Global Burden, Trends, and Inequalities of Clostridioides difficile Infections from 1990 to 2021 and Projections to 2040: A Systematic Analysis
Abstract
1. Introduction
2. Results
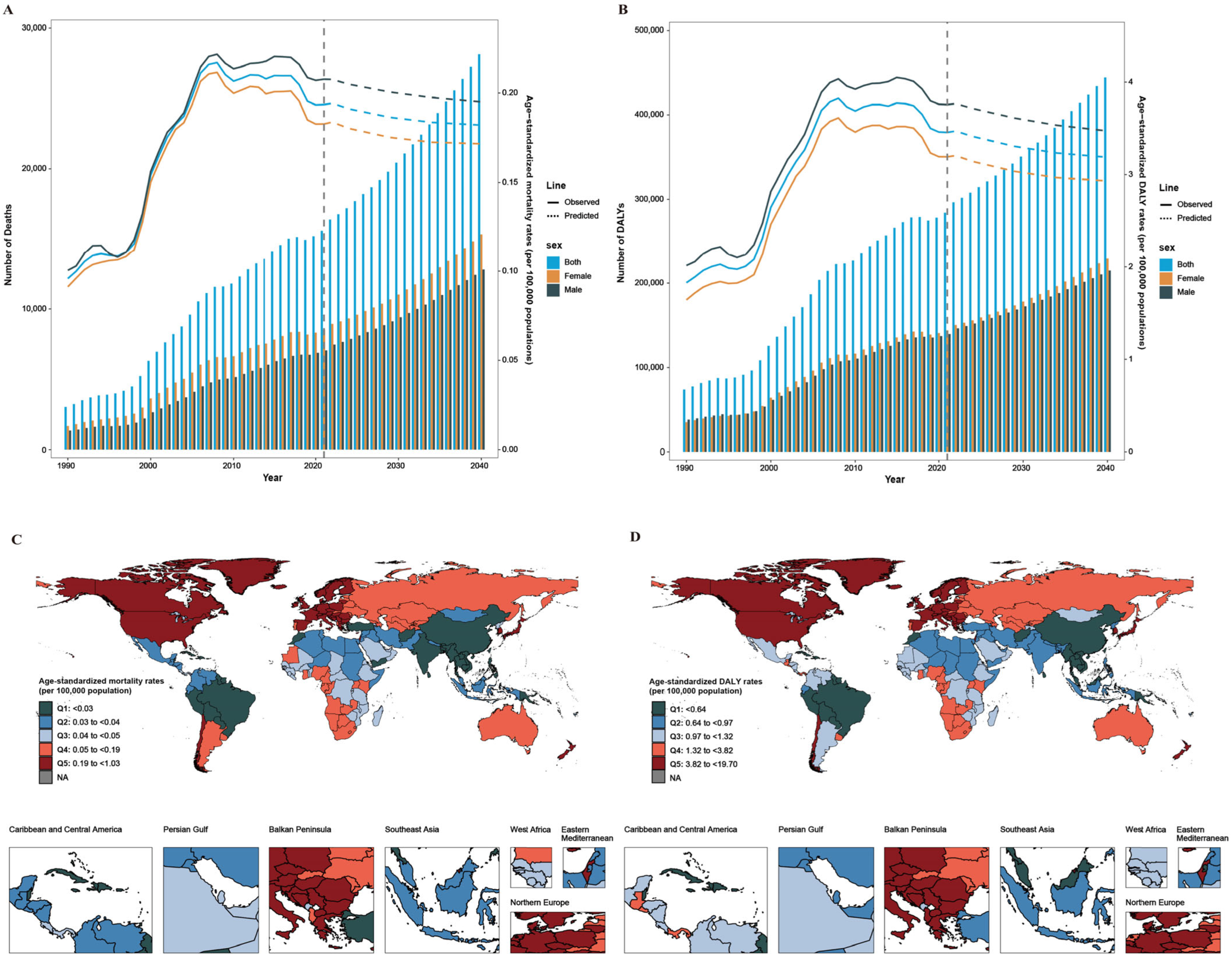
2.1. Global Burden and Trends of CDI from 1990 to 2021
2.2. Decomposition of Changes in the CDI Burden
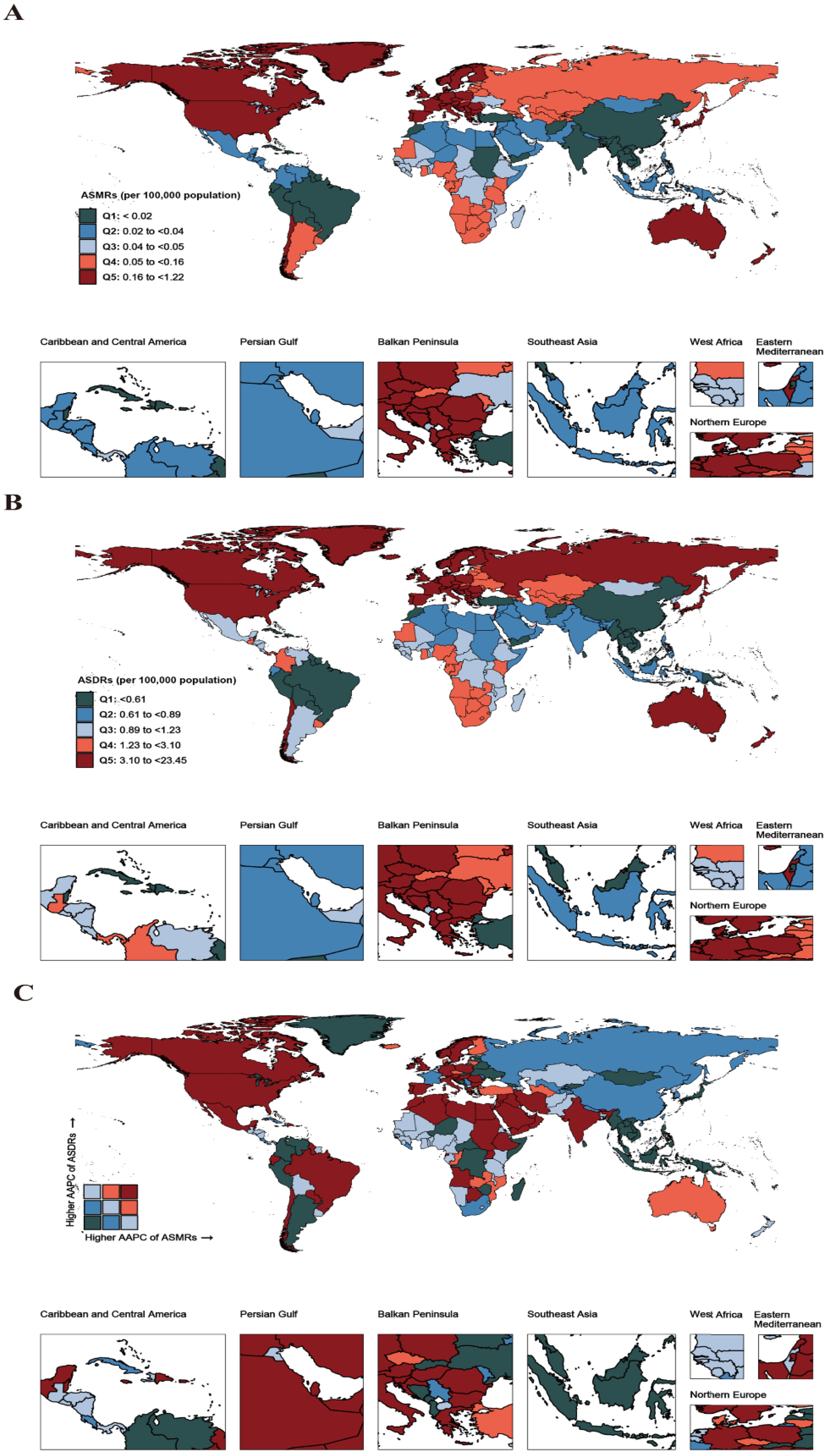
2.3. Inequality Analysis
2.4. Projected Burden and Trends in CDI
3. Discussion
4. Materials and Methods
4.1. Data Source and Definitions
4.2. Temporal Trends and Decomposition Analysis
- ai,y represents the proportion of the population in age category i for a given year y, corresponding to population aging.
- py represents the total population in a given year y, corresponding to population growth.
- ei,y represents the DALY rate for a given age category i in year y, corresponding to changes in age-specific rates (or epidemiologic changes).
