Modulation of Antimicrobial Resistance in Listeria monocytogenes via Synergistic Interactions Between Thymbra capitata L. (Cav.) Essential Oil and Conventional Antibiotics
Abstract
1. Introduction
2. Results
2.1. Chemical Volatile Composition
2.2. Antimicrobial Activity of T. capitata L. (Cav.) Essential Oil and Antibiotic Compounds Based on Minimum Inhibitory and Bactericidal Concentrations
2.3. Determination of Interactions Between TEO and Antibiotic Compounds
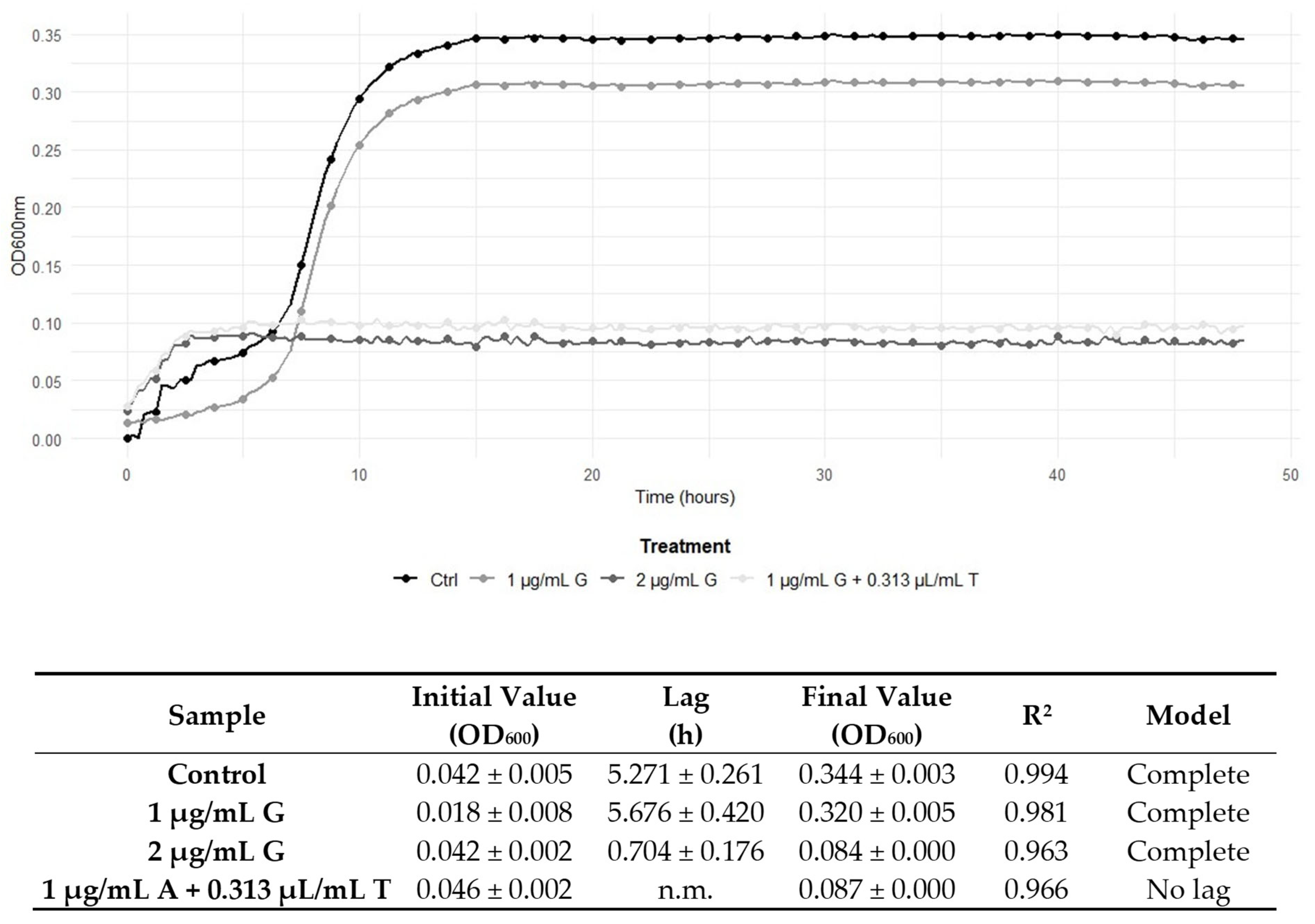
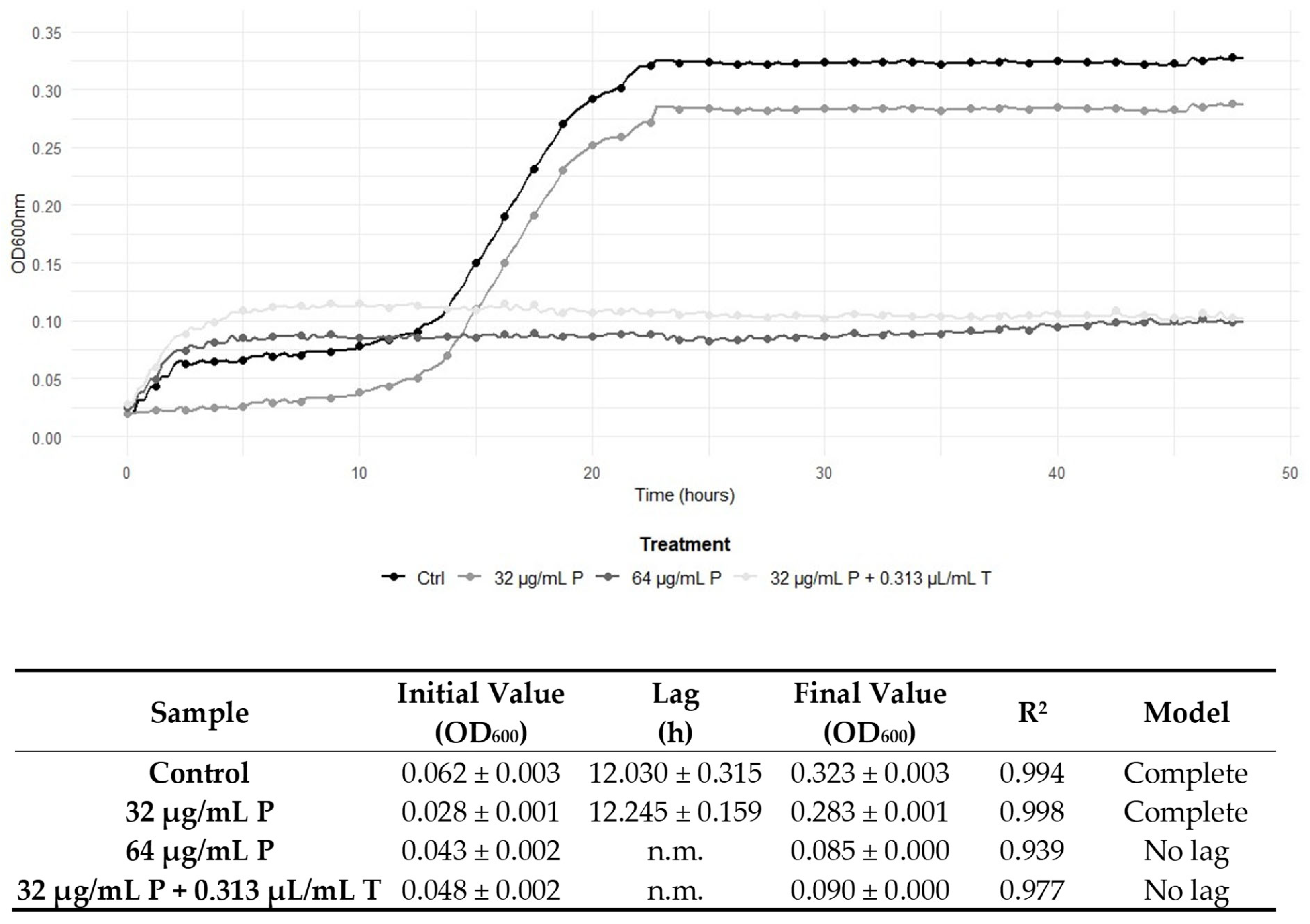
2.4. Determination of the Effect of the Pre-Exposure to a Sub-Inhibitory Concentration of TEO on Antibiotic Compounds’ Effectiveness
2.5. Graphical Representation of the Interactions Between TEO and Conventional Antibiotic Treatments
3. Discussion
4. Materials and Methods
4.1. Antimicrobic Compounds
4.2. Gas Chromatography-Based Analysis
4.3. Bacterial Strains and Culture Conditions
4.4. Determination of Minimum Inhibitory Concentrations and Minimum Bactericidal Concentrations
4.5. Determination of Synergy Between TEO and Antibiotic Compounds
4.6. Determination of the Effect of Sub-Lethal Concentrations of TEO Pre-Exposure on Antibiotic Compounds Treatments
4.7. Data Analysis
5. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ahmed, S.K.; Hussein, S.; Qurbani, K.; Ibrahim, R.H.; Fareeq, A.; Mahmood, K.A.; Mohamed, M.G. Antimicrobial resistance: Impacts, challenges, and future prospects. J. Med. Surg. Public Health 2024, 2, 100081. [Google Scholar] [CrossRef]
- GBD 2021 Antimicrobial Resistance Collaborators. Global burden of bacterial antimicrobial resistance 1990–2021: A systematic analysis with forecasts to 2050. Lancet 2024, 28, 1199–1226. [Google Scholar]
