Trends in Antimicrobial Resistance of Canine Otitis Pathogens in the Iberian Peninsula (2010–2021)
Abstract
1. Introduction
2. Results
2.1. Microbiological Diagnosis of Bacterial Infections
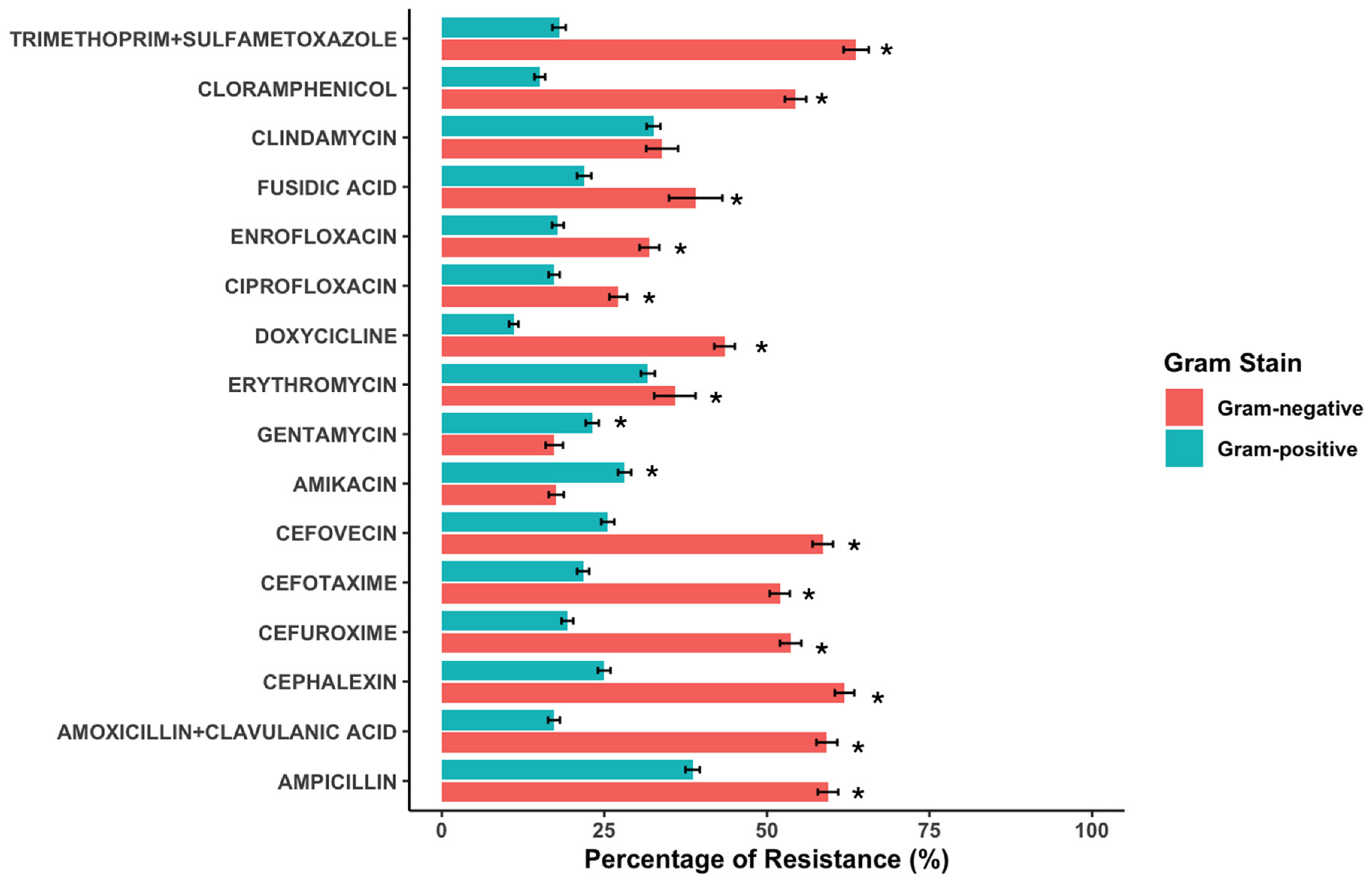
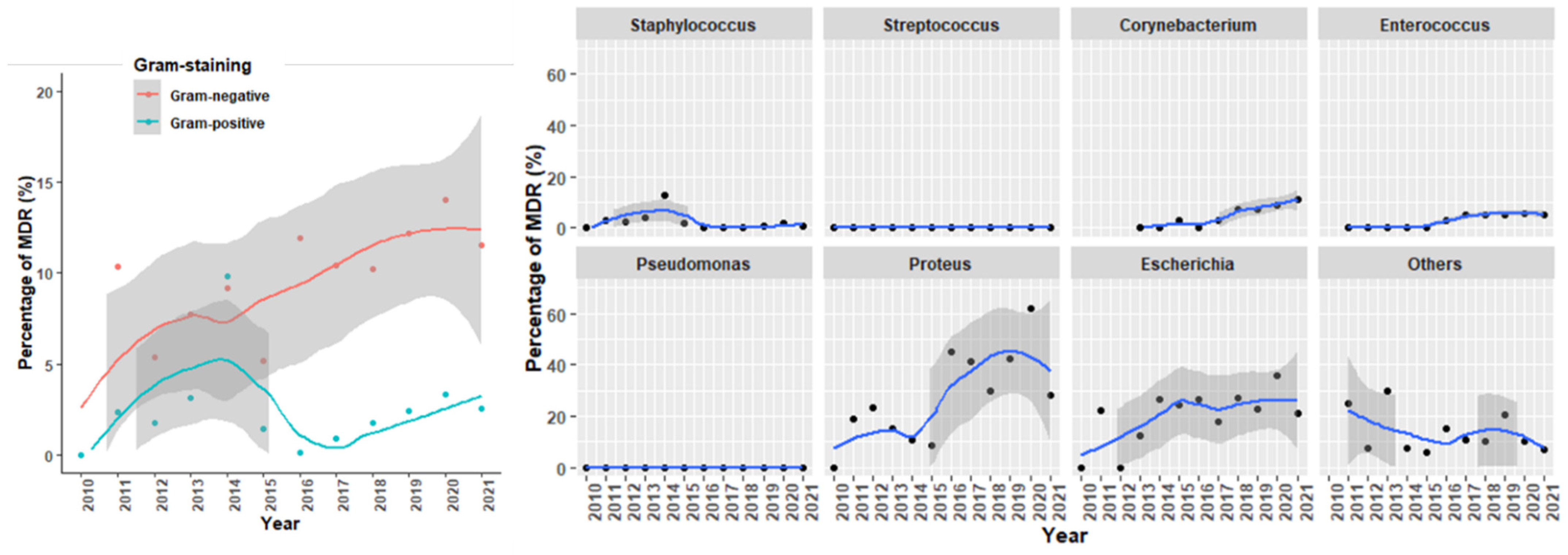
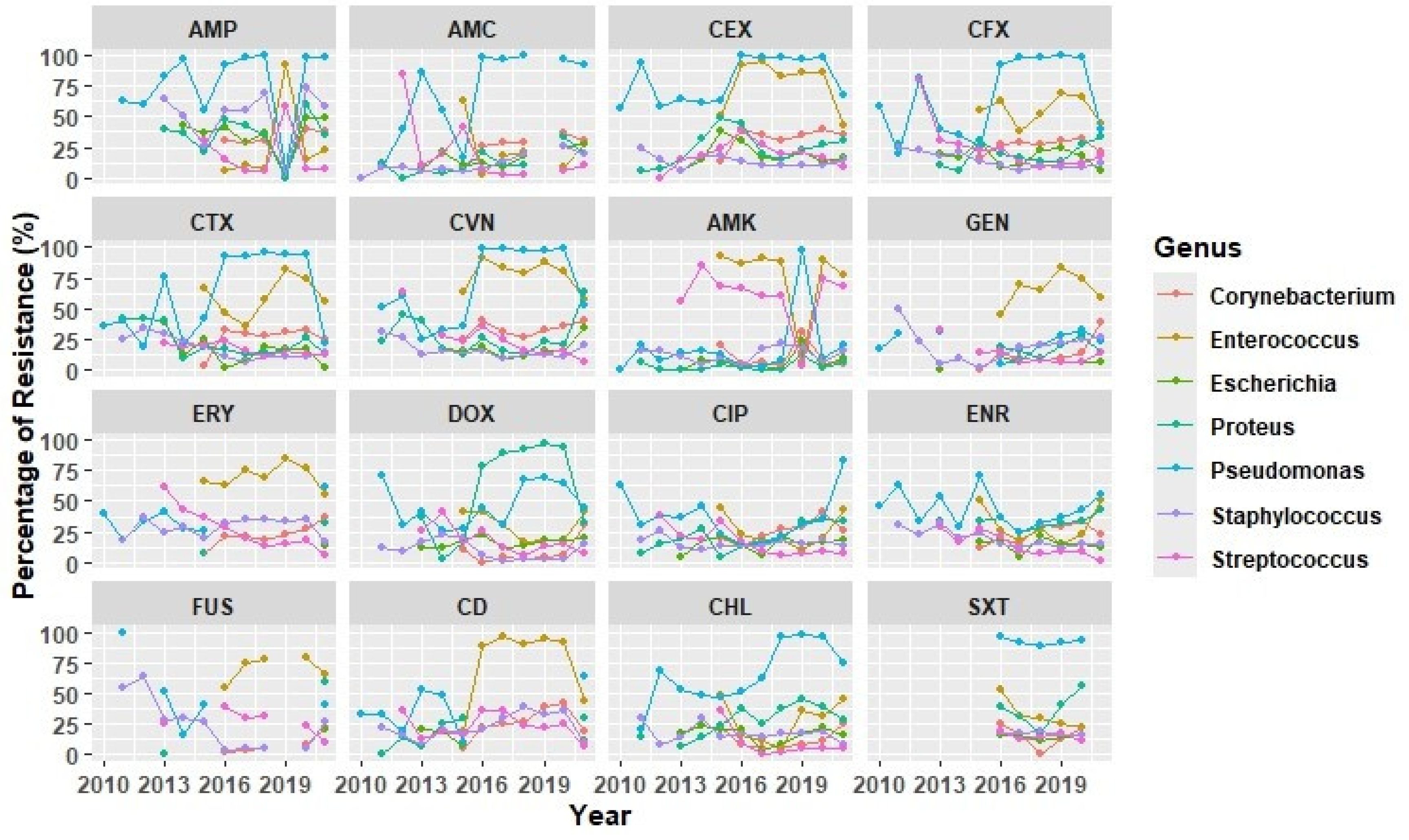
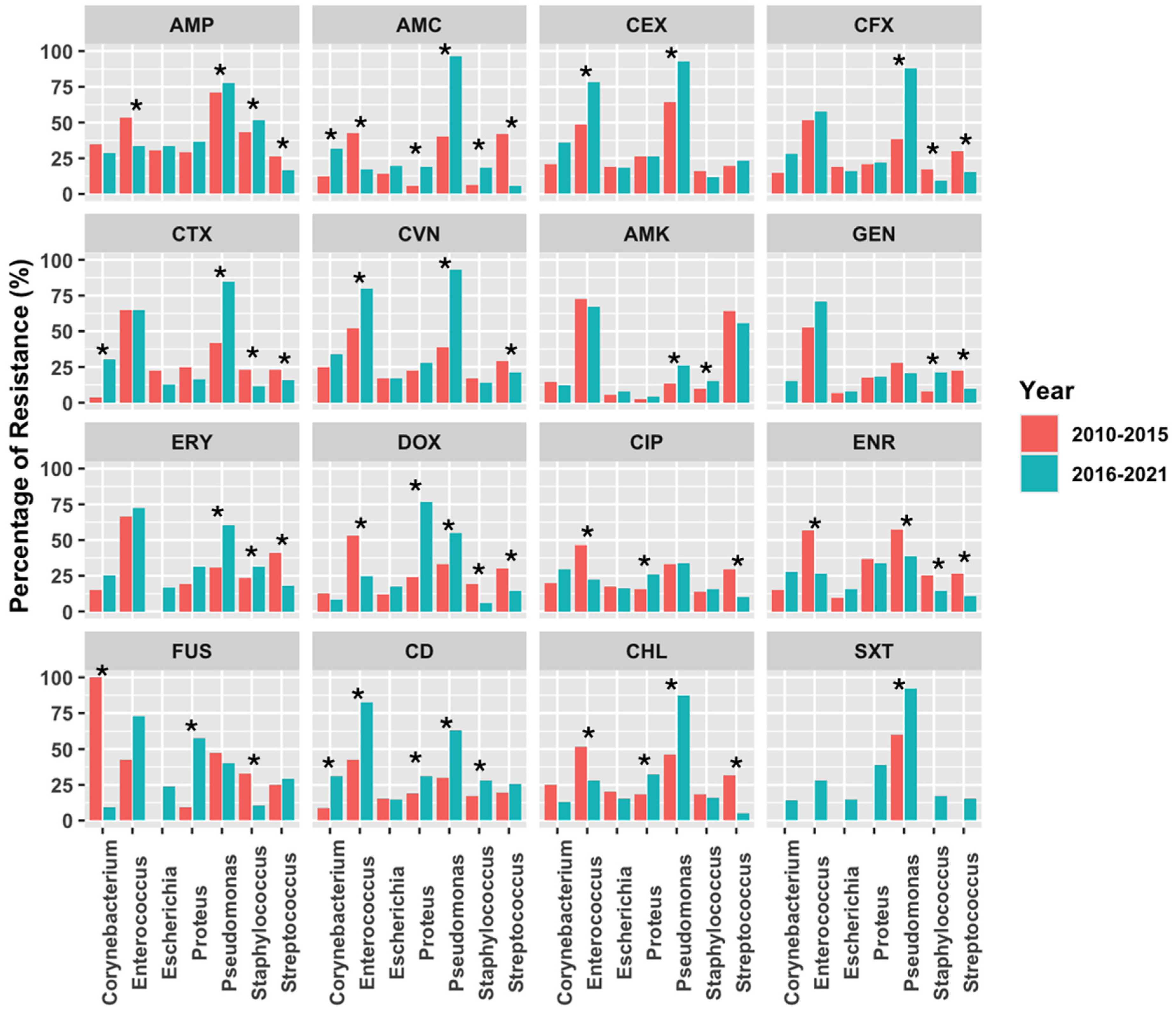
2.2. Antimicrobial Susceptibility Testing
3. Discussion
4. Materials and Methods
4.1. Data Source and Management
4.2. Microbiological Analysis and Antimicrobial Susceptibility Testing
4.3. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Naghavi, M.; Vollset, S.E.; Ikuta, K.S.; Swetschinski, L.R.; Gray, A.P.; Wool, E.E.; Robles Aguilar, G.; Mestrovic, T.; Smith, G.; Han, C.; et al. Global Burden of Bacterial Antimicrobial Resistance 1990–2021: A Systematic Analysis with Forecasts to 2050. Lancet 2024, 404, 1199–1226. [Google Scholar] [CrossRef]
- Murray, C.J.; Ikuta, K.S.; Sharara, F.; Swetschinski, L.; Robles Aguilar, G.; Gray, A.; Han, C.; Bisignano, C.; Rao, P.; Wool, E.; et al. Global Burden of Bacterial Antimicrobial Resistance in 2019: A Systematic Analysis. Lancet 2022, 399, 629–655. [Google Scholar] [CrossRef]
- Roncada, P.; Tilocca, B. Antimicrobial Resistance in Veterinary Medicine and Public Health. Animals 2022, 12, 3253. [Google Scholar] [CrossRef] [PubMed]
- Caneschi, A.; Bardhi, A.; Barbarossa, A.; Zaghini, A. The Use of Antibiotics and Antimicrobial Resistance in Veterinary Medicine, a Complex Phenomenon: A Narrative Review. Antibiotics 2023, 12, 487. [Google Scholar] [CrossRef]
