Global Prevalence of Antibiotic-Resistant Burkholderia pseudomallei in Melioidosis Patients: A Systematic Review and Meta-Analysis
Abstract
1. Introduction
2. Results
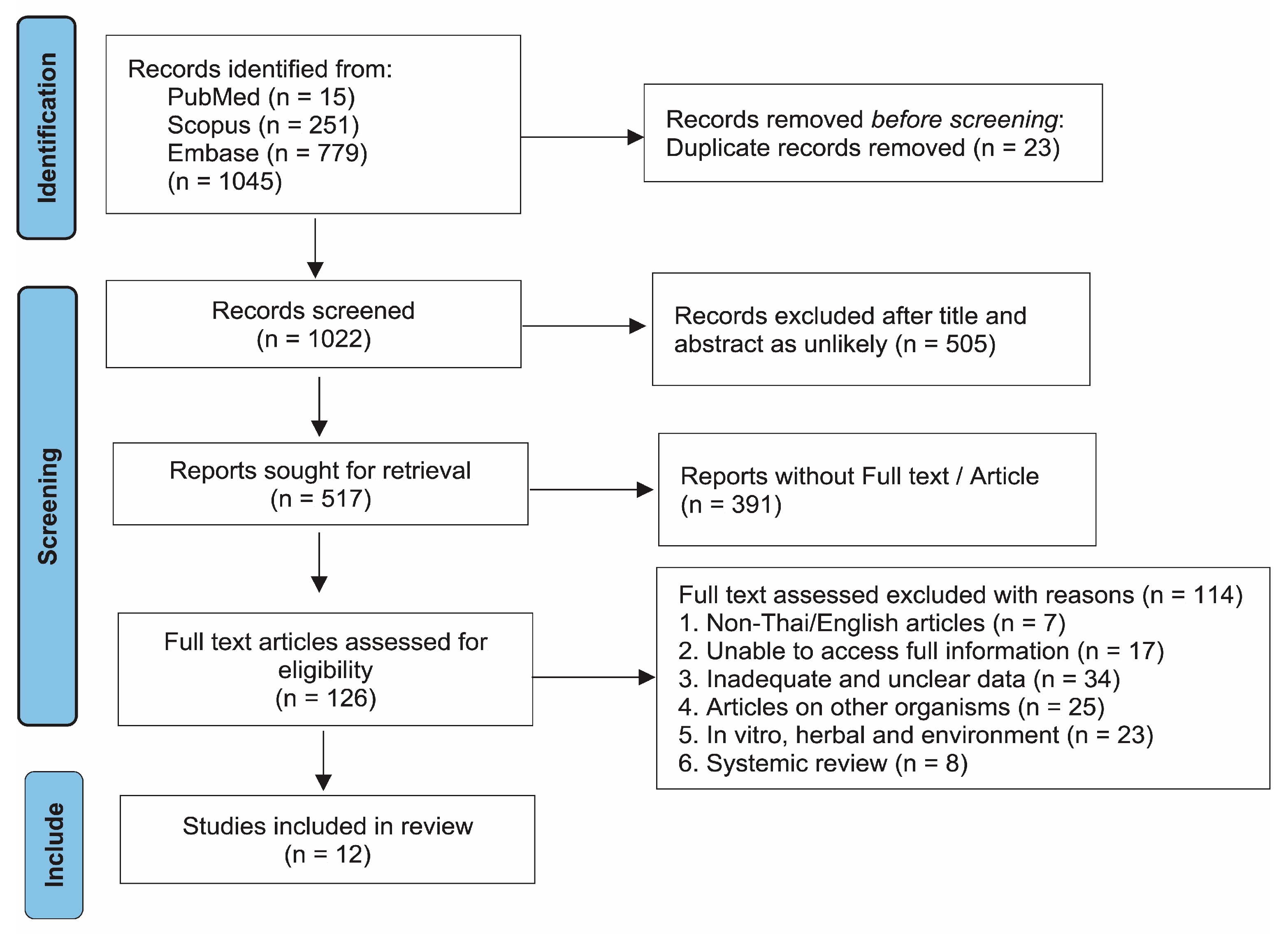
2.1. Search Results
2.2. Overview of the Included Studies
2.3. Quality of the Included Studies
2.4. Pool Prevalence of Antibiotic Resistance
3. Discussion
4. Materials and Methods
4.1. Protocol and Registration
4.2. Search Strategy and Study Selection
4.3. Data Extraction and Quality Assessment
4.4. Data Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AMC | Amoxicillin/clavulanic acid |
| AMX | Amoxicillin |
| AST | Antimicrobial susceptibility testing |
| ATM | Aztreonam |
| AZA | Aztreonam/avibactam |
| BMD | Broth microdilution |
| CAZ | Ceftazidime |
| CHL | Chloramphenicol |
| CIP | Ciprofloxacin |
| CLA | Clavulanic acid |
| CRO | Ceftriaxone |
| CTS | Cefoperazone/sulbactam |
| DOX | Doxycycline |
| DDF | Disk diffusion |
| IPM | Imipenem |
| MEM | Meropenem |
| MIC | Minimum inhibitory concentration |
| PIP | Piperacillin |
| SAM | Ampicillin/sulbactam |
| SMX | Trimethoprim |
| SUL | Sulfamethoxazole |
| SXT | Trimethoprim–sulfamethoxazole |
| TCY | Tetracycline |
| TGC | Tigecycline |
| TZP | Piperacillin/tazobactam |
References
- Stone, J.K.; DeShazer, D.; Brett, P.J.; Burtnick, M.N. Melioidosis: Molecular aspects of pathogenesis. Expert Rev. Anti-Infect. Ther. 2014, 12, 1487–1499. [Google Scholar] [CrossRef] [PubMed]
- Gassiep, I.; Armstrong, M.; Norton, R. Human Melioidosis. Clin. Microbiol. Rev. 2020, 33, 10–128. [Google Scholar] [CrossRef]
- White, N.J. Melioidosis. Lancet 2003, 361, 1715–1722. [Google Scholar] [CrossRef] [PubMed]
- Wiersinga, W.J.; Virk, H.S.; Torres, A.G.; Currie, B.J.; Peacock, S.J.; Dance, D.A.B.; Limmathurotsakul, D. Melioidosis. Nat. Rev. Dis. Primers 2018, 4, 17107. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Rhodes, K.A.; Schweizer, H.P. Antibiotic resistance in Burkholderia species. Drug Resist. Updates 2016, 28, 82–90. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Schweizer, H.P. Mechanisms of antibiotic resistance in Burkholderia pseudomallei: Implications for treatment of melioidosis. Future Microbiol. 2012, 7, 1389–1399. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ahmad, N.; Hashim, R.; Mohd Noor, A. The In Vitro Antibiotic Susceptibility of Malaysian Isolates of Burkholderia pseudomallei. Int. J. Microbiol. 2013, 2013, 121845. [Google Scholar] [CrossRef] [PubMed] [PubMed Central][Green Version]
- Bugrysheva, J.V.; Lascols, C.; McLaughlin, H.P.; Gee, J.E.; Elrod, M.G.; Sue, D. Antimicrobial Susceptibility of Western Hemisphere Isolates of Burkholderia pseudomallei: Phenotypic and Genomic Analyses. Microb. Drug Resist. 2021, 27, 1176–1185. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Fen, S.H.Y.; Tandhavanant, S.; Phunpang, R.; Ekchariyawat, P.; Saiprom, N.; Chewapreecha, C.; Seng, R.; Thiansukhon, E.; Morakot, C.; Sangsa, N.; et al. Antibiotic susceptibility of clinical Burkholderia pseudomallei isolates in northeast Thailand during 2015–2018 and the genomic characterization of β-lactam-resistant isolates. Antimicrob. Agents Chemother. 2023, 95, e02230-20. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hui, H.; Sheng, Y.; Han, X.; Wang, S.; Zhang, G.; Wei, X. A clinical study on clinical features, manifestations and drug resistance of melioidosis. Pak. J. Med. Sci. 2022, 38, 2301–2306. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Jenney, A.W.; Lum, G.; Fisher, D.A.; Currie, B.J. Antibiotic susceptibility of Burkholderia pseudomallei from tropical northern Australia and implications for therapy of melioidosis. Int. J. Antimicrob. Agents 2001, 17, 109–113. [Google Scholar] [CrossRef] [PubMed]
- Khosravi, Y.; Vellasamy, K.M.; Mariappan, V.; Ng, S.L.; Vadivelu, J. Antimicrobial susceptibility and genetic characterisation of Burkholderia pseudomallei isolated from Malaysian patients. Sci. World J. 2014, 2014, 132971. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Nhung, P.H.; Van, V.H.; Anh, N.Q.; Phuong, D.M. Antimicrobial susceptibility of Burkholderia pseudomallei isolates in Northern Vietnam. J. Glob. Antimicrob. Resist. 2019, 18, 34–36. [Google Scholar] [CrossRef] [PubMed]