- Vranakis, I.; Goniotakis, I.; Psaroulaki, A.; Sandalakis, V.; Tselentis, Y.; Gevaert, K.; Tsiotis, G. Proteome studies of bacterial antibiotic resistance mechanisms. J. Proteom. 2013, 97, 88–99. [Google Scholar] [CrossRef]
- Moura, A.; Leclercq, A.; Vales, G.; Tessaud-Rita, N.; Bracq-Dieye, H.; Thouvenot, P.; Madec, Y.; Charlier, C.; Lecuit, M. Phenotypic and genotypic antimicrobial resistance of Listeria monocytogenes: An observational study in France. Lancet Reg. Health Eur. 2024, 37, 100800. [Google Scholar] [CrossRef] [PubMed]
- D’Onofrio, F.; Butler, F.; Krasteva, I.; Schirone, M.; Iannetti, L.; Torresi, M.; Di Pancrazio, C.; Perletta, F.; Maggetti, M.; Marcacci, M.; et al. Integrative analysis of transcriptomic and immunoproteomic data reveals stress response mechanisms in Listeria monocytogenes. Heliyon 2024, 10, e39832. [Google Scholar] [CrossRef] [PubMed]
- EFSA and ECDC (European Food Safety Authority and European Centre for Disease Prevention and Control). The European Union One Health 2023 Zoonoses report. EFSA J. 2024, 22, e9106. [Google Scholar]
- Luque-Sastre, L.; Arroyo, C.; Fox, E.M.; McMahon, B.J.; Bai, L.I.; Li, F.; Fanning, S. Antimicrobial Resistance in Listeria Species. Microbiol. Spectr. 2018, 6. [Google Scholar] [CrossRef]
- Pagliano, P.; Arslan, F.; Ascione, T. Epidemiology and treatment of the commonest form of listeriosis: Meningitis and bacteraemia. Infez. Med. 2017, 3, 210–216. [Google Scholar]
- Baquero, F.F.; Lanza, V.; Duval, M.; Coque, T.M. Ecogenetics of antibiotic resistance in Listeria monocytogenes. Mol. Microbiol. 2020, 113, 570–579. [Google Scholar] [CrossRef]
