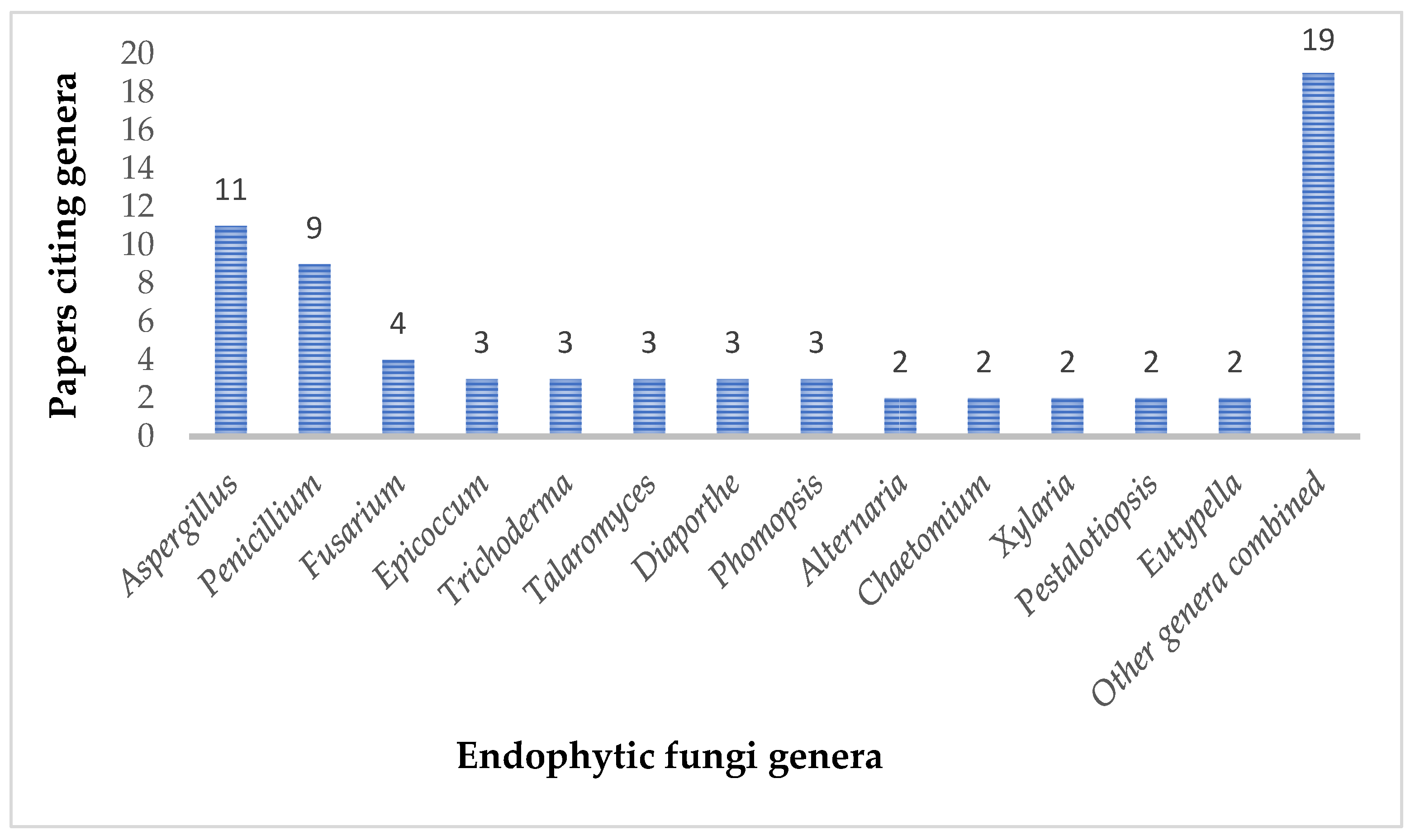
Antibacterial Compounds Isolated from Endophytic Fungi Reported from 2021 to 2024
Abstract
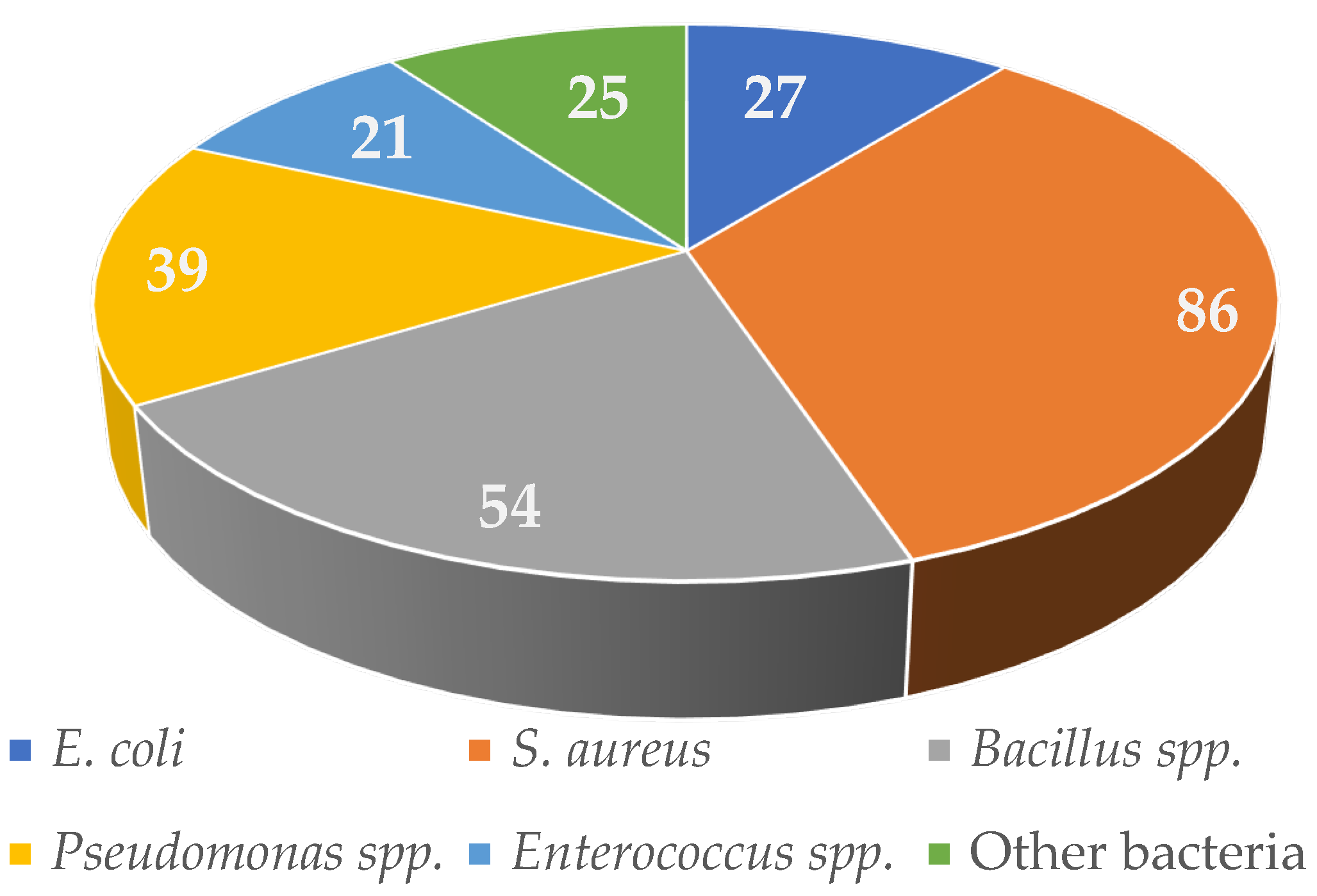
1. Introduction
2. Results
2.1. Polyketides
2.1.1. Quinones
2.1.2. Xanthones and Benzophenone Derivatives
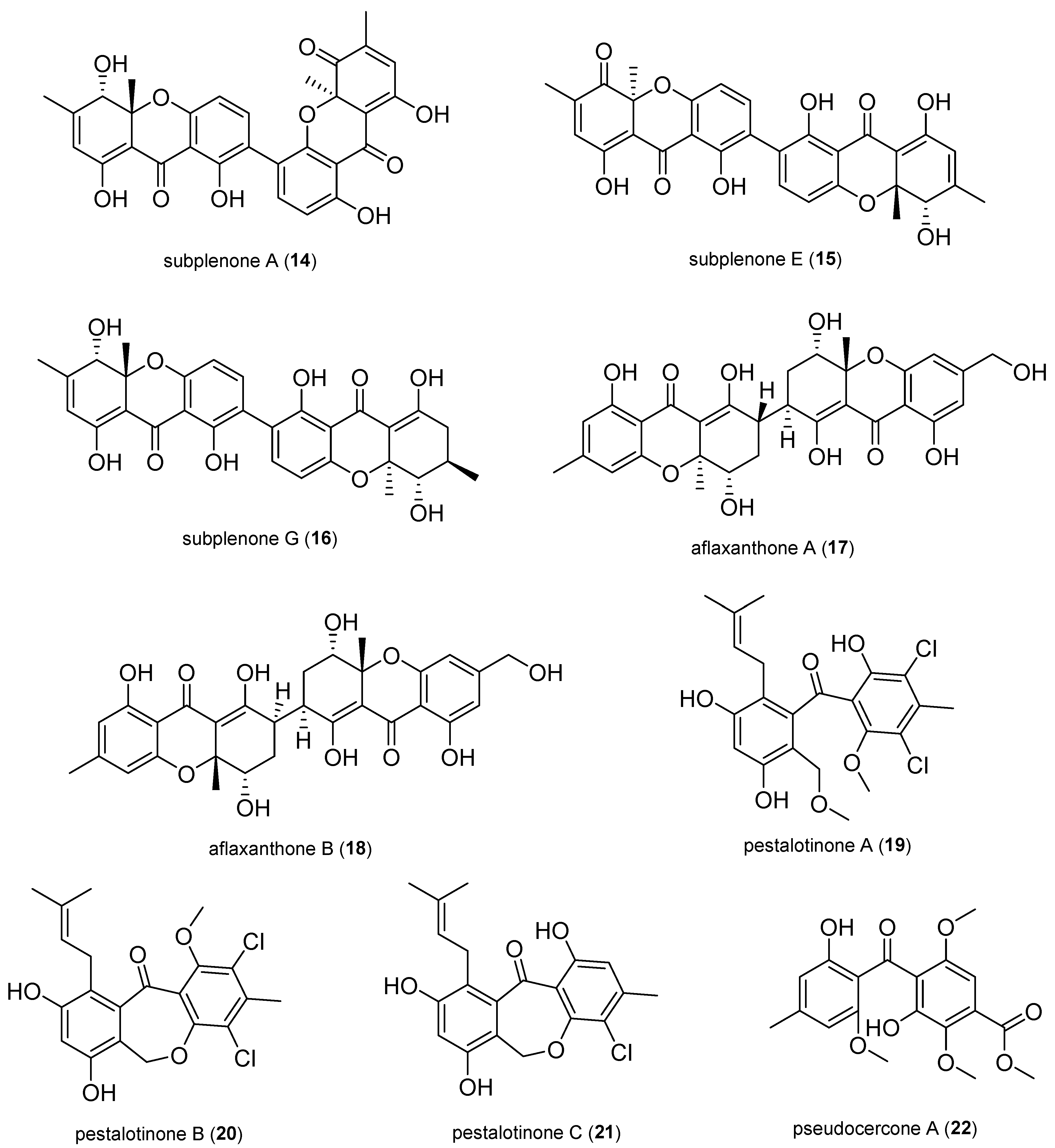
2.1.3. Pyrones and Lactones

2.1.4. Depsidones and Diphenyl Ether Derivatives
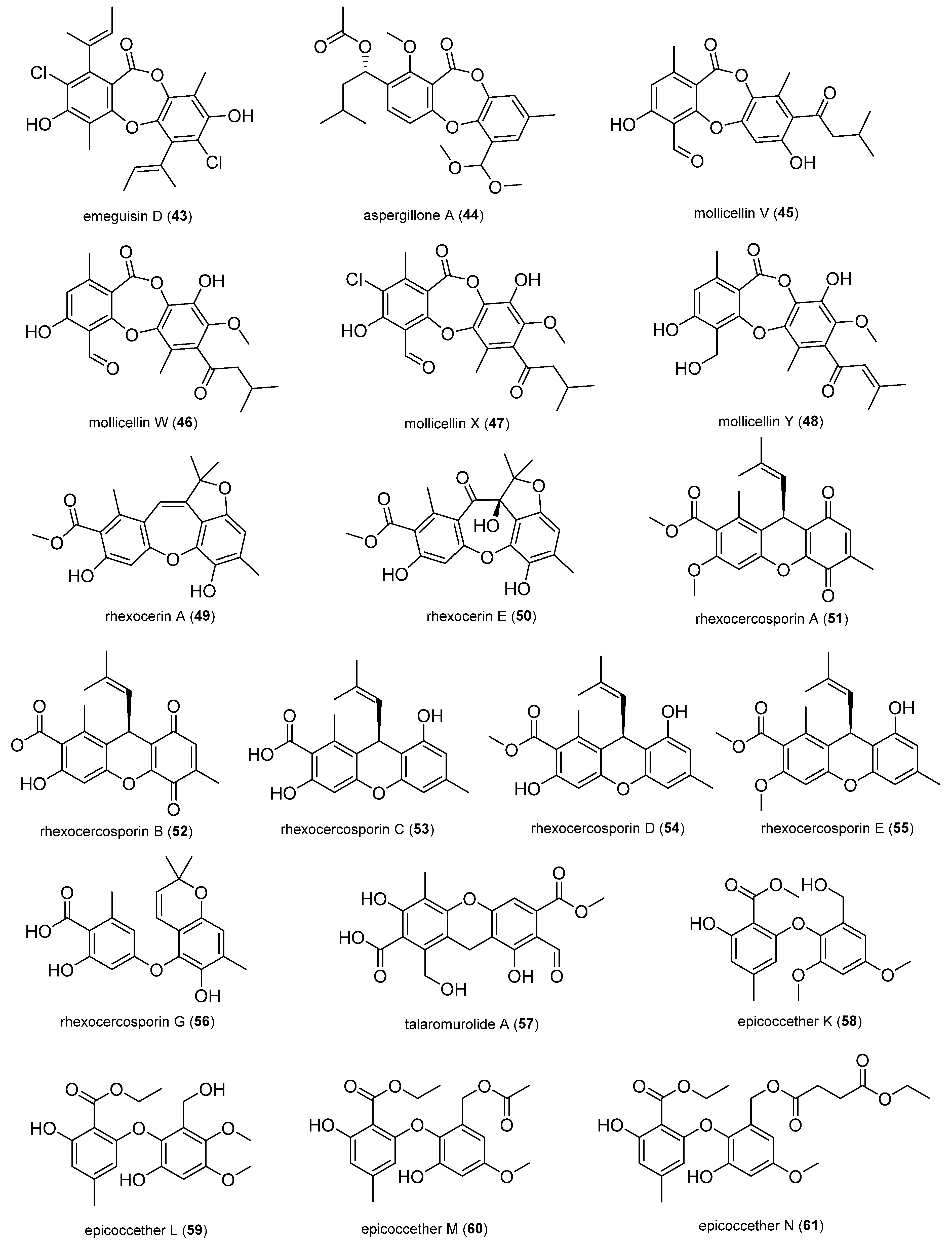
2.1.5. Naphthalene Derivatives
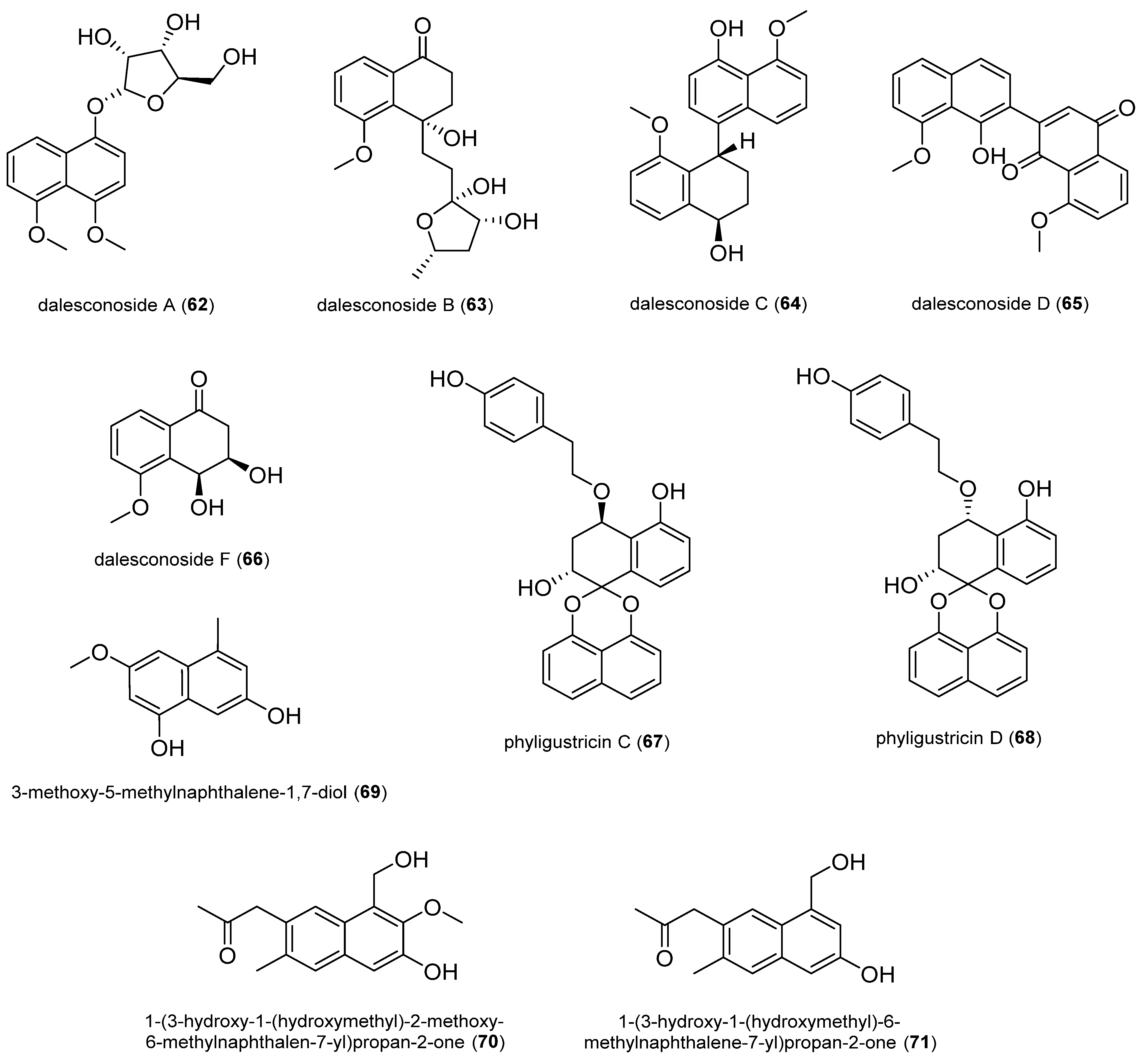
2.1.6. Other Polyketides
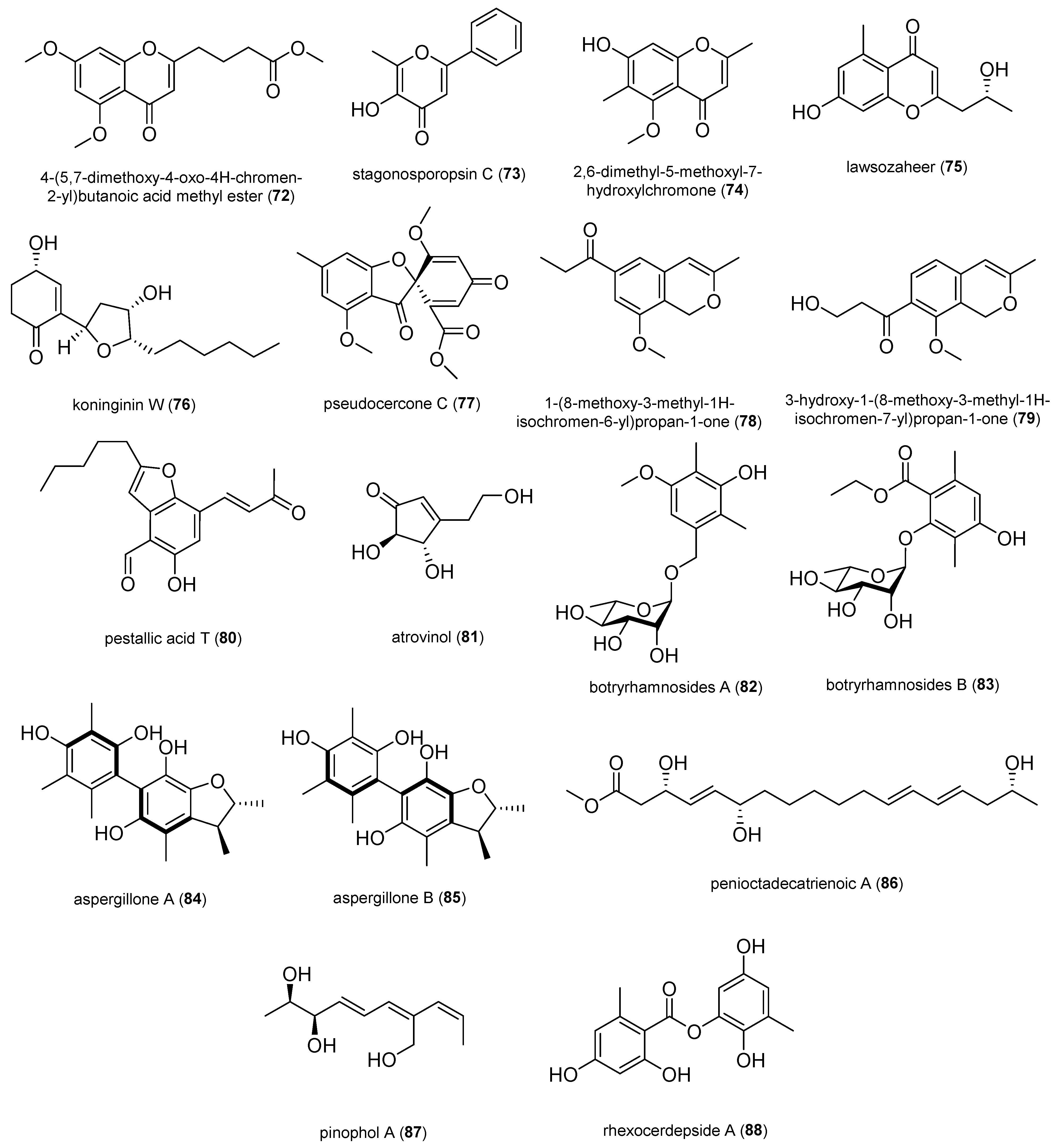
2.1.7. Nitrogen-Containing Compounds
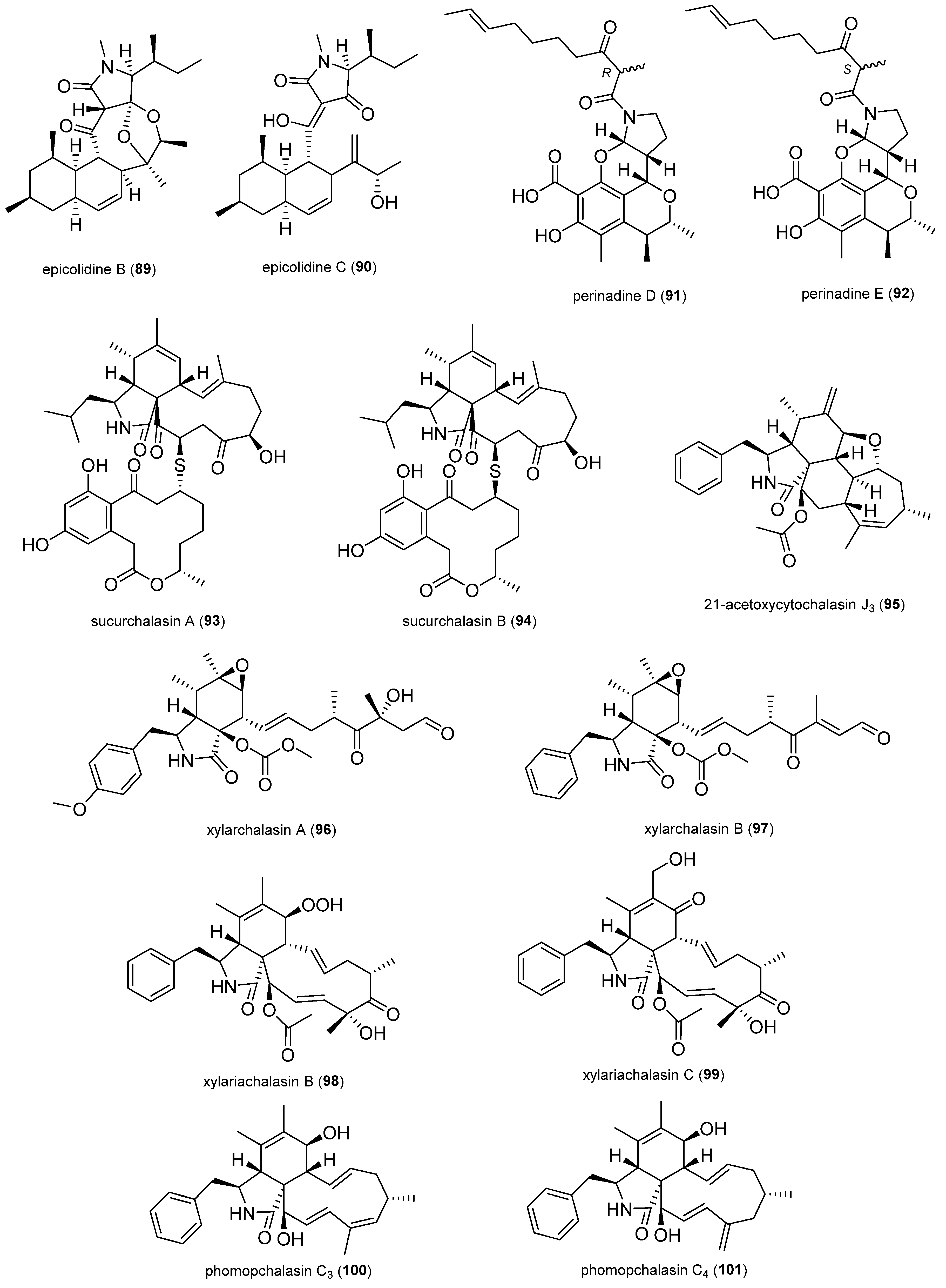
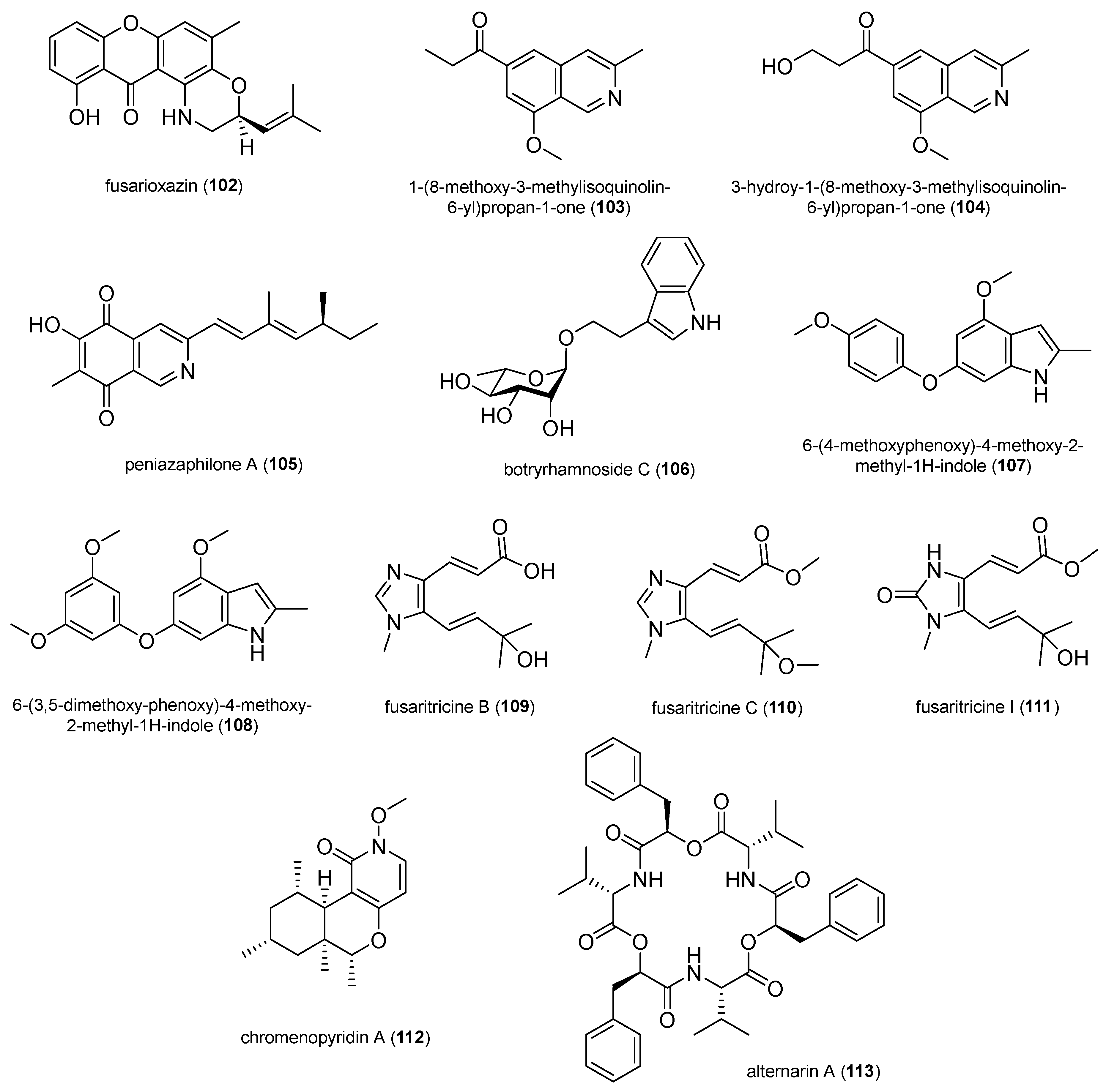
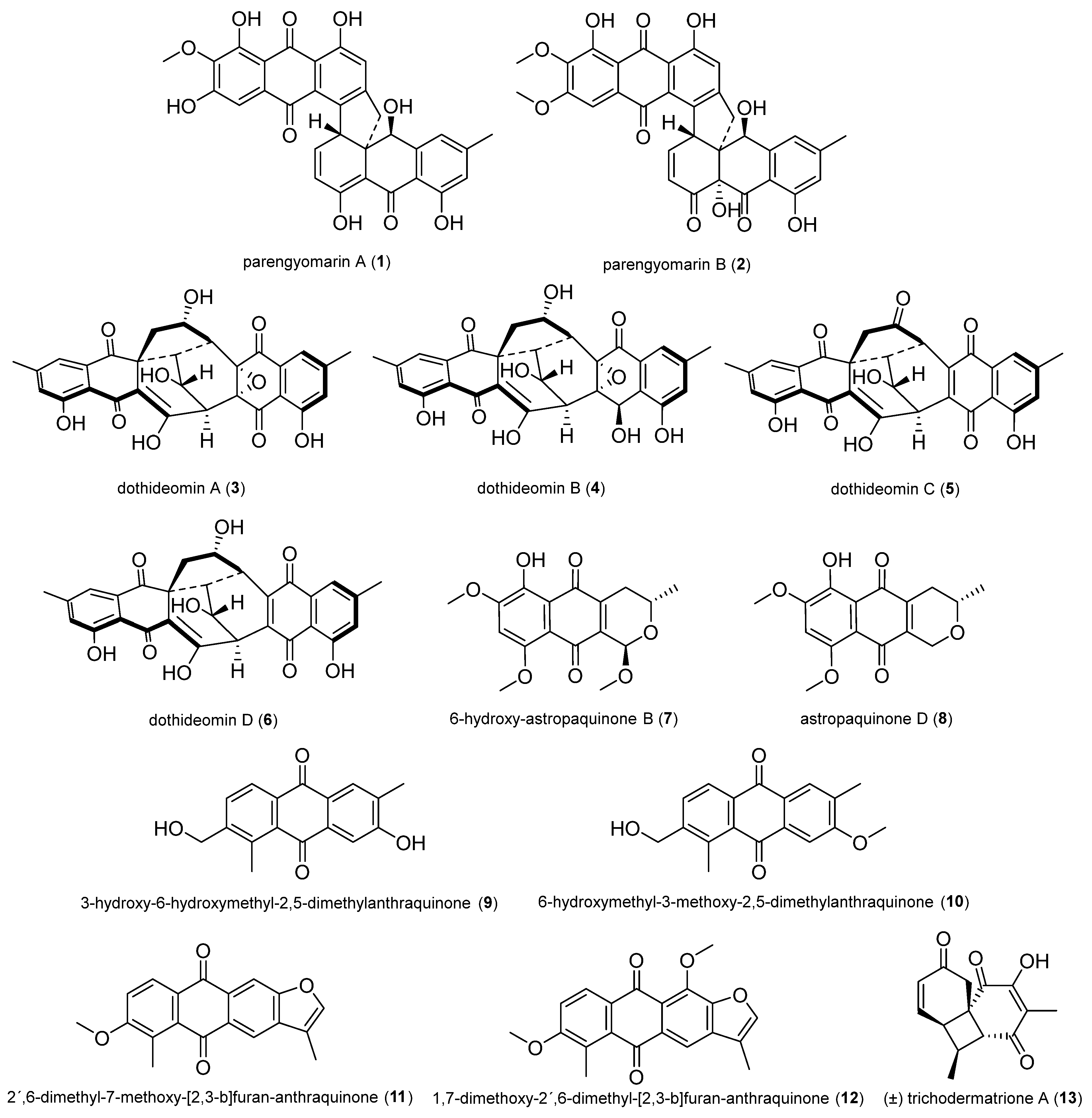
2.1.8. Terpenoids

3. Discussion
4. Materials and Methods
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Whittaker, R.H. New Concepts of Kingdoms or Organisms. Evolutionary Relations Are Better Represented by New Classifications than by the Traditional Two Kingdoms. Science 1969, 163, 150–160. [Google Scholar] [CrossRef] [PubMed]
- Lu, H.; Wei, T.; Lou, H.; Shu, X.; Chen, Q. A Critical Review on Communication Mechanism within Plant-Endophytic Fungi Interactions to Cope with Biotic and Abiotic Stresses. J. Fungi 2021, 7, 719. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, R.J.; White, J.F.; Arnold, A.E.; Redman, R.S. Fungal Endophytes: Diversity and Functional Roles: Tansley Review. New Phytol. 2009, 182, 314–330. [Google Scholar] [CrossRef] [PubMed]
- Arnold, A.E. Understanding the Diversity of Foliar Endophytic Fungi: Progress, Challenges, and Frontiers. Fungal Biol. Rev. 2007, 21, 51–66. [Google Scholar] [CrossRef]
- Abdelaziz, A.M.; Hashem, A.H.; Abd-Elsalam, K.A.; Attia, M.S. Biodiversity of Fungal Endophytes. In Fungal Endophytes Volume I; Springer Nature: Dordrecht, The Netherlands, 2025; pp. 43–61. [Google Scholar] [CrossRef]
- Abd-Elsalam, K.A.; Almoammar, H.; Hashem, A.H.; AbuQamar, S.F. Endophytic Fungi: Exploring Biodiversity and Bioactive Potential. In Fungal Endophytes Volume I; Springer Nature: Dordrecht, The Netherlands, 2025; pp. 1–42. [Google Scholar] [CrossRef]
- Wilson, R.A.; Talbot, N.J. Fungal Physiology—A Future Perspective. Microbiology 2009, 155, 3810–3815. [Google Scholar] [CrossRef]
- Schulz, B.; Boyle, C. The Endophytic Continuum. Mycol. Res. 2005, 109, 661–686. [Google Scholar] [CrossRef]
- Bandara, W.M.M.S.; Seneviratne, G.; Kulasooriya, S.A. Interactions among Endophytic Bacteria and Fungi: Effects and Potentials. J. Biosci. 2006, 31, 645–650. [Google Scholar] [CrossRef]
- Samreen, T.; Naveed, M.; Nazir, M.Z.; Asghar, H.N.; Khan, M.I.; Zahir, Z.A.; Kanwal, S.; Jeevan, B.; Sharma, D.; Meena, V.S.; et al. Seed Associated Bacterial and Fungal Endophytes: Diversity, Life Cycle, Transmission, and Application Potential. Appl. Soil. Ecol. 2021, 168, 104191. [Google Scholar] [CrossRef]
- Sieber, T.N. Endophytic Fungi in Forest Trees: Are They Mutualists? Fungal Biol. Rev. 2007, 21, 75–89. [Google Scholar] [CrossRef]
- Sarkar, S.; Dey, A.; Kumar, V.; Batiha, G.E.S.; El-Esawi, M.A.; Tomczyk, M.; Ray, P. Fungal Endophyte: An Interactive Endosymbiont with the Capability of Modulating Host Physiology in Myriad Ways. Front. Plant Sci. 2021, 12, 701800. [Google Scholar] [CrossRef]
- Waqar, S.; Bhat, A.A.; Khan, A.A. Endophytic Fungi: Unravelling Plant-Endophyte Interaction and the Multifaceted Role of Fungal Endophytes in Stress Amelioration. Plant Physiol. Biochem. 2024, 206, 108174. [Google Scholar] [CrossRef] [PubMed]
- Nath, P.; Kityania, S.; Nath, R.; Nath, D.; Talukdar, A. Das Endophytic Fungi Interactions with Plants. In Fungal Endophytes Volume I; Springer Nature: Dordrecht, The Netherlands, 2025; pp. 63–90. [Google Scholar] [CrossRef]