- Lloyd, D.H. Reservoirs of Antimicrobial Resistance in Pet Animals. Clin. Infect. Dis. 2007, 45, S148–S152. [Google Scholar] [CrossRef]
- Guardabassi, L.; Schwarz, S.; Lloyd, D.H. Pet Animals as Reservoirs of Antimicrobial-Resistant Bacteria. J. Antimicrob. Chemother. 2004, 54, 321–332. [Google Scholar]
- Marco-Fuertes, A.; Marin, C.; Lorenzo-Rebenaque, L.; Vega, S.; Montoro-Dasi, L. Antimicrobial Resistance in Companion Animals: A New Challenge for the One Health Approach in the European Union. Vet. Sci. 2022, 9, 208. [Google Scholar] [CrossRef]
- Secker, B.; Shaw, S.; Atterbury, R.J. Pseudomonas Spp. in Canine Otitis Externa. Microorganisms 2023, 11, 2650. [Google Scholar] [CrossRef]
- Terziev, G.; Borissov, I. Prevalence of Ear Diseases in Dogs—A Retrospective 5-Year Clinical Study. Bulg. J. Vet. Med. 2018, 21, 76–85. [Google Scholar] [CrossRef]
- O’Neill, D.G.; Volk, A.V.; Soares, T.; Church, D.B.; Brodbelt, D.C.; Pegram, C. Frequency and Predisposing Factors for Canine Otitis Externa in the UK—A Primary Veterinary Care Epidemiological View. Canine Med. Genet. 2021, 8, 7. [Google Scholar] [CrossRef]
- Ponn, P.C.; Tipold, A.; Volk, A.V. Can We Minimize the Risk of Dogs Developing Canine Otitis Externa?—A Retrospective Study on 321 Dogs. Animals 2024, 14, 2537. [Google Scholar] [CrossRef] [PubMed]
- Terziev, G.; Urumova, V. Retrospective Study on the Etiology and Clinical Signs of Canine Otitis. Comp. Clin. Pathol. 2018, 27, 7–12. [Google Scholar] [CrossRef]