- Rao, J.; Chen, B.; McClements, D.J. Improving the efficacy of essential oils as antimicrobials in foods: Mechanisms of Action. Annu. Rev. Food Sci. Technol. 2019, 10, 365–387. [Google Scholar] [CrossRef]
- Buccioni, F.; Purgatorio, C.; Maggio, F.; Garzoli, S.; Rossi, C.; Valbonetti, L.; Paparella, A.; Serio, A. Unraveling the Antimicrobial Effectiveness of Coridothymus capitatus Hydrolate against Listeria monocytogenes in Environmental Conditions Encountered in Foods: An In Vitro Study. Microorganisms 2022, 10, 920. [Google Scholar] [CrossRef] [PubMed]
- Maggio, F.; Rossi, C.; Chaves-López, C.; Valbonetti, L.; Desideri, G.; Paparella, A.; Serio, A. A single exposure to a sublethal concentration of Origanum vulgare essential oil initiates response against food stressors and restoration of antibiotic susceptibility in Listeria monocytogenes. Food Control 2022, 132, 108562. [Google Scholar] [CrossRef]
- Bhattacharya, R.; Rolta, R.; Dev, K.; Sourirajan, A. Synergistic potential of essential oils with antibiotics to combat fungal pathogens: Present status and future perspectives. Phytother Res. 2021, 35, 6089–6100. [Google Scholar] [CrossRef] [PubMed]
- Fokas, R.; Giormezis, N.; Vantarakis, A. Synergistic Approaches to Foodborne Pathogen Control: A Narrative Review of Essential Oils and Bacteriophages. Foods 2025, 14, 1508. [Google Scholar] [CrossRef]
- Drioiche, A.; Baammi, S.; Zibouh, K.; Al Kamaly, O.; Alnakhli, A.M.; Remok, F.; Saidi, S.; Amaiach, R.; El Makhoukhi, F.; Elomri, A.; et al. A Study of the Synergistic Effects of Essential Oils from Origanum compactum and Origanum elongatum with Commercial Antibiotics against Highly Prioritized Multidrug-Resistant Bacteria for the World Health Organization. Metabolites 2024, 14, 210. [Google Scholar] [CrossRef]