- Nath, R.; Mitra, J.; Kityania, S.; Nath, R.; Nath, D.; Talukdar, A. Das Foliar Endophytic Fungi: Interactions and Importance. In Fungal Endophytes Volume I; Springer Nature: Dordrecht, The Netherlands, 2025; pp. 119–144. [Google Scholar] [CrossRef]
- Liu, F.; Wang, S.H.; Cheewangkoon, R.; Zhao, R.L. Uneven Distribution of Prokaryote-Derived Horizontal Gene Transfer in Fungi: A Lifestyle-Dependent Phenomenon. mBio 2025, 16, e02855-24. [Google Scholar] [CrossRef] [PubMed]
- Filip, E.; Skuza, L. Horizontal Gene Transfer Involving Chloroplasts. Int. J. Mol. Sci. 2021, 22, 4484. [Google Scholar] [CrossRef]
- Shahzad, R.; Khan, A.L.; Bilal, S.; Asaf, S.; Lee, I.J. What Is There in Seeds? Vertically Transmitted Endophytic Resources for Sustainable Improvement in Plant Growth. Front. Plant Sci. 2018, 9, 00024. [Google Scholar] [CrossRef]
- Tiwari, P.; Bae, H. Horizontal Gene Transfer and Endophytes: An Implication for the Acquisition of Novel Traits. Plants 2020, 9, 305. [Google Scholar] [CrossRef]
- Panaccione, D.G.; Beaulieu, W.T.; Cook, D. Bioactive Alkaloids in Vertically Transmitted Fungal Endophytes. Funct. Ecol. 2014, 28, 299–314. [Google Scholar] [CrossRef]
- Jones, M.; Huynh, T.; Dekiwadia, C.; Daver, F.; John, S. Mycelium Composites: A Review of Engineering Characteristics and Growth Kinetics. J. Bionanosci. 2017, 11, 241–257. [Google Scholar] [CrossRef]
- Jo, C.; Zhang, J.; Tam, J.M.; Church, G.M.; Khalil, A.S.; Segrè, D.; Tang, T.C. Unlocking the Magic in Mycelium: Using Synthetic Biology to Optimize Filamentous Fungi for Biomanufacturing and Sustainability. Mater. Today Bio 2023, 19, 100560. [Google Scholar] [CrossRef]
- Bahram, M.; Netherway, T. Fungi as Mediators Linking Organisms and Ecosystems. FEMS Microbiol. Rev. 2022, 46, fuab058. [Google Scholar] [CrossRef]
- Hillman, E.T.; Readnour, L.R.; Solomon, K.V. Exploiting the Natural Product Potential of Fungi with Integrated—Omics and Synthetic Biology Approaches. Curr. Opin. Syst. Biol. 2017, 5, 50–56. [Google Scholar] [CrossRef]
- Tiwari, P.; Bae, H. Endophytic Fungi: Key Insights, Emerging Prospects, and Challenges in Natural Product Drug Discovery. Microorganisms 2022, 10, 360. [Google Scholar] [CrossRef] [PubMed]
- Hashem, A.H.; Attia, M.S.; Kandil, E.K.; Fawzi, M.M.; Abdelrahman, A.S.; Khader, M.S.; Khodaira, M.A.; Emam, A.E.; Goma, M.A.; Abdelaziz, A.M. Bioactive Compounds and Biomedical Applications of Endophytic Fungi: A Recent Review. Microb. Cell Fact. 2023, 22, 107. [Google Scholar] [CrossRef] [PubMed]
- Sagita, R.; Quax, W.J.; Haslinger, K. Current State and Future Directions of Genetics and Genomics of Endophytic Fungi for Bioprospecting Efforts. Front. Bioeng. Biotechnol. 2021, 9, 649906. [Google Scholar] [CrossRef]
- Choudhary, M.; Gupta, S.; Dhar, M.K.; Kaul, S. Endophytic Fungi-Mediated Biocatalysis and Biotransformations Paving the Way Toward Green Chemistry. Front. Bioeng. Biotechnol. 2021, 9, 664705. [Google Scholar] [CrossRef]
- Prescott, T.A.K.; Hill, R.; Mas-Claret, E.; Gaya, E.; Burns, E. Fungal Drug Discovery for Chronic Disease: History, New Discoveries and New Approaches. Biomolecules 2023, 13, 986. [Google Scholar] [CrossRef]
- Caioni, G.; Reyes, C.P.; Laurenti, D.; Chiaradia, C.; Dainese, E.; Mattioli, R.; Di Risola, D.; Santavicca, E.; Francioso, A. Biochemistry and Future Perspectives of Antibiotic Resistance: An Eye on Active Natural Products. Antibiotics 2024, 13, 1071. [Google Scholar] [CrossRef]
- Moussa, A.Y. Endophytes: A Uniquely Tailored Source of Potential Antibiotic Adjuvants. Arch. Microbiol. 2024, 206, 1–18. [Google Scholar] [CrossRef]
- Ahmed, S.K.; Hussein, S.; Qurbani, K.; Ibrahim, R.H.; Fareeq, A.; Mahmood, K.A.; Mohamed, M.G. Antimicrobial Resistance: Impacts, Challenges, and Future Prospects. J. Med. Surg. Public Health 2024, 2, 100081. [Google Scholar] [CrossRef]