- Kwon, J.; Ko, H.J.; Yang, M.H.; Park, C.; Park, S.C. Antibiotic Resistance and Species Profile of Enterococcus Species in Dogs with Chronic Otitis Externa. Vet. Sci. 2022, 9, 592. [Google Scholar] [CrossRef] [PubMed]
- Kwon, J.; Kim, S.G.; Kim, S.W.; Kim, H.J.; Kang, J.W.; Jo, S.J.; Giri, S.S.; Jeong, W.J.; Bin Lee, S.; Kim, J.H.; et al. Tailoring Formulation for Enhanced Phage Therapy in Canine Otitis Externa: A Cocktail Approach Targeting Pseudomonas Aeruginosa and Staphylococcus Pseudintermedius. Vet. Microbiol. 2025, 301, 110354. [Google Scholar] [CrossRef]
- Kasai, T.; Fukui, Y.; Aoki, K.; Ishii, Y.; Tateda, K. Changes in the Ear Canal Microbiota of Dogs with Otitis Externa. J. Appl. Microbiol. 2021, 130, 1084–1091. [Google Scholar] [CrossRef]
- Dégi, J.; Morariu, S.; Simiz, F.; Herman, V.; Beteg, F.; Dégi, D.M. Future Challenge: Assessing the Antibiotic Susceptibility Patterns of Staphylococcus Species Isolated from Canine Otitis Externa Cases in Western Romania. Antibiotics 2024, 13, 1162. [Google Scholar] [CrossRef]
- Lyskova, P.; Vydrzalova, M.; Mazurova, J. Identification and Antimicrobial Susceptibility of Bacteria and Yeasts Isolated from Healthy Dogs and Dogs with Otitis Externa. J. Vet. Med. Ser. A Physiol. Pathol. Clin. Med. 2007, 54, 559–563. [Google Scholar] [CrossRef]
- Bourély, C.; Cazeau, G.; Jarrige, N.; Leblond, A.; Madec, J.Y.; Haenni, M.; Gay, E. Antimicrobial Resistance Patterns of Bacteria Isolated from Dogs with Otitis. Epidemiol. Infect. 2019, 147, e121. [Google Scholar] [CrossRef]