- Neagu, R.; Popovici, V.; Ionescu, L.-E.; Ordeanu, V.; Biță, A.; Popescu, D.M.; Ozon, E.A.; Gîrd, C.E. Phytochemical Screening and Antibacterial Activity of Commercially Available Essential Oils Combinations with Conventional Antibiotics against Gram-Positive and Gram-Negative Bacteria. Antibiotics 2024, 13, 478. [Google Scholar] [CrossRef] [PubMed]
- Gan, C.; Langa, E.; Wang, G.; Van Bambeke, F.; Ballestero, D.; Pino-Otín, M.R. Mechanisms of action and resistance prevention of synergistic thymol and carvacrol combinations with antibiotics in Staphylococcus aureus and Acinetobacter baumannii. Nat. Prod. Bioprospect. 2025, 15, 36. [Google Scholar] [CrossRef]
- Gómez, N.C.; Manetsberger, J.; Lerma, L.L.; Martos, J.M.M.; Benomar, N.; Abriouel, H. Exploring synergistic effects of essential oils compounds with antibiotics and biocides against multidrug-resistant foodborne pathogens. Appl. Food Res. 2024, 4, 100581. [Google Scholar] [CrossRef]
- Al-Tawalbeh, D.; Alkhawaldeh, Y.; Sawan, H.M.; Al-Mamoori, F.; Al-Samydai, A.; Mayyas, A. Assessment of carvacrol-antibiotic combinations’ antimicrobial activity against methicillin-resistant Staphylococcus aureus. Front. Microbiol. 2024, 14, 1349550. [Google Scholar] [CrossRef]
- Noll, M.; Kleta, S.; Al Dahouk, S. Antibiotic susceptibility of 259 Listeria monocytogenes strains isolated from food, food-processing plants and human samples in Germany. J. Infect. Public Health 2018, 11, 572–577. [Google Scholar] [CrossRef]
- European Committee on Antimicrobial Susceptibility Testing. Clinical Breakpoints and Dosing of Antibiotics; EUCAST: Växjö, Sweden, 2024. Available online: https://www.eucast.org/clinical_breakpoints (accessed on 1 May 2025).