- Shields, R.K. Progress and New Challenges in Combatting the Threat of Antimicrobial Resistance: Perspective from an Infectious Diseases Pharmacist. J. Infect. Dis. 2024, 229, 303–306. [Google Scholar] [CrossRef]
- Nardulli, P.; Ballini, A.; Zamparella, M.; De Vito, D. The Role of Stakeholders’ Understandings in Emerging Antimicrobial Resistance: A One Health Approach. Microorganisms 2023, 11, 2797. [Google Scholar] [CrossRef]
- Ridley, C.P.; Ho, Y.L.; Khosla, C. Evolution of Polyketide Synthases in Bacteria. Proc. Natl. Acad. Sci. USA 2008, 105, 4595–4600. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Mao, Y.; Lu, H.; Zhao, Y.; Bilal, M.; Proksch, P.; Hu, P. Two New Torrubiellin Derivatives from the Mangrove Endophytic Fungus Parengyodontium album. Phytochem. Lett. 2021, 46, 149–152. [Google Scholar] [CrossRef]
- Wang, L.; Zong, S.; Wang, H.; Wu, C.; Wu, G.; Li, F.; Yu, G.; Li, D.; Zhu, M. Dothideomins A-D, Antibacterial Polycyclic Bisanthraquinones from the Endophytic Fungus Dothideomycetes sp. BMC-101. J. Nat. Prod. 2022, 85, 2789–2795. [Google Scholar] [CrossRef]
- Supratman, U.; Hirai, N.; Sato, S.; Watanabe, K.; Malik, A.; Annas, S.; Harneti, D.; Maharani, R.; Koseki, T.; Shiono, Y. New Naphthoquinone Derivatives from Fusarium napiforme of a Mangrove Plant. Nat. Prod. Res. 2021, 35, 1406–1412. [Google Scholar] [CrossRef] [PubMed]
- Wu, F.; Zhu, Y.N.; Hou, Y.T.; Mi, Q.L.; Chen, J.H.; Zhang, C.M.; Miao, D.; Zhou, M.; Wang, W.G.; Hu, Q.F.; et al. Two New Antibacterial Anthraquinones from Cultures of an Endophytic Fungus Phomopsis sp. Chem. Nat. Compd. 2021, 57, 823–827. [Google Scholar] [CrossRef]
- Dai, J.M.; Zhu, L.C.; Xiao, D.; Xie, J.; Wang, X.; Mi, Q.L.; Shi, J.Q.; Yin, G.Y.; Yang, Y.K.; Yang, G.Y.; et al. Two New Anthraquinones from the Cigar Tobacco-Derived Fungus Aspergillus versicolor and Their Bioactivities. Chem. Nat. Compd. 2022, 58, 1001–1005. [Google Scholar] [CrossRef]
- Yan, W.; Ji, W.; Ping, C.; Zhang, T.; Li, Y.; Wang, B.; Chen, T.; He, B.; Ye, Y. (+)- and (−)-Trichodermatrione A: A Pair of Enantiomers with a Cyclobutane-Containing Skeleton from the Endophytic Fungus Trichoderma sp. EFT2. Phytochemistry 2022, 196, 113087. [Google Scholar] [CrossRef]
- Cai, G.; Hu, X.; Zhang, R.; Wang, J.X.; Fang, X.; Pang, X.; Bai, J.; Zhang, T.; Zhang, T.; Lv, H.; et al. Subplenones A-J: Dimeric Xanthones with Antibacterial Activity from the Endophytic Fungus Subplenodomus sp. CPCC 401465. J. Nat. Prod. 2023, 86, 2474–2486. [Google Scholar] [CrossRef]
- Zang, Z.; Yang, W.; Cui, H.; Cai, R.; Li, C.; Zou, G.; Wang, B.; She, Z. Two Antimicrobial Heterodimeric Tetrahydroxanthones with a 7,7′-Linkage from Mangrove Endophytic Fungus Aspergillus flavus QQYZ. Molecules 2022, 27, 2691. [Google Scholar] [CrossRef]
- Jiang, Z.; Wu, P.; Li, H.; Xue, J.; Wei, X. Pestalotinones A–D, New Benzophenone Antibiotics from Endophytic Fungus Pestalotiopsis trachicarpicola SC-J551. J. Antibiot. 2022, 75, 207–212. [Google Scholar] [CrossRef]
- Peng, W.W.; Kuang, M.; Huang, Y.T.; Li, M.F.; Zheng, Y.T.; Xu, L.; Tan, J.B.; Kang, F.H.; Tan, H.B.; Zou, Z.X. Pseudocercones A-C, Three New Polyketide Derivatives from the Endophytic Fungus Pseudocercospora sp. TSS-1. Nat. Prod. Res. 2024, 38, 1248–1255. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.M.; Dai, G.C.; Zhou, B.B.; Chen, Y.; Tan, R.X. Three New Polyketide Derivatives of the Endophytic Fungus Aspergillus cristatus 2H1. J. Asian Nat. Prod. Res. 2022, 24, 722–730. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.T.; Qin, X.X.; Wang, X.; He, J. Pyranones from Kiwi-Associated Fungus Fusarium tricinctum and Their Antibacterial Activity. Phytochem. Lett. 2022, 49, 167–170. [Google Scholar] [CrossRef]
