In Vitro and In Silico Wound-Healing Activity of Two Cationic Peptides Derived from Cecropin D in Galleria mellonella
Abstract
1. Introduction
2. Results and Discussion
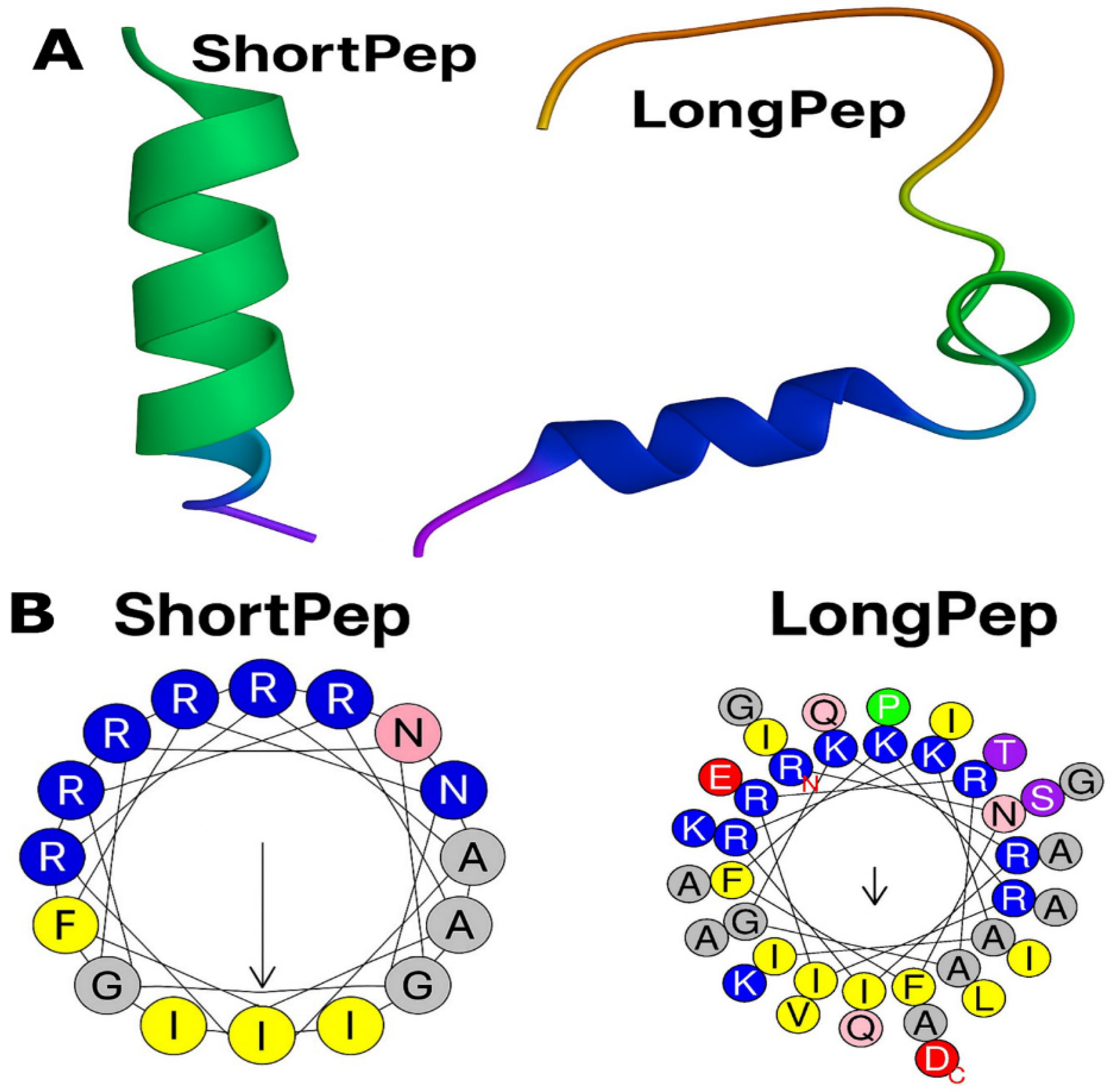
2.1. Design and Synthesis of Antimicrobial Peptides
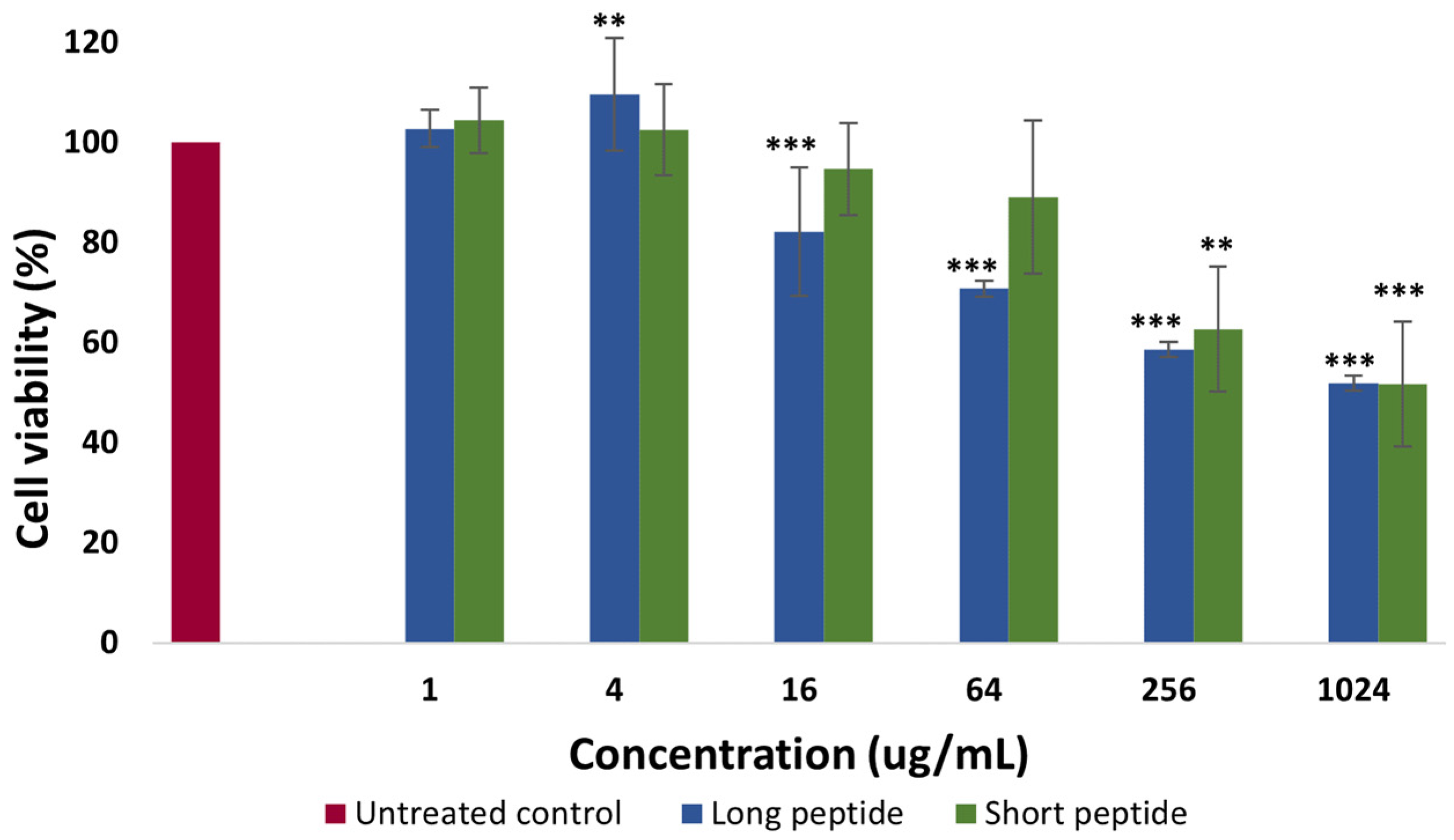
2.2. Cytotoxicity Assays
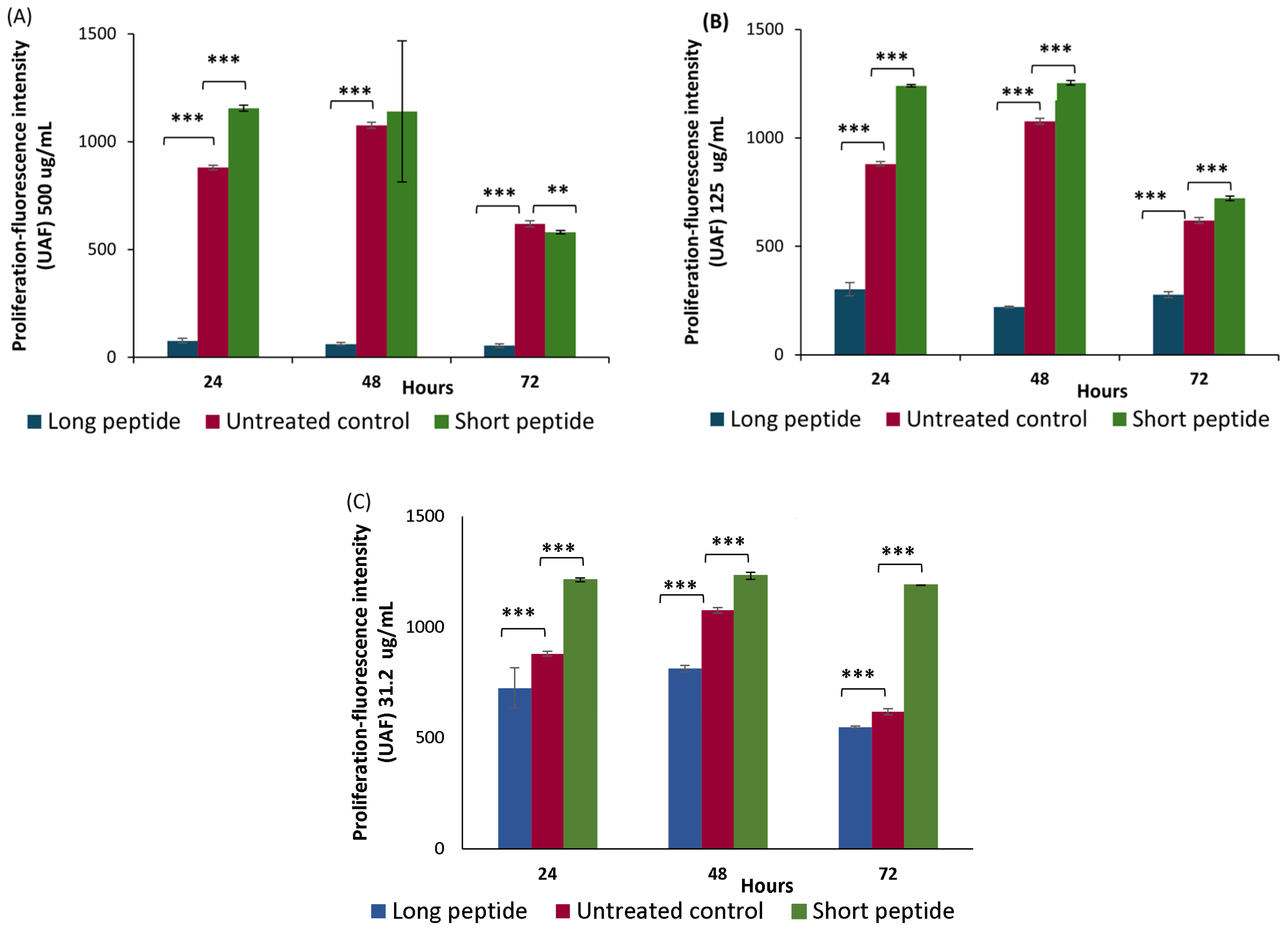
2.3. Proliferation Assays
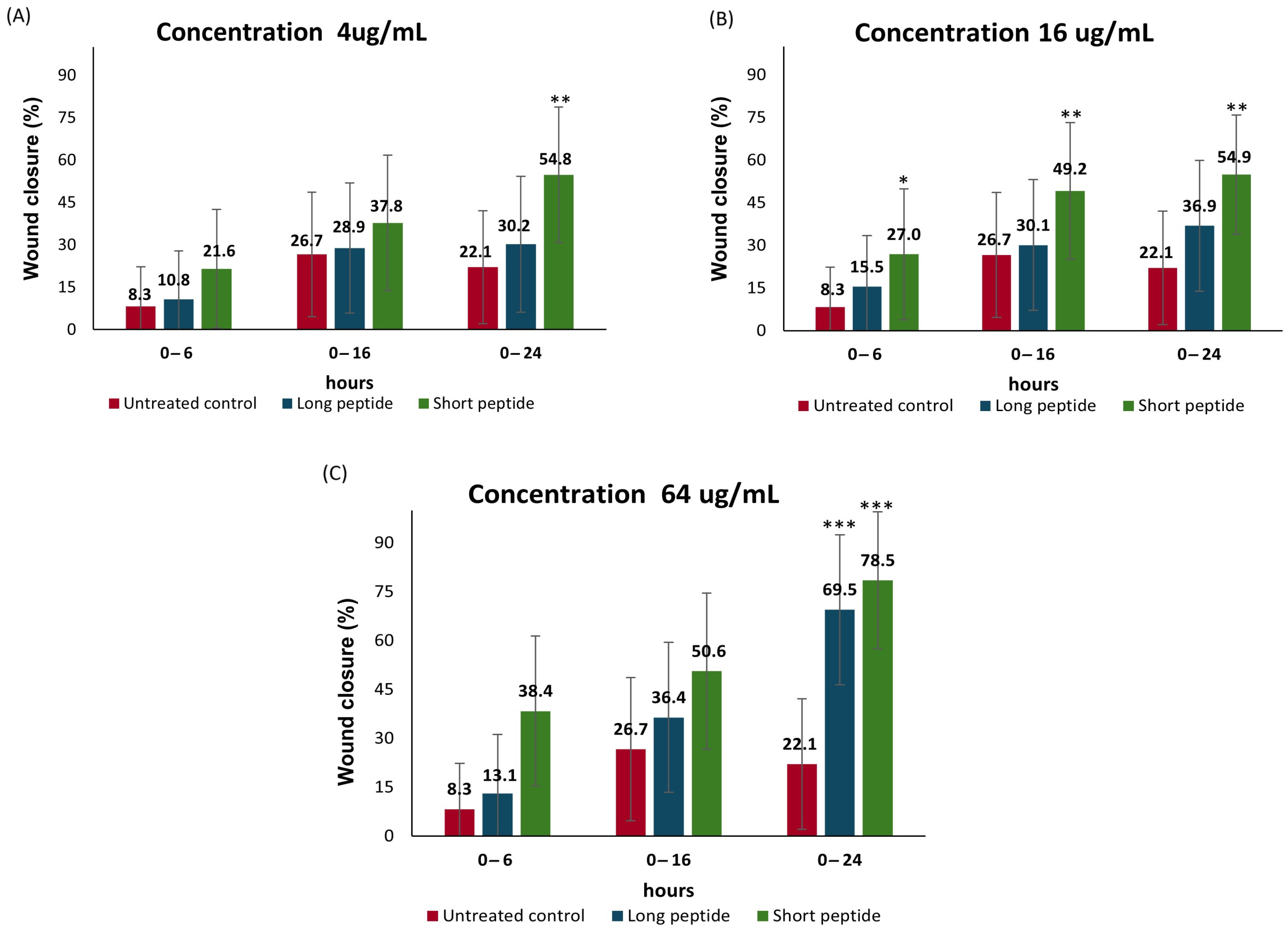
2.4. Cell Migration Assay
Wound-Healing Potential of the Antimicrobial Peptides
2.5. Molecular Docking Results
2.6. Integration of the In Vitro and In Silico Results
2.7. Clinical Implications
2.8. Limitations and Recommendations
3. Materials and Methods
3.1. Design and Synthesis of the AMPs
3.2. Cytotoxicity
3.3. Proliferation
3.4. Wound-Healing Potential of the Peptides
3.5. In Silico Peptide–Receptor Preparation
3.5.1. Receptor Preparation: TGFRB2
3.5.2. 3D Modeling Using AlphaFold
3.5.3. Validation of the Predicted Structure
3.6. Ligand Preparation
Construction and Optimization
3.7. Docking Configuration
3.7.1. Molecular Docking Process
3.7.2. Docking Validation
3.7.3. Post-Docking Analysis
3.7.4. Figures and Results
3.8. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AMPs | Antimicrobial peptides |
| CAMP | Cationic antimicrobial peptide |
| CAMP-CecD | Short peptide derived from cecropin D |
| DMEM | Dulbecco’s modified Eagle medium |
| DMSO | Dimethyl sulfoxide |
| EGF | Epidermal growth factor |
| EGFR | Epidermal Growth factor Receptor |
| FBS | Fetal bovine serum |
| IQR | Interquartile range |
| MTT | 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide |
| PBS | Phosphate-buffered saline |
| PDGF | Platelet-derived growth factor |
| RMSD | Root mean square deviation |
| TGFRβ2 | Transforming growth factor beta receptor 2 |
| TGF-β | Transforming growth factor beta |
| VEGF | Vascular endothelial growth factor |
| VEGFR | Vascular endothelial growth factor receptor |
| ∆M2 | Long peptide derived from cecropin D of Galleria mellonella |
References
- Demidova-Rice, T.N.; Hamblin, M.R.; Herman, I.M. Acute and Impaired Wound Healing: Pathophysiology and Current Methods for Drug Delivery, Part 1: Normal and Chronic Wounds: Biology, Causes, and Approaches to Care. Adv. Skin Wound Care 2012, 25, 304–314. [Google Scholar] [CrossRef] [PubMed]
- Dreifke, M.B.; Jayasuriya, A.A.; Jayasuriya, A.C. Current Wound Healing Procedures and Potential Care. Mater. Sci. Eng. C 2015, 48, 651–662. [Google Scholar] [CrossRef] [PubMed]
