Antimicrobials in Livestock Farming and Resistance: Public Health Implications
Abstract
1. Introduction
1.1. Antibiotics and Antibiotic Resistance
1.2. The Silent Pandemic
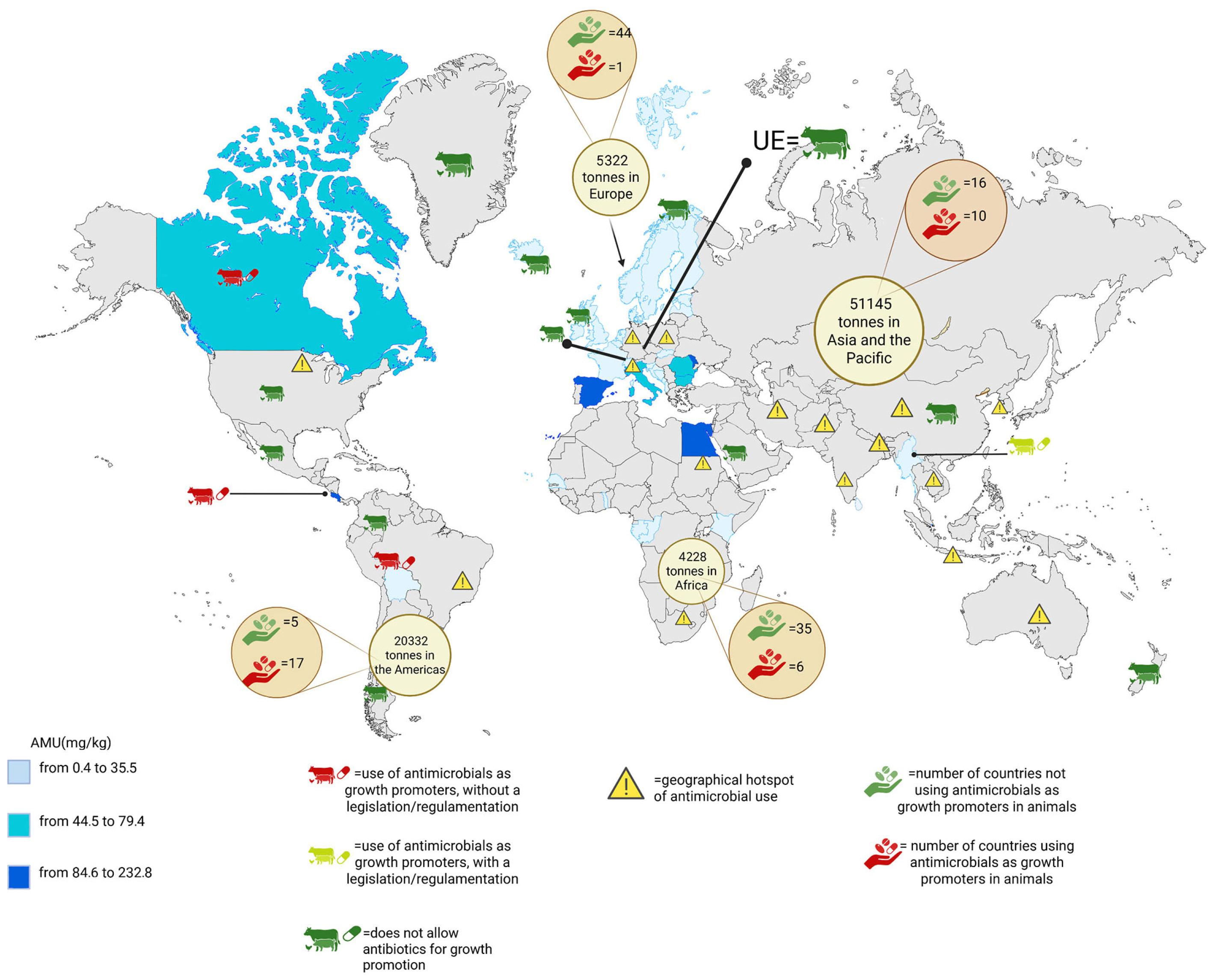
1.3. Global Antimicrobials Use in Livestock Farming
2. The Use of Antibiotics in the Veterinary Sector and in the Livestock Industry
3. The Key Reasons for Using Antibiotics in the Field of Animal Husbandry
3.1. Therapeutic Use
3.2. Antimicrobials for Metaphylactic and Prophylactic Use
3.3. Antimicrobials as Growth Promoters
3.4. Coordinate Action Against AMR: Global Action Plan
4. How Do Antibiotics in Livestock Impact Antibiotic-Resistant Human Infections?
5. Mechanisms of Antibiotic Resistance
5.1. Transmission Resistance
5.2. Role of the Livestock Farms in the Spread of Resistances
6. Policies and Strategies for Tackling Antimicrobial Resistance from Livestock to Humans
- -
- Asia: eastern China, southern India, Indonesia, central Thailand, the eastern coastline of Vietnam, western South Korea, eastern India and Bangladesh, Pakistan, and north-west Iran;
- -
- Europe: northern Italy, northern Germany, and central Poland;
- -
- The Americas: south of Brazil and the Midwest of the USA;
- -
- Africa: Nile delta and peri-urban areas of Johannesburg.
Alternative Strategies to Tackle Antimicrobial Resistance
- -
- The implementation of controlled access measures for people, vehicles, and animals that may potentially carry pathogens;
- -
- The maintenance of adequate farm fencing to prevent contact with stray and wild animals;
- -
- The adherence to stringent hygiene standards, including regular handwashing, boot disinfection, and thorough cleaning of equipment, to minimise the spread of pathogens within farm environments. Effective sanitation protocols are essential for disinfecting surfaces, tools, and equipment to minimise the risk of disease transmission (Figure 3). The regular monitoring of animal health and behaviour allows for the early identification of potential illnesses, enabling timely interventions to prevent infection [135]. Maintaining good ventilation and not overcrowding animals are important strategies to improve air quality and support animal health. Regular monitoring allows farmers to quickly report any issues to the relevant authorities. This helps to contain outbreaks. Surveillance and research are vital for guiding stakeholders on how to slow the spread of antimicrobial resistance. They provide reliable data on antimicrobial-resistant microorganisms, antimicrobial use, and the presence of antimicrobial residues in food and feed [8]. Meat slaughter plants are another vulnerable sector. A study of a chicken processing plant revealed that biofilms can diminish the efficacy of cleaning procedures involving various chemicals. Furthermore, while urban plants are subject to regulatory standards for pathogens in wastewater, in certain countries, restrictions on the treatment of waste from production animal farms are either ineffective or have only recently been prohibited (i.e., in China). Whilst storage and composting are effective methods for separating solid waste from liquid waste, they do not reduce pathogens or pollutants. Calcium oxide stabilises solid waste before using it as fertiliser. The composition of wastewater renders conventional treatment methods ineffective, as evidenced by the frequent presence of elevated concentrations of antibiotics in post-treated European samples. Recent advancements in wastewater treatment technologies have been demonstrated by the adoption of adsorption methods utilising wheat straw, which have achieved a 90–98% removal of ciprofloxacin [136]. A range of alternative techniques is also being reviewed, including membrane separation, microbial electrolysis, photocatalytic degradation, and advanced oxidation. However, the implementation of these methods requires efficiency per cycle, safety during use, storage, and transportation, and the treatment of secondary polluting products, which would lead to increased costs [136]. In recent years, there has been an increasing focus among researchers on the development of new strategies for tackling antibiotic resistance in animal husbandry. The objectives of these strategies are twofold: firstly, to improve animal health, and, secondly, to reduce the reliance on conventional antibiotics. These strategies include the use of peptides and nanoparticles with antimicrobial activity [137]. Antimicrobial peptides (AMPs) are a class of small molecules composed of fewer than 100 amino acid residues, which are naturally produced by most living organisms. These peptides have demonstrated broad-spectrum antimicrobial activity against bacteria, fungi, and protozoa. The mechanisms through which they exert their effects are diverse, targeting both intracellular and extracellular components, thereby strengthening the host’s immune defences against pathogens [138]. Research has shown that AMPs are effective at preventing infections. They have also been found to enhance the effectiveness of antibiotics, reducing the development of drug resistance [139]. In some cases, dietary supplementation with AMPs has also been observed to promote animal growth. For example, microcin J25 (MccJ25), a peptide produced by E. coli and found in animal faeces, has been shown to positively influence animal growth [140]. Another innovative and promising technology under investigation involves the synthesis of nanoparticles (NPs) [141]. The synthesis of NPs can be achieved through a variety of methodologies, including physical, chemical, or green synthesis methods. The classification of these particles can be categorised into two broad categories: organic and inorganic. The organic category includes liposomes, polymeric, and lipid nanoparticles, while the inorganic group includes quantum dots and metal/metal oxide nanoparticles. These can be used as animal feed additives, as disinfectants, or as vaccine adjuvants to boost immunity. Moreover, they can be utilised as standalone antimicrobial agents [142]. Several studies have demonstrated that NPs improve antimicrobial activity against antibiotic-resistant bacteria and inhibit biofilm formation [143]. However, although various nanoparticles are currently being used as feed preservatives, growth stimulants, and antibiotic replacements, important issues such as bioaccumulation in the food chain remain to be addressed [144].