- Panya, M.; Thirat, S.; Wanram, S.; Panomket, P.; Nilsakul, J. Prevalence of bla(PenA) and bla(OXA) in Burkholderia pseudomallei Isolated from Patients at Sappasitthiprasong Hospital and Their Susceptibility to Ceftazidime and Carbapenems. J. Med. Assoc. Thail. 2016, 99 (Suppl. S1), S12–S16. [Google Scholar] [PubMed]
- Paveenkittiporn, W.; Apisarnthanarak, A.; Dejsirilert, S.; Trakulsomboon, S.; Thongmali, O.; Sawanpanyalert, P.; Aswapokee, N. Five-year surveillance for Burkholderia pseudomallei in Thailand from 2000 to 2004: Prevalence and antimicrobial susceptibility. J. Med. Assoc. Thai. 2009, 92 (Suppl. S4), S46–S52. [Google Scholar] [PubMed]
- Sia, T.L.L.; Mohan, A.; Ooi, M.H.; Chien, S.L.; Tan, L.S.; Goh, C.; Pang, D.C.; Currie, B.J.; Wong, J.S.; Podin, Y. Epidemiological and Clinical Characteristics of Melioidosis Caused by Gentamicin-Susceptible Burkholderia pseudomallei in Sarawak, Malaysia. Open Forum Infect. Dis. 2021, 8, ofab460. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Sribenjalux, W.; Wonglakorn, L.; Meesing, A. In Vitro susceptibility of Burkholderia pseudomallei isolates from Thai patients to ceftolozane/tazobactam and ceftazidime/avibactam. J. Glob. Antimicrob. Resist. 2022, 28, 8–11. [Google Scholar] [CrossRef] [PubMed]
- Wuthiekanun, V.; Amornchai, P.; Saiprom, N.; Chantratita, N.; Chierakul, W.; Koh, G.C.; Chaowagul, W.; Day, N.P.; Limmathurotsakul, D.; Peacock, S.J. Survey of antimicrobial resistance in clinical Burkholderia pseudomallei isolates over two decades in Northeast Thailand. Antimicrob. Agents Chemother. 2011, 55, 5388–5391. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ministry of Public Health, Division of Communicable Disease. Guideline of Melioidosis. Nonthaburi: Ministry of Public Health, Division of Communicable Disease. 2021. Available online: http://odpc5ratchaburi.com/manual/uploads/other/file_3074rks5eytu12d86fwj9hg.pdf (accessed on 6 May 2025).