- Miao, F.; Li, Y.; Tai, Z.; Zhang, Y.; Gao, Y.; Hu, M.; Zhu, Q. Antimicrobial Peptides: The Promising Therapeutics for Cutaneous Wound Healing. Macromol. Biosci. 2021, 21, 2100103. [Google Scholar] [CrossRef]
- Trejos, M.; Aristizabal, Y.; Aragón-Muriel, A.; Oñate-Garzón, J.; Liscano, Y. Characterization and Classification In Silico of Peptides with Dual Activity (Antimicrobial and Wound Healing). Int. J. Mol. Sci. 2023, 24, 13091. [Google Scholar] [CrossRef] [PubMed]
- Tabas, I.; Glass, C.K. Anti-Inflammatory Therapy in Chronic Disease: Challenges and Opportunities. Science 2013, 339, 166–172. [Google Scholar] [CrossRef]
- Zhao, R.; Liang, H.; Clarke, E.; Jackson, C.; Xue, M. Inflammation in Chronic Wounds. Int. J. Mol. Sci. 2016, 17, 2085. [Google Scholar] [CrossRef]
- Shah, A.; Amini-Nik, S. The Role of Phytochemicals in the Inflammatory Phase of Wound Healing. Int. J. Mol. Sci. 2017, 18, 1068. [Google Scholar] [CrossRef]
- Mehrabi, T.; Mesgar, A.S.; Mohammadi, Z. Bioactive Glasses: A Promising Therapeutic Ion Release Strategy for Enhancing Wound Healing. ACS Biomater. Sci. Eng. 2020, 6, 5399–5430. [Google Scholar] [CrossRef]
- Veith, A.P.; Henderson, K.; Spencer, A.; Sligar, A.D.; Baker, A.B. Therapeutic Strategies for Enhancing Angiogenesis in Wound Healing. Adv. Drug Deliv. Rev. 2019, 146, 97–125. [Google Scholar] [CrossRef]
- Kaufman, N.E.M.; Dhingra, S.; Jois, S.D.; Vicente, M.D.G.H. Molecular Targeting of Epidermal Growth Factor Receptor (EGFR) and Vascular Endothelial Growth Factor Receptor (VEGFR). Molecules 2021, 26, 1076. [Google Scholar] [CrossRef]
- Mohammad, K.S.; Akhund, S.A. From Tumor to Bone: Growth Factor Receptors as Key Players in Cancer Metastasis. Front. Biosci. Landmark 2024, 29, 184. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.K.; Henen, M.A.; Hinck, A.P. Structural Biology of Betaglycan and Endoglin, Membrane-Bound Co-Receptors of the TGF-Beta Family. Exp. Biol. Med. 2019, 244, 1547–1558. [Google Scholar] [CrossRef] [PubMed]
- Baardsnes, J.; Hinck, C.S.; Hinck, A.P.; O’Connor-McCourt, M.D. TβR-II Discriminates the High- and Low-Affinity TGF-β Isoforms via Two Hydrogen-Bonded Ion Pairs. Biochemistry 2009, 48, 2146–2155. [Google Scholar] [CrossRef] [PubMed]
- Esquirol Caussa, J.; Herrero Vila, E. Un enfoque para el tratamiento de las úlceras de origen vascular: Revisión y papel del factor de crecimiento epidérmico. Angiología 2016, 68, 322–330. [Google Scholar] [CrossRef]