4.3. Cross-Country Inequality Analysis
4.4. Projection Analysis
4.5. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kordus, S.L.; Thomas, A.K.; Lacy, D.B. Clostridioides difficile toxins: Mechanisms of action and antitoxin therapeutics. Nat. Rev. Microbiol. 2022, 20, 285–298. [Google Scholar] [CrossRef] [PubMed]
- Dong, D.; Su, T.; Chen, W.; Wang, D.; Xue, Y.; Lu, Q.; Jiang, C.; Ni, Q.; Mao, E.; Peng, Y. Clostridioides difficile aggravates dextran sulfate solution (DSS)-induced colitis by shaping the gut microbiota and promoting neutrophil recruitment. Gut Microbes 2023, 15, 2192478. [Google Scholar] [CrossRef]
- Di Bella, S.; Sanson, G.; Monticelli, J.; Zerbato, V.; Principe, L.; Giuffrè, M.; Pipitone, G.; Luzzati, R.; Staley, C. Clostridioides difficile infection: History, epidemiology, risk factors, prevention, clinical manifestations, treatment, and future options. Clin. Microbiol. Rev. 2024, 37, e0013523. [Google Scholar] [CrossRef]
- Davies, K.A.; Longshaw, C.M.; Davis, G.L.; Bouza, E.; Barbut, F.; Barna, Z.; Delmée, M.; Fitzpatrick, F.; Ivanova, K.; Kuijper, E.; et al. Underdiagnosis of Clostridium difficile across Europe: The European, multicentre, prospective, biannual, point-prevalence study of Clostridium difficile infection in hospitalised patients with diarrhoea (EUCLID). Lancet Infect. Dis. 2014, 14, 1208–1219. [Google Scholar] [CrossRef]
- Marra, A.R.; Perencevich, E.N.; Nelson, R.E.; Samore, M.; Khader, K.; Chiang, H.Y.; Chorazy, M.L.; Herwaldt, L.A.; Diekema, D.J.; Kuxhausen, M.F.; et al. Incidence and Outcomes Associated with Clostridium difficile Infections: A Systematic Review and Meta-analysis. JAMA Netw. Open 2020, 3, e1917597. [Google Scholar] [CrossRef] [PubMed]
- Borren, N.Z.; Ghadermarzi, S.; Hutfless, S.; Ananthakrishnan, A.N.; Deshpande, A. The emergence of Clostridium difficile infection in Asia: A systematic review and meta-analysis of incidence and impact. PLoS ONE 2017, 12, e0176797. [Google Scholar] [CrossRef]
- Lessa, F.C.; Mu, Y.; Bamberg, W.M.; Beldavs, Z.G.; Dumyati, G.K.; Dunn, J.R.; Farley, M.M.; Holzbauer, S.M.; Meek, J.I.; Phipps, E.C.; et al. Burden of Clostridium difficile infection in the United States. N. Engl. J. Med. 2015, 372, 825–834. [Google Scholar] [CrossRef]
- Bauer, M.P.; Notermans, D.W.; van Benthem, B.H.; Brazier, J.S.; Wilcox, M.H.; Rupnik, M.; Monnet, D.L.; van Dissel, J.T.; Kuijper, E.J. Clostridium difficile infection in Europe: A hospital-based survey. Lancet 2011, 377, 63–73. [Google Scholar] [CrossRef]
- Zhang, S.; Palazuelos-Munoz, S.; Balsells, E.M.; Nair, H.; Chit, A.; Kyaw, M.H. Cost of hospital management of Clostridium difficile infection in United States—A meta-analysis and modelling study. BMC Infect. Dis. 2016, 16, 447. [Google Scholar] [CrossRef] [PubMed]
- Chitnis, A.S.; Holzbauer, S.M.; Belflower, R.M.; Winston, L.G.; Bamberg, W.M.; Lyons, C.; Farley, M.M.; Dumyati, G.K.; Wilson, L.E.; Beldavs, Z.G.; et al. Epidemiology of community-associated Clostridium difficile infection, 2009 through 2011. JAMA Intern. Med. 2013, 173, 1359–1367. [Google Scholar] [CrossRef]
- Ofori, E.; Ramai, D.; Dhawan, M.; Mustafa, F.; Gasperino, J.; Reddy, M. Community-acquired Clostridium difficile: Epidemiology, ribotype, risk factors, hospital and intensive care unit outcomes, and current and emerging therapies. J. Hosp. Infect. 2018, 99, 436–442. [Google Scholar] [CrossRef] [PubMed]
- Agnew, E.; Davies, K.A.; Viprey, V.F.; Evans, S.; Davis, G.L.; Hope, R.; Wilcox, M.H.; Wingen-Heimann, S.M.; Robotham, J.V. Impact of testing on Clostridioides difficile infection in hospitals across Europe: A mathematical model. Clin. Microbiol. Infect. 2023, 29, 796.e791–796.e796. [Google Scholar] [CrossRef] [PubMed]