7. Conclusions and Future Directions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mancuso, G.; De Gaetano, S.; Midiri, A.; Zummo, S.; Biondo, C. The Challenge of Overcoming Antibiotic Resistance in Carbapenem-Resistant Gram-Negative Bacteria: “Attack on Titan”. Microorganisms 2023, 11, 1912. [Google Scholar] [CrossRef]
- Adedeji, W.A. The Treasure Called Antibiotics. Ann. Ib. Postgrad. Med. 2016, 14, 56–57. [Google Scholar]
- Muteeb, G.; Rehman, M.T.; Shahwan, M.; Aatif, M. Origin of Antibiotics and Antibiotic Resistance, and Their Impacts on Drug Development: A Narrative Review. Pharmaceuticals 2023, 16, 1615. [Google Scholar] [CrossRef] [PubMed]
- Selvarajan, R.; Obize, C.; Sibanda, T.; Abia, A.L.K.; Long, H. Evolution and Emergence of Antibiotic Resistance in Given Ecosystems: Possible Strategies for Addressing the Challenge of Antibiotic Resistance. Antibiotics 2022, 12, 28. [Google Scholar] [CrossRef]
- Larsson, D.G.J.; Flach, C.F. Antibiotic resistance in the environment. Nat. Rev. Microbiol. 2022, 20, 257–269. [Google Scholar] [CrossRef]
- Biondo, C. Bacterial Antibiotic Resistance: The Most Critical Pathogens. Pathogens 2023, 12, 116. [Google Scholar] [CrossRef] [PubMed]
- Wisniewski, P.; Trymers, M.; Chajecka-Wierzchowska, W.; Tkacz, K.; Zadernowska, A.; Modzelewska-Kapitula, M. Antimicrobial Resistance in the Context of Animal Production and Meat Products in Poland-A Critical Review and Future Perspective. Pathogens 2024, 13, 1123. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, M.; Antunes, W.; Mota, S.; Madureira-Carvalho, A.; Dinis-Oliveira, R.J.; Dias da Silva, D. An Overview of the Recent Advances in Antimicrobial Resistance. Microorganisms 2024, 12, 1920. [Google Scholar] [CrossRef]
- Antimicrobial Resistance, C. Global burden of bacterial antimicrobial resistance in 2019: A systematic analysis. Lancet 2022, 399, 629–655. [Google Scholar] [CrossRef]
- Salam, M.A.; Al-Amin, M.Y.; Salam, M.T.; Pawar, J.S.; Akhter, N.; Rabaan, A.A.; Alqumber, M.A.A. Antimicrobial Resistance: A Growing Serious Threat for Global Public Health. Healthcare 2023, 11, 1946. [Google Scholar] [CrossRef]
- Ponzo, E.; De Gaetano, S.; Midiri, A.; Mancuso, G.; Giovanna, P.; Giuliana, D.; Zummo, S.; Biondo, C. The Antimicrobial Resistance Pandemic Is Here: Implementation Challenges and the Need for the One Health Approach. Hygiene 2024, 4, 297–316. [Google Scholar] [CrossRef]
- Sirota, M. Should we stop referring to the pandemic of antimicrobial resistance as silent? JAC-Antimicrob. Resist. 2024, 6, dlae018. [Google Scholar] [CrossRef]
- Porse, A.; Jahn, L.J.; Ellabaan, M.M.H.; Sommer, M.O.A. Dominant resistance and negative epistasis can limit the co-selection of de novo resistance mutations and antibiotic resistance genes. Nat. Commun. 2020, 11, 1199. [Google Scholar] [CrossRef]
- Ahmed, S.K.; Hussein, S.; Qurbani, K.; Ibrahim, R.H.; Fareeq, A.; Mahmood, K.A.; Mohamed, M.G. Antimicrobial resistance: Impacts, challenges, and future prospects. J. Med. Surg. Public Health 2024, 2, 100081. [Google Scholar] [CrossRef]
- Bengtsson-Palme, J.; Kristiansson, E.; Larsson, D.G.J. Environmental factors influencing the development and spread of antibiotic resistance. FEMS Microbiol. Rev. 2018, 42, fux053. [Google Scholar] [CrossRef] [PubMed]
- Iwu, C.D.; Korsten, L.; Okoh, A.I. The incidence of antibiotic resistance within and beyond the agricultural ecosystem: A concern for public health. Microbiologyopen 2020, 9, e1035. [Google Scholar] [CrossRef] [PubMed]
- Aslam, B.; Khurshid, M.; Arshad, M.I.; Muzammil, S.; Rasool, M.; Yasmeen, N.; Shah, T.; Chaudhry, T.H.; Rasool, M.H.; Shahid, A.; et al. Antibiotic Resistance: One Health One World Outlook. Front. Cell. Infect. Microbiol. 2021, 11, 771510. [Google Scholar] [CrossRef]
- Barathe, P.; Kaur, K.; Reddy, S.; Shriram, V.; Kumar, V. Antibiotic pollution and associated antimicrobial resistance in the environment. J. Hazard. Mater. Lett. 2024, 5, 100105. [Google Scholar] [CrossRef]
- Sambaza, S.S.; Naicker, N. Contribution of wastewater to antimicrobial resistance: A review article. J. Glob. Antimicrob. Resist. 2023, 34, 23–29. [Google Scholar] [CrossRef]
- Zhang, T.; Nickerson, R.; Zhang, W.; Peng, X.; Shang, Y.; Zhou, Y.; Luo, Q.; Wen, G.; Cheng, Z. The impacts of animal agriculture on One Health-Bacterial zoonosis, antimicrobial resistance, and beyond. One Health 2024, 18, 100748. [Google Scholar] [CrossRef]
- Mancuso, G.; Midiri, A.; Gerace, E.; Biondo, C. Bacterial Antibiotic Resistance: The Most Critical Pathogens. Pathogens 2021, 10, 1310. [Google Scholar] [CrossRef] [PubMed]
- Vercelli, C.; Gambino, G.; Amadori, M.; Re, G. Implications of Veterinary Medicine in the comprehension and stewardship of antimicrobial resistance phenomenon. From the origin till nowadays. Vet. Anim. Sci. 2022, 16, 100249. [Google Scholar] [CrossRef] [PubMed]
- Caneschi, A.; Bardhi, A.; Barbarossa, A.; Zaghini, A. The Use of Antibiotics and Antimicrobial Resistance in Veterinary Medicine, a Complex Phenomenon: A Narrative Review. Antibiotics 2023, 12, 487. [Google Scholar] [CrossRef] [PubMed]
- Teuber, M. Veterinary use and antibiotic resistance. Curr. Opin. Microbiol. 2001, 4, 493–499. [Google Scholar] [CrossRef] [PubMed]
- Shang, K.; Kim, J.H.; Park, J.Y.; Choi, Y.R.; Kim, S.W.; Cha, S.Y.; Jang, H.K.; Wei, B.; Kang, M. Comparative Studies of Antimicrobial Resistance in Escherichia coli, Salmonella, and Campylobacter Isolates from Broiler Chickens with and without Use of Enrofloxacin. Foods 2023, 12, 2239. [Google Scholar] [CrossRef]
- Verraes, C.; Van Boxstael, S.; Van Meervenne, E.; Van Coillie, E.; Butaye, P.; Catry, B.; de Schaetzen, M.A.; Van Huffel, X.; Imberechts, H.; Dierick, K.; et al. Antimicrobial resistance in the food chain: A review. Int. J. Environ. Res. Public Health 2013, 10, 2643–2669. [Google Scholar] [CrossRef]
- VT Nair, D.; Venkitanarayanan, K.; Kollanoor Johny, A. Antibiotic-Resistant Salmonella in the Food Supply and the Potential Role of Antibiotic Alternatives for Control. Foods 2018, 7, 167. [Google Scholar] [CrossRef]
- Tran-Dien, A.; Le Hello, S.; Bouchier, C.; Weill, F.X. Early transmissible ampicillin resistance in zoonotic Salmonella enterica serotype Typhimurium in the late 1950s: A retrospective, whole-genome sequencing study. Lancet Infect. Dis. 2018, 18, 207–214. [Google Scholar] [CrossRef]
- Schmerold, I.; van Geijlswijk, I.; Gehring, R. European regulations on the use of antibiotics in veterinary medicine. Eur. J. Pharm. Sci. Off. J. Eur. Fed. Pharm. Sci. 2023, 189, 106473. [Google Scholar] [CrossRef]
- Moulin, G.; Cavalie, P.; Pellanne, I.; Chevance, A.; Laval, A.; Millemann, Y.; Colin, P.; Chauvin, C.; Antimicrobial Resistance ad hoc Group of The French Agency for Food. A comparison of antimicrobial usage in human and veterinary medicine in France from 1999 to 2005. J. Antimicrob. Chemother. 2008, 62, 617–625. [Google Scholar] [CrossRef]
- Granados-Chinchilla, F.; Rodriguez, C. Tetracyclines in Food and Feedingstuffs: From Regulation to Analytical Methods, Bacterial Resistance, and Environmental and Health Implications. J. Anal. Methods Chem. 2017, 2017, 1315497. [Google Scholar] [CrossRef] [PubMed]
- Gray, P.; Jenner, R.; Norris, J.; Page, S.; Browning, G.; Australian Veterinary Association, L.; Animal Medicines, A. Antimicrobial prescribing guidelines for poultry. Aust. Vet. J. 2021, 99, 181–235. [Google Scholar] [CrossRef]
- Sani, A.A.; Rafiq, K.; Akter, F.; Islam, P.; Sachi, S.; Sultana, N.; Hayat, S.; Usman, U.B.; Islam, M.S.; Islam, M.Z.; et al. Effect of knowledge of informal poultry drug prescribers on their attitude and practice toward antimicrobial use, residues, and resistance in Bangladesh. Vet. World 2023, 16, 1821–1828. [Google Scholar] [CrossRef]
- Hofacre, C.L.; Fricke, J.A.; Inglis, T. Antimicrobial Drug Use in Poultry. In Antimicrobial Therapy in Veterinary Medicine; Giguère, S., Prescott, J.F., Dowling, P.M., Eds.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2013. [Google Scholar] [CrossRef]