- Constantino Rosa Santos, S.; Miguel, C.; Domingues, I.; Calado, A.; Zhu, Z.; Wu, Y.; Dias, S. VEGF and VEGFR-2 (KDR) Internalization Is Required for Endothelial Recovery during Wound Healing. Exp. Cell Res. 2007, 313, 1561–1574. [Google Scholar] [CrossRef]
- Stevens, M.; Oltean, S. Modulation of Receptor Tyrosine Kinase Activity through Alternative Splicing of Ligands and Receptors in the VEGF-A/VEGFR Axis. Cells 2019, 8, 288. [Google Scholar] [CrossRef]
- Repertinger, S.K.; Campagnaro, E.; Fuhrman, J.; El-Abaseri, T.; Yuspa, S.H.; Hansen, L.A. EGFR Enhances Early Healing After Cutaneous Incisional Wounding. J. Investig. Dermatol. 2004, 123, 982–989. [Google Scholar] [CrossRef]
- Tam, J.P.; Lv, S.N.; Gan, J.J. Epidermal Growth Factor Receptor (EGFR) Ligands. U.S. Patent Application US17/625,314, 18 August 2022. [Google Scholar]
- Oñate-Garzón, J.; Manrique-Moreno, M.; Trier, S.; Leidy, C.; Torres, R.; Patiño, E. Antimicrobial Activity and Interactions of Cationic Peptides Derived from Galleria Mellonella Cecropin D-like Peptide with Model Membranes. J. Antibiot. 2017, 70, 238–245. [Google Scholar] [CrossRef]
- Thapa, R.K.; Diep, D.B.; Tønnesen, H.H. Topical Antimicrobial Peptide Formulations for Wound Healing: Current Developments and Future Prospects. Acta Biomater. 2020, 103, 52–67. [Google Scholar] [CrossRef]
- Shaykhiev, R.; Beißwenger, C.; Kändler, K.; Senske, J.; Püchner, A.; Damm, T.; Behr, J.; Bals, R. Human Endogenous Antibiotic LL-37 Stimulates Airway Epithelial Cell Proliferation and Wound Closure. Am. J. Physiol. Lung Cell. Mol. Physiol. 2005, 289, L842–L848. [Google Scholar] [CrossRef]
- Grönberg, A.; Mahlapuu, M.; Ståhle, M.; Whately-Smith, C.; Rollman, O. Treatment with LL-37 Is Safe and Effective in Enhancing Healing of Hard-to-heal Venous Leg Ulcers: A Randomized, Placebo-controlled Clinical Trial. Wound Repair Regen. 2014, 22, 613–621. [Google Scholar] [CrossRef]
- Schmidt, N.W.; Jin, F.; Lande, R.; Curk, T.; Xian, W.; Lee, C.; Frasca, L.; Frenkel, D.; Dobnikar, J.; Gilliet, M.; et al. Liquid-Crystalline Ordering of Antimicrobial Peptide–DNA Complexes Controls TLR9 Activation. Nat. Mater. 2015, 14, 696–700. [Google Scholar] [CrossRef] [PubMed]
- Otvos, L.; Ostorhazi, E. Therapeutic Utility of Antibacterial Peptides in Wound Healing. Expert Rev. Anti Infect. Ther. 2015, 13, 871–881. [Google Scholar] [CrossRef]
