1. Introduction
Antimicrobial resistance (AMR) refers to the ability of microorganisms to withstand the effects of antimicrobial agents, either through inherent traits or acquired mechanisms. Over time, microorganisms can evolve these capabilities via Darwinian selection, allowing them to survive in the presence of otherwise toxic substances. This phenomenon can emerge when viruses, parasites, fungi, and bacteria undergo mutations in their genetic sequence, potentially resulting in resistance to one or multiple antimicrobial reagents [
1]. The treatment of multi-drug-resistant microorganisms, also known as “superbugs,” is a unique challenge actively working to be resolved by scientists [
2]. Bacteria with antimicrobial resistance have been identified in drinking water, soil, food products, the sea, and surprisingly, even in Antarctica. The widespread use of antimicrobials across various sectors is the leading cause of antimicrobial resistance globally [
3].
Antimicrobial resistance poses a major risk to human health worldwide. A predictive statistical model found that 4.95 million deaths in 2019 were caused by antimicrobial resistance. In a widely cited review commissioned by the UK government, it was claimed that without policies to prevent the spread of AMR, antimicrobial resistance could cause up to 10 million deaths annually by 2050 [
2].
The economic burden of antimicrobial resistance is substantial. The majority of studies show that healthcare costs in patients with infections resistant to antibiotics are higher than the care for patients due to an extended illness duration, prolonged length of hospital stay, additional diagnostic testing, and need for more expensive medications [
4,
5]. According to the CDC, treating six of the most alarming antimicrobial resistance threats results in an accumulation of healthcare costs of over USD 4.6 billion each year in the United States [
4,
6]. Antimicrobial resistance is projected to reduce global GDP by 1.1% [
7]. A study of 76 countries discovered that antibiotic consumption increased by 65% between the years 2000 and 2015. This increase may have been attributable to lower-income countries [
8]. Despite being under-recognized, socioeconomic factors contribute significantly to the spread of AMR. Several socioeconomic factors are believed to have a strong association with antimicrobial resistance, including but not limited to a lack of healthcare expenditure, along with poor infrastructure and governance [
9].
This study is worth developing as it addresses the urgent global health threat posed by antimicrobial resistance through a comprehensive examination of frequently overlooked socioeconomic factors that fuel its spread, particularly in resource-limited settings. This exploration aims to thoroughly assess the extent to which socioeconomic factors play a role in the rapid proliferation of antimicrobial resistance worldwide. Through a comprehensive review of the existing literature, we examine how the intersection of economic, social, and healthcare-related factors contributes to the development and spread of antimicrobial resistance, with a focus on the most resource-limited settings. Although previous studies have explored individual socioeconomic factors which have contributed to antimicrobial resistance in localized settings, comprehensive reviews examining a broad spectrum of socioeconomic determinants through a global perspective remain relatively scarce.
2. Methodology
The literature review for this study was conducted through a search of established public health databases including PubMed, Google Scholar, the Centers for Disease Control and Prevention, and the World Health Organization. Keywords of “antimicrobial resistance,” “socioeconomic factors,” “low- and middle-income countries,” “surveillance,” “healthcare access,” and “agriculture” were combined to optimize search efficiency. Preference was given to systematic reviews, large-scale observation studies, and policy analyses with regards to low- and middle-income countries. Articles pertaining solely to biological mechanisms of antimicrobial resistance and studies concentrated on animals without explicit linkage to human health were excluded. Studies were eligible for inclusion if they were tied to reputable global health organizations, published in peer-reviewed journals, addressed antimicrobial resistance with bridges to socioeconomic determinants, and were published in English. The selected articles were synthesized thematically to highlight patterns and interconnections among socioeconomic factors contributing to AMR, with particular attention to regulatory frameworks, healthcare infrastructure, and demographic disparities. Lastly, a collection of 86 studies were included for the purpose of this review.
3. Background
Intrinsic and acquired resistance mechanisms are the two methods used by bacteria to develop the ability to withstand antibiotics. Intrinsic resistance is independent of antibiotic exposure and is found universally within certain bacterial genomes. It is a chromosomally mediated mechanism of resistance and is predictable based on an organism’s identity [
10]. The second mechanism of antimicrobial resistance is acquired resistance, where a bacterium becomes resistant to an antibiotic it was previously susceptible to. This can result from genetic mutations or the uptake of plasmids carrying resistance genes from intrinsically resistant organisms [
11]. There are two methods by which bacteria can acquire resistance to antimicrobials. The first is horizontal gene transfer, in which bacteria transfer DNA from one bacterium to another. The second is the rapid reproduction of bacteria, causing evolutionary shifts and natural selection over time to favor bacteria which have resistance [
12].
A multitude of factors including vaccination rates, variations in healthcare systems, along with risks associated with tourism, population densities, migration, and sanitation practices can spread resistance among humans [
13]. While antibiotic use was once considered the most critical point in the development of antimicrobial resistance, recent research highlights the significant influence of socioeconomic factors in its global proliferation.
3.1. Significance of AMR
Antimicrobial resistance is an emerging global health crisis as it jeopardizes the effectiveness of antimicrobial drugs and poses extreme challenges from a One Health perspective. Resistant microorganisms in both the community and healthcare settings reduce survival rates, particularly in the case of infections acquired in the healthcare setting such as neonatal sepsis. As a result, drug-resistant infections limit the benefits of cancer treatments, surgeries, and transplants [
14]. Immediate attention and action are necessary to hinder the growing spread of AMR. Both higher-income and low–middle-income countries are susceptible to the growing threat of antimicrobial resistance worldwide. Data published in December 2019 have indicated that over 2.8 million individuals in the United States contract drug-resistant infections annually, leading to 35,000 deaths and an even greater number of hospitalizations [
15]. Healthcare-associated infections cost the US healthcare system between USD 28 and 45 billion annually [
16].
3.2. Causes of AMR
Bacteria inhabiting soil, despite mostly being nonpathogenic, are frequently exposed to different chemicals and are gradually able to develop mechanisms to interact with other microbes to defend themselves against threats [
17]. Gene transfer is a key factor in the spread of antimicrobial resistance. Bacteria reproduce quickly, which gives them the ability to undergo evolutionary changes through spontaneous genetic mutations that can emerge in short periods of time. De novo mutations, which are genetic variants appearing for the first time in a family tree, contribute significantly to the spread of antimicrobial resistance, often through plasmids that mediate the acquisition of resistance genes [
9]. AMR can proliferate due to uncontrollable horizontal gene transfer, a method in which circular plasmid DNA is shared between microbes, affecting the microbiota of microorganisms. Additionally, when resistant pathogenic microorganisms develop in one location, frequent travel and inadequate hygiene can upregulate their spread [
18]. In clinical settings, antimicrobial resistance can still be acquired when bacteria that were formerly susceptible to antibiotics develop resistance over time, complicating treatment options [
11].
Although antimicrobial agents have been an integral part of modern medicine for the last 80 years, AMR dates back much further in history. Interestingly, AMR existed well before antimicrobials were identified, synthesized, and commercialized. Bacteria isolated from glacial waters estimated to be 2000 years old have demonstrated resistance to Ampicillin [
19]. Sediment DNA dating back 30,000 years ago has revealed a cluster of genes that encode for resistance to tetracycline, glycopeptide, and β-lactam antibiotics [
20].