- Fatsis-Kavalopoulos, N.; Sánchez-Hevia, D.L.; Andernsson, D.I. Beyond the FIC index: The extended information from fractional inhibitory concentrations (FICs). J. Antimicrob. Chemoter. 2024, 79, 2394–2396. [Google Scholar] [CrossRef] [PubMed]
- Odds, F.C. Synergy, antagonism, and what the checkerboard puts between them. J. Antimicrob. Chemoter. 2003, 52, 1. [Google Scholar] [CrossRef]
- Fratini, F.; Mancini, S.; Turchi, B.; Friscia, E.; Pistelli, L.; Giusti, G.; Cerri, D. A novel interpretation of the fractional inhibitory concentration index: The case Origanum vulgare L. and Leptospermum scoparium J. R. et G. Forst essential oils against Staphylococcus aureus strains. Microbiol. Res. 2017, 195, 11–17. [Google Scholar] [CrossRef]
- Palma, E.; Tilocca, B.; Roncada, P. Antimicrobial resistance in veterinary medicine: An overview. Int. J. Mol. Sci. 2020, 21, 1914. [Google Scholar] [CrossRef] [PubMed]
- Ho, C.S.; Wong, C.T.H.; Aung, T.T.; Lakshminarayanan, R.; Mehta, J.S.; Rauz, S.; McNally, A.; Kintses, B.; Peacock, S.J.; de la Fuente-Nunez, C.; et al. Antimicrobial resistance: A concise update. Lancet Microbe. 2025, 6, 100947. [Google Scholar] [CrossRef] [PubMed]
- Rippa, A.; Bilei, S.; Peruzy, M.F.; Marrocco, M.G.; Leggeri, P.; Bossù, T.; Murru, N. Antimicrobial Resistance of Listeria monocytogenes Strains Isolated in Food and Food-Processing Environments in Italy. Antibiotics 2024, 13, 525. [Google Scholar] [CrossRef]
- Żurawik, A.; Kasperski, T.; Olechowska-Jarząb, A.; Szczesiul-Paszkiewicz, P.; Żak, I.; Wójcicki, M.; Maćkiw, E.; Chmielarczyk, A. Genetic Diversity, Virulence Factors and Antibiotic Resistance of Listeria monocytogenes from Food and Clinical Samples in Southern Poland. Pathogens 2024, 13, 725. [Google Scholar] [CrossRef]
- Maggio, F.; Lauteri, C.; Rossi, C.; Ferri, G.; Serio, A.; Vergara, A.; Paparella, A. Combined effect of Tetracycline compounds and essential oils on antimicrobial resistant Salmonella enterica isolated from the swine food chain. Front Microbiol. 2024, 15, 1439286. [Google Scholar] [CrossRef]
- Soulaimani, B. Comprehensive Review of the Combined Antimicrobial Activity of Essential Oil Mixtures and Synergism with Conventional Antimicrobials. Nat. Prod. Commun. 2025, 20, 1. [Google Scholar] [CrossRef]
- Yang, S.K.; Yusoff, K.; Mai, C.W.; Lim, W.M.; Lim, S.H.E.; Asmahani, A.; Lai, K.S. Mode of action of cinnamon bark (Cinnamomum verum) essential oil and the combinatory bactericidal activity with meropenem against KPC-producing Klebsiella pneumoniae. Asian J. Med. Biomed. 2018, 9, 1–7. [Google Scholar]
- Serio, A.; Chiarini, M.; Tettamanti, E.; Paparella, A. Electronic paramagnetic resonance investigation of the activity of Origanum vulgare L. essential oil on the Listeria monocytogenes membrane. Lett. Appl. Microbiol. 2010, 51, 149–157. [Google Scholar] [CrossRef] [PubMed]