- Ahmad, A.; Khan, J.M.; Bandy, A. A Systematic Review of the Design and Applications of Antimicrobial Peptides in Wound Healing. Cureus 2024, 16, e58178. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Yang, F.; Qin, X.; Yang, X.; Zhang, C.; Wan, Z.; Lin, H. Investigation of the In Vivo, In Vitro, and In Silico Wound Healing Potential of Pinctada Martensii Purified Peptides. Mar. Drugs 2022, 20, 417. [Google Scholar] [CrossRef]
- Severino, P.; Ariga, S.K.; Barbeiro, H.V.; De Lima, T.M.; De Paula Silva, E.; Barbeiro, D.F.; Machado, M.C.C.; Nizet, V.; Pinheiro Da Silva, F. Cathelicidin-Deficient Mice Exhibit Increased Survival and Upregulation of Key Inflammatory Response Genes Following Cecal Ligation and Puncture. J. Mol. Med. 2017, 95, 995–1003. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.-H.; Kim, Y.N.; Jang, Y.-S. Cutting Edge: LL-37–Mediated Formyl Peptide Receptor-2 Signaling in Follicular Dendritic Cells Contributes to B Cell Activation in Peyer’s Patch Germinal Centers. J. Immunol. 2017, 198, 629–633. [Google Scholar] [CrossRef]
- Gonzalez-Curiel, I.; Trujillo, V.; Montoya-Rosales, A.; Rincon, K.; Rivas-Calderon, B.; deHaro-Acosta, J.; Marin-Luevano, P.; Lozano-Lopez, D.; Enciso-Moreno, J.A.; Rivas-Santiago, B. 1,25-Dihydroxyvitamin D3 Induces LL-37 and HBD-2 Production in Keratinocytes from Diabetic Foot Ulcers Promoting Wound Healing: An In Vitro Model. PLoS ONE 2014, 9, e111355. [Google Scholar] [CrossRef]
- Kanaujia, K.A.; Mishra, N.; Rajinikanth, P.S.; Saraf, S.A. Antimicrobial Peptides as Antimicrobials for Wound Care Management: A Comprehensive Review. J. Drug Deliv. Sci. Technol. 2024, 95, 105570. [Google Scholar] [CrossRef]
- Pinzi, L.; Rastelli, G. Molecular Docking: Shifting Paradigms in Drug Discovery. Int. J. Mol. Sci. 2019, 20, 4331. [Google Scholar] [CrossRef]
- Rivera-Sánchez, S.P.; Agudelo-Góngora, H.A.; Oñate-Garzón, J.; Flórez-Elvira, L.J.; Correa, A.; Londoño, P.A.; Londoño-Mosquera, J.D.; Aragón-Muriel, A.; Polo-Cerón, D.; Ocampo-Ibáñez, I.D. Antibacterial Activity of a Cationic Antimicrobial Peptide against Multidrug-Resistant Gram-Negative Clinical Isolates and Their Potential Molecular Targets. Molecules 2020, 25, 5035. [Google Scholar] [CrossRef] [PubMed]
- Ocampo-Ibáñez, I.D.; Liscano, Y.; Rivera-Sánchez, S.P.; Oñate-Garzón, J.; Lugo-Guevara, A.D.; Flórez-Elvira, L.J.; Lesmes, M.C. A Novel Cecropin D-Derived Short Cationic Antimicrobial Peptide Exhibits Antibacterial Activity Against Wild-Type and Multidrug-Resistant Strains of Klebsiella pneumoniae and Pseudomonas aeruginosa. Evol. Bioinforma. 2020, 16, 1176934320936266. [Google Scholar] [CrossRef]