- Li, Y.; Fernández, R.; Durán, I.; Molina-López, R.A.; Darwich, L. Antimicrobial Resistance in Bacteria Isolated From Cats and Dogs From the Iberian Peninsula. Front. Microbiol. 2021, 11, 621597. [Google Scholar] [CrossRef]
- Tanveer, M.; Ntakiyisumba, E.; Hirwa, F.; Yoon, H.; Oh, S.I.; Kim, C.; Kim, M.H.; Yoon, J.S.; Won, G. Prevalence of Bacterial Pathogens Isolated from Canines with Pyoderma and Otitis Externa in Korea: A Systematic Review and Meta-Analysis. Vet. Sci. 2024, 11, 656. [Google Scholar] [CrossRef]
- Rosales, R.S.; Ramírez, A.S.; Moya-Gil, E.; de la Fuente, S.N.; Suárez-Pérez, A.; Poveda, J.B. Microbiological Survey and Evaluation of Antimicrobial Susceptibility Patterns of Microorganisms Obtained from Suspect Cases of Canine Otitis Externa in Gran Canaria, Spain. Animals 2024, 14, 742. [Google Scholar] [CrossRef] [PubMed]
- Darwich, L.; Seminati, C.; Burballa, A.; Nieto, A.; Durán, I.; Tarradas, N.; Molina-López, R.A. Antimicrobial Susceptibility of Bacterial Isolates from Urinary Tract Infections in Companion Animals in Spain. Vet. Rec. 2021, 188, e60. [Google Scholar] [CrossRef] [PubMed]
- Penna, B.; Varges, R.; Medeiros, L.; Martins, G.M.; Martins, R.R.; Lilenbaum, W. Species Distribution and Antimicrobial Susceptibility of Staphylococci Isolated from Canine Otitis Externa. Vet. Dermatol. 2010, 21, 292–296. [Google Scholar] [CrossRef] [PubMed]