- Constable, P.D.; Hinchcliff, K.W.; Done, S.H.; Grünberg, W. Veterinary Medicine. A Textbook of the Diseases of Cattle, Horses, Sheep, Pigs, and Goats; Elsevier Health Sciences: St Louis, MO, USA, 2017. [Google Scholar]
- Daniel, U.; Thomson, B.J.W. Veterinary Clinics of North America: Food Animal Practice; Elsevier: New York, NY, USA, 2015; Volume 31. [Google Scholar]
- Cutler, R.; Gleeson, B.; Page, S.; Norris, J.; Browning, G.; Australian Veterinary Association, L.; Animal Medicines, A. Antimicrobial prescribing guidelines for pigs. Aust. Vet. J. 2020, 98, 105–134. [Google Scholar] [CrossRef]
- Suojala, L.; Kaartinen, L.; Pyorala, S. Treatment for bovine Escherichia coli mastitis—An evidence-based approach. J. Vet. Pharmacol. Ther. 2013, 36, 521–531. [Google Scholar] [CrossRef] [PubMed]
- Springer, A.; Glass, A.; Topp, A.K.; Strube, C. Zoonotic Tick-Borne Pathogens in Temperate and Cold Regions of Europe-A Review on the Prevalence in Domestic Animals. Front. Vet. Sci. 2020, 7, 604910. [Google Scholar] [CrossRef]
- Butaye, P.; Devriese, L.A.; Haesebrouck, F. Antimicrobial growth promoters used in animal feed: Effects of less well known antibiotics on gram-positive bacteria. Clin. Microbiol. Rev. 2003, 16, 175–188. [Google Scholar] [CrossRef] [PubMed]
- Simjee, S.; Ippolito, G. European regulations on prevention use of antimicrobials from january 2022. Braz. J. Vet. Med. 2022, 44, e000822. [Google Scholar] [CrossRef]
- Yan, H.; Yu, B.; Degroote, J.; Spranghers, T.; Van Noten, N.; Majdeddin, M.; Van Poucke, M.; Peelman, L.; De Vrieze, J.; Boon, N.; et al. Antibiotic affects the gut microbiota composition and expression of genes related to lipid metabolism and myofiber types in skeletal muscle of piglets. BMC Vet. Res. 2020, 16, 392. [Google Scholar] [CrossRef]
- Santos, B.P.; Lisboa, J.A.N.; Bessegatto, J.A.; Montemor, C.H.; Paulino, L.R.; Alfieri, A.A.; Weese, J.S.; Costa, M.C. Impact of virginiamycin on the ruminal microbiota of feedlot cattle. Can. J. Vet. Res. 2024, 88, 114–122. [Google Scholar]
- Hayer, S.S.; Casanova-Higes, A.; Paladino, E.; Elnekave, E.; Nault, A.; Johnson, T.; Bender, J.; Perez, A.; Alvarez, J. Global Distribution of Extended Spectrum Cephalosporin and Carbapenem Resistance and Associated Resistance Markers in Escherichia coli of Swine Origin—A Systematic Review and Meta-Analysis. Front. Microbiol. 2022, 13, 853810. [Google Scholar] [CrossRef] [PubMed]
- Agga, G.E.; Schmidt, J.W.; Arthur, T.M. Effects of In-Feed Chlortetracycline Prophylaxis in Beef Cattle on Animal Health and Antimicrobial-Resistant Escherichia coli. Appl. Environ. Microbiol. 2016, 82, 7197–7204. [Google Scholar] [CrossRef] [PubMed]
- Ardakani, Z.; Canali, M.; Aragrande, M.; Tomassone, L.; Simoes, M.; Balzani, A.; Beber, C.L. Evaluating the contribution of antimicrobial use in farmed animals to global antimicrobial resistance in humans. One Health 2023, 17, 100647. [Google Scholar] [CrossRef]
- Wu, D.; Dai, S.; Feng, H.; Karunaratne, S.; Yang, M.; Zhang, Y. Persistence and potential risks of tetracyclines and their transformation products in two typical different animal manure composting treatments. Environ. Pollut. 2024, 341, 122904. [Google Scholar] [CrossRef]
- Mulchandani, R.; Wang, Y.; Gilbert, M.; Van Boeckel, T.P. Global trends in antimicrobial use in food-producing animals: 2020 to 2030. PLoS Glob. Public Health 2023, 3, e0001305. [Google Scholar] [CrossRef]
- Komarek, A.M.; Dunston, S.; Enahoro, D.; Godfray, H.C.J.; Herrero, M.; Mason-D’Croz, D.; Rich, K.M.; Scarborough, P.; Springmann, M.; Sulser, T.B.; et al. Income, consumer preferences, and the future of livestock-derived food demand. Glob. Environ. Change Hum. Policy Dimens. 2021, 70, 102343. [Google Scholar] [CrossRef]
- Abebe, E.; Gugsa, G.; Ahmed, M. Review on Major Food-Borne Zoonotic Bacterial Pathogens. J. Trop. Med. 2020, 2020, 4674235. [Google Scholar] [CrossRef] [PubMed]
- Shahzad, M.A.; Yousaf, A.; Ahsan, A.; Irshad, H.; Riaz, A.; Khan, A.; Ullah, I.; Sattar, S.; Bostan, N.; Javed, S. Virulence and resistance profiling of Staphylococcus aureus isolated from subclinical bovine mastitis in the Pakistani Pothohar region. Sci. Rep. 2024, 14, 14569. [Google Scholar] [CrossRef]
- Lees, P.; Pelligand, L.; Giraud, E.; Toutain, P.L. A history of antimicrobial drugs in animals: Evolution and revolution. J. Vet. Pharmacol. Ther. 2021, 44, 137–171. [Google Scholar] [CrossRef]
- Hosain, M.Z.; Kabir, S.M.L.; Kamal, M.M. Antimicrobial uses for livestock production in developing countries. Vet. World 2021, 14, 210–221. [Google Scholar] [CrossRef]
- Patel, S.J.; Wellington, M.; Shah, R.M.; Ferreira, M.J. Antibiotic Stewardship in Food-producing Animals: Challenges, Progress, and Opportunities. Clin. Ther. 2020, 42, 1649–1658. [Google Scholar] [CrossRef] [PubMed]
- Agren, E.; Backhans, A.; Lindberg, M.; Sjolund, M.; Bengtsson, B.; Comin, A. Antimicrobial resistance in Escherichia coli from Swedish piglets with diarrhoea and associations with potential risk factors. Acta Vet. Scand. 2025, 67, 16. [Google Scholar] [CrossRef] [PubMed]
- Watts, J.L.; Sweeney, M.T.; Lubbers, B.V. Antimicrobial Susceptibility Testing of Bacteria of Veterinary Origin. Microbiol. Spectr. 2018, 6. [Google Scholar] [CrossRef]
- Gens, K.D.; Singer, R.S.; Dilworth, T.J.; Heil, E.L.; Beaudoin, A.L. Antimicrobials in Animal Agriculture in the United States: A Multidisciplinary Overview of Regulation and Utilization to Foster Collaboration: On Behalf Of the Society of Infectious Diseases Pharmacists. Open Forum Infect. Dis. 2022, 9, ofac542. [Google Scholar] [CrossRef]
- Amenu, K.; McIntyre, K.M.; Moje, N.; Knight-Jones, T.; Rushton, J.; Grace, D. Approaches for disease prioritization and decision-making in animal health, 2000-2021: A structured scoping review. Front. Vet. Sci. 2023, 10, 1231711. [Google Scholar] [CrossRef] [PubMed]
- Odey, T.O.J.; Tanimowo, W.O.; Afolabi, K.O.; Jahid, I.K.; Reuben, R.C. Antimicrobial use and resistance in food animal production: Food safety and associated concerns in Sub-Saharan Africa. Int. Microbiol. Off. J. Span. Soc. Microbiol. 2024, 27, 1–23. [Google Scholar] [CrossRef]
- El-Fateh, M.; Bilal, M.; Zhao, X. Effect of antibiotic growth promoters (AGPs) on feed conversion ratio (FCR) of broiler chickens: A meta-analysis. Poult. Sci. 2024, 103, 104472. [Google Scholar] [CrossRef]
- Peng, C.; Ghanbari, M.; May, A.; Abeel, T. Effects of antibiotic growth promoter and its natural alternative on poultry cecum ecosystem: An integrated analysis of gut microbiota and host expression. Front. Microbiol. 2024, 15, 1492270. [Google Scholar] [CrossRef]
- Theodoridou Oxinou, D.; Lamnisos, D.; Filippou, C.; Spernovasilis, N.; Tsioutis, C. Antimicrobial Use and Antimicrobial Resistance in Food-Producing Animals: Cross-Sectional Study on Knowledge, Attitudes, and Practices Among Veterinarians and Operators of Establishments in the Republic of Cyprus. Antibiotics 2025, 14, 251. [Google Scholar] [CrossRef]
- Kimera, Z.I.; Mshana, S.E.; Rweyemamu, M.M.; Mboera, L.E.G.; Matee, M.I.N. Antimicrobial use and resistance in food-producing animals and the environment: An African perspective. Antimicrob. Resist. Infect. Control 2020, 9, 37. [Google Scholar] [CrossRef]
- Graham, D.W.; Bergeron, G.; Bourassa, M.W.; Dickson, J.; Gomes, F.; Howe, A.; Kahn, L.H.; Morley, P.S.; Scott, H.M.; Simjee, S.; et al. Complexities in understanding antimicrobial resistance across domesticated animal, human, and environmental systems. Ann. N. Y. Acad. Sci. 2019, 1441, 17–30. [Google Scholar] [CrossRef] [PubMed]
- Argudin, M.A.; Deplano, A.; Meghraoui, A.; Dodemont, M.; Heinrichs, A.; Denis, O.; Nonhoff, C.; Roisin, S. Bacteria from Animals as a Pool of Antimicrobial Resistance Genes. Antibiotics 2017, 6, 12. [Google Scholar] [CrossRef] [PubMed]