- Dubberke, E.R.; Rohde, J.M.; Saint, S.; Jones, K.; Snyder, A.; Rolle, A.J.; Chopra, V. Quantitative Results of a National Intervention to Prevent Clostridioides difficile Infection: A Pre-Post Observational Study. Ann. Intern. Med. 2019, 171, S52–S58. [Google Scholar] [CrossRef] [PubMed]
- Brown, K.; Valenta, K.; Fisman, D.; Simor, A.; Daneman, N. Hospital ward antibiotic prescribing and the risks of Clostridium difficile infection. JAMA Intern. Med. 2015, 175, 626–633. [Google Scholar] [CrossRef]
- Choi, H.Y.; Park, S.Y.; Kim, Y.A.; Yoon, T.Y.; Choi, J.M.; Choe, B.K.; Ahn, S.H.; Yoon, S.J.; Lee, Y.R.; Oh, I.H. The epidemiology and economic burden of Clostridium difficile infection in Korea. Biomed Res. Int. 2015, 2015, 510386. [Google Scholar] [CrossRef]
- Finn, E.; Andersson, F.L.; Madin-Warburton, M. Burden of Clostridioides difficile infection (CDI)—A systematic review of the epidemiology of primary and recurrent CDI. BMC Infect. Dis. 2021, 21, 456. [Google Scholar] [CrossRef]
- Balsells, E.; Shi, T.; Leese, C.; Lyell, I.; Burrows, J.; Wiuff, C.; Campbell, H.; Kyaw, M.H.; Nair, H. Global burden of Clostridium difficile infections: A systematic review and meta-analysis. J. Glob. Health 2019, 9, 010407. [Google Scholar] [CrossRef]
- Guh, A.Y.; Mu, Y.; Winston, L.G.; Johnston, H.; Olson, D.; Farley, M.M.; Wilson, L.E.; Holzbauer, S.M.; Phipps, E.C.; Dumyati, G.K.; et al. Trends in U.S. Burden of Clostridioides difficile Infection and Outcomes. N. Engl. J. Med. 2020, 382, 1320–1330. [Google Scholar] [CrossRef]
- Roldan, G.A.; Cui, A.X.; Pollock, N.R.; Kraft, C.S. Assessing the Burden of Clostridium difficile Infection in Low- and Middle-Income Countries. J. Clin. Microbiol. 2018, 56, 10–1128. [Google Scholar] [CrossRef]
- Keeley, A.J.; Beeching, N.J.; Stott, K.E.; Roberts, P.; Watson, A.J.; Beadsworth, M.B. Clostridium difficile: A healthcare-associated infection of unknown significance in adults in sub-Saharan Africa. Malawi Med. J. 2016, 28, 66–69. [Google Scholar] [CrossRef]
- Chalmers, J.D.; Akram, A.R.; Singanayagam, A.; Wilcox, M.H.; Hill, A.T. Risk factors for Clostridium difficile infection in hospitalized patients with community-acquired pneumonia. J. Infect. 2016, 73, 45–53. [Google Scholar] [CrossRef] [PubMed]
- Vollset, S.E.; Ababneh, H.S.; Abate, Y.H.; Abbafati, C.; Abbasgholizadeh, R.; Abbasian, M.; Abbastabar, H.; Magied, A.H.A.A.A.; ElHafeez, S.A.; Abdelkader, A.; et al. Burden of disease scenarios for 204 countries and territories, 2022–2050: A forecasting analysis for the Global Burden of Disease Study 2021. Lancet 2024, 403, 2204–2256. [Google Scholar] [CrossRef] [PubMed]
- Lim, S.C.; Knight, D.R.; Riley, T.V. Clostridium difficile and One Health. Clin. Microbiol. Infect. 2020, 26, 857–863. [Google Scholar] [CrossRef] [PubMed]
- Naghavi, M.; Ong, K.L.; Aali, A.; Ababneh, H.S.; Abate, Y.H.; Abbafati, C.; Abbasgholizadeh, R.; Abbasian, M.; Abbasi-Kangevari, M.; Abbastabar, H.; et al. Global burden of 288 causes of death and life expectancy decomposition in 204 countries and territories and 811 subnational locations, 1990–2021: A systematic analysis for the Global Burden of Disease Study 2021. Lancet 2024, 403, 2100–2132. [Google Scholar] [CrossRef]
- GBD 2016 Diarrhoeal Disease Collaborators. Estimates of the global, regional, and national morbidity, mortality, and aetiologies of diarrhoea in 195 countries: A systematic analysis for the Global Burden of Disease Study 2016. Lancet Infect. Dis. 2018, 18, 1211–1228. [Google Scholar] [CrossRef]
- Lopman, B.; Hall, A. Incomplete use of global data for aetiological attribution of diarrhoeal disease in the Global Burden of Disease study. Lancet Infect. Dis. 2019, 19, 128. [Google Scholar] [CrossRef]