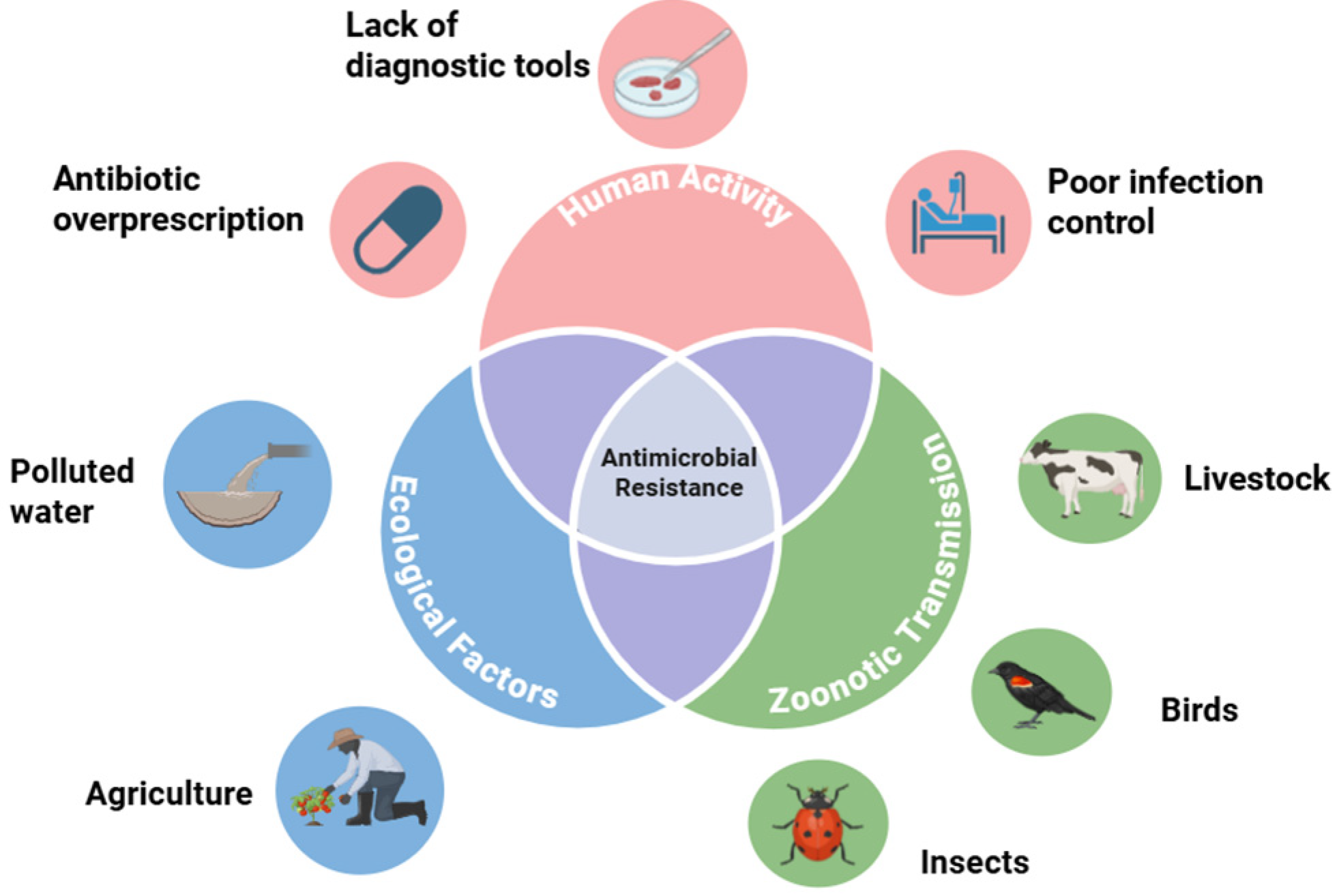
Antimicrobial resistance contagion, which is the spread of bacterial strains resistant to antimicrobial formulas, can spread via an assortment of vectors. The biological transmission of these antimicrobial-resistant strains can be traced to interactions between birds, insects, humans, water, and agriculture [
9]. Hence, combatting the threat of antimicrobial resistance requires a comprehensive One Health approach. The environment should be considered as having an underappreciated role in the proliferation of AMR. Yet, it is essential to understand that antibiotic consumption is increasing around the world. The One Health framework underlying the drivers of antimicrobial resistance is depicted in
Figure 1.
3.3. Global Increase in Antibiotic Consumption
Surveillance data on national antibiotic use is crucial for tracking trends, comparing countries, establishing baselines for reduction efforts, and analyzing the link between use and resistance over time. Antibiotic consumption is exponentially increasing all around the world, especially in low- and middle-income countries. A study projected that global antibiotic consumption will increase by 200% in 2030 compared to 2015 [
8]. It is estimated that approximately 50% of antimicrobials in healthcare are considered to be unnecessary [
21].
3.4. Demographic Susceptibility
The health burden caused by antimicrobial resistance is heavily related to the income of the country. Low- and low–middle-income countries tend to carry the largest AMR burden. Western Sub-Saharan Africa and South Asia exhibit the greatest antimicrobial resistance, with a death rate due to AMR in 2019 of 27.3 per 100,000 and 21.5 per 100,000, respectively [
22]. This is significantly higher than in Western Europe, where the death rate attributed to AMR was 11.7 per 100,000. However, it should be noted that a multitude of socioeconomic factors result in the proliferation of AMR.
4. Socioeconomic Factors Contributing to Antimicrobial Resistance
4.1. Challenges in Low- and Low–Middle-Income Countries
There is significant evidence pointing to variations within higher- and lower-income countries when it comes to residual active pharmaceutical ingredients (APIs) found in nature. The manufacturing activities of active pharmaceutical ingredients lead to residues via industrial waste discharge, which is prevalent in the riverine environment [
23]. While many higher-income countries can monitor their waste streams for capture and treatment, this practice is not as commonplace in low- and low–middle-income countries. In the Hyderabad region of India, water from lakes and areas downstream from treatment plants was found to have abnormally high levels of API residue when compared with levels commonly detected in North America and Europe [
5]. Additionally, it has been reported that the API market in Asia is expanding at twice the rate of G7 countries [
24]. While approximately 78% of the global population lives in Africa and Asia, only around 16% live in North America and Europe. This becomes a concern as the global pharmaceutical manufacturing base shifts to Asia and other low-income regions of the world.
Additionally, another factor contributing to the spread of AMR is poor sewer connectivity in LMICs. A lack of sewer connectivity in low-income countries leads to the release of a bulk of APIs into the environment, promoting a high degree of antimicrobial resistance. Even in urban areas, some lower-income countries in southern Asia have less than 30% sewer connectivity. Indonesia and Vietnam only have 2 and 4 percent sewer connectivity, respectively, largely as a result of their dependence on septic systems [
25]. In high-income countries, over 90% of the population is linked by sewers, highlighting how a lack of sewer connectivity in poorer regions can facilitate the growth of AMR.
In certain countries, the absence of wastewater treatment facilities results in the contamination of surface and groundwater resources [
26]. A study on New Delhi’s sewer treatment plants found that only 50% of the capacity was utilized, and effluent frequently failed fecal coliform standards [
27]. According to a study published in 2009, only 22% of the daily sewage was treated in 71 Indian cities, while 78% of the untreated sewage had to be discharged into large bodies of water [
28]. As a result, these large bodies of water become environmental reservoirs for antimicrobial resistance.
In many poorer regions of the world, most antimicrobials can be ordered without prescription, also referred to as self-medication [
29]. Additionally, overprescription due to the perceived patient demand becomes an unresolved issue in lower-income countries. The pressure applied by pharmaceutical companies has unseen impacts on the prescription practices of medical providers [
30].
Income inequality, even in developed European nations, has been found to be correlated with AMR [
31]. Higher-income countries still consume greater levels of antibiotics, though the consumption in LMICs has been converging in recent years. Contrary to the popular belief that income inequality leads to increased antibiotic usage, improving the income level has been found to be a key driver of antibiotic consumption [
8]. This highlights how AMR proliferation is not only a symptom of poverty or inequality, but also of growing wealth and access, pointing towards the complexity of the relationship between economic development, inequality, and antimicrobial resistance.