- Pitman, M.C.; Luck, T.; Marshall, C.S.; Anstey, N.M.; Ward, L.; Currie, B.J. Intravenous therapy duration and outcomes in melioidosis: A new treatment paradigm. PLoS Negl. Trop. Dis. 2015, 9, e0003586. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Greer, N.D. Tigecycline (Tygacil): The first in the glycylcycline class of antibiotics. Bayl. Univ. Med. Cent. Proc. 2006, 19, 155–161. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Russell, P.; Eley, S.M.; Ellis, J.; Green, M.; Bell, D.L.; Kenny, D.J.; Titball, R.W. Comparison of efficacy of ciprofloxacin and doxycycline against experimental melioidosis and glanders. J. Antimicrob. Chemother. 2000, 45, 813–818. [Google Scholar] [CrossRef] [PubMed][Green Version]
- White, N.J.; Dance, D.A.; Chaowagul, W.; Wattanagoon, Y.; Wuthiekanun, V.; Pitakwatchara, N. Halving of mortality of severe melioidosis by ceftazidime. Lancet 1989, 2, 697–701. [Google Scholar] [CrossRef] [PubMed]
- Dance, D.A.; Davong, V.; Soeng, S.; Phetsouvanh, R.; Newton, P.N.; Turner, P. Trimethoprim/sulfamethoxazole resistance in Burkholderia pseudomallei. Int. J. Antimicrob. Agents 2014, 44, 368–369. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Simpson, A.J.; Suputtamongkol, Y.; Smith, M.D.; Angus, B.J.; Rajanuwong, A.; Wuthiekanun, V.; Howe, P.A.; Walsh, A.L.; Chaowagul, W.; White, N.J. Comparison of imipenem and ceftazidime as therapy for severe melioidosis. Clin. Infect. Dis. 1999, 29, 381–387. [Google Scholar] [CrossRef] [PubMed]
- Chea, S.; Thonglao, N.; Wonglakorn, L.; Kuwatjanakul, W.; Suttiprapa, S.; Boonjaraspinyo, S.; Yordpratum, U.; Panomket, P.; Chareonsudjai, S. A Comparison of Disk Diffusion and Etest with Broth Microdilution Methods for Susceptibility Testing of Ceftazidime and Trimethoprim/Sulfamethoxazole Against Clinical Isolates of Burkholderia pseudomallei in Northeast Thailand. Srinagarind Med. J. 2024, 39, 167–177. [Google Scholar]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- von Elm, E.; Altman, D.G.; Egger, M.; Pocock, S.J.; Gøtzsche, P.C.; Vandenbroucke, J.P.; STROBE Initiative. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: Guidelines for reporting observational studies. J. Clin. Epidemiol. 2008, 61, 344–349. [Google Scholar] [CrossRef] [PubMed]
- Hunter, J.P.; Saratzis, A.; Sutton, A.J.; Boucher, R.H.; Sayers, R.D.; Bown, M.J. In meta-analyses of proportion studies, funnel plots were found to be an inaccurate method of assessing publication bias. J. Clin. Epidemiol. 2014, 67, 897–903. [Google Scholar] [CrossRef] [PubMed]
| Author, Year | Ref. | Country | Method | Test Number (Total 10,391) | Antibiotic Susceptibility Test (Resistance Number) |
|---|---|---|---|---|---|
| Ahmad, 2013 | [7] | Malaysia | MIC (E-test) | 170 | AMC (1), CAZ (1), CHL (1), CIP (98), DOX (1), IPM (1), MEM (0), SAM (1), SXT (17), TGC (60), TZP (0) |
| Bugrysheva, 2021 | [8] | USA | MIC (BMD) | 26 | AMC (1), AMX (0), ATM (26), AZA (25), CAZ (0), CHL (0), CLA (0), DOX (0), IPM (0), SMX (3), SUL (0), SXT (0), TCY (0) |