- Miettinen, O.S. Proportion of disease caused or prevented by a given exposure, trait or intervention. Am. J. Epidemiol. 1974, 99, 325–332. [Google Scholar] [CrossRef]
- Rao, K.; Malani, P.N. Diagnosis and Treatment of Clostridioides (Clostridium) difficile Infection in Adults in 2020. Jama 2020, 323, 1403–1404. [Google Scholar] [CrossRef]
- Baur, D.; Gladstone, B.P.; Burkert, F.; Carrara, E.; Foschi, F.; Döbele, S.; Tacconelli, E. Effect of antibiotic stewardship on the incidence of infection and colonisation with antibiotic-resistant bacteria and Clostridium difficile infection: A systematic review and meta-analysis. Lancet Infect. Dis. 2017, 17, 990–1001. [Google Scholar] [CrossRef]
- Sholeh, M.; Krutova, M.; Forouzesh, M.; Mironov, S.; Sadeghifard, N.; Molaeipour, L.; Maleki, A.; Kouhsari, E. Antimicrobial resistance in Clostridioides (Clostridium) difficile derived from humans: A systematic review and meta-analysis. Antimicrob. Resist. Infect. Control 2020, 9, 158. [Google Scholar] [CrossRef]
- Lv, T.; Zheng, L.; Wu, T.; Shen, P.; Chen, Y. Molecular characterization and antibiotic resistance of Clostridioides difficile in patients with inflammatory bowel disease from two hospitals in China. J. Glob. Antimicrob. Resist. 2022, 30, 252–258. [Google Scholar] [CrossRef] [PubMed]
- Valiente, E.; Cairns, M.D.; Wren, B.W. The Clostridium difficile PCR ribotype 027 lineage: A pathogen on the move. Clin. Microbiol. Infect. 2014, 20, 396–404. [Google Scholar] [CrossRef] [PubMed]
- Clements, A.C.; Magalhães, R.J.; Tatem, A.J.; Paterson, D.L.; Riley, T.V. Clostridium difficile PCR ribotype 027: Assessing the risks of further worldwide spread. Lancet Infect. Dis. 2010, 10, 395–404. [Google Scholar] [CrossRef]
- Liu, C.; Monaghan, T.; Yadegar, A.; Louie, T.; Kao, D. Insights into the Evolving Epidemiology of Clostridioides difficile Infection and Treatment: A Global Perspective. Antibiotics 2023, 12, 1141. [Google Scholar] [CrossRef]
- Curcio, D.; Cané, A.; Fernández, F.A.; Correa, J. Clostridium difficile-associated Diarrhea in Developing Countries: A Systematic Review and Meta-Analysis. Infect. Dis. Ther. 2019, 8, 87–103. [Google Scholar] [CrossRef]
- Lim, S.S.; Mokdad, A.H. Socioeconomic inequalities and infectious disease burden. Lancet 2012, 379, 1080–1081. [Google Scholar] [CrossRef]
- Browne, A.J.; Chipeta, M.G.; Haines-Woodhouse, G.; Kumaran, E.P.A.; Hamadani, B.H.K.; Zaraa, S.; Henry, N.J.; Deshpande, A.; Reiner, R.C., Jr.; Day, N.P.J.; et al. Global antibiotic consumption and usage in humans, 2000–2018: A spatial modelling study. Lancet Planet. Health 2021, 5, e893–e904. [Google Scholar] [CrossRef]
- Gavazzi, G.; Krause, K.H. Ageing and infection. Lancet Infect. Dis. 2002, 2, 659–666. [Google Scholar] [CrossRef]
- Tamez-Torres, K.M.; Torres-González, P.; Leal-Vega, F.; García-Alderete, A.; López García, N.I.; Mendoza-Aguilar, R.; Galindo-Fraga, A.; Bobadilla-Del Valle, M.; Ponce de León, A.; Sifuentes-Osornio, J. Impact of Clostridium difficile infection caused by the NAP1/RT027 strain on severity and recurrence during an outbreak and transition to endemicity in a Mexican tertiary care center. Int. J. Infect. Dis. 2017, 65, 44–49. [Google Scholar] [CrossRef]
- Ojemolon, P.E.; Shaka, H.; Kwei-Nsoro, R.; Laswi, H.; Ebhohon, E.; Shaka, A.; Abusalim, A.R.; Mba, B. Trends and Disparities in Outcomes of Clostridioides difficile Infection Hospitalizations in the United States: A Ten-Year Joinpoint Trend Analysis. J. Clin. Med. Res. 2022, 14, 474–486. [Google Scholar] [CrossRef]
- Sarma, J.B.; Marshall, B.; Cleeve, V.; Tate, D.; Oswald, T.; Woolfrey, S. Effects of fluoroquinolone restriction (from 2007 to 2012) on Clostridium difficile infections: Interrupted time-series analysis. J. Hosp. Infect. 2015, 91, 74–80. [Google Scholar] [CrossRef] [PubMed]