- Mojsoska, B.; Jenssen, H. Peptides and Peptidomimetics for Antimicrobial Drug Design. Pharmaceuticals 2015, 8, 366–415. [Google Scholar] [CrossRef] [PubMed]
- Luan, X.; Wu, Y.; Shen, Y.-W.; Zhang, H.; Zhou, Y.-D.; Chen, H.-Z.; Nagle, D.G.; Zhang, W.-D. Cytotoxic and Antitumor Peptides as Novel Chemotherapeutics. Nat. Prod. Rep. 2021, 38, 7–17. [Google Scholar] [CrossRef] [PubMed]
- Rivera-Sanchez, S.P.; Ocampo-Ibáñez, I.D.; Liscano, Y.; Martínez, N.; Muñoz, I.; Manrique-Moreno, M.; Martinez-Martinez, L.; Oñate-Garzon, J. Integrating In Vitro and In Silico Analysis of a Cationic Antimicrobial Peptide Interaction with Model Membranes of Colistin-Resistant Pseudomonas Aeruginosa Strains. Pharmaceutics 2022, 14, 1248. [Google Scholar] [CrossRef]
- Stone, T.A.; Cole, G.B.; Ravamehr-Lake, D.; Nguyen, H.Q.; Khan, F.; Sharpe, S.; Deber, C.M. Positive Charge Patterning and Hydrophobicity of Membrane-Active Antimicrobial Peptides as Determinants of Activity, Toxicity, and Pharmacokinetic Stability. J. Med. Chem. 2019, 62, 6276–6286. [Google Scholar] [CrossRef]
- Sano, M.; Fukuda, K. Activation of Mitochondrial Biogenesis by Hormesis. Circ. Res. 2008, 103, 1191–1193. [Google Scholar] [CrossRef]
- Machado, I.F.; Teodoro, J.S.; Castela, A.C.; Palmeira, C.M.; Rolo, A.P. Mitohormesis. In Mitochondrial Physiology and Vegetal Molecules: Therapeutic Potential of Natural Compounds on Mitochondrial Health; de Oliveira, M.R., Ed.; Elsevier: Amsterdam, The Netherlands, 2021; Chapter 33; pp. 729–746. [Google Scholar]
- Hunt, M.; Torres, M.; Bachar-Wikström, E.; Wikström, J.D. Multifaceted Roles of Mitochondria in Wound Healing and Chronic Wound Pathogenesis. Front. Cell Dev. Biol. 2023, 11, 1252318. [Google Scholar] [CrossRef]
- Martens, M.A.; Bleeke, M.S.; Leopold, V.A.; Farmer, D.R. Toxicology and Human Health Risk Assessment of Polyethoxylated Tallow Amine Surfactant Used in Glyphosate Formulations. Regul. Toxicol. Pharmacol. 2019, 107, 104347. [Google Scholar] [CrossRef]
- Heil, N.; Bravo, K.; Montoya, A.; Robledo, S.; Osorio, E. Wound Healing Activity of Ullucus Tuberosus, an Andean Tuber Crop. Asian Pac. J. Trop. Biomed. 2017, 7, 538–543. [Google Scholar] [CrossRef]