4.2. Living Conditions
Living conditions increase the risk of antimicrobial resistance. Poor water, sanitation, and hygiene practices can promote the risk of the dissemination of infectious diseases and AMR genes [
32]. Household overcrowding is associated with increased antimicrobial use and antimicrobial resistance [
33,
34,
35]. In addition, those who are unhoused or incarcerated have greater susceptibility to AMR carriage [
36]. In urban regions of a lower socioeconomic status in New York City, groups susceptible to overcrowding seemed to be at an elevated risk of community-associated methicillin-resistant
S. aureus, especially in households with more than three persons [
37]. In another study conducted in emergency department patients in California, homelessness was identified as a determinant for antimicrobial resistance [
38]. The insufficient living conditions of many LMICs contribute to greater AMR susceptibility.
4.3. Access to Healthcare
Limited access to healthcare is associated with an increase in self-medication with antibiotics [
39]. In resource-confined settings, a prolonged shortage of antibiotics may lead to AMR through selection of ineffective pharmaceuticals, suboptimal broad-spectrum antibiotics, and substandard antimicrobial practices [
40,
41]. Substandard road conditions and long distances hinder patient access to drug-dispensing facilities and testing centers, exacerbating the AMR crisis [
42]. Medication shortages resulting from procurement delays restrict healthcare access and cause the implementation of inappropriate alternatives, contributing to the rise in AMR [
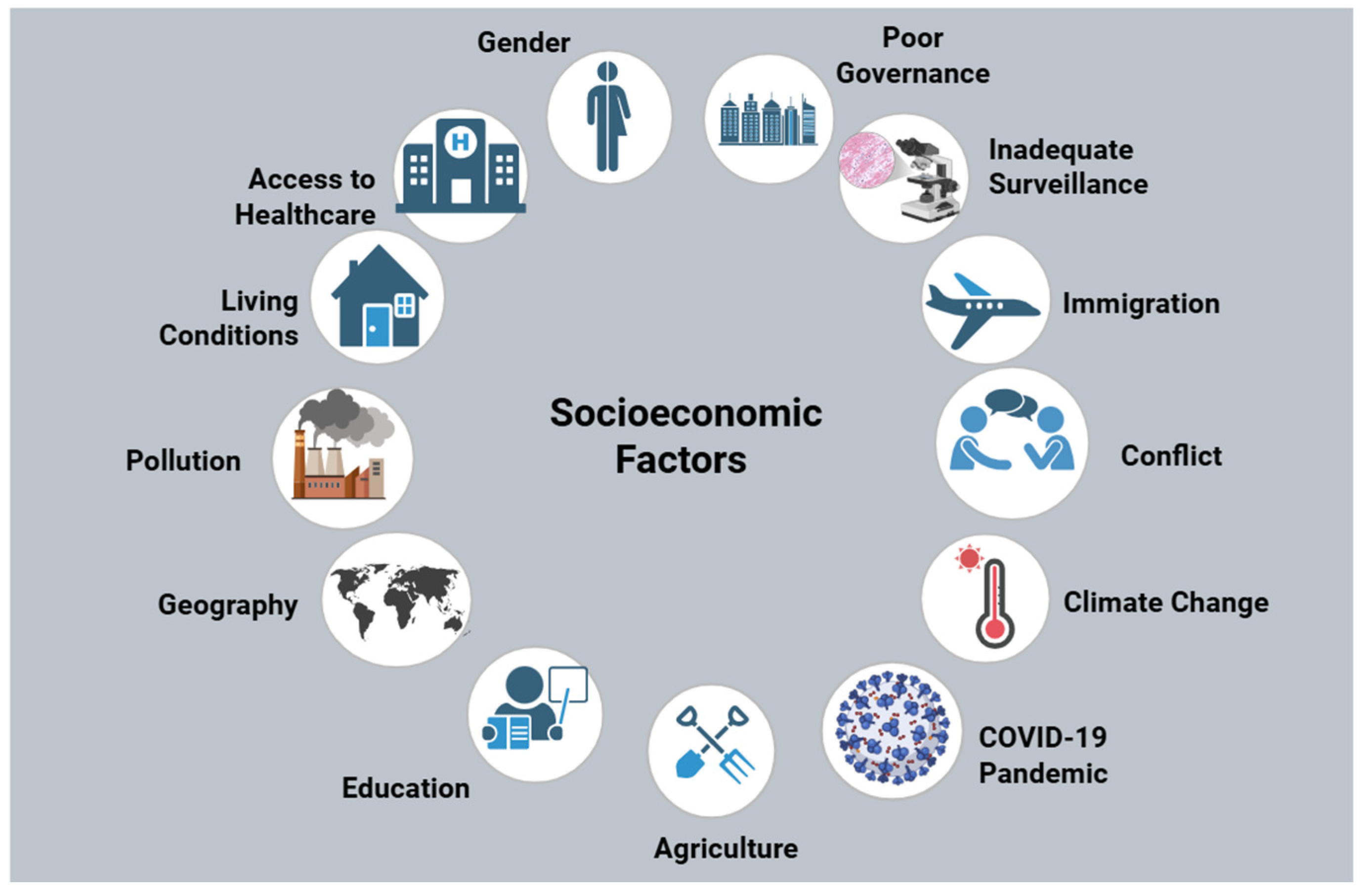
43]. Inadequate healthcare access proliferates AMR due to inappropriate antibiotic selection. Access to healthcare is one of the many socioeconomic determinants that has been illustrated in
Figure 2.
4.4. Gender
Several biological factors pertaining to women may expose them to greater risks of infections than their male counterparts. A study has shown that women have a 27% higher chance than men to receive an antimicrobial prescription [
44]. In the same study, it was found that women receive a 25% greater quantity of antibiotics compared to men. Some studies have also indicated that gender bias influences more antimicrobial prescribing to women than to men for the same illness [
45]. The gender gap may be able to be traced to adult women being significantly more likely to consult primary care, as found by a large study of the British healthcare system [
46]. Understanding the intersection of gender disparity and socioeconomic factors is key, as women often face unique challenges in healthcare access, affordability, and cultural expectations.
4.5. Poor Governance and Limited Regulatory Frameworks
Lower standards of governance, evaluated via indicators like accountability, voice, the rule of law, regulatory quality, and corruption control, have strong associations with increased antimicrobial usage and resistance rates [
9,
47]. A study which analyzed data from 43 countries in sub-Saharan Africa between 1996 and 2011 found that healthcare spending was significantly higher in countries that had better qualities of governance [
48]. Low- and low–middle-income countries may struggle to establish regulatory agencies that are willing to implement stricter standards to import, produce, and distribute antimicrobials [
49]. In fact, LMICs have been known to promise the adoption of public health policies which they are incapable of replicating [
50]. Government bodies should encourage the public to raise awareness for improved policies, especially in LMICs which often exhibit unregulated antibiotic distribution and consumption.
4.6. Inadequate Surveillance Systems
Antimicrobial resistance surveillance is crucial in LMICs because of the prevalence of bacterial infections. However, LMICs generally lack the ability to maintain surveillance on the proliferation of antimicrobial resistance. Many LMICs do not possess adequate laboratories with the necessary resources to conduct comprehensive AMR testing. In a study conducted in Tanzania in 2012, it was found that only 27% of public hospitals could perform tuberculosis microscopy and only 40% of dispensaries had a malaria diagnostic capacity [
51]. In addition, data collection methods tend to be inadequate and standardized protocols are few and far between. Other limitations in low- and low–middle-income countries include an inability to practice standard laboratory techniques, the insufficient use of microbiology services, and low representation [
52]. A shortage of skilled personnel prevents accurate and timely antimicrobial susceptibility testing [
53]. In many low- and low–middle-income countries, there is a lack of standardized reporting systems, which hinders the ability of researchers to gather data regarding the risks of antibiotic resistance [
54].