- Chen, Q.; Yu, J.J.; He, J.; Feng, T.; Liu, J.K. Isobenzofuranones and Isocoumarins from Kiwi Endophytic Fungus Paraphaeosphaeria sporulosa and Their Antibacterial Activity against Pseudomonas syringae pv. actinidiae. Phytochemistry 2022, 195, 113050. [Google Scholar] [CrossRef]
- Zhang, W.; Lu, X.; Wang, H.; Chen, Y.; Zhang, J.; Zou, Z.; Tan, H. Antibacterial Secondary Metabolites from the Endophytic Fungus Eutypella scoparia SCBG-8. Tetrahedron Lett. 2021, 79, 153314. [Google Scholar] [CrossRef]
- Pan, X.; Ma, J.; Wang, Z.; Wang, X.; Ma, Z.; Sun, J.; Zhang, Y. Isolation and Identification of an Undescribed Isocoumarin Derivative from Endophytic Fungus Thermothielavioides terrestris YB4. Phytochem. Lett. 2024, 60, 163–166. [Google Scholar] [CrossRef]
- Chen, H.W.; Jiang, C.X.; Li, J.; Li, N.; Zang, Y.; Wu, X.Y.; Chen, W.X.; Xiong, J.; Li, J.; Hu, J.F. Beshanzoides A–D, Unprecedented Cycloheptanone-Containing Polyketides from Penicillium commune P-4-1, an Endophytic Fungus of the Endangered Conifer Abies beshanzuensis. RSC Adv. 2021, 11, 39781–39789. [Google Scholar] [CrossRef]
- Zhao, S.; Wang, B.; Tian, K.; Ji, W.; Zhang, T.; Ping, C.; Yan, W.; Ye, Y. Novel Metabolites from the Cercis Chinensis Derived Endophytic Fungus Alternaria alternata ZHJG5 and Their Antibacterial Activities. Pest. Manag. Sci. 2021, 77, 2264–2271. [Google Scholar] [CrossRef]
- Hu, Y.; Zheng, Y.L.; Zhou, Z.D.; Li, M.; Wang, M.L.; Kong, L.Y.; Yang, M.H. Aromatic Polyketide Aspergillones A-D from the Endophytic Fungus Aspergillus sclerotiorum. Phytochem. Lett. 2023, 55, 131–136. [Google Scholar] [CrossRef]
- Qin, Y.; Liu, X.; Lin, J.; Huang, J.; Jiang, X.; Mo, T.; Xu, Z.; Li, J.; Yang, R. Two New Phthalide Derivatives from the Endophytic Fungus Penicillium vulpinum Isolated from Sophora tonkinensis. Nat. Prod. Res. 2021, 35, 421–427. [Google Scholar] [CrossRef]
- Song, H.C.; Qin, D.; Liu, H.Y.; Dong, J.Y.; You, C.; Wang, Y.M. Resorcylic Acid Lactones Produced by an Endophytic Penicillium ochrochloron Strain from Kadsura angustifolia. Planta Med. 2021, 87, 225–235. [Google Scholar] [CrossRef] [PubMed]
- Shamim, A.H.M.; Mondol, M.A.M.; Hossain, M.; Shovo, T.I.; Uddin, M.; Nur-e-Alam, M.; Alam, I.; Alharbi, H.A.; Rahman, A.F.M.M. New Antibacterial Penicimenolide G with Unusual 12-Membered Resorcylic Acid Lactone Ring Isolated from Endophytic Fungus Aspergillus giganteus. Phytochem. Lett. 2024, 62, 18–23. [Google Scholar] [CrossRef]
- Sadorn, K.; Saepua, S.; Bunbamrung, N.; Boonyuen, N.; Komwijit, S.; Rachtawee, P.; Pittayakhajonwut, P. Diphenyl Ethers and Depsidones from the Endophytic Fungus Aspergillus unguis BCC54176. Tetrahedron 2022, 105, 132612. [Google Scholar] [CrossRef]
- Wang, M.L.; Chen, R.; Sun, F.J.; Cao, P.R.; Chen, X.R.; Yang, M.H. Three Alkaloids and One Polyketide from Aspergillus cristatus Harbored in Pinellia ternate Tubers. Tetrahedron Lett. 2021, 68, 152914. [Google Scholar] [CrossRef]
- Promgool, T.; Kanokmedhakul, K.; Leewijit, T.; Song, J.; Soytong, K.; Yahuafai, J.; Kudera, T.; Kokoska, L.; Kanokmedhakul, S. Cytotoxic and Antibacterial Depsidones from the Endophytic Fungus Chaetomium brasiliense Isolated from Thai Rice. Nat. Prod. Res. 2022, 36, 4599–4607. [Google Scholar] [CrossRef]
- Gu, G.; Hou, X.; Xue, M.; Pan, X.; Dong, J.; Yang, Y.; Amuzu, P.; Xu, D.; Lai, D.; Zhou, L. Diphenyl Ethers from Endophytic Fungus Rhexocercosporidium sp. Dzf14 and Their Antibacterial Activity by Affecting Homeostasis of Cell Membranes. Pest. Manag. Sci. 2024, 80, 2658–2667. [Google Scholar] [CrossRef]
- Gu, G.; Gong, X.; Xu, D.; Yang, Y.; Yin, R.; Dai, J.; Zhu, K.; Lai, D.; Zhou, L. Diphenyl Ether Derivative Rhexocerins and Rhexocercosporins from the Endophytic Fungus Rhexocercosporidium sp. Dzf14 Active against Gram-Positive Bacteria with Multidrug-Resistance. J. Nat. Prod. 2023, 86, 1931–1938. [Google Scholar] [CrossRef]