- Donskey, C.J. Clostridium difficile in Older Adults. Infect. Dis. Clin. North Am. 2017, 31, 743–756. [Google Scholar] [CrossRef] [PubMed]
- Asempa, T.E.; Nicolau, D.P. Clostridium difficile infection in the elderly: An update on management. Clin. Interv. Aging 2017, 12, 1799–1809. [Google Scholar] [CrossRef]
- Kelly, C.R.; Fischer, M.; Allegretti, J.R.; LaPlante, K.; Stewart, D.B.; Limketkai, B.N.; Stollman, N.H. ACG Clinical Guidelines: Prevention, Diagnosis, and Treatment of Clostridioides difficile Infections. Am. J. Gastroenterol. 2021, 116, 1124–1147. [Google Scholar] [CrossRef]
- Mounsey, A.; Lacy Smith, K.; Reddy, V.C.; Nickolich, S. Clostridioides difficile Infection: Update on Management. Am. Fam. Physician 2020, 101, 168–175. [Google Scholar]
- GBD 2021 Diseases and Injuries Collaborators. Global incidence, prevalence, years lived with disability (YLDs), disability-adjusted life-years (DALYs), and healthy life expectancy (HALE) for 371 diseases and injuries in 204 countries and territories and 811 subnational locations, 1990–2021: A systematic analysis for the Global Burden of Disease Study 2021. Lancet 2024, 403, 2133–2161. [Google Scholar] [CrossRef]
- Brauer, M.; Roth, G.A.; Aravkin, A.Y.; Zheng, P.; Abate, K.H.; Abate, Y.H.; Abbafati, C.; Abbasgholizadeh, R.; Abbasi, M.A.; Abbasian, M.; et al. Global burden and strength of evidence for 88 risk factors in 204 countries and 811 subnational locations, 1990–2021: A systematic analysis for the Global Burden of Disease Study 2021. Lancet 2024, 403, 2162–2203. [Google Scholar] [CrossRef]
- Schumacher, A.E.; Kyu, H.H.; Aali, A.; Abbafati, C.; Abbas, J.; Abbasgholizadeh, R.; Abbasi, M.A.; Abbasian, M.; ElHafeez, S.A.; Abdelmasseh, M.; et al. Global age-sex-specific mortality, life expectancy, and population estimates in 204 countries and territories and 811 subnational locations, 1950–2021, and the impact of the COVID-19 pandemic: A comprehensive demographic analysis for the Global Burden of Disease Study 2021. Lancet 2024, 403, 1989–2056. [Google Scholar] [CrossRef]
- Kim, H.J.; Fay, M.P.; Feuer, E.J.; Midthune, D.N. Permutation tests for joinpoint regression with applications to cancer rates. Stat. Med. 2000, 19, 335–351. [Google Scholar] [CrossRef]
- Das Gupta, P. Standardization and decomposition of rates from cross-classified data. Genus 1994, 50, 171–196. [Google Scholar]
- Schlotheuber, A.; Hosseinpoor, A.R. Summary Measures of Health Inequality: A Review of Existing Measures and Their Application. Int. J. Environ. Res. Public Health 2022, 19, 3697. [Google Scholar] [CrossRef] [PubMed]
- Gastwirth, J.L. The estimation of the Lorenz curve and Gini index. Rev. Econ. Stat. 1972, 54, 306–316. [Google Scholar] [CrossRef]
- Zhao, H.; Geng, G.; Zhang, Q.; Davis, S.J.; Li, X.; Liu, Y.; Peng, L.; Li, M.; Zheng, B.; Huo, H.; et al. Inequality of household consumption and air pollution-related deaths in China. Nat. Commun. 2019, 10, 4337. [Google Scholar] [CrossRef]
- Arnold, M.; Park, J.Y.; Camargo, M.C.; Lunet, N.; Forman, D.; Soerjomataram, I. Is gastric cancer becoming a rare disease? A global assessment of predicted incidence trends to 2035. Gut 2020, 69, 823–829. [Google Scholar] [CrossRef] [PubMed]
- Vollset, S.E.; Goren, E.; Yuan, C.W.; Cao, J.; Smith, A.E.; Hsiao, T.; Bisignano, C.; Azhar, G.S.; Castro, E.; Chalek, J.; et al. Fertility, mortality, migration, and population scenarios for 195 countries and territories from 2017 to 2100: A forecasting analysis for the Global Burden of Disease Study. Lancet 2020, 396, 1285–1306. [Google Scholar] [CrossRef]
- Joinpoint Regression Program, 5.1.0.0; Statistical Methodology and Applications Branch, Surveillance Research Program; National Cancer Institute: Bethesda, MD, USA, 2024.