4.7. Immigration and Human Mobility
The movement of individuals across borders can elevate the spread of novel antimicrobial resistance genes internationally. Travelers are susceptible to acquiring resistant infections along their journeys, which can facilitate the spread of resistant strains into new countries [
55]. When studying causes of bacterial infections resistant to antibiotics, Chatterjee et al. discovered that travel abroad was responsible for 3% of AMR transmission in the studies examined [
56]. In a large meta-analysis, it was found that migration is associated with the development of antimicrobial resistance, as 25.4% of migrants examined carried or had AMR infections [
57]. Immigration resulting from poor socioeconomic conditions can, therefore, lead to the mass movement of populations across borders, enhancing the ability of AMR infections to proliferate. Hence, immigration serves as a vector for AMR transmission.
4.8. Conflict
Conflict may hinder access to healthcare systems and the delivery of basic health services for those impacted. Specifically, conflict can damage infrastructure such as lab equipment in regions with already insufficient microbial testing facilities, preventing the identification of drug-resistant pathogens [
58]. It may also cause disruptions in antibiotic stewardship practices, leading to unoptimized antibiotic choices and a decline in water, sanitation, and hygiene availability [
59]. During the Syrian conflict, healthcare infrastructure was severely damaged, healthcare personnel moved out of the country, inappropriate antibiotic use was escalated and overcrowding and poor sanitation accelerated the spread of AMR [
60]. Thus, prominent issues in LMICs only become exacerbated during conflict, especially in war-torn regions. As conflict is more likely to arise in countries of lower income, the socioeconomic status intersects with the prevalence of AMR in these regions.
4.9. Climate Change
The World Health Organization has identified climate change as the most formidable health threat facing humanity, with 250,000 more deaths expected annually from 2030 to 2050 [
61]. Due to global warming, several microorganisms have crossed over to humans, including
Salmonella,
Vibrio cholera, and
Campylobacter. This is a result of increasing temperatures in water systems [
62]. Climate change and seasonal fluctuations can impact bacterial survival and antibiotic resistance rates, as exemplified by
S. aureus resistance rates across South African provinces; this was attributed to the enhancement of biofilm formation during warmer and wetter seasons [
63]. There is growing evidence that climate change brings humans and animals closer together, increasing the chance for the zoonotic transmission of antimicrobial-resistant infections [
1]. In essence, the movement of animals due to the climate has driven zoonotic infection rates of AMR. As a result, LMICs are burdened with mitigating climate change while simultaneously attempting to manage the resulting health effects with an already limited supply of resources.
4.10. COVID-19 Pandemic
Studies have found correlations between poverty and low-income households and an increased risk of COVID-19 [
64]. The COVID-19 pandemic has spread AMR due to elevated antibiotic usage and nosocomial drug-resistant infections [
65,
66]. On the contrary, the implementation of stricter regulations on travel worldwide and elective procedures in hospitals, along with increases in hand hygiene, may have reduced the spread of AMR in the short term. Nevertheless, in 2020, it is estimated that AMR led to a third as many deaths as COVID-19. As nearly 70% of COVID-19 patients were given antimicrobials in outpatient or inpatient settings, this likely exacerbated AMR [
67,
68]. One particular instance of AMR emerging due to COVID-19 antibiotic use is chloroquine, an antimalarial drug; the frequent administration of this drug may encourage chloroquine resistance in non-
Plasmodium falciparum [
69]. Another aspect of inappropriate antibiotic prescription during the peak of the COVID-19 era may have been triggered by an overlap in the symptomatology of COVID-19 and bacterial infections. A lack of adequate materials for testing can result in incorrect diagnoses of COVID-19 in LMICs and the subsequent prescription of antibiotic agents when they are unnecessary. Essentially, the scarcity of resources in the healthcare system of LMICs upregulates the spread of AMR.
4.11. Agriculture
In many populated parts of the world, farming is associated with a low socioeconomic status [
70]. Farmers are found to have disproportionately high rates of methicillin-resistant
Staphylococcus aureus (MRSA) infections compared to the general population [
40,
71]. The use of antibiotics in agriculture is thought to have potentially exceeded antibiotic consumption by humans in 2010, when roughly 63,200 tons worth of antibiotic usage was found in livestock [
6]. Intensive agricultural practices have been shown to be drivers of antimicrobial resistance. More interestingly, conventionally farmed sites have been found to harbor more antimicrobial resistance genes than organically farmed sites. Agricultural chemicals and pollutants can inflict stress upon intensively farmed soils. These stressors may lead to cross-resistance in bacteria [
72,
73].
4.12. Education
Educational level has been proven to be a factor in terms of the proliferation of AMR. In a Japanese study, 6982 participants were evaluated via a questionnaire, and it was determined that the strongest indicator of awareness of proper antibiotic useand AMR was educational level [
74]. Poor access to education has been linked to resistant
S. pneumoniae and
E. coli infections [
33]. Those with stronger education levels are able to better understand the risks of inappropriate antibiotic use and the emergence of AMR [
75]. However, there are studies refuting this claim. The authors of a meta-analysis claim that the misuse of antibiotics lacks a strong correlation to educational levels. This meta-analysis surprisingly found that individuals with higher education in Europe had 25% greater odds of antibiotic misuse [
76]. Contrasting evidence has manifested in another study. Contrary to popular belief, a study conducted in Lao PDR found that educational level alone is not a reliable indicator of safer antibiotic practices to mitigate the proliferation of AMR [
77]. Hence, the connection between education and the optimal use of antibiotics is supported by significant evidence, but still a subject of controversy.
4.13. Geography
Non-prescription antibiotic use is well known to increase antimicrobial-resistant bacteria in the community. In a large meta-analysis by Morgan et al., it was found that there was a clear connection between geographical location and non-prescription antibiotic use. This study found non-prescription antibiotic use to be 100% in Africa, 58% in Asia, and 3% in northern Europe [
78]. In a separate study, it was found that self-medication with antibiotics ranged from 19% to 82% in different Middle Eastern countries [
79]. Antibiotic consumption rates vary significantly by geographic region, where LMICs have exhibited increases in usage driven by rising income levels and healthcare access. In a 2015 study of 76 countries based on the income level, four of the six countries with the highest antibiotic usage rates were LMICs, specifically Turkey, Tunisia, Algeria, and Romania [
8]. Moreover, geographic variation significantly influences AMR in
Salmonella Enteritidis, as exhibited in the high multidrug resistance rates in isolates from China (up to 81%) and sub-Saharan Africa (42%), in contrast to much lower rates in the United States (2.2%) and Europe (3.2%), with distinct resistance gene profiles and plasmid variations found in each region [
80]. Geographical location may be linked to socioeconomic status; the wealthiest regions tend to have the least nonprescription antibiotic use.
4.14. Pollution
Antibiotic resistance in bacteria can develop via mutations in the bacterial genome or by acquiring foreign DNA. Low-quality infrastructure to manage human and animal waste streams can lead to an increase in residual antibiotics and fecal bacteria found in the environment [
29,
81]. China and India produce the most antibiotics, but struggle to discard them properly, as the ineffective waste management and excessive emission of antibiotics have been reported in both countries. A lack of resources in LMICs has exacerbated the uncontrolled deposition of antibiotic residues in nature [
82,
83]. Pollution in countries of all socioeconomic statuses can result in the prevalence of antibiotics and antibiotic resistance genes (ARGs) in marine habitats. Antibiotics and ARGs can be found in riverine runoff, wastewater treatment plants, sewage discharge, and aquaculture. Antibiotic spread into the environment can be attributed to environmental variables, such as heavy metals, organic pollutants, and nutrients. ARG dissemination can be affected by antibiotic residues, environmental factors, mobile genetic elements, and bacterial communities. A global proliferation of antibiotics and ARGs within marine environments poses an unseen threat to both marine and human life, not to mention the disruption they cause to microbial communities and biogeochemical cycles. Most antibiotics consumed by humans end up excreted in an unmetabolized form, especially in LMICs with limited wastewater-processing facilities [
4]. It is crucial to garner public awareness to alter human behaviors to be able to limit the disposal of unwanted antibiotics into natural habitats. In lower-income regions, there is a dire need for the improved regulation of waste excreted into the environment which often contains unforeseen amounts of ARGs, enhancing AMR proliferation. In a large study surveying the 11 most populous cities in Catalonia, Spain, short-term exposure to increased levels of ambient air pollutants was significantly associated with a rise in antibiotic prescriptions for acute respiratory symptoms, suggesting that air pollution may indirectly drive antimicrobial resistance [
84].