- Mandujano-Hernandez, A.; Martinez-Vazquez, A.V.; Paz-Gonzalez, A.D.; Herrera-Mayorga, V.; Sanchez-Sanchez, M.; Lara-Ramirez, E.E.; Vazquez, K.; de Jesus de Luna-Santillana, E.; Bocanegra-Garcia, V.; Rivera, G. The Global Rise of ESBL-Producing Escherichia coli in the Livestock Sector: A Five-Year Overview. Animals 2024, 14, 2490. [Google Scholar] [CrossRef]
- Sorum, M.; Holstad, G.; Lillehaug, A.; Kruse, H. Prevalence of vancomycin resistant enterococci on poultry farms established after the ban of avoparcin. Avian Dis. 2004, 48, 823–828. [Google Scholar] [CrossRef]
- Simm, R.; Slettemeas, J.S.; Norstrom, M.; Dean, K.R.; Kaldhusdal, M.; Urdahl, A.M. Significant reduction of vancomycin resistant E. faecium in the Norwegian broiler population coincided with measures taken by the broiler industry to reduce antimicrobial resistant bacteria. PLoS ONE 2019, 14, e0226101. [Google Scholar] [CrossRef]
- Wernli, D.; Harbarth, S.; Levrat, N.; Pittet, D. A ‘whole of United Nations approach’ to tackle antimicrobial resistance? A mapping of the mandate and activities of international organisations. BMJ Glob. Health 2022, 7, e008181. [Google Scholar] [CrossRef] [PubMed]
- Majumder, M.A.A.; Rahman, S.; Cohall, D.; Bharatha, A.; Singh, K.; Haque, M.; Gittens-St Hilaire, M. Antimicrobial Stewardship: Fighting Antimicrobial Resistance and Protecting Global Public Health. Infect. Drug Resist. 2020, 13, 4713–4738. [Google Scholar] [CrossRef]
- Rahman, M.M.; Alam Tumpa, M.A.; Zehravi, M.; Sarker, M.T.; Yamin, M.; Islam, M.R.; Harun-Or-Rashid, M.; Ahmed, M.; Ramproshad, S.; Mondal, B.; et al. An Overview of Antimicrobial Stewardship Optimization: The Use of Antibiotics in Humans and Animals to Prevent Resistance. Antibiotics 2022, 11, 667. [Google Scholar] [CrossRef]
- Barata, R.; Saavedra, M.J.; Almeida, G. A Decade of Antimicrobial Resistance in Human and Animal Campylobacter spp. Isolates. Antibiotics 2024, 13, 904. [Google Scholar] [CrossRef]
- Ramos, S.; Silva, V.; Dapkevicius, M.L.E.; Canica, M.; Tejedor-Junco, M.T.; Igrejas, G.; Poeta, P. Escherichia coli as Commensal and Pathogenic Bacteria Among Food-Producing Animals: Health Implications of Extended Spectrum beta-lactamase (ESBL) Production. Animals 2020, 10, 2239. [Google Scholar] [CrossRef]
- Havelaar, A.H.; Kirk, M.D.; Torgerson, P.R.; Gibb, H.J.; Hald, T.; Lake, R.J.; Praet, N.; Bellinger, D.C.; de Silva, N.R.; Gargouri, N.; et al. World Health Organization Global Estimates and Regional Comparisons of the Burden of Foodborne Disease in 2010. PLoS Med. 2015, 12, e1001923. [Google Scholar] [CrossRef] [PubMed]
- Elbehiry, A.; Abalkhail, A.; Marzouk, E.; Elmanssury, A.E.; Almuzaini, A.M.; Alfheeaid, H.; Alshahrani, M.T.; Huraysh, N.; Ibrahem, M.; Alzaben, F.; et al. An Overview of the Public Health Challenges in Diagnosing and Controlling Human Foodborne Pathogens. Vaccines 2023, 11, 725. [Google Scholar] [CrossRef]
- Van, T.T.H.; Yidana, Z.; Smooker, P.M.; Coloe, P.J. Antibiotic use in food animals worldwide, with a focus on Africa: Pluses and minuses. J. Glob. Antimicrob. Resist. 2020, 20, 170–177. [Google Scholar] [CrossRef] [PubMed]
- Manyi-Loh, C.; Mamphweli, S.; Meyer, E.; Okoh, A. Antibiotic Use in Agriculture and Its Consequential Resistance in Environmental Sources: Potential Public Health Implications. Molecules 2018, 23, 795. [Google Scholar] [CrossRef]
- Pandey, S.; Doo, H.; Keum, G.B.; Kim, E.S.; Kwak, J.; Ryu, S.; Choi, Y.; Kang, J.; Kim, S.; Lee, N.R.; et al. Antibiotic resistance in livestock, environment and humans: One Health perspective. J. Anim. Sci. Technol. 2024, 66, 266–278. [Google Scholar] [CrossRef] [PubMed]
- Bava, R.; Castagna, F.; Lupia, C.; Poerio, G.; Liguori, G.; Lombardi, R.; Naturale, M.D.; Mercuri, C.; Bulotta, R.M.; Britti, D.; et al. Antimicrobial Resistance in Livestock: A Serious Threat to Public Health. Antibiotics 2024, 13, 551. [Google Scholar] [CrossRef]
- Aidara-Kane, A.; Angulo, F.J.; Conly, J.M.; Minato, Y.; Silbergeld, E.K.; McEwen, S.A.; Collignon, P.J.; Group, W.H.O.G.D. World Health Organization (WHO) guidelines on use of medically important antimicrobials in food-producing animals. Antimicrob. Resist. Infect. Control 2018, 7, 7. [Google Scholar] [CrossRef]
- Berman, T.S.; Barnett-Itzhaki, Z.; Berman, T.; Marom, E. Antimicrobial resistance in food-producing animals: Towards implementing a one health based national action plan in Israel. Isr. J. Health Policy Res. 2023, 12, 18. [Google Scholar] [CrossRef]
- Scott, H.M.; Acuff, G.; Bergeron, G.; Bourassa, M.W.; Gill, J.; Graham, D.W.; Kahn, L.H.; Morley, P.S.; Salois, M.J.; Simjee, S.; et al. Critically important antibiotics: Criteria and approaches for measuring and reducing their use in food animal agriculture. Ann. N. Y. Acad. Sci. 2019, 1441, 8–16. [Google Scholar] [CrossRef]
- Michaelis, C.; Grohmann, E. Horizontal Gene Transfer of Antibiotic Resistance Genes in Biofilms. Antibiotics 2023, 12, 328. [Google Scholar] [CrossRef]
- Galgano, M.; Pellegrini, F.; Catalano, E.; Capozzi, L.; Del Sambro, L.; Sposato, A.; Lucente, M.S.; Vasinioti, V.I.; Catella, C.; Odigie, A.E.; et al. Acquired Bacterial Resistance to Antibiotics and Resistance Genes: From Past to Future. Antibiotics 2025, 14, 222. [Google Scholar] [CrossRef] [PubMed]
- Beshiru, A.; Igbinosa, I.H.; Akinnibosun, O.; Ogofure, A.G.; Dunkwu-Okafor, A.; Uwhuba, K.E.; Igbinosa, E.O. Characterization of resistance and virulence factors in livestock-associated methicillin-resistant Staphylococcus aureus. Sci. Rep. 2024, 14, 13235. [Google Scholar] [CrossRef] [PubMed]
- Zalewska, M.; Blazejewska, A.; Gawor, J.; Adamska, D.; Goryca, K.; Szelag, M.; Kalinowski, P.; Popowska, M. The IncC and IncX1 resistance plasmids present in multi-drug resistant Escherichia coli strains isolated from poultry manure in Poland. Environ. Sci. Pollut. Res. Int. 2024, 31, 47727–47741. [Google Scholar] [CrossRef]
- Lerner, A.; Matthias, T.; Aminov, R. Potential Effects of Horizontal Gene Exchange in the Human Gut. Front. Immunol. 2017, 8, 1630. [Google Scholar] [CrossRef] [PubMed]
- Ghimpeteanu, O.M.; Pogurschi, E.N.; Popa, D.C.; Dragomir, N.; Dragotoiu, T.; Mihai, O.D.; Petcu, C.D. Antibiotic Use in Livestock and Residues in Food-A Public Health Threat: A Review. Foods 2022, 11, 1430. [Google Scholar] [CrossRef]
- Chen, Y.; Xiao, L.; Zhou, M.; Zhang, H. The microbiota: A crucial mediator in gut homeostasis and colonization resistance. Front. Microbiol. 2024, 15, 1417864. [Google Scholar] [CrossRef]
- Berendsen, B.J.; Wegh, R.S.; Memelink, J.; Zuidema, T.; Stolker, L.A. The analysis of animal faeces as a tool to monitor antibiotic usage. Talanta 2015, 132, 258–268. [Google Scholar] [CrossRef]
- Tripathi, A.; Jaiswal, A.; Kumar, D.; Pandit, R.; Blake, D.; Tomley, F.; Joshi, M.; Joshi, C.G.; Dubey, S.K. Whole genome sequencing revealed high occurrence of antimicrobial resistance genes in bacteria isolated from poultry manure. Int. J. Antimicrob. Agents 2025, 65, 107452. [Google Scholar] [CrossRef]
- Cortes, P.; Pokrant, E.; Yevenes, K.; Maddaleno, A.; Flores, A.; Vargas, M.B.; Trincado, L.; Maturana, M.; Lapierre, L.; Cornejo, J. Antimicrobial Residues in Poultry Litter: Assessing the Association of Antimicrobial Persistence with Resistant Escherichia coli Strains. Antibiotics 2025, 14, 89. [Google Scholar] [CrossRef]
- Yevenes, K.; Pokrant, E.; Trincado, L.; Lapierre, L.; Galarce, N.; Martin, B.S.; Maddaleno, A.; Hidalgo, H.; Cornejo, J. Detection of Antimicrobial Residues in Poultry Litter: Monitoring a Risk through a Selective and Sensitive HPLC-MS/MS Method. Animals 2021, 11, 1399. [Google Scholar] [CrossRef]
- Hazards, E.P.o.B.; Koutsoumanis, K.; Allende, A.; Alvarez-Ordonez, A.; Bolton, D.; Bover-Cid, S.; Chemaly, M.; Davies, R.; De Cesare, A.; Herman, L.; et al. Role played by the environment in the emergence and spread of antimicrobial resistance (AMR) through the food chain. EFSA J. Eur. Food Saf. Auth. 2021, 19, e06651. [Google Scholar] [CrossRef]
