinty, M.N.; Peres, K.G.; Do, L.G.; Horta, B.L.; Gigante, D.P.; Corrêa, M.B.; Demarco, F.F. Diet-Induced Overweight and Obesity and Periodontitis Risk: An Application of the Parametric G-Formula in the 1982 Pelotas Birth Cohort. Am. J. Epidemiol. 2017, 185, 442–451. [Google Scholar] [CrossRef] [PubMed]
- Suvan, J.E.; Petrie, A.; Nibali, L.; Darbar, U.; Rakmanee, T.; Donos, N.; D’AIuto, F. Association between overweight/obesity and increased risk of periodontitis. J. Clin. Periodontol. 2015, 42, 733–739. [Google Scholar] [CrossRef] [PubMed]
- Adawi, H.; Aggarwal, A.; Jain, S.; Othman, M.A.; Othman, A.A.A.; Zakri, R.A.; Namazi, S.A.M.; Sori, S.A.Y.; Abuzawah, L.H.A.; Madkhali, Z.M. Influence of Bariatric Surgery on Oral Microbiota: A Systematic Review. Eur. J. Dent. 2023, 3, 602–614. [Google Scholar] [CrossRef] [PubMed]
- Pataro, A.L.; Cortelli, S.C.; Abreu, M.H.; Cortelli, J.R.; Franco, G.C.; Aquino, D.R.; Cota, L.O.; Costa, F.O. Frequency of periodontal pathogens and Helicobacter pylori in the mouths and stomachs of obese individuals submitted to bariatric surgery: A cross-sectional study. J. Appl. Oral Sci. 2016, 24, 229–238. [Google Scholar] [CrossRef]
- Schultze, L.B.; Maldonado, A.; Lussi, A.; Sculean, A.; Eick, S. The Impact of the pH Value on Biofilm Formation. Monogr. Oral Sci. 2021, 29, 19–29. [Google Scholar] [CrossRef]
- Takahashi, N.; Schachtele, C.F. Effect of pH on the growth and proteolytic activity of Porphyromonas gingivalis and Bacteroides intermedius. J. Dent. Res. 1990, 69, 1266–1269. [Google Scholar] [CrossRef]
- Mikx, F.H. Environmental effects on the growth and proteolysis of Treponema denticola ATCC 33520. Oral Microbiol. Immunol. 1997, 12, 249–253. [Google Scholar] [CrossRef]
- Fontanille, I.; Boillot, A.; Rangé, H.; Carra, M.C.; Sales-Peres, S.H.C.; Czernichow, S.; Bouchard, P. Bariatric surgery and periodontal status: A systematic review with meta-analysis. Surg. Obes. Relat. Dis. 2018, 14, 1618–1631. [Google Scholar] [CrossRef]
- Janus, M.M.; Crielaard, W.; Volgenant, C.M.C.; Van Der Veen, M.H.; Brandt, B.W.; Krom, B.P. Candida albicans alters the bacterial microbiome of early in vitro oral biofilms. J. Oral Microbiol. 2017, 9, 1270613. [Google Scholar] [CrossRef]
- Singh, S.; Fatima, Z.; Hameed, S. Predisposing factors endorsing Candida infections. Infez. Med. 2015, 23, 211–223. [Google Scholar]
- Nguyen, P.T.; Baldeck, J.D.; Olsson, J.; Marquis, R.E. Antimicrobial actions of benzimidazoles against oral streptococci. Oral Microbiol. Immunol. 2005, 20, 93–100. [Google Scholar] [CrossRef] [PubMed]
- Fischer, M.I.; Dias, C.; Stein, A.; Meinhardt, N.G.; Heineck, I. Antibiotic prophylaxis in obese patients submitted to bariatric surgery. A systematic review. Acta Cir. Bras. 2014, 29, 209–217. [Google Scholar] [CrossRef] [PubMed]
- Freeman, J.T.; Anderson, D.J.; Hartwig, M.G.; Sexton, D.J. Surgical site infections following bariatric surgery in community hospitals: A weighty concern? Obes. Surg. 2011, 21, 836–840. [Google Scholar] [CrossRef] [PubMed]
- Nalluri, H.; Kizy, S.; Ewing, K.; Luthra, G.; Leslie, D.B.; Bernlohr, D.A.; Sadowsky, M.J.; Ikramuddin, S.; Khoruts, A.; Staley, C.; et al. Peri-operative antibiotics acutely and significantly impact intestinal microbiota following bariatric surgery. Sci. Rep. 2020, 10, 20340. [Google Scholar] [CrossRef]

| Author/Year /Design | Country | Study Population (Number, M/F Ratio, Mean Age [Years]) | Intervention | Follow-Up | Microbiologic Evaluation | Results |
|---|---|---|---|---|---|---|
| Balogh et al., 2020 Follow-up study [28] | Hungary | 57 total, M/F 33/24, 39.1 18 obese controls, M/F 5/13, 44.1 17 obese BS patients, M/F 10/7, 39.4 22 healthy controls, M/F 9/13, 33.9 | Gastric bypass | 12 months | Inoculation of clinical specimens onto selective media (blood agar, chocolate agar, Sabouraud dextrose agar) and identification with MALDI-TOF MS (crevicular fluid) | After surgery and weight loss, the mean germ count increased, but not significantly. Candida albicans and non-albicans Candida species appeared after surgery; Neisseria was either absent throughout or eliminated after surgery. |