- Raikwar, G.; Kumar, D.; Mohan, S.; Dahiya, P. Synergistic potential of essential oils with antibiotics for antimicrobial resistance with emphasis on mechanism of action: A review. Biocatal. Agric. Biotechnol. 2024, 61, 103384. [Google Scholar] [CrossRef]
- Moo, C.L.; Yang, S.K.; Osman, M.A.; Yuswan, M.H.; Loh, J.Y.; Lim, W.M.; Lim, S.H.; Lai, K.S. Antibacterial Activity and Mode of Action of β-caryophyllene on Bacillus cereus. Pol. J. Microbiol. 2020, 69, 49–54. [Google Scholar] [CrossRef] [PubMed]
- Marchese, A.; Arciola, C.R.; Barbieri, R.; Silva, A.S.; Nabavi, S.F.; Tsetegho Sokeng, A.J.; Izadi Jafari, N.J.; Suntar, I.; Daglia, M.; Nabavi, S.M. Update on Monoterpenes as Antimicrobial Agents: A Particular Focus on p-Cymene. Materials 2017, 10, 947. [Google Scholar] [CrossRef]
- D’Amato, S.; Rossi, C.; Maggio, F.; Valbonetti, L.; Savini, V.; Paparella, A.; Serio, A. Antilisterial Effectiveness of Origanum vulgare var. hirtum and Coridothymus capitatus Essential Oils and Hydrolates Alone and in Combination. Foods 2024, 13, 860. [Google Scholar] [CrossRef]
- Ng, H.; Ng May Ling, J.; Fong, N. Mechanisms of Gentamicin Resistance in Listeria monocytogenes: A Mini Review (Mekanisme Rintangan Gentamisin dalam Listeria monocytogenes: Suatu Kajian Mini). Sains Malaysiana 2023, 52, 2625–2632. [Google Scholar] [CrossRef]
- Zhi, Z.; Zhou, P.; He, T.; Chen, S.; Qian, X.; Ye, Y.; Wong, W.L.; Li, S.; Sun, N.; Yuan, W. Study of the antimicrobial activity of carvacrol and its mechanism of action against drug-resistant bacteria. Biochem. Biophys. Res. Commun. 2025, 757, 151643. [Google Scholar] [CrossRef]
- Sharma, K.; Gulerioa, S.; Razdan, V.K.; Babu, V. Synergistic antioxidant and antimicrobial activities of essential oils of some selected medicinal plants in combination and with synthetic compounds. Ind. Crops Prod. 2020, 154, 112569. [Google Scholar] [CrossRef]
- Oliveira Ribeiro, S.; Fontaine, V.; Mathieu, V.; Zhiri, A.; Baudoux, D.; Stévigny, C.; Souard, F. Antibacterial and Cytotoxic Activities of Ten Commercially Available Essential Oils. Antibiotics 2020, 9, 717. [Google Scholar] [CrossRef]
- CLSI. Performance Standards for Antimicrobial Susceptibility Testing, 30th ed.; CLSI supplement M100S; CLSI: Malvern, PA, USA, 2020. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2023. [Google Scholar]
- RStudio Team. RStudio: Integrated Development Environment for R; Posit Software; PBC: Boston, MA, USA, 2025. [Google Scholar]
- Wickham, H.; Bryan, J. R package, version 1.4.4. readxl: Read Excel Files. R Foundation for Statistical Computing: Vienna, Austria, 2025.
- Wickham, H.; François, R.; Henry, L.; Müller, K.; Vaughan, D. R package, version 1.1.2. dplyr: A Grammar of Data Manipulation. R Foundation for Statistical Computing: Vienna, Austria, 2023.