- Zhu, S.; Xu, T.C.; Huang, R.; Gao, Y.; Wu, S.H. Four New Polyketides from an Endophytic Fungus Talaromyces muroii. Fitoterapia 2024, 177, 106073. [Google Scholar] [CrossRef]
- Zhu, J.J.; Huang, Q.S.; Liu, S.Q.; Ding, W.J.; Xiong, Y.H.; LI, C.Y. Four New Diphenyl Ether Derivatives from a Mangrove Endophytic Fungus Epicoccum sorghinum. Chin. J. Nat. Med. 2022, 20, 537–540. [Google Scholar] [CrossRef]
- Xu, Z.; Feng, T.; Wen, Z.; Li, Q.; Chen, B.; Liu, P.; Xu, J. New Naphthalene Derivatives from the Mangrove Endophytic Fungus Daldinia eschscholzii MCZ-18. Mar. Drugs 2024, 22, 242. [Google Scholar] [CrossRef]
- Chen, H.W.; Jiang, C.X.; Ma, G.L.; Wu, X.Y.; Jiang, W.; Li, J.; Zang, Y.; Li, J.; Xiong, J.; Hu, J.F. Unprecedented Spirodioxynaphthalenes from the Endophytic Fungus Phyllosticta ligustricola HDF-L-2 Derived from the Endangered Conifer Pseudotsuga gaussenii. Phytochemistry 2023, 211, 113687. [Google Scholar] [CrossRef] [PubMed]
- Towett, K.E.; Joyce, J.K.; Josphat, M. New Naphthalene Derivative Isolated from Diaporthe sp. Host to Syzygium cordatum Hochst.Ex Krauss Plant. J. Med. Plants Res. 2021, 15, 196–205. [Google Scholar] [CrossRef]
- Li, X.M.; Mi, Q.L.; Gao, Q.; Li, J.; Song, C.M.; Zeng, W.L.; Xiang, H.Y.; Liu, X.; Chen, J.H.; Zhang, C.M.; et al. Antibacterial Naphthalene Derivatives from the Fermentation Products of the Endophytic Fungus Phomopsis fukushii. Chem. Nat. Compd. 2021, 57, 293–296. [Google Scholar] [CrossRef]
- Liao, L.X.; Huang, Z.D.; Wei, F.T.; Wang, W.J.; Yang, X.L. New Chromone Analog and Pyrrole Alkaloid Produced by Penicillium sclerotiorum and Their Antibacterial Activity. J. Asian Nat. Prod. Res. 2023, 25, 225–230. [Google Scholar] [CrossRef]
- Wang, J.T.; Li, H.Y.; Rao, R.; Yue, J.Y.; Wang, G.K.; Yu, Y. (±)-Stagonosporopsin A, Stagonosporopsin B and Stagonosporopsin C, Antibacterial Metabolites Produced by Endophytic Fungus Stagonosporopsis oculihominis. Phytochem. Lett. 2021, 45, 157–160. [Google Scholar] [CrossRef]
- Lai, D.; Li, J.; Zhao, S.; Gu, G.; Gong, X.; Proksch, P.; Zhou, L. Chromone and Isocoumarin Derivatives from the Endophytic Fungus Xylomelasma sp. Samif07, and Their Antibacterial and Antioxidant Activities. Nat. Prod. Res. 2021, 35, 4616–4620. [Google Scholar] [CrossRef]
- Abbas, Z.; Siddiqui, B.S.; Shahzad, S.; Sattar, S.; Begum, S.; Batool, A.; Choudhary, M.I. Lawsozaheer, a New Chromone Produced by an Endophytic Fungus Paecilomyces variotii Isolated from Lawsonia alba Lam. Inhibits the Growth of Staphylococcus aureus. Nat. Prod. Res. 2021, 35, 4448–4453. [Google Scholar] [CrossRef]
- Wang, Y.L.; Hu, B.Y.; Qian, M.A.; Wang, Z.H.; Zou, J.M.; Sang, X.Y.; Li, L.; Luo, X.D.; Zhao, L.X. Koninginin W, a New Polyketide from the Endophytic Fungus Trichoderma koningiopsis YIM PH30002. Chem. Biodivers. 2021, 18, e2100460. [Google Scholar] [CrossRef]
- Liu, W.Y.; Zhang, P.; Su, Y.L.; Li, W.; Song, X.R.; Gong, D.P.; Kong, G.H.; Wang, W.G.; Li, Y.K.; Hu, Q.F.; et al. Bioactivity Isochromenes from Cigar Tobacco-Derived Endophytic Fungus Aspergillus fumigatus. Chem. Nat. Compd. 2024, 60, 435–439. [Google Scholar] [CrossRef]
- Dong, F.; Jiang, Z.; Wu, P.; Duan, F.; Xue, J.; Tan, H.; Wei, X. Bioactive Ambuic Acid Congeners from Endophytic Fungus Pestalotiopsis trachicarpicola SC-J551. J. Antibiot. 2024, 77, 21–29. [Google Scholar] [CrossRef]
- Zhu, L.; Wang, J.P.; Hao, F.; Gan, D.; Zhang, X.R.; Li, C.Z.; Wang, C.Y.; Zhang, L.; Cai, L. A New Cyclopentenone Derivative from Trichoderma atroviride HH-01. Nat. Prod. Res. 2023, 37, 1205–1211. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.-R.; Li, K.-X.; Chen, H.-W.; He, Y.-H.; Wang, H.-Y.; MAO, Y.; Li, J.; Hu, J.-F.; Xiong, J. Bioactive Polyketides and Tryptophol Alkaloids from the Endophytic Fungus Botryosphaeria dothidea Le-07 of Chinese Tulip Tree. Fitoterapia 2024, 179, 106229. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; He, J.; Liu, X.; Hong, B.; Yu, M.; Niu, S. Penioctadecatrienoic A: A New Polyketide from Endophytic Fungus Penicillium pinophilum J70. Rec. Nat. Prod. 2023, 17, 742. [Google Scholar] [CrossRef]