| Fen, 2021 | [9] | Thailand | MIC (BMD) | 1317 | AMC (2), CAZ (3), IPM (0), MEM (0), SXT (1) |
| Hui, 2022 | [10] | China | MIC (VITEK 2) | 45 | CAZ (3), CIP (5), IPM (0), MEM (0), SXT (5) |
| Jenney, 2001 | [11] | Australia | MIC (BMD) | 170 | AMC (0), CAZ (7), CHL (0), CRO (0), DOX (7), IPM (7), MEM (7), PIP (0), SXT (7) |
| Khosravi, 2014 | [12] | Malaysia | MIC (E-test) | 69 | AMC (25), CAZ (4), CHL (12), CLA (14), DOX (6), IPM (7), MEM (6), SXT (13), TGC (41) |
| Nhung, 2019 | [13] | Vietnam | MIC (BMD) | 312 | AMC (0), CAZ (0), DOZ (2), IPM (0), SXT (34) |
| Panya, 2016 | [14] | Thailand | MIC (Sensititre) | 85 | CAZ (0), CIP (46), CRO (78), IPM (0) |
| Paveenkittiporn, 2009 | [15] | Thailand | Disk diffusion | 4019 | AMC (201), CAZ (60), CTS (80), IPM (60), MEM (80), SXT (1,889) |
| Sia, 2021 | [16] | Malaysia | Disk diffusion | 129 | GEN (45) |
| Sribenjalux, 2022 | [17] | Thailand | MIC (BMD) | 28 | CAZ (0), IPM (0), MEM (0), SXT (0) |
| Wuthiekanun, 2011 | [18] | Thailand | Disk diffusion | 4021 | AMC (2), CAZ (2), IPM (0), MEM (0), |
| Antibiotic | n of Study (n of Isolates) | Prevalence (95% CI) | I2 | p-Value |
|---|---|---|---|---|
| AMC | 8 (10,104) | 0.0048 (0.0006–0.0371) | 95.7 | <0.0001 |
| Region | 0.4572 | |||
| Asia | 6 (9908) | 0.0054 (0.0005–0.0565) | 96.9 | <0.0001 |
| America | 1 (26) | 0.0385 (0.0010–0.1964) | ||
| Australia | 1 (170) | 0.0000 (0.0000–0.0215) | ||
| Method of testing | 0.9627 | |||
| MIC | 6 (2064) | 0.0046 (0.0003–0.0595) | 93.7 | <0.0001 |
| DDF | 2 (8040) | 0.0050 (0.0002–0.1295) | 97.7 | <0.0001 |
| CAZ | 11 (10,262) | 0.053 (0.0014–0.0192) | 82.2 | <0.0001 |
| Region | 0.0208 | |||
| Asia | 9 (10,066) | 0.0043 (0.0010–0.0177) | 83.6 | <0.0001 |
| America | 1 (26) | 0.0000 (0.0000–0.1323) | ||
| Australia | 1 (170) | 0.0412 (0.0167–0.0830) | ||
| Method of testing | 0.5343 | |||
| MIC | 9 (2222) | 0.0069 (0.0016–0.0289) | 70.6 | 0.0007 |
| DDF | 2 (8040) | 0.0028 (0.0002–0.0329) | 95.6 | <0.0001 |
| CHL | 4 (435) | 0.0130 (0.0007–0.1992) | 72.3 | 0.0107 |
| Region | 1.0000 | |||
| Asia | 2 (239) | 0.0797 (0.0247–0.2287) | 91.1 | 0.0008 |
| America | 1 (26) | 0.0000 (0.0000–0.1323) | ||
| Australia | 1 (170) | 0.0000 (0.0000–0.0215) | ||
| Method of testing | NA | |||
| MIC | 4 (435) | 0.0130 (0.0007–0.1992) | 72.3 | 0.0107 |
| CIP | 3 (300) | 0.3826 (0.1562–0.6747) | 91.3 | <0.0001 |
| Region | NA | |||
| Asia | 3 (300) | 0.3826 (0.1562–0.6747) | 91.3 | <0.0001 |
| Method of testing | NA | |||
| MIC | 3 (300) | 0.3826 (0.1562–0.6747) | 91.3 | <0.0001 |
| CLA | 2 (95) | 0.1474 (0.0893–0.2336) a | 0 | 0.9996 |
| Region | 0 | 0.9996 | ||
| Asia | 1 (69) | 0.0000 (0.0000–0.3169) | ||
| America | 1 (26) | 0.0000 (0.0000–0.1323) | ||
| Method of testing | NA | |||
| MIC | 2 (95) | 0.1474 (0.0893–0.2336) a | 0 | 0.9996 |
| CRO | 2 (255) | 0.3059 (0.2524–0.3652) a | 0 | 0.9995 |
| Region | 0.9995 | |||
| Asia | 1 (85) | 0.9176 (0.8377–0.9662) | ||
| Australia | 1 (170) | 0.0000 (0.0000–0.0215) | ||
| Method of testing | NA | |||
| MIC | 2 (255) | 0.3059 (0.2524–0.3652) a | 0 | 0.9995 |