5. Conclusions and Future Directions
Antimicrobial resistance is regarded as a worldwide threat to human health. The rise in antimicrobial resistance is a multifaceted global challenge influenced heavily by socioeconomic factors, particularly in LMICs. Although the primary driver of AMR is the consumption of antibiotics, socioeconomic factors play a largely unseen role in the proliferation of antimicrobial resistance [
85]. Key contributors include inadequate infrastructure, poor sanitation, and insufficient healthcare access, which promote the excessive and incorrect use of antibiotics. The environmental impact of pharmaceutical waste, especially from growing manufacturing bases in regions like Asia, further exacerbates the spread of AMR [
23]. Middle- and low-income communities are found to have higher rates of resistant bacterial colonization [
86]. The lack of regulatory frameworks, weak governance, poor laboratory practices, and ineffective surveillance systems hinder efforts to control antibiotic resistance in these regions, leading to an unchecked proliferation of resistant bacteria in the environment and the community.
Poor living conditions, overcrowding, and inadequate water and sanitation facilities in LMICs create breeding grounds for the spread of AMR genes [
33]. Limited healthcare access often drives self-medication, leading to inappropriate antibiotic use and furthering the development of resistant strains. Furthermore, gender inequalities and limited awareness regarding the appropriate use of antibiotics exacerbate the problem, as women are disproportionately prescribed antibiotics without medical necessity [
44]. Without targeted interventions to address these socioeconomic disparities, AMR will continue to present a formidable danger to global health.
In conclusion, socioeconomic factors significantly contribute to the proliferation of antimicrobial resistance in healthcare. Addressing AMR requires a holistic, coordinated approach that tackles the underlying socioeconomic drivers. Strengthening healthcare systems, improving the water and sanitation infrastructure, and enforcing stricter regulations on antibiotic use are crucial steps toward mitigating the spread of resistance. Raising public awareness, promoting education on the responsible use of antibiotics, and implementing stronger surveillance systems in LMICs remain vital to combat the threat of AMR. Future authors should aim not only to document disparities, but also to explore measurable data on income inequality, health expenditure, educational levels, immigration, as well as pharmaceutical lobbying expenditures through comparative longitudinal studies across LMICs and high-income countries.
Author Contributions
Writing—original draft preparation, S.M.E.; writing—review and editing, S.M.E. and A.C. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data are contained within this article.
Acknowledgments
Selim Eke would like to extend his appreciation to infectious disease expert Arnold Cua for editing and giving suggestions for the manuscript. He would like to express his appreciation for his father, Sancar Eke, in supporting the creation of the manuscript throughout the writing process.
Conflicts of Interest
The authors declare no conflicts of interest. Arnold Cua is employed by MultiCare Health Systems. Arnold Cua has not received research grants from MultiCare Health Systems. The remaining author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- Roberta, M.S.L.; Favara, G.; Maugeri, A.; Barchitta, M.; Agodi, A. How Antimicrobial Resistance Is Linked to Climate Change: An Overview of Two Intertwined Global Challenges. Int. J. Environ. Res. Public Health 2023, 20, 17. [Google Scholar] [CrossRef]
- O’Neill, J. Tackling Drug-Resistant Infections Globally: Final Report and Recommendations; Wellcome Trust: London, UK, 2016. [Google Scholar]
- Holmes, A.H.; Moore, L.S.; Sundsfjord, A.; Steinbakk, M.; Regmi, S.; Karkey, A.; Guerin, P.J.; Piddock, L.J. Understanding the Mechanisms and Drivers of Antimicrobial Resistance. Lancet 2016, 387, 176–187. [Google Scholar] [CrossRef]
- World Health Organization. World Antimicrobial Awareness Week 2023; World Health Organization: Geneva, Switzerland, 2023; Available online: www.who.int/campaigns/world-amr-awareness-week/2023 (accessed on 1 November 2024).
- World Health Organization. The Socioeconomic Drivers and Impacts of Antimicrobial Resistance (AMR): Implications for Policy and Research; World Health Organization: Geneva, Switzerland, 2024; Available online: https://eurohealthobservatory.who.int/publications/i/the-socioeconomic-drivers-and-impacts-of-antimicrobial-resistance-(amr)-implications-for-policy-and-research (accessed on 1 November 2024).
- World Bank. Drug-Resistant Infections: A Threat to Our Economic Future; World Bank Group: Washington, DC, USA, 2017; Available online: www.worldbank.org/en/topic/health/publication/drug-resistant-infections-a-threat-to-our-economic-future (accessed on 1 November 2024).
- De Angelis, G. Burden of Antibiotic Resistant Gram Negative Bacterial Infections: Evidence and Limits. Available online: www.researchgate.net/publication/314173950_Burden_of_Antibiotic_Resistant_Gram_Negative_Bacterial_Infections_Evidence_and_Limits (accessed on 2 November 2024).
- Klein, E.Y.; Van Boeckel, T.P.; Martinez, E.M.; Pant, S.; Gandra, S.; Levin, S.A.; Goossens, H.; Laxminarayan, R. Global Increase and Geographic Convergence in Antibiotic Consumption between 2000 and 2015. Proc. Natl. Acad. Sci. USA 2018, 115, E3463–E3470. [Google Scholar] [CrossRef] [PubMed]
- Collignon, P.; Beggs, J.J.; Walsh, T.R.; Gandra, S.; Laxminarayan, R. Anthropological and Socioeconomic Factors Contributing to Global Antimicrobial Resistance: A Univariate and Multivariable Analysis. Lancet Planet. Health 2018, 2, e398–e405. [Google Scholar] [CrossRef] [PubMed]
- Cox, G.; Wright, G.D. Intrinsic Antibiotic Resistance: Mechanisms, Origins, Challenges and Solutions. Int. J. Med. Microbiol. 2013, 303, 287–292. [Google Scholar] [CrossRef]
- Munita, J.M.; Arias, C.A. Mechanisms of Antibiotic Resistance. Microbiol. Spectr. 2016, 4, 481–511. [Google Scholar] [CrossRef]
- Oz, T.; Guvenek, A.; Yildiz, S.; Karaboga, E.; Tamer, Y.T.; Mumcuyan, N.; Ozan, V.B.; Senturk, G.H.; Cokol, M.; Yeh, P.; et al. Strength of Selection Pressure Is an Important Parameter Contributing to the Complexity of Antibiotic Resistance Evolution. Mol. Biol. Evol. 2014, 31, 2387–2401. [Google Scholar] [CrossRef]
- Turnidge, J.; Christiansen, K. Antibiotic Use and Resistance—Proving the Obvious. Lancet 2005, 365, 548–549. [Google Scholar] [CrossRef]
- Laxminarayan, R.; Matsoso, P.; Pant, S.; Brower, C.; Røttingen, J.A.; Klugman, K.; Davies, S. Access to Effective Antimicrobials: A Worldwide Challenge. Lancet 2016, 387, 168–175. [Google Scholar] [CrossRef]
- Antibiotic Resistance Threats Report. Centers for Disease Control and Prevention. 2019. Available online: www.cdc.gov/antimicrobial-resistance/data-research/threats/index.html (accessed on 1 November 2024).