- Aalbæk, B.; Bemis, D.A.; Schjærff, M.; Kania, S.A.; Frank, L.A.; Guardabassi, L. Coryneform Bacteria Associated with Canine Otitis Externa. Vet. Microbiol. 2010, 145, 292–298. [Google Scholar] [CrossRef]
- Malayeri, H.Z.; Jamshidi, S.; Salehi, T.Z. Identification and Antimicrobial Susceptibility Patterns of Bacteria Causing Otitis Externa in Dogs. Vet. Res. Commun. 2010, 34, 435–444. [Google Scholar] [CrossRef]
- Ma, S.; Chen, S.; Lyu, Y.; Huang, W.; Liu, Y.; Dang, X.; An, Q.; Song, Y.; Jiao, Y.; Gong, X.; et al. China Antimicrobial Resistance Surveillance Network for Pets (CARPet), 2018 to 2021. One Health Adv. 2023, 1, 7. [Google Scholar] [CrossRef]
- Reygaert, W.C. An Overview of the Antimicrobial Resistance Mechanisms of Bacteria. AIMS Microbiol. 2018, 4, 482–501. [Google Scholar] [CrossRef]
- Fernández, M.; Garcias, B.; Duran, I.; Molina-López, R.A.; Darwich, L. Current Situation of Bacterial Infections and Antimicrobial Resistance Profiles in Pet Rabbits in Spain. Vet. Sci. 2023, 10, 352. [Google Scholar] [CrossRef]
- Tejedor Junco, M.T.; Martín Barrasa, J.L. Identification and Antimicrobial Susceptibility of Coagulase Positive Staphylococci Isolated from Healthy Dogs and Dogs Suffering from Otitis Externa. J. Vet. Med. Ser. B 2002, 49, 419–423. [Google Scholar] [CrossRef]
- Glen, K.A.; Lamont, I.L. β-Lactam Resistance in Pseudomonas Aeruginosa: Current Status, Future Prospects. Pathogens 2021, 10, 1638. [Google Scholar] [CrossRef]