| DOX | 5 (747) | 0.0174 (0.0052–0.0563) | 72.2 | 0.0061 |
| Region | 0.5283 | |||
| Asia | 3 (551) | 0.0149 (0.0029–0.0726) | 86 | 0.0008 |
| America | 1 (26) | 0.0000 (0.0000–0.1323) | ||
| Australia | 1 (170) | 0.0412 (0.0167–0.0830) | ||
| Method of testing | NA | |||
| MIC | 5 (747) | 0.0174 (0.0052–0.0563) | 72.2 | 0.0061 |
| IPM | 11 (10,262) | 0.0009 (0.0000–0.0161) | 65.1 | 0.0014 |
| Region | <0.0001 | |||
| Asia | 9 (10,066) | 0.0004 (0.0004–0.0004) | 67 | 0.0021 |
| America | 1 (26) | 0.0000 (0.0000–0.1323) | ||
| Australia | 1 (170) | 0.0412 (0.0167–0.830) | ||
| Method of testing | 0.7273 | |||
| MIC | 9 (2222) | 0.0012 (0.0000–0.0291) a | 6.7 | 0.3793 |
| DDF | 2 (8040) | 0.0075 (0.0058–0.0096) a | 0 | 0.9996 |
| MEM | 8 (9839) | 0.0008 (0.0000–0.0341) | 52.8 | 0.0382 |
| Region | 0.0587 | |||
| Asia | 7 (9669) | 0.0002 (0.0000–0.0473) | 51 | 0.0568 |
| Australia | 1 (170) | |||
| Method of testing | 0.2825 | |||
| MIC | 6 (1799) | 0.0072 (0.0042–0.0124) a | 0 | 0.861 |
| DDF | 2 (8040) | 0.0100 (0.0080–0.0124) a | 0 | 0.9996 |
| SXT | 9 (6156) | 0.0423 (0.0097–0.1660) | 97.4 | <0.0001 |
| Region | 0.953 | |||
| Asia | 7 (5960) | 0.0547 (0.0120–0.2463) | 97.7 | <0.0001 |
| America | 1 (26) | 0.0000 (0.0000–0.1323) | ||
| Australia | 1 (170) | 0.0412 (0.067–0.0830) | ||
| Method of testing | <0.0001 | |||
| MIC | 8 (2137) | 0.0296 (0.0069–0.1175) | 81.1 | <0.0001 |
| DDF | 1 (4019) | 0.47000 (0.4525–0.4856) | ||
| TGC | 2 (239) | 0.4634 (0.3025–0.6323) | 91.2 | 0.0008 |
| Region | NA | |||
| Asia | 2 (239) | 0.4634 (0.3025–0.6323) | 91.2 | 0.0008 |
| Method of testing | NA | |||
| MIC | 2 (239) | 0.4634 (0.3025–0.6323) | 91.2 | 0.0008 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Thanasai, J.; Laklaeng, S.-N.; Khemla, S.; Ratanavong, K.; Chatatikun, M.; Tangpong, J.; Klangbud, W.K. Global Prevalence of Antibiotic-Resistant Burkholderia pseudomallei in Melioidosis Patients: A Systematic Review and Meta-Analysis. Antibiotics 2025, 14, 647. https://doi.org/10.3390/antibiotics14070647
Thanasai J, Laklaeng S-N, Khemla S, Ratanavong K, Chatatikun M, Tangpong J, Klangbud WK. Global Prevalence of Antibiotic-Resistant Burkholderia pseudomallei in Melioidosis Patients: A Systematic Review and Meta-Analysis. Antibiotics. 2025; 14(7):647. https://doi.org/10.3390/antibiotics14070647
Chicago/Turabian StyleThanasai, Jongkonnee, Sa-Ngob Laklaeng, Supphachoke Khemla, Khonesavanh Ratanavong, Moragot Chatatikun, Jitbanjong Tangpong, and Wiyada Kwanhian Klangbud. 2025. "Global Prevalence of Antibiotic-Resistant Burkholderia pseudomallei in Melioidosis Patients: A Systematic Review and Meta-Analysis" Antibiotics 14, no. 7: 647. https://doi.org/10.3390/antibiotics14070647
APA StyleThanasai, J., Laklaeng, S.-N., Khemla, S., Ratanavong, K., Chatatikun, M., Tangpong, J., & Klangbud, W. K. (2025). Global Prevalence of Antibiotic-Resistant Burkholderia pseudomallei in Melioidosis Patients: A Systematic Review and Meta-Analysis. Antibiotics, 14(7), 647. https://doi.org/10.3390/antibiotics14070647