- Greco, I.; Molchanova, N.; Holmedal, E.; Jenssen, H.; Hummel, B.D.; Watts, J.L.; Håkansson, J.; Hansen, P.R.; Svenson, J. Correlation between Hemolytic Activity, Cytotoxicity and Systemic in Vivo Toxicity of Synthetic Antimicrobial Peptides. Sci. Rep. 2020, 10, 13206. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.; Li, S.; Bjorklund, M.; Xu, S. Mitochondrial Fragmentation and ROS Signaling in Wound Response and Repair. Cell Regen. 2022, 11, 38. [Google Scholar] [CrossRef] [PubMed]
- Gagat, P.; Ostrówka, M.; Duda-Madej, A.; Mackiewicz, P. Enhancing Antimicrobial Peptide Activity through Modifications of Charge, Hydrophobicity, and Structure. Int. J. Mol. Sci. 2024, 25, 10821. [Google Scholar] [CrossRef]
- Fathi, F.; Alizadeh, B.; Tabarzad, M.V.; Tabarzad, M. Important Structural Features of Antimicrobial Peptides towards Specific Activity: Trends in the Development of Efficient Therapeutics. Bioorganic Chem. 2024, 149, 107524. [Google Scholar] [CrossRef]
- Aragón-Muriel, A.; Liscano, Y.; Morales-Morales, D.; Polo-Cerón, D.; Oñate-Garzón, J. A Study of the Interaction of a New Benzimidazole Schiff Base with Synthetic and Simulated Membrane Models of Bacterial and Mammalian Membranes. Membranes 2021, 11, 449. [Google Scholar] [CrossRef]
- Kumar, P.; Nagarajan, A.; Uchil, P.D. Analysis of Cell Viability by the alamarBlue Assay. Cold Spring Harb. Protoc. 2018, 2018, pdb.prot095489. [Google Scholar] [CrossRef] [PubMed]
- Grada, A.; Otero-Vinas, M.; Prieto-Castrillo, F.; Obagi, Z.; Falanga, V. Research Techniques Made Simple: Analysis of Collective Cell Migration Using the Wound Healing Assay. J. Investig. Dermatol. 2017, 137, e11–e16. [Google Scholar] [CrossRef] [PubMed]
- desJardins-Park, H.E.; Foster, D.S.; Longaker, M.T. Fibroblasts and Wound Healing: An Update. Regen. Med. 2018, 13, 491–495. [Google Scholar] [CrossRef]
- Kang, H.K.; Lee, H.H.; Seo, C.H.; Park, Y. Antimicrobial and Immunomodulatory Properties and Applications of Marine-Derived Proteins and Peptides. Mar. Drugs 2019, 17, 350. [Google Scholar] [CrossRef]
- Carretero, M.; Escámez, M.J.; García, M.; Duarte, B.; Holguín, A.; Retamosa, L.; Jorcano, J.L.; Río, M.D.; Larcher, F. In Vitro and In Vivo Wound Healing-Promoting Activities of Human Cathelicidin LL-37. J. Investig. Dermatol. 2008, 128, 223–236. [Google Scholar] [CrossRef]
- Ramírez, D.; Caballero, J. Is It Reliable to Take the Molecular Docking Top Scoring Position as the Best Solution without Considering Available Structural Data? Mol. J. Synth. Chem. Nat. Prod. Chem. 2018, 23, 1038. [Google Scholar] [CrossRef] [PubMed]