- Delgado, M.; Neto, I.; Correia, J.H.D.; Pomba, C. Antimicrobial Resistance and Evaluation of Susceptibility Testing among Pathogenic Enterococci Isolated from Dogs and Cats. Int. J. Antimicrob. Agents 2007, 30, 98–100. [Google Scholar] [CrossRef] [PubMed]
- Thomas, C.M.; Nielsen, K.M. Mechanisms of, and Barriers to, Horizontal Gene Transfer between Bacteria. Nat. Rev. Microbiol. 2005, 3, 711–721. [Google Scholar] [PubMed]
- Sher, A.A.; VanAllen, M.E.; Ahmed, H.; Whitehead-Tillery, C.; Rafique, S.; Bell, J.A.; Zhang, L.; Mansfield, L.S. Conjugative RP4 Plasmid-Mediated Transfer of Antibiotic Resistance Genes to Commensal and Multidrug-Resistant Enteric Bacteria In Vitro. Microorganisms 2023, 11, 193. [Google Scholar] [CrossRef] [PubMed]
- Magiorakos, A.-P.; Srinivasan, A.; Carey, R.B.; Carmeli, Y.; Falagas, M.E.; Giske, C.G.; Harbarth, S.; Hindler, J.F.; Kahlmeter, G.; Olsson-Liljequist, B.; et al. Multidrug-Resistant, Extensively Drug-Resistant and Pandrug-Resistant Bacteria: An International Expert Proposal for Interim Standard Definitions for Acquired Resistance. Clin. Microbiol. Infect. 2012, 18, 268–281. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2020. [Google Scholar]
- Wickham, H. Ggplot2. Wiley Interdiscip. Rev. Comput. Stat. 2011, 3, 180–185. [Google Scholar] [CrossRef]
- Kassambara, A. Visualization of a Correlation Matrix Using “Ggplot2”. Package ‘ggcorrplot’. R Package Version 0.1.4.999. 2022. Available online: https://github.com/kassambara/ggcorrplot (accessed on 1 March 2025).