| Džunková et al., 2020 Cohort study [6] | Czech Republic | 35, M/F 18/17, 48.0 | Sleeve gastrectomy, Roux-en-Y gastric bypass, Omega loop gastric bypass, laparoscopic gastric plication | 12 months | 16S rRNA gene sequencing (saliva) | Increased proportion of Veillonella species after the decrease of BMI. Streptococcus oralis had a positive correlation with BMI. Megasphaera micronuciformis proportion increased when the BMI decreased. |
| Hashizume et al., 2015 Cohort study [24] | Brazil | 27, M/F 1/26, 45.0 | Roux-en-Y gastric bypass | 6 months | Inoculation of clinical specimens onto selective media (Mitis salivarius bacitracin Agar, Rogosa SL agar, Sabouraud dextrose agar with chloramphenicol) and identification based on colony morphology and biochemical tests (saliva) | Salivary levels of Streptococcus mutans increased following BS. |
| Kim et al., 2025 Case-control study [23] | Republic of Korea | 55 total, M/F 55/0, 36.0 31 obese BS patients, M/F 31/0, 37.0 24 lean controls, M/F 24/0, 35.0 | Sleeve gastrectomy | 6 months | 16S rRNA gene sequencing (subgingival plaque, saliva, and oral swab) | Distinct species associated with periodontal disease found in the obese, surgically treated group in subgingival plaque (Filifactor alocis, Peptostreptococcaceae spp., Prevotella spp., and Treponema maltophilum). Microbiomes associated with a healthy state increased over time (Streptococcus salivarius and various Veillonella spp.). Clusters containing periodontal pathogens, including Porphyromonas spp., tended to diminish. |
| Ribeiro et al., 2023 Case-control study [26] | Brazil | 40 total, 20 obese BS patients, M/F 5/15, 34.9 20 obese controls, M/F 5/15, 31.7 | Roux-en-Y gastric bypass | 6 months | 16S rRNA gene sequencing (saliva) | Both interventions changed in different degrees the salivary inflammatory biomarkers and microbiota but did not improve the periodontal status after 6 months. |
| Sales-Peres et al., 2015 Cohort study [25] | Brazil | 50, M/F 8/42, 38.9 | Roux-en-Y gastric bypass | 12 months | RTq-PCR (crevicular fluid) | Porphyromonas gingivalis increased after BS. |
| Shillitoe et al., 2012 Cohort study [22] | USA | 29 M/F 7/22, 41.0 | Roux-en-Y gastric bypass | 12 weeks | RTq-PCR (saliva) | No changes in the levels of bacteria that exceeded 2-fold, except for the Bifidobacteria species, which showed a 2.4-fold increase in patients without DM type-2 and a 10-fold increase in DM patients. The levels of circulating endotoxin and TNF-α had decreased. |
| Stefura et al., 2022 Cohort study [27] | Poland | 45 M/F 18/27, 43.5 | Sleeve gastrectomy, Roux-en-Y gastric bypass | 6 months | 16S rRNA gene sequencing (oral swab) | Bacteria from phylum Bacteroidetes increased in abundance in the oral cavity 6 months after BS. Patients achieving at least 50% of excess weight loss presented similar results to the entire study group. Patients with less favorable outcomes presented an increase in the phylum Fusobacteria and a decrease in the phylum Firmicutes in the oral cavity. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ślebioda, Z.; Rangé, H.; Strózik-Wieczorek, M.; Wyganowska, M.L. Potential Shifts in the Oral Microbiome Induced by Bariatric Surgery—A Scoping Review. Antibiotics 2025, 14, 695. https://doi.org/10.3390/antibiotics14070695
Ślebioda Z, Rangé H, Strózik-Wieczorek M, Wyganowska ML. Potential Shifts in the Oral Microbiome Induced by Bariatric Surgery—A Scoping Review. Antibiotics. 2025; 14(7):695. https://doi.org/10.3390/antibiotics14070695
Chicago/Turabian StyleŚlebioda, Zuzanna, Hélène Rangé, Marta Strózik-Wieczorek, and Marzena Liliana Wyganowska. 2025. "Potential Shifts in the Oral Microbiome Induced by Bariatric Surgery—A Scoping Review" Antibiotics 14, no. 7: 695. https://doi.org/10.3390/antibiotics14070695
APA StyleŚlebioda, Z., Rangé, H., Strózik-Wieczorek, M., & Wyganowska, M. L. (2025). Potential Shifts in the Oral Microbiome Induced by Bariatric Surgery—A Scoping Review. Antibiotics, 14(7), 695. https://doi.org/10.3390/antibiotics14070695