- Gomes, A.; Teixeira, C.; Ferraz, R.; Prudêncio, C.; Gomes, P.A.C. Wound-Healing Peptides for Treatment of Chronic Diabetic Foot Ulcers and Other Infected Skin Injuries. Mol. J. Synth. Chem. Nat. Prod. Chem. 2017, 22, 1743. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Feng, Z.; Yang, M.; Zeng, L.; Qi, B.; Yin, S.; Li, B.; Li, Y.; Fu, Z.; Shu, L.; et al. Discovery of a Novel Short Peptide with Efficacy in Accelerating the Healing of Skin Wounds. Pharmacol. Res. 2020, 163, 105296. [Google Scholar] [CrossRef]
- Garvey, M. Antimicrobial Peptides Demonstrate Activity against Resistant Bacterial Pathogens. Infect. Dis. Rep. 2023, 15, 454–469. [Google Scholar] [CrossRef] [PubMed]
- Pfalzgraff, A.; Brandenburg, K.; Weindl, G. Antimicrobial Peptides and Their Therapeutic Potential for Bacterial Skin Infections and Wounds. Front. Pharmacol. 2018, 9, 281. [Google Scholar] [CrossRef]
- Souza, G.S.d.; Sonego, L.d.J.; Mundim, A.C.S.; Moraes, J.d.M.; Sales-Campos, H.; Lorenzón, E.N. Antimicrobial-Wound Healing Peptides: Dual-Function Molecules for the Treatment of Skin Injuries. Peptides 2021, 148, 170707. [Google Scholar] [CrossRef]
- Oñate-Garzón, J.F.; Manrique-Moreno, M.; Patiño González, E. Actividad antimicrobiana de péptidos catiónicos diseñados a partir de un péptido neutro. Acta Biológica Colomb. 2017, 22, 35. [Google Scholar] [CrossRef]
- Kupcsik, L. Estimation of Cell Number Based on Metabolic Activity: The MTT Reduction Assay. In Mammalian Cell Viability; Stoddart, M.J., Ed.; Methods in Molecular Biology; Humana Press: Totowa, NJ, USA, 2011; Volume 740, pp. 13–19. ISBN 978-1-61779-107-9. [Google Scholar]
- Tolosa, L.; Donato, M.T.; Gómez-Lechón, M.J. General Cytotoxicity Assessment by Means of the MTT Assay. In Protocols in In Vitro Hepatocyte Research; Vinken, M., Rogiers, V., Eds.; Springer: New York, NY, USA, 2015; pp. 333–348. ISBN 978-1-4939-2074-7. [Google Scholar]
- Cappiello, F.; Casciaro, B.; Mangoni, M.L. A Novel In Vitro Wound Healing Assay to Evaluate Cell Migration. J. Vis. Exp. 2018, 56825. [Google Scholar] [CrossRef]
- Aragón-Muriel, A.; Liscano-Martínez, Y.; Rufino-Felipe, E.; Morales-Morales, D.; Oñate-Garzón, J.; Polo-Cerón, D. Synthesis, Biological Evaluation and Model Membrane Studies on Metal Complexes Containing Aromatic N,O-Chelate Ligands. Heliyon 2020, 6, e04126. [Google Scholar] [CrossRef]
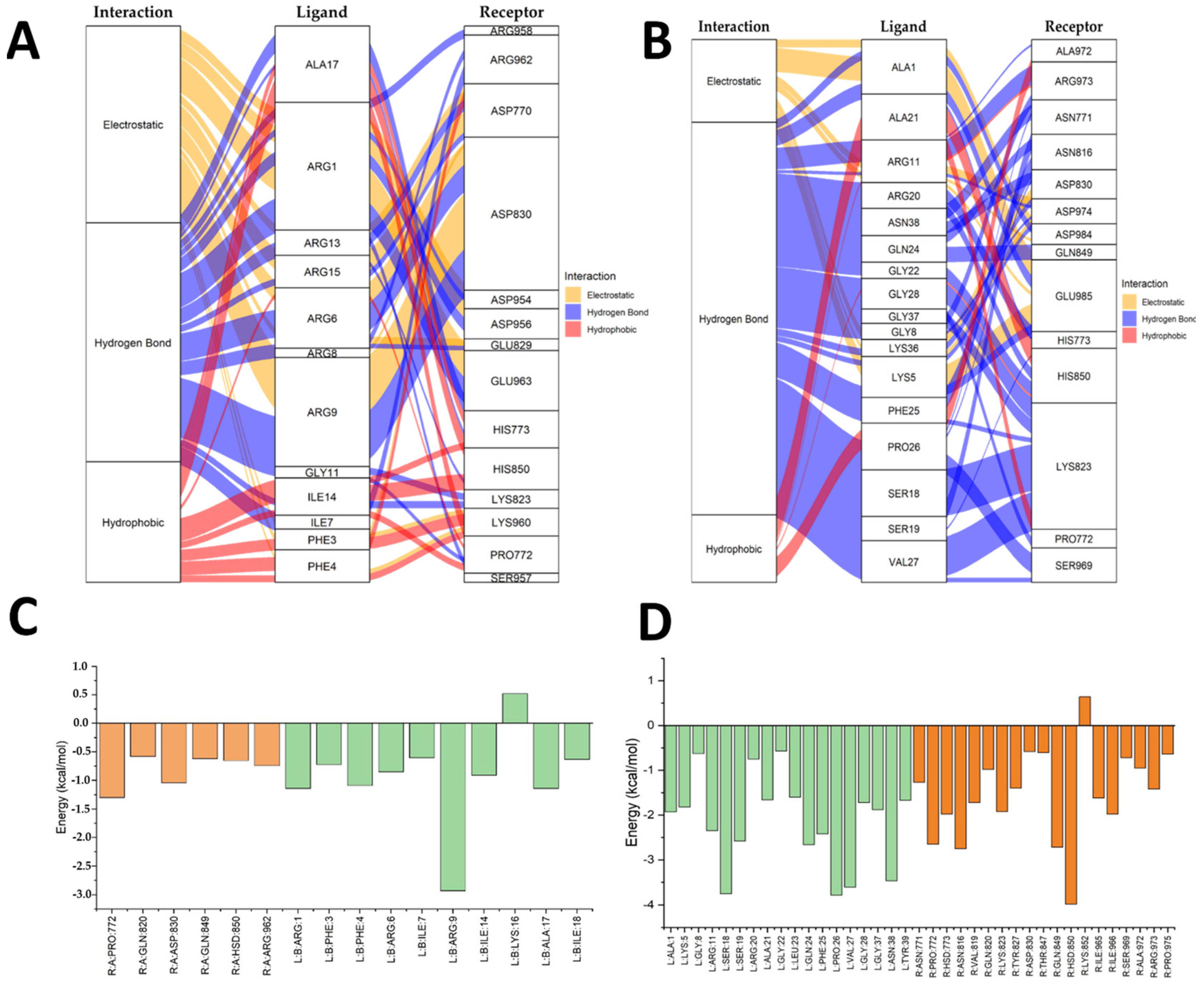
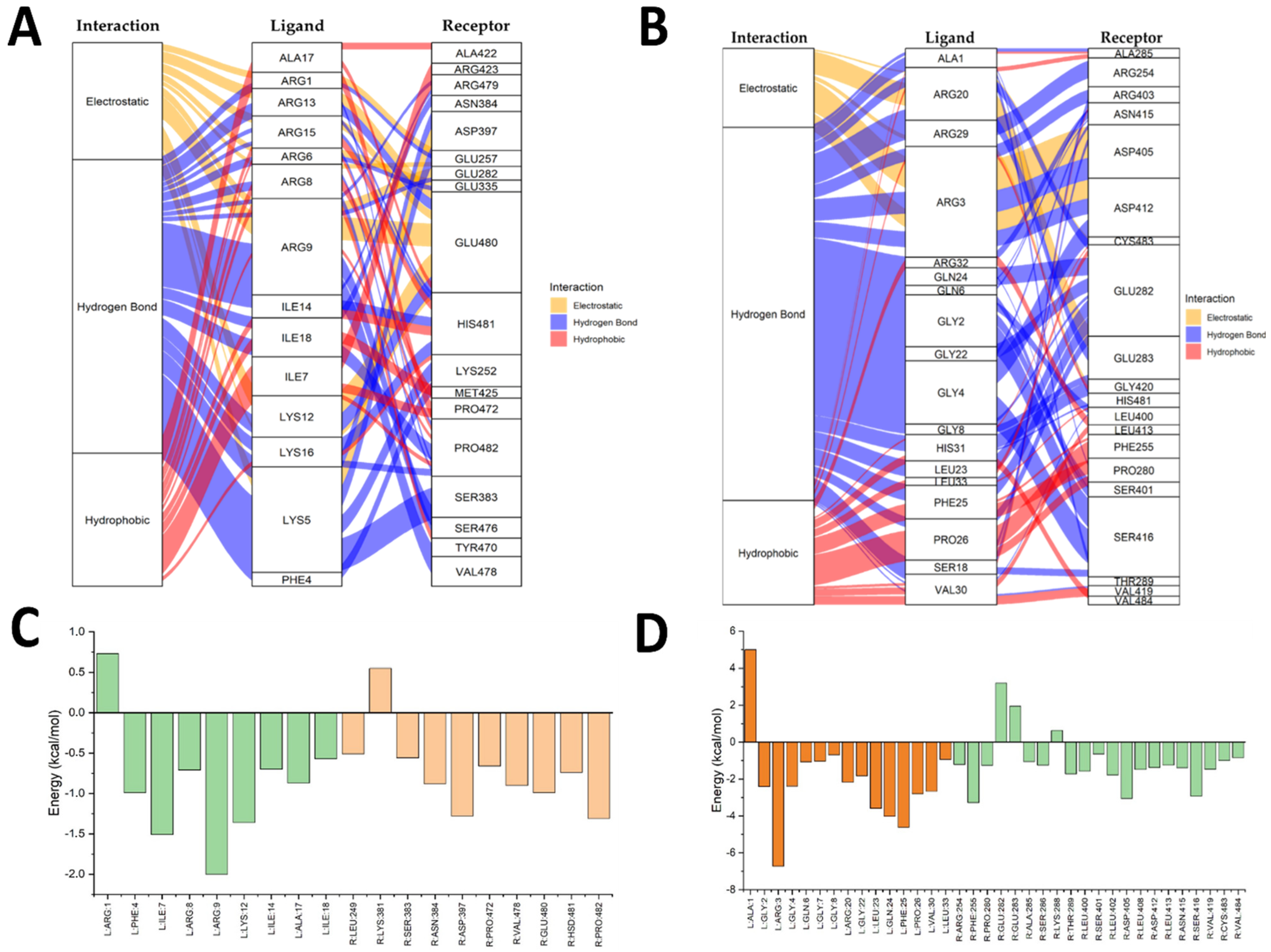