- Gwenzi, W.; Chaukura, N.; Muisa-Zikali, N.; Teta, C.; Musvuugwa, T.; Rzymski, P.; Abia, A.L.K. Insects, Rodents, and Pets as Reservoirs, Vectors, and Sentinels of Antimicrobial Resistance. Antibiotics 2021, 10, 68. [Google Scholar] [CrossRef] [PubMed]
- Fouz, N.; Pangesti, K.N.A.; Yasir, M.; Al-Malki, A.L.; Azhar, E.I.; Hill-Cawthorne, G.A.; Abd El Ghany, M. The Contribution of Wastewater to the Transmission of Antimicrobial Resistance in the Environment: Implications of Mass Gathering Settings. Trop. Med. Infect. Dis. 2020, 5, 33. [Google Scholar] [CrossRef]
- La Rosa, M.C.; Maugeri, A.; Favara, G.; La Mastra, C.; Magnano San Lio, R.; Barchitta, M.; Agodi, A. The Impact of Wastewater on Antimicrobial Resistance: A Scoping Review of Transmission Pathways and Contributing Factors. Antibiotics 2025, 14, 131. [Google Scholar] [CrossRef]
- Arnold, K.E.; Laing, G.; McMahon, B.J.; Fanning, S.; Stekel, D.J.; Pahl, O.; Coyne, L.; Latham, S.M.; McIntyre, K.M. The need for One Health systems-thinking approaches to understand multiscale dissemination of antimicrobial resistance. Lancet Planet. Health 2024, 8, e124–e133. [Google Scholar] [CrossRef]
- Xu, C.; Kong, L.; Gao, H.; Cheng, X.; Wang, X. A Review of Current Bacterial Resistance to Antibiotics in Food Animals. Front. Microbiol. 2022, 13, 822689. [Google Scholar] [CrossRef] [PubMed]
- Che, M.; Birk, T.; Hansen, L.T. Prevalence and Transmission of Extended-Spectrum Cephalosporin (ESC) Resistance Genes in Escherichia coli Isolated from Poultry Production Systems and Slaughterhouses in Denmark. Antibiotics 2023, 12, 1602. [Google Scholar] [CrossRef] [PubMed]
- European Food Safety Authority (EFSA); European Centre for Disease Prevention and Control (ECDC). The European Union summary report on antimicrobial resistance in zoonotic and indicator bacteria from humans, animals and food in 2021–2022. EFSA J. Eur. Food Saf. Auth. 2024, 22, e8583. [Google Scholar] [CrossRef]
- McEwen, S.A.; Collignon, P.J. Antimicrobial Resistance: A One Health Perspective. Microbiol. Spectr. 2018, 6. [Google Scholar] [CrossRef]
- Teklemariam, A.D.; Al-Hindi, R.R.; Albiheyri, R.S.; Alharbi, M.G.; Alghamdi, M.A.; Filimban, A.A.R.; Al Mutiri, A.S.; Al-Alyani, A.M.; Alseghayer, M.S.; Almaneea, A.M.; et al. Human Salmonellosis: A Continuous Global Threat in the Farm-to-Fork Food Safety Continuum. Foods 2023, 12, 1756. [Google Scholar] [CrossRef]
- Delahoy, M.J.; Shah, H.J.; Weller, D.L.; Ray, L.C.; Smith, K.; McGuire, S.; Trevejo, R.T.; Scallan Walter, E.; Wymore, K.; Rissman, T.; et al. Preliminary Incidence and Trends of Infections Caused by Pathogens Transmitted Commonly Through Food—Foodborne Diseases Active Surveillance Network, 10 U.S. Sites, 2022. MMWR. Morb. Mortal. Wkly. Rep. 2023, 72, 701–706. [Google Scholar] [CrossRef]
- Pourzand, F.; Kim, A.H.M.; Chambers, T.; Grout, L.; Baker, M.G.; Hales, S. Examining campylobacteriosis disease notification rates: Association with water supply characteristics. Environ. Res. 2025, 271, 121064. [Google Scholar] [CrossRef] [PubMed]
- Pietsch, M.; Simon, S.; Meinen, A.; Trost, E.; Banerji, S.; Pfeifer, Y.; Flieger, A. Third generation cephalosporin resistance in clinical non-typhoidal Salmonella enterica in Germany and emergence of bla(CTX-M)-harbouring pESI plasmids. Microb. Genom. 2021, 7, 000698. [Google Scholar] [CrossRef]
- Matuszewska, M.; Murray, G.G.R.; Ba, X.; Wood, R.; Holmes, M.A.; Weinert, L.A. Stable antibiotic resistance and rapid human adaptation in livestock-associated MRSA. eLife 2022, 11, e74819. [Google Scholar] [CrossRef] [PubMed]
- Larsen, J.; Raisen, C.L.; Ba, X.; Sadgrove, N.J.; Padilla-Gonzalez, G.F.; Simmonds, M.S.J.; Loncaric, I.; Kerschner, H.; Apfalter, P.; Hartl, R.; et al. Emergence of methicillin resistance predates the clinical use of antibiotics. Nature 2022, 602, 135–141. [Google Scholar] [CrossRef]
- Larsen, J.; Stegger, M.; Andersen, P.S.; Petersen, A.; Larsen, A.R.; Westh, H.; Agerso, Y.; Fetsch, A.; Kraushaar, B.; Kasbohrer, A.; et al. Evidence for Human Adaptation and Foodborne Transmission of Livestock-Associated Methicillin-Resistant Staphylococcus aureus. Clin. Infect. Dis. 2016, 63, 1349–1352. [Google Scholar] [CrossRef] [PubMed]
- Kock, R.; Schaumburg, F.; Mellmann, A.; Koksal, M.; Jurke, A.; Becker, K.; Friedrich, A.W. Livestock-associated methicillin-resistant Staphylococcus aureus (MRSA) as causes of human infection and colonization in Germany. PLoS ONE 2013, 8, e55040. [Google Scholar] [CrossRef]
- Cuny, C.; Wieler, L.H.; Witte, W. Livestock-Associated MRSA: The Impact on Humans. Antibiotics 2015, 4, 521–543. [Google Scholar] [CrossRef]
- Patel, J.; Harant, A.; Fernandes, G.; Mwamelo, A.J.; Hein, W.; Dekker, D.; Sridhar, D. Measuring the global response to antimicrobial resistance, 2020-21: A systematic governance analysis of 114 countries. Lancet Infect. Dis. 2023, 23, 706–718. [Google Scholar] [CrossRef]
- Hammerum, A.M.; Heuer, O.E.; Emborg, H.D.; Bagger-Skjot, L.; Jensen, V.F.; Rogues, A.M.; Skov, R.L.; Agerso, Y.; Brandt, C.T.; Seyfarth, A.M.; et al. Danish integrated antimicrobial resistance monitoring and research program. Emerg. Infect. Dis. 2007, 13, 1632–1639. [Google Scholar] [CrossRef]
- Yang, Y.; Feye, K.M.; Shi, Z.; Pavlidis, H.O.; Kogut, M.; Ashworth, J.A.; Ricke, S.C. A Historical Review on Antibiotic Resistance of Foodborne Campylobacter. Front. Microbiol. 2019, 10, 1509. [Google Scholar] [CrossRef] [PubMed]
- Mader, R.; Jarrige, N.; Haenni, M.; Bourely, C.; Madec, J.Y.; Amat, J.P.; Eu, J. OASIS evaluation of the French surveillance network for antimicrobial resistance in diseased animals (RESAPATH): Success factors underpinning a well-performing voluntary system. Epidemiol. Infect. 2021, 149, e104. [Google Scholar] [CrossRef] [PubMed]
- Dutil, L.; Irwin, R.; Finley, R.; Ng, L.K.; Avery, B.; Boerlin, P.; Bourgault, A.M.; Cole, L.; Daignault, D.; Desruisseau, A.; et al. Ceftiofur resistance in Salmonella enterica serovar Heidelberg from chicken meat and humans, Canada. Emerg. Infect. Dis. 2010, 16, 48–54. [Google Scholar] [CrossRef]
- Zhang, R.; Eggleston, K.; Rotimi, V.; Zeckhauser, R.J. Antibiotic resistance as a global threat: Evidence from China, Kuwait and the United States. Glob. Health 2006, 2, 6. [Google Scholar] [CrossRef]
- Wen, R.; Li, C.; Zhao, M.; Wang, H.; Tang, Y. Withdrawal of antibiotic growth promoters in China and its impact on the foodborne pathogen Campylobacter coli of swine origin. Front. Microbiol. 2022, 13, 1004725. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Han, Z.; Song, K.; Zhen, T. Antibiotic Consumption Trends in China: Evidence From Six-Year Surveillance Sales Records in Shandong Province. Front. Pharmacol. 2020, 11, 491. [Google Scholar] [CrossRef]
- Tiseo, K.; Huber, L.; Gilbert, M.; Robinson, T.P.; Van Boeckel, T.P. Global Trends in Antimicrobial Use in Food Animals from 2017 to 2030. Antibiotics 2020, 9, 918. [Google Scholar] [CrossRef]
- Grave, K.; Torren-Edo, J.; Muller, A.; Greko, C.; Moulin, G.; Mackay, D.; Group, E. Variations in the sales and sales patterns of veterinary antimicrobial agents in 25 European countries. J. Antimicrob. Chemother. 2014, 69, 2284–2291. [Google Scholar] [CrossRef]
- Idoko-Akoh, A.; Goldhill, D.H.; Sheppard, C.M.; Bialy, D.; Quantrill, J.L.; Sukhova, K.; Brown, J.C.; Richardson, S.; Campbell, C.; Taylor, L.; et al. Creating resistance to avian influenza infection through genome editing of the ANP32 gene family. Nat. Commun. 2023, 14, 6136. [Google Scholar] [CrossRef]
- Jeffery, A.; Gilbert, M.; Corsaut, L.; Gaudreau, A.; Obradovic, M.R.; Cloutier, S.; Frenette, M.C.; Surprenant, C.; Lacouture, S.; Arnal, J.L.; et al. Immune response induced by a Streptococcus suis multi-serotype autogenous vaccine used in sows to protect post-weaned piglets. Vet. Res. 2024, 55, 57. [Google Scholar] [CrossRef]
- Duan, Y.; Hao, Y.; Feng, H.; Shu, J.; He, Y. Research progress on Haemophilus parasuis vaccines. Front. Vet. Sci. 2025, 12, 1492144. [Google Scholar] [CrossRef] [PubMed]