| 1990 | 2021 | 1990–2021 AAPC (95% CI), % | |||
|---|---|---|---|---|---|
| Death Count (95% UIs) | ASMRs (95% UIs) | Death Count (95% UIs) | ASMRs (95% UIs) | ||
| Global | |||||
| Males | 1362 (1130 to 1639) | 0.10 (0.08 to 0.12) | 7060 (6068 to 8224) | 0.21 (0.18 to 0.24) | 2.36 (1.88 to 2.85) |
| Females | 1685 (1406 to 1988) | 0.09 (0.08 to 0.11) | 8538 (7276 to 10056) | 0.18 (0.16 to 0.21) | 2.22 (1.69 to 2.75) |
| Both | 3047 (2550 to 3609) | 0.1 (0.08 to 0.11) | 15598 (13418 to 18222) | 0.19 (0.16 to 0.23) | 2.26 (1.77 to 2.76) |
| SDI quintiles | |||||
| High SDI | 1969 (1687 to 2299) | 0.19 (0.16 to 0.22) | 12378 (10799 to 14323) | 0.53 (0.47 to 0.61) | 3.27 (2.6 to 3.94) |
| High–Middle SDI | 699 (586 to 832) | 0.09 (0.07 to 0.1) | 2119 (1695 to 2628) | 0.11 (0.09 to 0.14) | 1.01 (0.08 to 1.94) |
| Middle SDI | 192 (127 to 279) | 0.02 (0.01 to 0.03) | 566 (378 to 842) | 0.02 (0.02 to 0.03) | 0.69 (0.53 to 0.84) |
| Low–Middle SDI | 121 (75 to 175) | 0.02 (0.01 to 0.03) | 342 (226 to 495) | 0.02 (0.02 to 0.04) | 0.97 (0.66 to 1.28) |
| Low SDI | 61 (38 to 89) | 0.03 (0.02 to 0.04) | 177 (118 to 255) | 0.03 (0.02 to 0.05) | 0.78 (0.65 to 0.9) |
| Health system Grouping Levels | |||||
| Advanced Health System | 2583 (2213 to 2995) | 0.17 (0.15 to 0.2) | 14253 (12298 to 16600) | 0.45 (0.4 to 0.52) | 3.17 (2.69 to 3.66) |
| Basic Health System | 269 (176 to 390) | 0.02 (0.01 to 0.03) | 751 (486 to 1120) | 0.02 (0.02 to 0.03) | 0.43 (0.14 to 0.72) |
| Limited Health System | 171 (109 to 247) | 0.02 (0.01 to 0.03) | 528 (353 to 765) | 0.03 (0.02 to 0.04) | 0.91 (0.61 to 1.22) |
| Minimal Health System | 19 (12 to 28) | 0.03 (0.02 to 0.05) | 51 (33 to 74) | 0.04 (0.03 to 0.06) | 0.67 (0.61 to 0.73) |
| GBD regions | |||||
| Andean Latin America | 3 (2 to 4) | 0.01 (0.01 to 0.02) | 10 (6 to 13) | 0.02 (0.01 to 0.02) | 1.09 (0.33 to 1.86) |
| Australasia | 39 (28 to 52) | 0.17 (0.12 to 0.22) | 130 (103 to 160) | 0.22 (0.18 to 0.27) | 1.05 (0.14 to 1.97) |
| Caribbean | 3 (2 to 4) | 0.01 (0.01 to 0.01) | 6 (4 to 9) | 0.01 (0.01 to 0.02) | 0.8 (−0.06 to 1.67) |
| Central Asia | 22 (16 to 31) | 0.05 (0.04 to 0.07) | 37 (28 to 48) | 0.05 (0.04 to 0.07) | 0.14 (−0.04 to 0.31) |
| Central Europe | 221 (199 to 242) | 0.18 (0.16 to 0.19) | 812 (649 to 1030) | 0.36 (0.29 to 0.45) | 2.14 (0.87 to 3.43) |
| Central Latin America | 25 (16 to 38) | 0.02 (0.01 to 0.03) | 78 (51 to 117) | 0.03 (0.02 to 0.05) | 1.28 (0.64 to 1.92) |
| Central Sub-Saharan Africa | 8 (5 to 12) | 0.04 (0.03 to 0.06) | 26 (17 to 38) | 0.05 (0.03 to 0.07) | 0.65 (0.34 to 0.96) |
| East Asia | 121 (74 to 183) | 0.02 (0.01 to 0.03) | 399 (254 to 609) | 0.02 (0.01 to 0.03) | 0.83 (0.25 to 1.41) |