- Stone Patricia, W. Economic Burden of Healthcare-Associated Infections: An American Perspective. Expert Rev. Pharmacoeconomics Outcomes Res. 2009, 9, 417–422. [Google Scholar] [CrossRef]
- Morrison, L.; Zembower, T.R. Antimicrobial Resistance. Gastrointest. Endosc. Clin. N. Am. 2020, 30, 619–635. [Google Scholar] [CrossRef] [PubMed]
- Ashbolt, N.J.; Amézquita, A.; Backhaus, T.; Borriello, P.; Brandt, K.K.; Collignon, P.; Coors, A.; Finley, R.; Gaze, W.H.; Heberer, T.; et al. Human Health Risk Assessment (HHRA) for Environmental Development and Transfer of Antibiotic Resistance. Environ. Health Perspect. 2013, 121, 993–1001. [Google Scholar] [CrossRef] [PubMed]
- Dancer, S.J.; Shears, P.; Platt, D.J. Isolation and characterization of coliforms from glacial ice and water in Canada’s High Arctic. J. Appl. Microbiol. 1997, 82, 597–609. [Google Scholar] [CrossRef] [PubMed]
- D’Costa, V.M.; King, C.E.; Kalan, L.; Morar, M.; Sung, W.W.; Schwarz, C.; Froese, D.; Zazula, G.; Calmels, F.; Debruyne, R.; et al. Antibiotic Resistance Is Ancient. Nature 2011, 477, 457–461. [Google Scholar] [CrossRef]
- Antibiotic Resistance Threats in the United States, 2013. Centers for Disease Control and Prevention. Available online: https://www.cdc.gov/antimicrobial-resistance/data-research/threats/index.html#:~:text=The%202013%20report%20stated%20that,for%20Combating%20Antibiotic%2DResistant%20Bacteria.&text=CDC’s%202013%20AR%20Threats%20Report. (accessed on 1 November 2024).
- Ranjbar, R.; Alam, M. Antimicrobial Resistance Collaborators. Global burden of bacterial antimicrobial resistance in 2019: A systematic analysis. Evid. Based Nurs. 2023, ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Fick, J.; Söderström, H.; Lindberg, R.H.; Phan, C.; Tysklind, M.; Larsson, D.G. Contamination of Surface, Ground, and Drinking Water from Pharmaceutical Production. Environ. Toxicol. Chem. 2009, 28, 2522–2527. [Google Scholar] [CrossRef]
- United States Agency for International Development. A Rapid Assessment of SEPTAGE Management in Asia: Policies and Practices in India, Indonesia, Malaysia, the Philippines, Sri Lanka, Thailand, and Vietnam; United States Agency for International Development: Washington, DC, USA, 2012; Available online: https://www.ircwash.org/sites/default/files/AECOM-2010-Rapid.pdf (accessed on 1 November 2024).
- Kookana, R.S.; Drechsel, P.; Jamwal, P.; Vanderzalm, J. Urbanisation and emerging economies: Issues and potential solutions for water and food security. Sci. Total Environ. 2020, 732, 139057. [Google Scholar] [CrossRef] [PubMed]
- Rehman, M.S.; Rashid, N.; Ashfaq, M.; Saif, A.; Ahmad, N.; Han, J.I. Global Risk of Pharmaceutical Contamination from Highly Populated Developing Countries. Chemosphere 2015, 138, 1045–1055. [Google Scholar] [CrossRef]
- Jamwal, P.; Mittal, A.K.; Mouchel, J.M. Efficiency Evaluation of Sewage Treatment Plants with Different Technologies in Delhi (India). Environ. Monit. Assess. 2009, 153, 293–305. [Google Scholar] [CrossRef]
- Agarwal, S.; Darbar, S.; Saha, S. Chapter 25—Challenges in management of domestic wastewater for sustainable development. Curr. Dir. Water Scarcity Res. 2022, 6, 531–552. [Google Scholar] [CrossRef]
- Kookana, R.S.; Williams, M.; Boxall, A.B.; Larsson, D.J.; Gaw, S.; Choi, K.; Yamamoto, H.; Thatikonda, S.; Zhu, Y.G.; Carriquiriborde, P. Potential Ecological Footprints of Active Pharmaceutical Ingredients: An Examination of Risk Factors in Low-, Middle- and High-Income Countries. Philos. Trans. R. Soc. London. Ser. B Biol. Sci. 2014, 369, 20130586. [Google Scholar] [CrossRef]
- Radyowijati, A.; Haak, H. Improving Antibiotic Use in Low-Income Countries: An Overview of Evidence on Determinants. Soc. Sci. Med. 2003, 57, 733–744. [Google Scholar] [CrossRef] [PubMed]
- Kirby, A.; Herbert, A. Correlations between Income inequality and antimicrobial resistance. PLoS ONE 2013, 8, e73115. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Fuhrmeister, E.R.; Harvey, A.P.; Nadimpalli, M.L.; Gallandat, K.; Ambelu, A.; Arnold, B.F.; Brown, J.; Cumming, O.; Earl, A.M.; Kang, G.; et al. Evaluating the Relationship between Community Water and Sanitation Access and the Global Burden of Antibiotic Resistance: An Ecological Study. Lancet Microbe 2023, 4, e591–e600. [Google Scholar] [CrossRef] [PubMed]
- Vivian, A.L.; Mariano, V.; Ahmad, R.; Charani, E.; Rawson, T.M.; Holmes, A.H.; Castro-Sánchez, E. Investigating the Impact of Poverty on Colonization and Infection with Drug-Resistant Organisms in Humans: A Systematic Review. Infect. Dis. Poverty 2018, 7, 76. [Google Scholar]
- Fabrizio, B.; Previti, C.; Calabrese, F.; Scaioli, G.; Siliquini, R. Antibiotics Self Medication among Children: A Systematic Review. Antibiotics 2022, 111, 1583. [Google Scholar] [CrossRef]
- Caroline, C.; Schneider, A.; Zhang, T.; Kadetz, P.; Feng, R.; Lambert, H.; Wang, D.; Oliver, I.; Michie, S.; Cabral, C. Identifying Key Influences on Antibiotic Use in China: A Systematic Scoping Review and Narrative Synthesis. BMJ Open 2022, 12, e056348. [Google Scholar]
- Mitevska, E.; Wong, B.; Surewaard, B.G.; Jenne, C.N. The Prevalence, Risk, and Management of Methicillin-Resistant Staphylococcus Aureus Infection in Diverse Populations across Canada: A Systematic Review. Pathogens 2021, 10, 393. [Google Scholar] [CrossRef]
- Bratu, S.; Landman, D.; Gupta, J.; Trehan, M.; Panwar, M.; Quale, J. A population-based study examining the emergence of community-associated methicillin-resistant Staphylococcus aureus USA300 in New York City. Ann. Clin. Microbiol. Antimicrob. 2006, 5, 29. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Frazee, B.W.; Lynn, J.; Charlebois, E.D.; Lambert, L.; Lowery, D.; Perdreau-Remington, F. High prevalence of methicillin-resistant Staphylococcus aureus in emergency department skin and soft tissue infections. Ann. Emerg. Med. 2005, 45, 311–320. [Google Scholar] [CrossRef] [PubMed]