- Wickham, H. ggplot2: Elegant Graphics for Data Analysis; Springer: New York, NY, USA, 2016. [Google Scholar]
- Baranyi, J.; Roberts, T.A. A dynamic approach to predicting bacterial growth in food. Int. J. Food Microbiol. 1994, 12, 277–294. [Google Scholar] [CrossRef]


| N° | Components 1 | LRI 2 | LRI 3 | % |
|---|---|---|---|---|
| 1 | α-pinene | 925 | 928 | 1.2 ± 0.06 |
| 2 | camphene | 958 | 961 | 0.4 ± 0.02 |
| 3 | β-thujene | 971 | 968 | 1.8 ± 0.07 |
| 4 | sabinene | 975 | 972 | 0.7 ± 0.03 |
| 5 | β-pinene | 982 | 980 | 0.1 ± 0.01 |
| 6 | β-myrcene | 993 | 992 | 2.4 ± 0.04 |
| 7 | α-phellandrene | 995 | 998 | 0.6 ± 0.03 |
| 8 | 3-carene | 1012 | 1014 | 0.2 ± 0.02 |
| 9 | p-cymene | 1020 | 1016 | 7.7 ± 0.10 |
| 10 | α-terpinene | 1015 | 1019 | 2.6 ± 0.07 |
| 11 | limonene | 1025 | 1022 | 0.6 ± 0.03 |
| 12 | 1,8-cineole | 1036 | 1033 | 0.1 ± 0.01 |
| 13 | cis-β-ocimene | 1041 | 1037 | 0.1 ± 0.02 |
| 14 | γ-terpinene | 1071 | 1065 | 7.6 ± 0.11 |
| 15 | p-cymenene | 1086 | 1083 | 0.1 ± 0.01 |
| 16 | terpinolene | 1088 | 1085 | 0.4 ± 0.02 |
| 17 | linalool | 1096 | 1095 | 2.4 ± 0.05 |
| 18 | terpinen-4-ol | 1158 | 1161 | 2.3 ± 0.08 |
| 19 | (E)-dihydrocarvone | 1210 | 1207 | 0.3 ± 0.02 |
| 20 | neral | 1218 | 1220 | 0.1 ± 0.01 |
| 21 | carvacrol | 1282 | 1278 | 55.4 ± 6.02 |
| 22 | β-caryophyllene | 1422 | 1424 | 8.6 ± 0.15 |
| 23 | aromadendrene | 1465 | 1460 | 0.1 ± 0.01 |
| 24 | humulene | 1471 | 1473 | 0.3 ± 0.02 |
| 25 | β-bisabolene | 1498 | 1495 | 1.5 ± 0.08 |
| 26 | δ-cadinene | 1517 | 1515 | 0.1 ± 0.02 |
| 27 | trans-α-bisabolene | 1541 | 1536 | 1.2 ± 0.05 |
| 28 | caryophyllene oxide | 1579 | 1580 | 1.0 ± 0.06 |
| 29 | isoaromadendrene epoxide | 1591 | 1594 | 0.1 ± 0.02 |
| SUM | 100.0 | |||
| Monoterpenes | 87.1 | |||
| Sesquiterpenes | 12.9 | |||
| Others |
| A | G | P | T | A/T | G/T | P/T | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| MIC | MBC | MIC | MBC | MIC | MBC | MIC | MBC | MIC | MBC | MIC | MBC | MIC | MBC | |
| 48 h | 48 h | 48 h | 48 h | 48 h | 48 h | 48 h | 48 h | 48 h | 48 h | 48 h | 48 h | 48 h | 48 h | |
| L. m. ATCC 7644 | 0.5 | 0.5 | 0.5 | 0.5 | 0.5 | 0.5 | 0.63 | 0.63 | 0.5–0.63 | 0.5–0.63 | 0.5–0.31 | 0.5–0.31 | 0.5–0.31 | 0.5–0.31 |
| L. m. 6 | 2 | 2 | 2 | 2 | 64 | 64 | 2.5 | 2.5 | 2−0.63 | 2−0.63 | 2−0.31 | 2−0.31 | 32−0.31 | 32−0.31 |
| L. m. 17 | 8 | 8 | 2 | 2 | 2 | 2 | 1.25 | 1.25 | 8−0.31 | 8−0.31 | 2−0.31 | 2−0.31 | 2−0.31 | 2−0.31 |
| L. m. 120 | 2 | 2 | 2 | 2 | 2 | 2 | 1.25 | 1.25 | 1−0.31 | 1−0.31 | 1−0.31 | 1−0.31 | 2−0.63 | 2−0.63 |
| L. m. 229 | 8 | 8 | 2 | 2 | 4 | 4 | 0.63 | 0.63 | 1−0.63 | 1−0.63 | 1−0.31 | 1−0.31 | 1−0.31 | 1−0.31 |