- Blake, D.P.; Vrba, V.; Xia, D.; Jatau, I.D.; Spiro, S.; Nolan, M.J.; Underwood, G.; Tomley, F.M. Genetic and biological characterisation of three cryptic Eimeria operational taxonomic units that infect chickens (Gallus gallus domesticus). Int. J. Parasitol. 2021, 51, 621–634. [Google Scholar] [CrossRef] [PubMed]
- Petro-Turnquist, E.; Pekarek, M.J.; Weaver, E.A. Swine influenza A virus: Challenges and novel vaccine strategies. Front. Cell. Infect. Microbiol. 2024, 14, 1336013. [Google Scholar] [CrossRef]
- Hoelzer, K.; Bielke, L.; Blake, D.P.; Cox, E.; Cutting, S.M.; Devriendt, B.; Erlacher-Vindel, E.; Goossens, E.; Karaca, K.; Lemiere, S.; et al. Vaccines as alternatives to antibiotics for food producing animals. Part 2: New approaches and potential solutions. Vet. Res. 2018, 49, 70. [Google Scholar] [CrossRef]
- Graham, A.E.; Ledesma-Amaro, R. The microbial food revolution. Nat. Commun. 2023, 14, 2231. [Google Scholar] [CrossRef]
- Radhi, K.S.; Arif, M.; Rehman, A.U.; Faizan, M.; Almohmadi, N.H.; Youssef, I.M.; Swelum, A.A.; Suliman, G.M.; Tharwat, M.; Ebrahim, A.; et al. Growth performance of broiler chickens fed diets supplemented with amylase and protease enzymes individually or combined. Open Vet. J. 2023, 13, 1425–1435. [Google Scholar] [CrossRef]
- Latif, A.; Shehzad, A.; Niazi, S.; Zahid, A.; Ashraf, W.; Iqbal, M.W.; Rehman, A.; Riaz, T.; Aadil, R.M.; Khan, I.M.; et al. Probiotics: Mechanism of action, health benefits and their application in food industries. Front. Microbiol. 2023, 14, 1216674. [Google Scholar] [CrossRef]
- Mazziotta, C.; Tognon, M.; Martini, F.; Torreggiani, E.; Rotondo, J.C. Probiotics Mechanism of Action on Immune Cells and Beneficial Effects on Human Health. Cells 2023, 12, 184. [Google Scholar] [CrossRef] [PubMed]
- Davani-Davari, D.; Negahdaripour, M.; Karimzadeh, I.; Seifan, M.; Mohkam, M.; Masoumi, S.J.; Berenjian, A.; Ghasemi, Y. Prebiotics: Definition, Types, Sources, Mechanisms, and Clinical Applications. Foods 2019, 8, 92. [Google Scholar] [CrossRef]
- Gupta, R.; Sharma, S. Role of alternatives to antibiotics in mitigating the antimicrobial resistance crisis. Indian J. Med. Res. 2022, 156, 464–477. [Google Scholar] [CrossRef]
- Klous, G.; Huss, A.; Heederik, D.J.J.; Coutinho, R.A. Human-livestock contacts and their relationship to transmission of zoonotic pathogens, a systematic review of literature. One Health 2016, 2, 65–76. [Google Scholar] [CrossRef] [PubMed]
- Makovska, I.; Chantziaras, I.; Caekebeke, N.; Dhaka, P.; Dewulf, J. Assessment of Cleaning and Disinfection Practices on Pig Farms across Ten European Countries. Animals 2024, 14, 593. [Google Scholar] [CrossRef] [PubMed]
- Alawa, B.; Singh, S.; Chakma, S.; Kishor, R.; Stalsby Lundborg, C.; Diwan, V. Development of novel biochar adsorbent using agricultural waste biomass for enhanced removal of ciprofloxacin from water: Insights into the isotherm, kinetics, and thermodynamic analysis. Chemosphere 2025, 375, 144252. [Google Scholar] [CrossRef]
- Qadeer, A.; Khan, A.; Khan, N.M.; Wajid, A.; Ullah, K.; Skalickova, S.; Chilala, P.; Slama, P.; Horky, P.; Alqahtani, M.S.; et al. Use of nanotechnology-based nanomaterial as a substitute for antibiotics in monogastric animals. Heliyon 2024, 10, e31728. [Google Scholar] [CrossRef]
- Abreu, R.; Semedo-Lemsaddek, T.; Cunha, E.; Tavares, L.; Oliveira, M. Antimicrobial Drug Resistance in Poultry Production: Current Status and Innovative Strategies for Bacterial Control. Microorganisms 2023, 11, 953. [Google Scholar] [CrossRef]
- Mhlongo, J.T.; Waddad, A.Y.; Albericio, F.; de la Torre, B.G. Antimicrobial Peptide Synergies for Fighting Infectious Diseases. Adv. Sci. 2023, 10, e2300472. [Google Scholar] [CrossRef]
- Wang, G.; Song, Q.; Huang, S.; Wang, Y.; Cai, S.; Yu, H.; Ding, X.; Zeng, X.; Zhang, J. Effect of Antimicrobial Peptide Microcin J25 on Growth Performance, Immune Regulation, and Intestinal Microbiota in Broiler Chickens Challenged with Escherichia coli and Salmonella. Animals 2020, 10, 345. [Google Scholar] [CrossRef] [PubMed]
- Mancuso, G.; Trinchera, M.; Midiri, A.; Zummo, S.; Vitale, G.; Biondo, C. Novel Antimicrobial Approaches to Combat Bacterial Biofilms Associated with Urinary Tract Infections. Antibiotics 2024, 13, 154. [Google Scholar] [CrossRef]
- Burlec, A.F.; Corciova, A.; Boev, M.; Batir-Marin, D.; Mircea, C.; Cioanca, O.; Danila, G.; Danila, M.; Bucur, A.F.; Hancianu, M. Current Overview of Metal Nanoparticles’ Synthesis, Characterization, and Biomedical Applications, with a Focus on Silver and Gold Nanoparticles. Pharmaceuticals 2023, 16, 1410. [Google Scholar] [CrossRef]
- Moradi, F.; Ghaedi, A.; Fooladfar, Z.; Bazrgar, A. Recent advance on nanoparticles or nanomaterials with anti-multidrug resistant bacteria and anti-bacterial biofilm properties: A systematic review. Heliyon 2023, 9, e22105. [Google Scholar] [CrossRef]
- Danchuk, O.; Levchenko, A.; da Silva Mesquita, R.; Danchuk, V.; Cengiz, S.; Cengiz, M.; Grafov, A. Meeting Contemporary Challenges: Development of Nanomaterials for Veterinary Medicine. Pharmaceutics 2023, 15, 2326. [Google Scholar] [CrossRef] [PubMed]
- Bacanli, M.G. The two faces of antibiotics: An overview of the effects of antibiotic residues in foodstuffs. Arch. Toxicol. 2024, 98, 1717–1725. [Google Scholar] [CrossRef] [PubMed]


| Antimicrobial Agents | Species | Use | Mode of Action | Bacterial Resistance |
|---|---|---|---|---|
| AMINOCOUMARIN Novobiocin | Poultry [32,33,34], Caprine [35,36], Ovine [35,36] | Novobiocin is for treating mastitis in animals. | Novobiocin blocks the action of the bacterial DNA gyrase enzyme. Its action is bacteriostatic at lower concentrations and bactericidal at higher concentrations. | Resistance is linked to mutations in the topoisomerase II gene. |
| AMINOCYCLITOL Spectinomycin | Bovine [36], Poultry [32,33,34], Caprine [35,36], Ovine [35,36], Swine [35,37] | Used for respiratory infections in cattle and enteric infections in multiple species. | Inhibition of protein synthesis by binding to the 30S ribosomal subunit. Spectinomycin exerts bacteriostatic activity. | Resistance has been linked to aminoglycoside 3’-adenylate transferase. |
| AMINOGLYCOSIDES Dihydrostreptomycin | Bovine [35,36], Caprine [35,36], Ovine [35,36], Swine [35,37] | Aminoglycosides have a broad spectrum of applications, including the treatment of the following:
| After internalisation, aminoglycosides bind to the 30S and 50S subunits, stopping protein synthesis. They are effective against rapidly multiplying organisms. At low concentrations, they exhibit bacteriostatic activity. | The resistance mechanism consists of an enzymatic modification of the antibiotic molecule, which may be either plasmid-encoded or chromosomally mediated, into three major classes:
|
| Streptomycin | Bovine [35,36], Poultry [32,33,34], Caprine [35,36], Ovine [35,36], Swine [35,37] | |||
| AMINOGLYCOSIDES + 2 DEOXYSTREPTAMINE Apramycin | Bovine [35,36], Poultry [32,33,34], Ovine [35,36], Swine [35,37] | |||
| Fortimycin Kanamycin | Bovine [35,36], Ovine [35,36], Swine [35,37] | |||
| Framycetin | Bovine [35,36], Caprine [35,36], Ovine [35,36] | |||
| Gentamicin | Bovine [35,36], Poultry [32,33,34] Caprine [35,36] | |||
| Neomycin Paromomycin | Poultry [32,33,34] Bovine [35,36], Caprine [35,36], Ovine [35,36], Swine [35,37] | |||
| AMPHENICOLS Florfenicol Thiamphenicol | Bovine [35,36], Caprine [35,36], Poultry [32,33,34], Ovine [35,36], Swine [35,37] | They are used in the treatment of respiratory infections in cattle, pigs, and poultry. The florfenicol used to treat pasteurellosis as well. | These antibiotics are bacteriostatic. They bind to the 50S ribosomal subunit, stopping protein production in bacteria. May be bactericidal against S. pseudopneumoniae | Resistance is mediated by an efflux pump system, due to a specific transporter (Flo-R). These genes are encoded on mobile genetic elements such as plasmids, transposons, or cassette genes. |