- Zhao, W.T.; Shi, X.; Xian, P.J.; Feng, Z.; Yang, J.; Yang, X.L. A New Fusicoccane Diterpene and a New Polyene from the Plant Endophytic Fungus Talaromyces pinophilus and Their Antimicrobial Activities. Nat. Prod. Res. 2021, 35, 124–130. [Google Scholar] [CrossRef]
- Chang, S.; Li, Y.; Huang, X.; He, N.; Wang, M.; Wang, J.; Luo, M.; Li, Y.; Xie, Y. Bioactivity-Based Molecular Networking-Guided Isolation of Epicolidines A-C from the Endophytic Fungus Epicoccum sp. 1-042. J. Nat. Prod. 2024, 87, 1582–1590. [Google Scholar] [CrossRef]
- Wang, D.; Wang, H.; Chen, X.; Xu, Y.; He, W.; Wu, D.; Zuo, M.; Zhu, W.; Wang, L. Five Previously Undescribed Citrinin Derivatives from the Endophytic Fungus Penicillium citrinum GZWMJZ-836. Phytochemistry 2024, 220, 114032. [Google Scholar] [CrossRef]
- Zhou, J.T.; Wu, Q.; Zhao, J.X.; Wu, L.L.; He, X.H.; Liang, L.Q.; Zhang, G.H.; Li, J.; Xu, W.F.; Yang, R.Y. Sucurchalasins A and B, Sulfur-Containing Heterodimers of a Cytochalasan and a Macrolide from the Endophytic Fungus Aspergillus spelaeus GDGJ-286. J. Nat. Prod. 2024, 87, 2327–2334. [Google Scholar] [CrossRef]
- Huang, X.; Zhou, D.; Liang, Y.; Liu, X.; Cao, F.; Qin, Y.; Mo, T.; Xu, Z.; Li, J.; Yang, R. Cytochalasins from Endophytic Diaporthe sp. GDG-118. Nat. Prod. Res. 2021, 35, 3396–3403. [Google Scholar] [CrossRef]
- Xu, Z.L.; Li, B.C.; Huang, L.L.; Lv, L.X.; Luo, Y.; Xu, W.F.; Yang, R.Y. Two New Cytochalasins from the Endophytic Fungus Xylaria sp. GDGJ-77B. Nat. Prod. Res. 2024, 38, 1503–1509. [Google Scholar] [CrossRef]
- Tan, Q.; Ye, X.; Fu, S.; Yin, Y.; Liu, Y.; Wu, J.; Cao, F.; Wang, B.; Zhu, T.; Yang, W.; et al. The Cytochalasins and Polyketides from a Mangrove Endophytic Fungus Xylaria arbuscula QYF. Mar. Drugs 2024, 22, 407. [Google Scholar] [CrossRef]
- Huang, G.; Lin, W.; Li, H.; Tang, Q.; Hu, Z.; Huang, H.; Deng, X.; Xu, Q. Pentacyclic Cytochalasins and Their Derivatives from the Endophytic Fungus Phomopsis sp. Xz-18. Molecules 2021, 26, 6505. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, G.A.; Ibrahim, S.R.M.; Alhakamy, N.A.; Aljohani, O.S. Fusaroxazin, a Novel Cytotoxic and Antimicrobial Xanthone Derivative from Fusarium oxysporum. Nat. Prod. Res. 2022, 36, 952–960. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.S.; Qiu, W.Y.; Lin, Z.L.; Wu, Y.P.; Li, W.; Gong, D.P.; Dong, M.; Wang, W.G.; Li, Y.K.; Yao, H.; et al. Two New Isoquinolines from Cigar Tobacco-Derived Endophytic Fungi Aspergillus puniceus and Their Antibacterial Activity. Chem. Nat. Compd. 2023, 59, 1142–1146. [Google Scholar] [CrossRef]
- Yang, W.; Yuan, J.; Tan, Q.; Chen, Y.; Zhu, Y.; Jiang, H.; Zou, G.; Zang, Z.; Wang, B.; She, Z. Peniazaphilones A—I, Produced by Co-Culturing of Mangrove Endophytic Fungi, Penicillium sclerotiorum THSH-4 and Penicillium sclerotiorum ZJHJJ-18. Chin. J. Chem. 2021, 39, 3404–3412. [Google Scholar] [CrossRef]
- Li, M.; Xiao, D.; Zhu, L.C.; Liu, L.; Zheng, J.N.; Gu, X.J.; Zhu, Y.N.; Xie, J.; Wang, X.; Dai, J.M.; et al. Indole Alkaloids from the Cigar Tobacco-Derived Endophytic Fungus Aspergillus oryzae and Their Antibacterial Activity. Chem. Nat. Compd. 2022, 58, 1093–1097. [Google Scholar] [CrossRef]
- Ma, J.T.; Du, J.X.; Zhang, Y.; Liu, J.K.; Feng, T.; He, J. Natural Imidazole Alkaloids as Antibacterial Agents against Pseudomonas syringae pv. actinidiae Isolated from Kiwi Endophytic Fungus Fusarium tricinctum. Fitoterapia 2022, 156, 105070. [Google Scholar] [CrossRef]