| A/T | G/T | P/T | A/T | G/T | P/T | |
|---|---|---|---|---|---|---|
| FICI | FICI | FICI | Effect | Effect | Effect | |
| 48 h | 48 h | 48 h | 48 h | 48 h | 48 h | |
| L. monocytogenes ATCC 7644 | 2 | 1.5 | 1.5 | IndE | IndE | IndE |
| L. monocytogenes 6 | 1.25 | 1.13 | 0.63 | IndE | IndE | SynE |
| L. monocytogenes 17 | 1.25 | 1.25 | 1.25 | IndE | IndE | IndE |
| L. monocytogenes 120 | 0.75 | 0.75 | 1.5 | SynE | SynE | IndE |
| L. monocytogenes 229 | 1.12 | 1 | 1 | IndE | AddE | AddE |
| T | A | G | P | ||||
|---|---|---|---|---|---|---|---|
| MIC/2 | MIC | MBC | MIC | MBC | MIC | MBC | |
| 48 h | 48 h | 48 h | 48 h | 48 h | 48 h | 48 h | |
| L. monocytogenes ATCC 7644 | 0.31 | 0.5 | 0.5 | 0.5 | 0.5 | 0.5 | 0.5 |
| L. monocytogenes 6 | 1.25 | 2 | 2 | 2 | 2 | 2 | 2 |
| L. monocytogenes 17 | 0.63 | 1 | 1 | 4 | 4 | 2 | 2 |
| L. monocytogenes 120 | 0.63 | 1 | 1 | 1 | 1 | 1 | 1 |
| L. monocytogenes 229 | 0.31 | 1 | 1 | 1 | 1 | 1 | 1 |
| Species | Strain Code | Origin |
|---|---|---|
| L. monocytogenes | ATCC 7644 | Type strain |
| L. monocytogenes | 6 | Pork ribs |
| L. monocytogenes | 17 | Fermented pork meat-based product |
| L. monocytogenes | 120 | Clinical isolation from humans |
| L. monocytogenes | 229 | Clinical isolation from humans |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maggio, F.; Buccioni, F.; Garzoli, S.; Paparella, A.; Serio, A. Modulation of Antimicrobial Resistance in Listeria monocytogenes via Synergistic Interactions Between Thymbra capitata L. (Cav.) Essential Oil and Conventional Antibiotics. Antibiotics 2025, 14, 623. https://doi.org/10.3390/antibiotics14060623
Maggio F, Buccioni F, Garzoli S, Paparella A, Serio A. Modulation of Antimicrobial Resistance in Listeria monocytogenes via Synergistic Interactions Between Thymbra capitata L. (Cav.) Essential Oil and Conventional Antibiotics. Antibiotics. 2025; 14(6):623. https://doi.org/10.3390/antibiotics14060623
Chicago/Turabian StyleMaggio, Francesca, Francesco Buccioni, Stefania Garzoli, Antonello Paparella, and Annalisa Serio. 2025. "Modulation of Antimicrobial Resistance in Listeria monocytogenes via Synergistic Interactions Between Thymbra capitata L. (Cav.) Essential Oil and Conventional Antibiotics" Antibiotics 14, no. 6: 623. https://doi.org/10.3390/antibiotics14060623
APA StyleMaggio, F., Buccioni, F., Garzoli, S., Paparella, A., & Serio, A. (2025). Modulation of Antimicrobial Resistance in Listeria monocytogenes via Synergistic Interactions Between Thymbra capitata L. (Cav.) Essential Oil and Conventional Antibiotics. Antibiotics, 14(6), 623. https://doi.org/10.3390/antibiotics14060623