| ANSAMYCIN—RIFAMYCINS Rifaximin | Bovine [35], Caprine [35,36], Ovine [35,36], Swine [35,37] | This class of antibiotics is indicated for a limited number of cases (mastitis), and there are few alternatives. | Rifamycins inhibit the synthesis of RNA in microorganisms by binding to DNA-dependent RNA polymerase subunits. It is particularly efficacious in the treatment of intracellular microorganisms. | Resistance is due to a chromosomal mutation in the rpoB gene. This normally codes for the bacterium’s RNA polymerase. |
| BICYCLOMYCIN Bicozamycin | Bovine [36] | They are used in the treatment of digestive and respiratory diseases in cattle. | NA | NA |
| CEPHALOSPORINS FIRST GENERATION Cefacetrile Cefapyrin | Bovine [35,36,38] | Cephalosporins are employed in the treatment of the following conditions:
| These antibiotics disrupt the synthesis of bacterial cell wall formation by interacting with a group of proteins known as the penicillin-binding proteins (PBPs). Antibiotic activity of beta-lactams is confined to organisms in the log phase of growth or during active multiplication. The different generations of cephalosporins vary with respect to their antibacterial spectra, beta-lactamase sensitivities, and pharmacokinetics. | The mechanisms of antimicrobial resistance are multifactorial. These mechanisms may comprise:
|
| Cefalexin | Bovine [35,36,38], Poultry [32,33,34], Caprine [35,36], Ovine [35,36], Swine [35,37] | |||
| Cefalonium Cefazolin | Bovine [35,36,38], Caprine [35,36], Ovine [35,36] | |||
| CEPHALOSPORINS SECOND GENERATION Cefuroxime | Bovine [35,36,38] | |||
| CEPHALOSPORINS THIRD GENERATION Cefoperazone | Bovine [35,36,38], Caprine [35,36], Ovine [35,36] | |||
| Ceftiofur | Bovine [35,36,38], Poultry [32,33,34], Caprine [35,36], Ovine [35,36], Swine [35,37] | |||
| Ceftriaxone | Bovine [35,36,38], Ovine [35,36], Swine [35,37] | |||
| CEPHALOSPORINS FOURTH GENERATION Cefquinome | Bovine [35,36,38], Caprine [35,36], Ovine [35,36], Swine [35,37] | |||
| FUSIDANE Fusidic acid | Bovine [35] | Fusidic acid is employed in the treatment of ophthalmic diseases in cattle. | It interferes with the function of elongation factor and inhibits protein synthesis at the 50S subunit of the ribosome. The action of fusidic acid is bacteriostatic against Gram-positive organisms, and bactericidal against Staphylococcus aureus. | The resistance mechanism consists in the binding of the FusB protein to both EF-G, associated with a molecule of fusidic acid, and to the ribosome. This creates conformational changes with release of both EF-G and the antibiotic. |
| IONOPHORES Lasalocid | Bovine [35], Ovine [35,36], Poultry [32,33,34] | Ionophores are used in the context of controlling intestinal parasitic coccidiosis (Eimeria spp.). | They are lipid-soluble molecules that can bind and transport ions across cell membranes. They can change the concentration of different types of ions, including calcium, potassium, hydrogen, and sodium. This can block protein transport, reduce metabolic activity, and kill the organism. | Resistance to ionophores is probably adaptive, not due to mutation or gene acquisition. |
| Monensin | Bovine [35], Poultry [32,33,34], Caprine [35,36] | |||
| Narasin | Bovine [35], Poultry [32,33,34] | |||
| Salinomycin | Bovine [35], Poultry [32,33,34], Swine [35,37] | |||
| LINCOSAMIDES Lincomycin | Swine [35,37], Poultry [32,33,34] | Lincosamides are used for treating the following conditions:
| Bind to the 50S subunit of bacterial ribosomes, suppressing protein synthesis via inhibition of peptidyl transferases. Present as bacteriostatic at low concentrations and bactericidal at high concentrations. | The resistance mechanism consists of methylation of the ribosomal subunit, by an enzyme of plasmid or chromosomal origin. Other mechanisms include increased activation of the efflux pump and destruction of the drug. |
| Pirlimycin | Swine [35,37] | |||
| MACROLIDES 14- MEMBERED RING Erythromycin | Bovine [35,36], Poultry [32,33,34], Caprine [35,36], Ovine [35,36], Swine [35,37] | The following conditions are treated with macrolides:
| They inhibit protein synthesis by binding to the 50S ribosomal subunit. The action of these substances is bacteriostatic; however, at elevated concentrations, it can be bactericidal. | Resistance is observed at multiple levels:
|
| Oleandomycin | Bovine [35,36] | |||
| MACROLIDES 15- MEMBERED RING Gamithromycin | Bovine [35,36] | |||
| Tulathromycin | Bovine [35,36], Swine [35,37] | |||
| MACROLIDES 16- MEMBERED RING Josamycin Terdecamycin | Swine [35,37] | |||
| Kitasamycin Mirosamycin Tylvalosin | Poultry [32,33,34] Swine [35,37] | |||
| Spiramycin Tilmicosin Tylosin | Bovine [35,36], Poultry [32,33,34], Caprine [35,36], Ovine [35,36], Swine [35,37] | |||
| Tildipirosin | Bovine [35,36], Swine [35,37] | |||
| MACROLIDES C17 Sedecamycin | Swine [35,37] | |||
| ORTHOSOMYCINS Avilamycin | Swine [35,37], Poultry [32,33,34] | Avilamycin is employed in the treatment of enteric diseases affecting poultry and swine. | It inhibits the proteins being built by blocking the A-tRNA site of ribosomal RNA 50S. It works against Gram-positive bacteria but not Gram-negative ones. | The molecular mechanisms underlying the resistance appear to be associated with mutations in single nucleotides at positions H89 and H91 of the ribosomal RNA 50S helices. |
| NATURAL PENICILLINS (including esters and salts) Benethamine penicillin Penethamate (hydroiodide) | Bovine [35,36] | This group of antibiotics has been proven effective in treating the following:
| These antibiotics disrupt the synthesis of bacterial cell wall formation by interacting with a group of proteins known as the penicillin-binding proteins (PBPs). This class of antibiotics is active against Gram-positive bacteria, and a limited number of Gram-negative bacteria. | Penicillins are susceptible to hydrolysis by beta-lactamases, also known as penicillinases. The combination of broad-spectrum penicillins and beta-lactamase inhibitors enhances the efficacy of treatment against both Gram-positive and Gram-negative pathogens. |
| Benzylpenicillin Benzylpenicillin procaine/ Benzathine penicillin | Bovine [35,36], Poultry [32,34], Caprine [35,36], Ovine [35,36], Swine [35,37] | |||
| AMDINOPENICILLINS Mecillinam | Bovine [35,36], Swine [35,37] | |||
| AMINOPENICILLINS AmoxicillinAmpicillin | Bovine [35,36], Poultry [32,34], Caprine [35,36], Ovine [35,36], Swine [35,37] | |||
| Hetacillin | Bovine [35,36] | |||
| AMINOPENICILLIN + BETALACTAMASE INHIBITOR Amoxicillin + Clavulanic | Bovine [35,36], Poultry [32,34], Caprine [35,36], Ovine [35,36], Swine [35,37] | |||
| Acid Ampicillin + Sulbactam | Bovine [35,36], Swine [35,37] | |||
| UREIDOPENICILLIN Aspoxicillin | Bovine [35,36], Swine [35,37] | |||
| PHENOXYPENICILLINS Phenoxymethylpenicillin | Poultry [32,34], Swine [35,37] | |||
| ANTISTAPHYLOCOCCAL PENICILLINS Cloxacillin Oxacillin | Bovine [35,36], Caprine [35,36], Ovine [35,36], Swine [35,37] | |||
| Dicloxacillin | Bovine [35,36], Poultry [32,34], Caprine [35,36], Ovine [35,36], Swine [35,37] | |||
| Nafcillin | Bovine [35,36], Caprine [35,36], Ovine [35,36] | |||
| PHOSPHONIC ACID DERIVATIVES Fosfomycin | Bovine [35,36], Poultry [32,34], Swine [35,37] | The limited availability of this pharmaceutical product in numerous countries leads to its classification as a Very Hard to Import (VHIA) medication. | Fosfomycin functions as a competitive inhibitor of the substrate phosphoenolpyruvate (PEP) binding to the enzyme UDP-GlcNAc enolpyruvyl transferase (MurA). MurA catalyses the first committed step in bacterial peptidoglycan biosynthesis, which is thereby inhibited. | The following mechanisms are representative of resistance:
|
| PLEUROMUTILINS Tiamulin | Caprine [35,36], Poultry [32,34], Ovine [35,36], Swine [35,37] | They are used in respiratory infections in both pigs and poultry, as well as for the treatment of swine dysentery. | They act on the bacterial cell wall synthesis mechanism, inhibiting protein synthesis at the level of bacterial ribosomes. These antibiotics are active against Gram-positive bacteria, mycoplasmas, and anaerobes. | The development of resistance occurs through mutations to chromosomal targets. These occur in the 23S rRNA and rplC genes linked to bacterial ribosomes. |