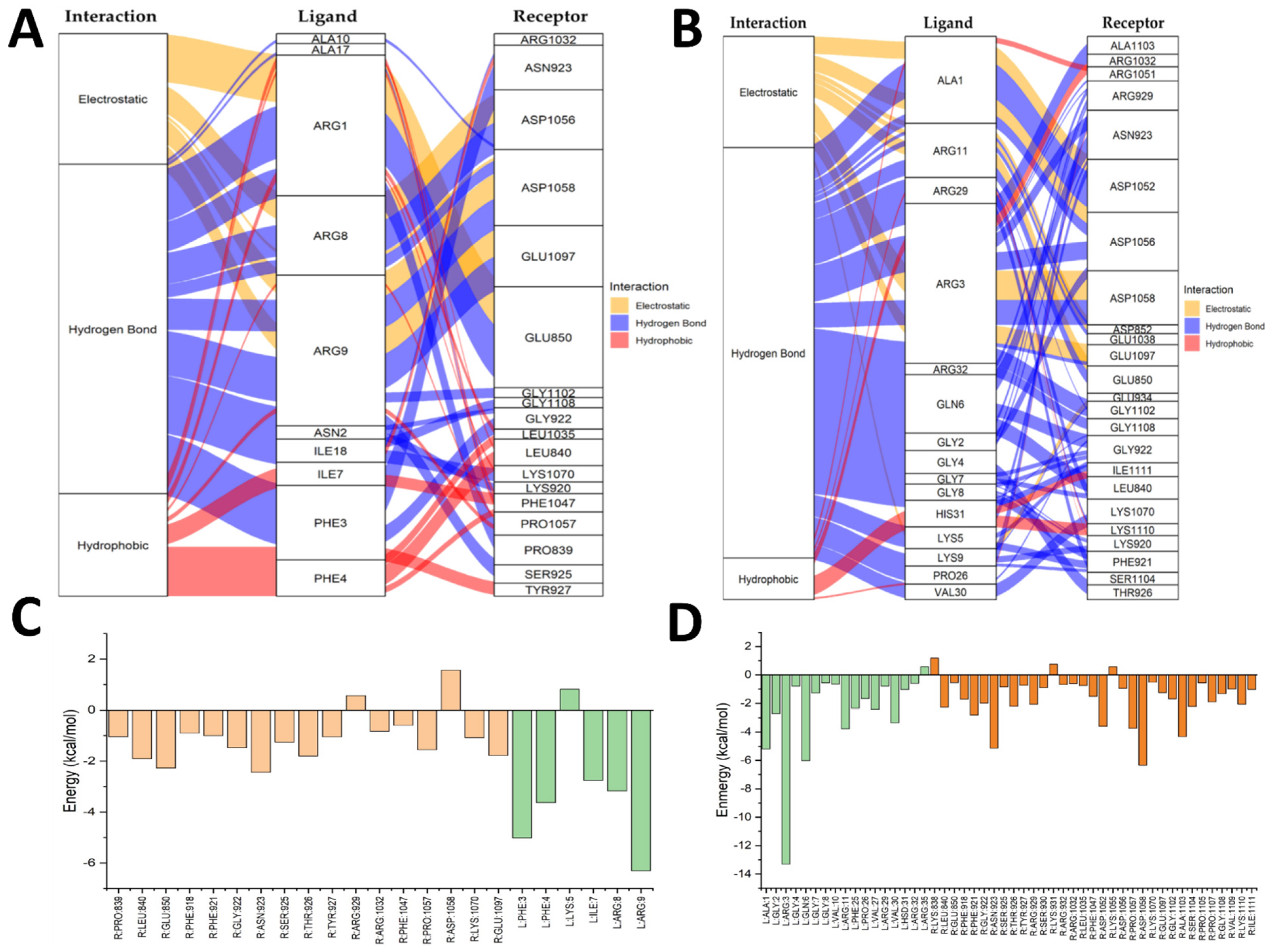
| Receptor | Ligand | Affinity (kcal/mol) ± SD | RMSD (Å) |
|---|---|---|---|
| VEGFR | Axitinib | −7.8 ± 0.3 | 1.2 ± 0.1 |
| VEGFR | ShortPep | −6.7 ± 0.4 | 1.6 ± 0.2 |
| VEGFR | LongPep | −5.3 ± 0.2 | 1.8 ± 0.2 |
| EGFR | Osimertinib | −7.6 ± 0.3 | 1.2 ± 0.1 |
| EGFR | ShortPep | −7.2 ± 0.5 | 1.7 ± 0.2 |
| EGFR | LongPep | −3.7 ± 0.4 | 1.5 ± 0.1 |
| TGFRβ2 | Galunisertib | −6.8 ± 0.3 | 1.1 ± 0.1 |
| TGFRβ2 | ShortPep | −5.6 ± 0.2 | 1.4 ± 0.2 |
| TGFRβ2 | LongPep | −4.0 ± 0.3 | 1.7 ± 0.3 |
| Peptide | Sequence | Length | Net Charge | Aliphatic Index | Grand Average of Hydropathicity (GRAVY) | Hydrophobicity <H> | Hydrophobic Moment <µH> |
|---|---|---|---|---|---|---|---|
| Short Pep | RNFFKRIRRAGKRIRKAI | 18 | 9 | 76.11 | −1.106 | −0.002 | 0.723 |
| Long Pep | RNFFKRIRRAGKRIRKAIISAAPAVETLAQAQKIIKGGD | 39 | 9 | 95.38 | −0.387 | 0.178 | 0.296 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rivera-Sanchez, S.P.; Ocampo-Ibáñez, I.D.; Moncaleano, M.C.; Liscano, Y.; Elvira, L.J.F.; Aristizabal Salazar, Y.A.; Martínez-Martínez, L.; Oñate-Garzon, J. In Vitro and In Silico Wound-Healing Activity of Two Cationic Peptides Derived from Cecropin D in Galleria mellonella. Antibiotics 2025, 14, 651. https://doi.org/10.3390/antibiotics14070651
Rivera-Sanchez SP, Ocampo-Ibáñez ID, Moncaleano MC, Liscano Y, Elvira LJF, Aristizabal Salazar YA, Martínez-Martínez L, Oñate-Garzon J. In Vitro and In Silico Wound-Healing Activity of Two Cationic Peptides Derived from Cecropin D in Galleria mellonella. Antibiotics. 2025; 14(7):651. https://doi.org/10.3390/antibiotics14070651
Chicago/Turabian StyleRivera-Sanchez, Sandra Patricia, Iván Darío Ocampo-Ibáñez, Maria Camila Moncaleano, Yamil Liscano, Liliana Janeth Flórez Elvira, Yesid Armando Aristizabal Salazar, Luis Martínez-Martínez, and Jose Oñate-Garzon. 2025. "In Vitro and In Silico Wound-Healing Activity of Two Cationic Peptides Derived from Cecropin D in Galleria mellonella" Antibiotics 14, no. 7: 651. https://doi.org/10.3390/antibiotics14070651
APA StyleRivera-Sanchez, S. P., Ocampo-Ibáñez, I. D., Moncaleano, M. C., Liscano, Y., Elvira, L. J. F., Aristizabal Salazar, Y. A., Martínez-Martínez, L., & Oñate-Garzon, J. (2025). In Vitro and In Silico Wound-Healing Activity of Two Cationic Peptides Derived from Cecropin D in Galleria mellonella. Antibiotics, 14(7), 651. https://doi.org/10.3390/antibiotics14070651