- Sulis, G.; Gandra, S. Access to Antibiotics: Not a Problem in Some Lmics. Lancet Global Health 2021, 9, e561–e562. [Google Scholar] [CrossRef]
- Chokshi, A.; Sifri, Z.; Cennimo, D.; Horng, H. Global Contributors to Antibiotic Resistance. J. Glob. Infect. Dis. 2019, 11, 36–42. [Google Scholar] [CrossRef] [PubMed]
- Iskandar, K.; Molinier, L.; Hallit, S.; Sartelli, M.; Catena, F.; Coccolini, F.; Hardcastle, T.C.; Roques, C.; Salameh, P. Drivers of Antibiotic Resistance Transmissionin Low- and Middle-Income Countriesfrom a ‘One Health’ Perspective-A Review. Antibiotics 2020, 9, 372. [Google Scholar] [CrossRef] [PubMed]
- Rutta, E.; Kibassa, B.; McKinnon, B.; Liana, J.; Mbwasi, R.; Mlaki, W.; Embrey, M.; Gabra, M.; Shekalaghe, E.; Kimatta, S.; et al. Increasing Access to Subsidized Artemisinin-based Combination, Therapy through Accredited Drug Dispensing Outlets in Tanzania. Health Res. Policy 2011, 9, 22. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kangwana, B.B.; Njogu, J.; Wasunna, B.; Kedenge, S.V.; Memusi, D.N.; Goodman, C.A.; Zurovac, D.; Snow, R.W. Malaria drug shortages in Kenya: A major failure to provide access to effective treatment. Am. J. Trop. Med. Hyg. 2009, 80, 737–738. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Schröder, W.; Sommer, H.; Gladstone, B.P.; Foschi, F.; Hellman, J.; Evengard, B.; Tacconelli, E. Gender Differences in Antibiotic Prescribing in the Community: A Systematic Review and Meta-Analysis. J. Antimicrob. Chemother. 2016, 71, 1800–1806. [Google Scholar] [CrossRef]
- Eggermont, D.; Smit, M.A.M.; Kwestroo, G.A.; Verheij, R.A.; Hek, K.; Kunst, A.E. The Influence of Gender Concordance between General Practitioner and Patient on Antibiotic Prescribing for Sore Throat Symptoms: A Retrospective Study. BMC Fam. Pract. 2018, 19, 175. [Google Scholar] [CrossRef]
- Smith, D.R.M.; Dolk, F.C.K.; Smieszek, T.; Robotham, J.V.; Pouwels, K.B. Understanding the gender gap in antibiotic prescribing: A cross-sectional analysis of English primary care. BMJ Open 2018, 8, e020203. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Andrea, M.; Barchitta, M.; Puglisi, F.; Agodi, A. Socio-Economic, Governance and Health Indicators Shaping Antimicrobial Resistance: An Ecological Analysis of 30 European Countries. Glob. Health 2023, 19, 12. [Google Scholar]
- Makuta, I.; O’Hare, B. Quality of Governance, Public Spending on Health and Health Status in Sub Saharan Africa: A Panel Data Regression Analysis. BMC Public Health 2015, 15, 932. [Google Scholar] [CrossRef]
- Iwu, C.D.; Korsten, L.; Okoh, A.I. The Incidence of Antibiotic Resistance within and beyond the Agricultural Ecosystem: A Concern for Public Health. MicrobiologyOpen 2020, 9, e1035. [Google Scholar] [CrossRef]
- Jeleff, M.; Haddad, C.; Kutalek, R. Making sense of ‘implementation gaps’ as a governance problem of antimicrobial resistance. Eur. J. Public Health 2023, 33 (Suppl. S2), ckad160-171. [Google Scholar] [CrossRef] [PubMed Central]
- Ministry of Health and Social Welfare. Tanzania Service Availability and Readiness Assessment (SARA); Ministry of Health and Social Welfare: Sejong-si, Republic of Korea, 2012. [Google Scholar]
- Gandra, S.; Alvarez-Uria, G.; Turner, P.; Joshi, J.; Limmathurotsakul, D.; van Doorn, H.R. Antimicrobial Resistance Surveillance in Low- and Middle-Income Countries: Progress and Challenges in Eight South Asian and Southeast Asian Countries. Clin. Microbiol. Rev. 2020, 33, 10–1128. [Google Scholar] [CrossRef]
- Hoque, R.; Ahmed, S.M.; Naher, N.; Islam, M.A.; Rousham, E.K.; Islam, B.Z.; Hassan, S. Tackling Antimicrobial Resistance in Bangladesh: A Scoping Review of Policy and Practice in Human, Animal and Environment Sectors. PLoS ONE 2020, 15, e0227947. [Google Scholar] [CrossRef] [PubMed]
- Antimicrobial Resistance Collaborators. Global burden of bacterial antimicrobial resistance in 2019: A systematic analysis. Lancet 2022, 399, 629–655, Erratum in Lancet 2022, 400, 1102. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wuerz, T.C.; Kassim, S.S.; Atkins, K.E. Acquisition of Extended-Spectrum Beta-Lactamase-Producing Enterobacteriaceae (ESBL-PE) Carriage after Exposure to Systemic Antimicrobials during Travel: Systematic Review and Meta-Analysis. Travel Med. Infect. Dis. 2020, 37, 101823. [Google Scholar] [CrossRef]
- Chatterjee, A.; Modarai, M.; Naylor, N.R.; Boyd, S.E.; Atun, R.; Barlow, J.; Holmes, A.H.; Johnson, A.; Robotham, J.V. Quantifying Drivers of Antibiotic Resistance in Humans: A Systematic Review. Lancet Infect. Dis. 2018, 18, e368–e378. [Google Scholar] [CrossRef]
- Nellums, L.B.; Thompson, H.; Holmes, A.; Castro-Sánchez, E.; Otter, J.A.; Norredam, M.; Friedland, J.S.; Hargreaves, S. Antimicrobial Resistance among Migrants in Europe: A Systematic Review and Meta-Analysis. Lancet Infect. Dis. 2018, 18, 796–811. [Google Scholar] [CrossRef]
- Pallett, S.J.C.; Boyd, S.E.; O’Shea, M.K.; Martin, J.; Jenkins, D.R.; Hutley, E.J. The Contribution of Human Conflict to the Development of Antimicrobial Resistance. Commun. Med. 2023, 3, 153. [Google Scholar] [CrossRef]
- Petrosillo, N.; Petersen, E.; Antoniak, S. Ukraine War and Antimicrobial Resistance. Lancet Infect. Dis. 2023, 23, 653–654. [Google Scholar] [CrossRef]
- Abbara, A.; Rawson, T.M.; Karah, N.; El-Amin, W.; Hatcher, J.; Tajaldin, B.; Dar, O.; Dewachi, O.; Abu Sitta, G.; Uhlin, B.E.; et al. Antimicrobial resistance in the context of the Syrian conflict: Drivers before and after the onset of conflict and key recommendations. Int. J. Infect. Dis. 2018, 73, 1–6. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Who Health and Climate Change Survey Report; World Health Organization: Geneva, Switzerland, 2021; Available online: www.who.int/publications/i/item/9789240038509 (accessed on 1 November 2024).