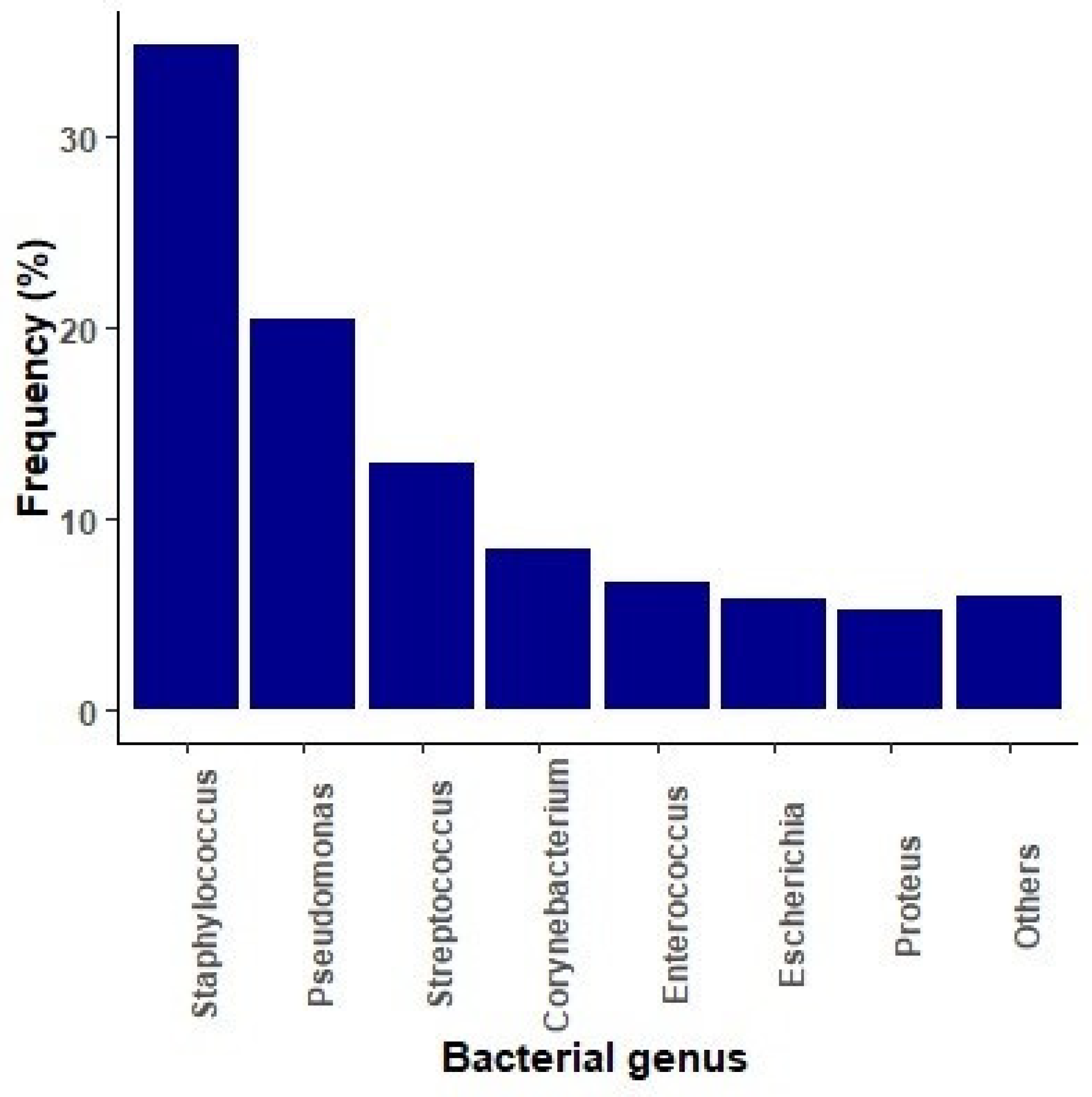


| Microbiological Isolates | Number (% Within spp.) | Overall (N = 12,498) % (95% CI) |
|---|---|---|
| Staphylococcus spp. | n = 4339 | 34.7 (33.9–35.6) |
| S. pseudintermedius | 2024 (46.7) | 16.2 (15.6–16.9) |
| S.schleiferi | 451 (10.4) | 3.6 (3.3–4) |
| S. intermedius | 356 (8.2) | 2.8 (2.6–3.2) |
| S. aureus | 320 (7.4) | 2.6 (2.3–2.9) |
| S. epidermidis | 100 (2.3) | 0.8 (0.7–1) |
| S. simulans | 28 (0.6) | 0.2 (0.2–0.3) |
| S. chromogenes | 21 (0.5) | 0.2 (0.1–0.3) |
| S. warneri | 17 (0.4) | 0.1 (0.08–0.2) |
| S. hominis | 16 (0.4) | 0.1 (0.07–0.2) |
| S. haemolyticus | 14 (0.3) | 0.1 (0.06–0.2) |
| Others | 993 (22.9) | 7.9 (7.5–8.4) |
| Pseudomonas spp. | n = 2541 | 20.3 (19.6–21.1) |
| P. aeruginosa | 2365 (93.1) | 18.9 (18.2–19.6) |
| P. putida | 25 (1.0) | 0.2 (0.1–0.3) |
| P. oryzihabitams | 15 (0.6) | 0.1 (0.07–0.2) |
| Others | 176 (6.9) | 1.4 (1.2–1.6) |
| Streptococcus spp. | n = 1609 | 12.9 (12.3–13.5) |
| S. canis | 661 (41.1) | 5.3 (4.9–5.7) |
| S. halichoeri | 42 (2.6) | 0.3 (0.2–0.5) |
| S. dysgalacticae | 31 (1.9) | 0.2 (0.2–0.4) |
| Others | 875 (54.4) | 7.0 (6.6–7.5) |
| Corynebacterium spp. | n = 1050 | 8.4 (7.9–8.9) |
| C. auriscanis | 545 (51.9) | 4.4 (4–4.7) |
| C. amycolatum | 201 (19.1) | 1.6 (1.4–1.8) |
| Others | 304 (29.0) | 2.4 (2.2–2.7) |
| Enterococcus spp. | n = 842 | 6.7 (6.3–7.2) |
| E. faecalis | 538 (63.9) | 4.3 (4–4.7) |
| E. faecium | 66 (7.9) | 0.5 (0.4–0.7) |