- Zhu, Y.X.; Peng, C.; Ding, W.; Hu, J.F.; Li, J. Chromenopyridin A, a New N-Methoxy-1-Pyridone Alkaloid from the Endophytic Fungus Penicillium nothofagi P-6 Isolated from the Critically Endangered Conifer Abies beshanzuensis. Nat. Prod. Res. 2022, 36, 2049–2055. [Google Scholar] [CrossRef]
- Ruan, W.; Ma, Q.Y.; Guo, J.C.; Xie, Q.Y.; Yang, L.; Dai, H.F.; Wu, Y.G.; Zhao, Y.X. A New Cyclic Peptide from Betel Nut Endophytic Fungus Alternaria sp. RW-AL. Chem. Nat. Compd. 2024, 60, 693–696. [Google Scholar] [CrossRef]
- Khanam, S.; Mishra, P.; Faruqui, T.; Alam, P.; Albalawi, T.; Siddiqui, F.; Rafi, Z.; Khan, S. Plant-Based Secondary Metabolites as Natural Remedies: A Comprehensive Review on Terpenes and Their Therapeutic Applications. Front. Pharmacol. 2025, 16, 1587215. [Google Scholar] [CrossRef]
- Qin, Y.Y.; Huang, X.S.; Liu, X.B.; Mo, T.X.; Xu, Z.L.; Li, B.C.; Qin, X.Y.; Li, J.; Schӓberle, T.F.; Yang, R.Y. Three New Andrastin Derivatives from the Endophytic Fungus Penicillium vulpinum. Nat. Prod. Res. 2022, 36, 3262–3270. [Google Scholar] [CrossRef]
- Zhang, Z.B.; Du, S.Y.; Ji, B.; Ji, C.J.; Xiao, Y.W.; Yan, R.M.; Zhu, D. New Helvolic Acid Derivatives with Antibacterial Activities from Sarocladium oryzae DX-THL3, an Endophytic Fungus from Dongxiang Wild Rice (Oryza rufipogon Griff.). Molecules 2021, 26, 1828. [Google Scholar] [CrossRef] [PubMed]
- Chen, N.; Chen, S.W.; Qiu, S.Y.; Lu, S.M.; Wei, J.R.; Yang, F.W.; Geng, H.C.; Zhou, M. Ganodermasides E-H, Four New Ergosterol Derivatives from the Endophytic Fungus Epicoccum poae DJ-F Associated with Euphorbia royleana. Fitoterapia 2023, 168, 105562. [Google Scholar] [CrossRef] [PubMed]
- ZHAO, S.; JING, Z. New Pimarane Diterpenoids with Antibacterial Activity from Fungus Arthrinium sp. ZS03. Chin. J. Nat. Med. 2024, 22, 356–364. [Google Scholar] [CrossRef]
- Intaraudom, C.; Bunbamrung, N.; Dramae, A.; Boonyuen, N.; Srichomthong, K.; Pittayakhajonwut, P. Antimicrobial Properties of Unusual Eremophilanes from the Endophytic Diaporthe sp. BCC69512. Phytochemistry 2024, 222, 114078. [Google Scholar] [CrossRef]
- Yu, J.J.; Wei, W.K.; Zhang, Y.; Cox, R.J.; He, J.; Liu, J.K.; Feng, T. Terpenoids from Kiwi Endophytic Fungus Bipolaris Sp. and Their Antibacterial Activity against Pseudomonas syringae pv. actinidiae. Front. Chem. 2022, 10, 990734. [Google Scholar] [CrossRef]
- Elnaggar, M.S.; Mostafa, N.M.; Elissawy, A.M.; Phutthacharoen, K.; Eckhardt, P.; Sandargo, B.; van Geelen, L.; Ebada, S.S.; Opatz, T.; Singab, A.N.B.; et al. Acremochlorin S and Other Prenylated Chlorophenol Antimicrobial Metabolites from the Fungus Acremonium sp. Strain MNA-F-1. Fitoterapia 2024, 179, 106254. [Google Scholar] [CrossRef]
- Huang, X.; Li, D.; Long, B.; Li, H.; Li, J.; Wang, W.; Xu, K.; Yu, X. Activation of a Silent Gene Cluster from the Endophytic Fungus Talaromyces sp. Unearths Cryptic Azaphilone Metabolites. J. Agric. Food Chem. 2024, 72, 15801–15810. [Google Scholar] [CrossRef]
- Zhang, W.; Lu, X.; Huo, L.; Zhang, S.; Chen, Y.; Zou, Z.; Tan, H. Sesquiterpenes and Steroids from an Endophytic Eutypella scoparia. J. Nat. Prod. 2021, 84, 1715–1724. [Google Scholar] [CrossRef]
- Gan, D.; Wang, C.Y.; Li, C.Z.; Zhu, L.; Zhang, X.R.; Ding, H.; Cai, L.; Ding, Z.T. Secondary Metabolites from Annulohypoxylon sp. and Structural Revision of Emericellins A and B. J. Nat. Prod. 2022, 85, 828–837. [Google Scholar] [CrossRef]
- Li-Bin, L.; Xiao, J.; Zhang, Q.; Han, R.; Xu, B.; Yang, S.X.; Han, W.B.; Tang, J.J.; Gao, J.M. Eremophilane Sesquiterpenoids with Antibacterial and Anti-Inflammatory Activities from the Endophytic Fungus Septoria rudbeckiae. J. Agric. Food Chem. 2021, 69, 11878–11889. [Google Scholar] [CrossRef]
- Morehouse, N.J.; Clark, T.N.; Kerr, R.G.; Johnson, J.A.; Gray, C.A. Caryophyllene Sesquiterpenes from a Chaetomium globosum Endophyte of the Canadian Medicinal Plant Empetrum nigrum. J. Nat. Prod. 2023, 86, 1615–1619. [Google Scholar] [CrossRef] [PubMed]