| Eastern Europe | 247 (195 to 313) | 0.1 (0.08 to 0.13) | 322 (275 to 376) | 0.1 (0.08 to 0.11) | 0.41 (−1.4 to 2.25) |
| Eastern Sub-Saharan Africa | 28 (18 to 40) | 0.04 (0.02 to 0.05) | 82 (56 to 119) | 0.05 (0.03 to 0.07) | 0.85 (0.78 to 0.91) |
| High-Income Asia Pacific | 328 (245 to 432) | 0.19 (0.15 to 0.25) | 1236 (911 to 1650) | 0.22 (0.17 to 0.28) | 0.48 (−0.16 to 1.12) |
| High-Income North America | 646 (573 to 692) | 0.18 (0.16 to 0.19) | 8011 (7202 to 8860) | 1.16 (1.04 to 1.27) | 6.03 (5.41 to 6.66) |
| North Africa and Middle East | 29 (18 to 43) | 0.02 (0.01 to 0.02) | 102 (67 to 150) | 0.03 (0.02 to 0.04) | 1.39 (1.08 to 1.7) |
| Oceania | 1 (0 to 1) | 0.02 (0.01 to 0.03) | 2 (1 to 2) | 0.03 (0.02 to 0.04) | 0.33 (0.18 to 0.48) |
| South Asia | 98 (60 to 147) | 0.02 (0.01 to 0.02) | 313 (203 to 464) | 0.02 (0.01 to 0.03) | 1.25 (0.86 to 1.64) |
| Southeast Asia | 53 (34 to 79) | 0.02 (0.01 to 0.03) | 129 (81 to 198) | 0.02 (0.01 to 0.03) | 0.29 (0.1 to 0.47) |
| Southern Latin America | 28 (19 to 39) | 0.07 (0.05 to 0.09) | 102 (79 to 132) | 0.12 (0.09 to 0.15) | 1.87 (0.27 to 3.49) |
| Southern Sub-Saharan Africa | 16 (11 to 22) | 0.06 (0.04 to 0.08) | 35 (25 to 50) | 0.07 (0.05 to 0.09) | 0.55 (0.5 to 0.61) |
| Tropical Latin America | 8 (5 to 12) | 0.02 (0.02 to 0.03) | 30 (20 to 43) | 0.01 (0.01 to 0.02) | 2.27 (1.77 to 2.77) |
| Western Europe | 1085 (879 to 1318)) | 0.19 (0.16 to 0.23) | 3628 (2813 to 4600) | 0.33 (0.26 to 0.41) | 1.77 (0.64 to 2.92) |
| Western Sub-Saharan Africa | 36 (24 to 52) | 0.04 (0.03 to 0.06) | 107 (75 to 152) | 0.05 (0.04 to 0.07) | 0.79 (0.7 to 0.89) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, Z.; Wu, J.; Ye, X.; Jin, J.; Zhang, W. Global Burden, Trends, and Inequalities of Clostridioides difficile Infections from 1990 to 2021 and Projections to 2040: A Systematic Analysis. Antibiotics 2025, 14, 652. https://doi.org/10.3390/antibiotics14070652
Chen Z, Wu J, Ye X, Jin J, Zhang W. Global Burden, Trends, and Inequalities of Clostridioides difficile Infections from 1990 to 2021 and Projections to 2040: A Systematic Analysis. Antibiotics. 2025; 14(7):652. https://doi.org/10.3390/antibiotics14070652
Chicago/Turabian StyleChen, Zhihui, Jing Wu, Xiangru Ye, Jialin Jin, and Wenhong Zhang. 2025. "Global Burden, Trends, and Inequalities of Clostridioides difficile Infections from 1990 to 2021 and Projections to 2040: A Systematic Analysis" Antibiotics 14, no. 7: 652. https://doi.org/10.3390/antibiotics14070652
APA StyleChen, Z., Wu, J., Ye, X., Jin, J., & Zhang, W. (2025). Global Burden, Trends, and Inequalities of Clostridioides difficile Infections from 1990 to 2021 and Projections to 2040: A Systematic Analysis. Antibiotics, 14(7), 652. https://doi.org/10.3390/antibiotics14070652