| Valnemulin | Swine [35,37] | |||
| POLYPEPTIDES Bacitracin | Bovine [35,36], Poultry [32,34], Swine [35,37], Ovine [35,36] | Bacitracin is employed in the treatment of necrotic enteritis in poultry. It is also indicated in the following:
| The mechanism of action of this antibiotic involves the following: interference with cell membrane function; and suppression of peptidoglycan synthesis. Possess bactericidal activity. | Rare resistance cases reported. |
| Gramicidin | Poultry [32,34], Swine [35,37] | |||
| POLYMYXINS Polymixin B | Bovine [35,36], Caprine [35,36], Ovine [35,36] | Polymyxin E (colistin) is employed in the treatment of Gram-negative enteric infections. | They are able to alter the phospholipid composition of bacterial cell membranes, compromising their permeability and functionality. It has been shown that polymyxins are more effective against Gram-negative bacteria than Gram-positive bacteria, with bactericidal action. | Polymyxin resistance is an exceptionally rare phenomenon, and is dependent on chromosomal mutations. However, a new plasmid-mediated gene, called mcr-1, has been discovered that confers resistance to colistin. |
| Polymixin E (colistin) | Bovine [35,36], Poultry [32,34], Caprine [35,36], Ovine [35,36], Swine [35,37] | |||
| QUINOLONES FIRST GENERATION Flumequin | Bovine [35,36,38], Poultry [32,34], Caprine [35,36], Ovine [35,36], Swine [35,37] | They are used in the following conditions:
| Quinolones inhibit bacterial topoisomerase enzymes, in particular, the following:
It has bactericidal activity. | Several resistance mechanisms have been detected:
|
| Nalidixic acid | Bovine [35,36,38] | |||
| Oxolinic acid | Bovine [35,36,38], Poultry [32,34], Swine [35,37], Ovine [35,36] | |||
| QUINOLONES SECOND GENERATION (FLUOROQUINOLONES) Ciprofloxacin Difloxacin | Bovine [35,36,38], Poultry [32,34], Swine [35,37] | Fluoroquinolones are critically important in the treatment of the following:
| ||
| Danofloxacin | Bovine [35,36,38], Caprine [35,36], Ovine [35,36], Swine [35,37] | |||
| Enrofloxacin Norfloxacin | Bovine [35,36,38], Poultry [32,34], Caprine [35,36], Ovine [35,36], Swine [35,37] | |||
| Marbofloxacin Orbifloxacin | Bovine [35,36,38], Swine [35,37] | |||
| Ofloxacin | Poultry [32,34], Swine [35,37] | |||
| QUINOXALINES Carbadox Olaquindox | Swine [35,37] | Quinoxalines (carbadox) is employed in the treatment of digestive diseases affecting swine, including swine dysentery. | NA | NA |
| SULFONAMIDES Phthalylsulfathiazole Sulfamonomethoxine Sulfamethoxine | Swine [35,37] | These classes alone or in combination are critically important in the treatment of a wide range of infections:
| Sulfonamides are structurally similar to PABA (para-aminobenzoic acid). They competitively inhibit dihydropterate synthetase enzyme (DPS), interrupting the synthesis of dihydrofolic acid, precursor of the folic acid. They have bacteriostatic action, but can have bactericidal action at high concentrations. Diaminopyrimidines, such as trimethoprim, are able to inhibit dihydrofolate reductase. The combination with sulfonamide is synergistic, and enhances the antibiotic action. | Resistance mechanisms to sulfonamides can be divided into the following:
|
| Sulfacetamide | Bovine [35,36,39], Ovine [35,36] | |||
| Sulfachlorpyridazine Sulfadimethoxazole | Bovine [35,36,39], Swine [35,37] | |||
| Sulfadiazine Sulfadimethoxine | Bovine [35,36,39], Caprine [35,36], Ovine [35,36], Swine [35,37] | |||
| Sulfadimidine Sulfamerazine | Bovine [35,36,39], Poultry [32,34], Caprine [35,36], Ovine [35,36], Swine [35,37] | |||
| (Sulfamethazine, Sulfadimerazine) Sulfadoxine | Bovine [35,36,39], Ovine [35,36], Swine [35,37] | |||
| Sulfafurazole | Bovine [35,36,39] | |||
| Sulfaguanidine | Caprine [35,36], Ovine [35,36] | |||
| Sulfanilamide Sulfaquinoxaline | Bovine [35,36,39], Caprine [35,36], Ovine [35,36] | |||
| Sulfapyridine | Bovine [35,36,39], Poultry [32,34], Swine [35,37] | |||
| SULFONAMIDES+ DIAMINOPYRIMIDINES Sulfamethoxypyridazine | Bovine [35,36,39], Swine [35,37] | |||
| Trimethoprim+ Sulfonamide | Bovine [35,36,39], Caprine [35,36], Ovine [35,36], Swine [35,37] | |||
| DIAMINOPYRIMIDINES Baquiloprim | Bovine [35,36,39], Swine [35,37] | |||
| Trimethoprim | Bovine [35,36,39], Caprine [35,36], Ovine [35,36], Swine [35,37] | |||
| STREPTOGRAMINS Virginiamycin | Bovine [35,36], Poultry [32,34], Ovine [35,36], Swine [35,37] | It plays an important role in preventing necrotic enteritis (Clostridium perfringens). | These antibiotics bind to the 50S subunit of bacterial ribosomes to stop protein production by blocking peptidyl transferases. | Cross-resistance with macrolides and lincosamides is classified as macrolide–lincosamide–streptogramin B (MLSB) resistance due to their similar mechanisms. |
| TETRACYCLINES Chlortetracycline Doxycycline Oxytetracycline Tetracycline | Bovine [35,36], Poultry [35,36], Caprine [35,36], Ovine [35,36], Swine [35,37] | They are used in the treatment of chlamydial infections. Tetracyclines are of critical importance in the treatment of animals against heartwater and anaplasmosis. | The have the capacity to bind reversibly to the bacterial 30S ribosomal subunit, impeding the process of ribosomal translation at the aminoacyl-tRNA acceptor (A) site on the mRNA ribosomal complex. Possess bacteriostatic activity. They have bacteriostatic action, while, at high concentrations, they can have bactericidal capacity. | The phenomenon of resistance to antibiotics is associated with two distinct mechanisms. The first is characterised by the acquisition of efflux pumps, plasmid- or transposon-mediated. The second is attributed to the synthesis of a protective protein, which functions by either preventing binding at the ribosomal target. |
| THIOSTREPTON Nosiheptide | Swine [35,37] | This class is currently used in the treatment of some dermatological conditions. | Functions by impeding the formation of the 70S initiation complex. It has an inhibitory effect: - The initiation G protein IF2; - Elongation, by interfering with the G proteins EF-Tu, which is required for the rapid binding of the aminoacyl-tRNA to the ribosome; - EF-G, which catalyses the translocation of the tRNA-mRNA complex from the A and P sites to the P and E sites. | The mechanism of resistance consists of the target site modification, a process catalysed by an enzyme action of a methyltransferase (NHR) belonging to the class SpoU. In particular, we are witnessing 2’-O-methylation of the 23S rRNA, where the elongation (F-G) factor performs its function. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Trinchera, M.; De Gaetano, S.; Sole, E.; Midiri, A.; Silvestro, S.; Mancuso, G.; Catalano, T.; Biondo, C. Antimicrobials in Livestock Farming and Resistance: Public Health Implications. Antibiotics 2025, 14, 606. https://doi.org/10.3390/antibiotics14060606
Trinchera M, De Gaetano S, Sole E, Midiri A, Silvestro S, Mancuso G, Catalano T, Biondo C. Antimicrobials in Livestock Farming and Resistance: Public Health Implications. Antibiotics. 2025; 14(6):606. https://doi.org/10.3390/antibiotics14060606
Chicago/Turabian StyleTrinchera, Marilena, Silvia De Gaetano, Elenoire Sole, Angelina Midiri, Serena Silvestro, Giuseppe Mancuso, Teresa Catalano, and Carmelo Biondo. 2025. "Antimicrobials in Livestock Farming and Resistance: Public Health Implications" Antibiotics 14, no. 6: 606. https://doi.org/10.3390/antibiotics14060606
APA StyleTrinchera, M., De Gaetano, S., Sole, E., Midiri, A., Silvestro, S., Mancuso, G., Catalano, T., & Biondo, C. (2025). Antimicrobials in Livestock Farming and Resistance: Public Health Implications. Antibiotics, 14(6), 606. https://doi.org/10.3390/antibiotics14060606