- Ho, C.S.; Wong, C.T.H.; Aung, T.T.; Lakshminarayanan, R.; Mehta, J.S.; Rauz, S.; McNally, A.; Kintses, B.; Peacock, S.J.; de la Fuente-Nunez, C.; et al. Antimicrobial Resistance: A Concise Update. Lancet Microbe 2024, 6, 100947. [Google Scholar] [CrossRef] [PubMed]
- Eshboev, F.; Mamadalieva, N.; Nazarov, P.A.; Hussain, H.; Katanaev, V.; Egamberdieva, D.; Azimova, S. Antimicrobial Action Mechanisms of Natural Compounds Isolated from Endophytic Microorganisms. Antibiotics 2024, 13, 271. [Google Scholar] [CrossRef] [PubMed]
- Mouton, J.W.; Brown, D.F.J.; Apfalter, P.; Cantón, R.; Giske, C.G.; Ivanova, M.; MacGowan, A.P.; Rodloff, A.; Soussy, C.J.; Steinbakk, M.; et al. The Role of Pharmacokinetics/Pharmacodynamics in Setting Clinical MIC Breakpoints: The EUCAST Approach. Clin. Microbiol. Infect. 2012, 18, E37–E45. [Google Scholar] [CrossRef]
- Clatworthy, A.E.; Pierson, E.; Hung, D.T. Targeting Virulence: A New Paradigm for Antimicrobial Therapy. Nat. Chem. Biol. 2007, 3, 541–548. [Google Scholar] [CrossRef]
- Dimasi, J.A.; Feldman, L.; Seckler, A.; Wilson, A. Trends in Risks Associated with New Drug Development: Success Rates for Investigational Drugs. Clin. Pharmacol. Ther. 2010, 87, 272–277. [Google Scholar] [CrossRef]

| Compound | Antimicrobial Activity (μg/mL) * | Positive Control (μg/mL) * |
|---|---|---|
| parengyomarin A (1) | S. aureus 0.39 * MRSA 0.39 * | moxifloxacin 0.78 * moxifloxacin 6.25 * |
| dothideomin A (3) | B. subtilis 0.4 S. aureus 0.4 | chloramphenicol 0.3–1.5 |
| dothideomin C (5) | B. subtilis 0.4 S. aureus 0.4 | chloramphenicol 0.3–1.5 |
| dothideomin D (6) | S. aureus 1.6 | chloramphenicol 0.3–1.5 |
| subplenone A (14) | ATCC 700698 MRSA 0.25 vancomicin-resistant E. faecalis 2.0 | levofloxacin 0.125 levofloxacin 1.0 |
| subplenone E (15) | ATCC 700698 MRSA 0.25 vancomicin-resistant E. faecalis 2.0 | levofloxacin 0.125 levofloxacin 1.0 |
| pestalotinone A (19) | MRSA 2.5 | kanamycin 1.25 |
| mollicellin V (45) | ATCC 25923 MRSA 64 | oxacillin 32–128 |
| mollicellin W (46) | B. subtilis 4.0 ATCC 25923 32 | kanamycin 2.0 oxacillin 32–128 |
| rhexocerin (50) | B. subtilis 64 | streptomycin sulphate 32 |
| rhexocercosporin E (55) | MRSA T144 4.0 | vancomycin 2.0 |
| epicoccether K (58) | E. coli serotype 06 25 | cefradine 12.5 |
| 4-(5,7-dimethoxy-4-oxo-4H-chromen-2-yl)butanoic acid methyl ester (72) | carbapenem-resistant P. aeruginosa 3.13 | ciprofloxacin 0.78 |
| koninginin W (76) | E. coli 128 | ampicillin 128–256 |
| pseudocercone C (77) | S. aureus 3.9 | amikacin 4.0 |
| botryrhamnoside A (82) | E. coli 8.0 | chloramphenicol 2.0 |
| botryrhamnoside B (83) | E. coli 8.0 | chloramphenicol 2.0 |
| xylariacinol D (130) | E. coli 2.0 | kanamycin 1.0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ortega, H.E.; Torres-Mendoza, D.; Cubilla-Rios, L. Antibacterial Compounds Isolated from Endophytic Fungi Reported from 2021 to 2024. Antibiotics 2025, 14, 644. https://doi.org/10.3390/antibiotics14070644
Ortega HE, Torres-Mendoza D, Cubilla-Rios L. Antibacterial Compounds Isolated from Endophytic Fungi Reported from 2021 to 2024. Antibiotics. 2025; 14(7):644. https://doi.org/10.3390/antibiotics14070644
Chicago/Turabian StyleOrtega, Humberto E., Daniel Torres-Mendoza, and Luis Cubilla-Rios. 2025. "Antibacterial Compounds Isolated from Endophytic Fungi Reported from 2021 to 2024" Antibiotics 14, no. 7: 644. https://doi.org/10.3390/antibiotics14070644
APA StyleOrtega, H. E., Torres-Mendoza, D., & Cubilla-Rios, L. (2025). Antibacterial Compounds Isolated from Endophytic Fungi Reported from 2021 to 2024. Antibiotics, 14(7), 644. https://doi.org/10.3390/antibiotics14070644