| E. canintestini | 55 (6.5) | 0.4 (0.3–0.6) |
| E. avium | 26 (3.0) | 0.2 (0.1–0.3) |
| E. hirae | 10 (1.2) | 0.1 (0.04–0.2) |
| Others | 147 (17.5) | 1.1 (1–1.4) |
| Escherichia spp. | n = 725 | 5.8 (5.4–6.2) |
| E. coli | 711 (98.1) | 5.7 (5.3–6.1) |
| Others | 14 (1.9) | 0.1 (0.06–0.2) |
| Proteus spp. | n = 656 | 5.3 (4.9–5.7) |
| P. mirabilis | 622 (94.8) | 5.0 (4.6–5.4) |
| P. vulgaris | 18 (2.6) | 0.1 (0.08–0.2) |
| Others | 16 (2.4) | 0.1 (0.08–0.2) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Garcias, B.; Batalla, M.; Vidal, A.; Durán, I.; Darwich, L. Trends in Antimicrobial Resistance of Canine Otitis Pathogens in the Iberian Peninsula (2010–2021). Antibiotics 2025, 14, 328. https://doi.org/10.3390/antibiotics14040328
Garcias B, Batalla M, Vidal A, Durán I, Darwich L. Trends in Antimicrobial Resistance of Canine Otitis Pathogens in the Iberian Peninsula (2010–2021). Antibiotics. 2025; 14(4):328. https://doi.org/10.3390/antibiotics14040328
Chicago/Turabian StyleGarcias, Biel, Mar Batalla, Anna Vidal, Inma Durán, and Laila Darwich. 2025. "Trends in Antimicrobial Resistance of Canine Otitis Pathogens in the Iberian Peninsula (2010–2021)" Antibiotics 14, no. 4: 328. https://doi.org/10.3390/antibiotics14040328
APA StyleGarcias, B., Batalla, M., Vidal, A., Durán, I., & Darwich, L. (2025). Trends in Antimicrobial Resistance of Canine Otitis Pathogens in the Iberian Peninsula (2010–2021). Antibiotics, 14(4), 328. https://doi.org/10.3390/antibiotics14040328