- Anna, O.; Bylund, H.; Boqvist, S.; Högberg, A.; Björkman, C.; Tryland, M.; Evengård, B.; Koch, A.; Berggren, C.; Malogolovkin, A.; et al. Identifying Climate-Sensitive Infectious Diseases in Animals and Humans in Northern Regions. Acta Vet. Scand. 2019, 61, 53. [Google Scholar]
- Karzis, J.; Petzer, I.M.; Donkin, E.F.; Naidoo, V.; Etter, E.M.C. Climatic and regional antibiotic resistance patterns of Staphylococcus aureus in South African dairy herds. Onderstepoort J. Vet. Res. 2019, 86, e1–e9. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Khanijahani, A.; Iezadi, S.; Gholipour, K.; Azami-Aghdash, S.; Naghibi, D. A Systematic Review of Racial/Ethnic and Socioeconomic Disparities in COVID-19. Int. J. Equity Health 2021, 20, 248. [Google Scholar] [CrossRef]
- Hyacinth, O.U. The Interrelationships between Antimicrobial Resistance, COVID-19, Past, and Future Pandemics. J. Infect. Public Health 2021, 14, 53–60. [Google Scholar]
- Emma, P.; Smith, E.; Taylor, J.; Davies, S.; Ali, G.C.; d’Angelo, C. Global Action on Antimicrobial Resistance: Lessons from the History of Climate Change and Tobacco Control Policy. Mendeley 1970, 7, e009283. [Google Scholar]
- Langford, B.J.; So, M.; Raybardhan, S.; Leung, V.; Soucy, J.R.; Westwood, D.; Daneman, N.; MacFadden, D.R. Antibiotic Prescribing in Patients with COVID-19: Rapid Review and Meta-Analysis. Clin. Microbiol. Infect. Off. Publ. Eur. Soc. Clin. Microbiol. Infect. Dis. 2021, 27, 520–531. [Google Scholar] [CrossRef]
- Isaric. Available online: https://isaric.org/wp-content/uploads/2021/01/ISARIC-Clinical-Data-Report-20.11.2020.pdf (accessed on 2 November 2024).
- Wootton, J.C.; Feng, X.; Ferdig, M.T.; Cooper, R.A.; Mu, J.; Baruch, D.I.; Magill, A.J.; Su, X.Z. Genetic diversity and chloroquine selective sweeps in Plasmodium falciparum. Nature 2002, 418, 320–323. [Google Scholar] [CrossRef] [PubMed]
- Emran, S.A.; Krupnik, T.J.; Aravindakshan, S.; Kumar, V.; Pittelkow, C.M. Factors Contributing to Farm-Level Productivity and Household Income Generation in Coastal Bangladesh’s Rice-Based Farming Systems. PLoS ONE 2021, 16, e0256694. [Google Scholar] [CrossRef]
- Chen, C.-J.; Huang, Y.-C. New Epidemiology of Staphylococcus Aureus Infection in Asia. Clin. Microbiol. Infect. Off. Publ. Eur. Soc. Clin. Microbiol. Infect. Dis. 2014, 20, 605–623. [Google Scholar] [CrossRef]
- Abdullah, K.A.; Balasundram, S.K.; Azizi, S.; Afsharyzad, Y.; Zarei, M.; Etesami, H.; Shamshiri, R.R. An Overview of Antibiotic Resistance and Abiotic Stresses Affecting Antimicrobial Resistance in Agricultural Soils. Int. J. Environ. Res. Public Health 2022, 19, 4666. [Google Scholar]
- Bassitta, R.; Nottensteiner, A.; Bauer, J.; Straubinger, R.K.; Hölzel, C.S. Spread of Antimicrobial Resistance Genes via Pig Manure from Organic and Conventional Farms in the Presence or Absence of Antibiotic Use. J. Appl. Microbiol. 2022, 133, 2457–2465. [Google Scholar] [CrossRef] [PubMed]
- Tsuzuki, S.; Fujitsuka, N.; Horiuchi, K.; Ijichi, S.; Gu, Y.; Fujitomo, Y.; Takahashi, R.; Ohmagari, N. Factors Associated with Sufficient Knowledge of Antibiotics and Antimicrobial Resistance in the Japanese General Population. Sci. Rep. 2020, 10, 3502. [Google Scholar] [CrossRef]
- Michael, A. Challenges to Tackling Antimicrobial Resistance; Cambridge Core; Cambridge University Press: Cambridge, UK, 2020; Available online: www.cambridge.org/core/books/challenges-to-tackling-antimicrobial-resistance/7A666C209981B53263C4B59C83A719E9 (accessed on 1 November 2024).
- Narmeen, M.; Orsini, N.; Figueiras, A.; Takkouche, B. Education Level and Misuse of Antibiotics in the General Population: A Systematic Review and Dose-Response Meta-Analysis. Antimicrob. Resist. Infect. Control. 2022, 11, 24. [Google Scholar]
- Haenssgen, M.J.; Xayavong, T.; Charoenboon, N.; Warapikuptanun, P.; Khine Zaw, Y. The Consequences of AMR Education and Awareness Raising: Outputs, Outcomes, and Behavioural Impacts of an Antibiotic-Related Educational Activity in Lao PDR. Antibiotics 2018, 7, 95. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Morgan, D.J.; Okeke, I.N.; Laxminarayan, R.; Perencevich, E.N.; Weisenberg, S. Non-Prescription Antimicrobial Use Worldwide: A Systematic Review. Lancet Infect. Dis. 2011, 11, 692–701. [Google Scholar] [CrossRef] [PubMed]
- Alhomoud, F.; Aljamea, Z.; Almahasnah, R.; Alkhalifah, K.; Basalelah, L.; Alhomoud, F.K. Self-Medication and Self-Prescription with Antibiotics in the Middle East-Do They Really Happen? A Systematic Review of the Prevalence, Possible Reasons, and Outcomes. Int. J. Infect. Dis. IJID Off. Publ. Int. Soc. Infect. Dis. 2017, 57, 3–12. [Google Scholar] [CrossRef]
- Cao, G.; Zhao, S.; Kuang, D.; Hsu, C.H.; Yin, L.; Luo, Y.; Chen, Z.; Xu, X.; Strain, E.; McDermott, P.; et al. Geography shapes the genomics and antimicrobial resistance of Salmonella enterica Serovar Enteritidis isolated from humans. Sci. Rep. 2023, 13, 1331. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ahammad, Z.S.; Sreekrishnan, T.R.; Hands, C.L.; Knapp, C.W.; Graham, D.W. Increased Waterborne BLANDM-1 Resistance Gene Abundances Associated with Seasonal Human Pilgrimages to the Upper Ganges River. Environ. Sci. Technol. 2014, 48, 3014–3020. [Google Scholar] [CrossRef]
- Joakim, L.D.G. Pollution from Drug Manufacturing: Review and Perspectives. Philos. Trans. R. Soc. Lond. Ser. B Biol. Sci. 2014, 369, 1656. [Google Scholar]
- Bielen, A.; Šimatović, A.; Kosić-Vukšić, J.; Senta, I.; Ahel, M.; Babić, S.; Jurina, T.; González Plaza, J.J.; Milaković, M.; Udiković-Kolić, N. Negative Environmental Impacts of Antibiotic-Contaminated Effluents from Pharmaceutical Industries. Water Res. 2017, 126, 79–87. [Google Scholar] [CrossRef]
- Abelenda-Alonso, G.; Satorra, P.; Marí-Dell’Olmo, M.; Tebé, C.; Padullés, A.; Vergara, A.; Gudiol, C.; Pujol, M.; Carratalà, J. Short-Term Exposure to Ambient Air Pollution and Antimicrobial Use for Acute Respiratory Symptoms. JAMA Netw Open 2024, 7, e2432245. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Fridkin, S.K.; Edwards, J.R.; Courval, J.M.; Hill, H.; Tenover, F.C.; Lawton, R.; Gaynes, R.P.; McGowan, J.E. The Effect of Vancomycin and Third-Generation Cephalosporins on Prevalence of Vancomycin-Resistant Enterococci in 126 U.S. Adult Intensive Care Units. Ann. Intern. Med. 2001, 135, 175–183. [Google Scholar] [CrossRef] [PubMed]
- Neves, F.P.G.; Marlow, M.A.; Rezende-Pereira, G.; Pinheiro, M.G.; Dos Santos, A.F.M.; de Fátima Nogueira de Freitas, M.; Barros, R.R.; Aguiar-Alves, F.; Cardoso, C.A.A.; Riley, L.W. Differences in Gram-Positive Bacterial Colonization and Antimicrobial Resistance among Children in a High Income Inequality Setting. BMC Infect. Dis. 2020, 19, 478. [Google Scholar] [CrossRef] [PubMed]
| Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).