The Role of Livestock Antibiotic Use in Microbiota Dysbiosis and Neuroinflammation
Abstract
1. Introduction
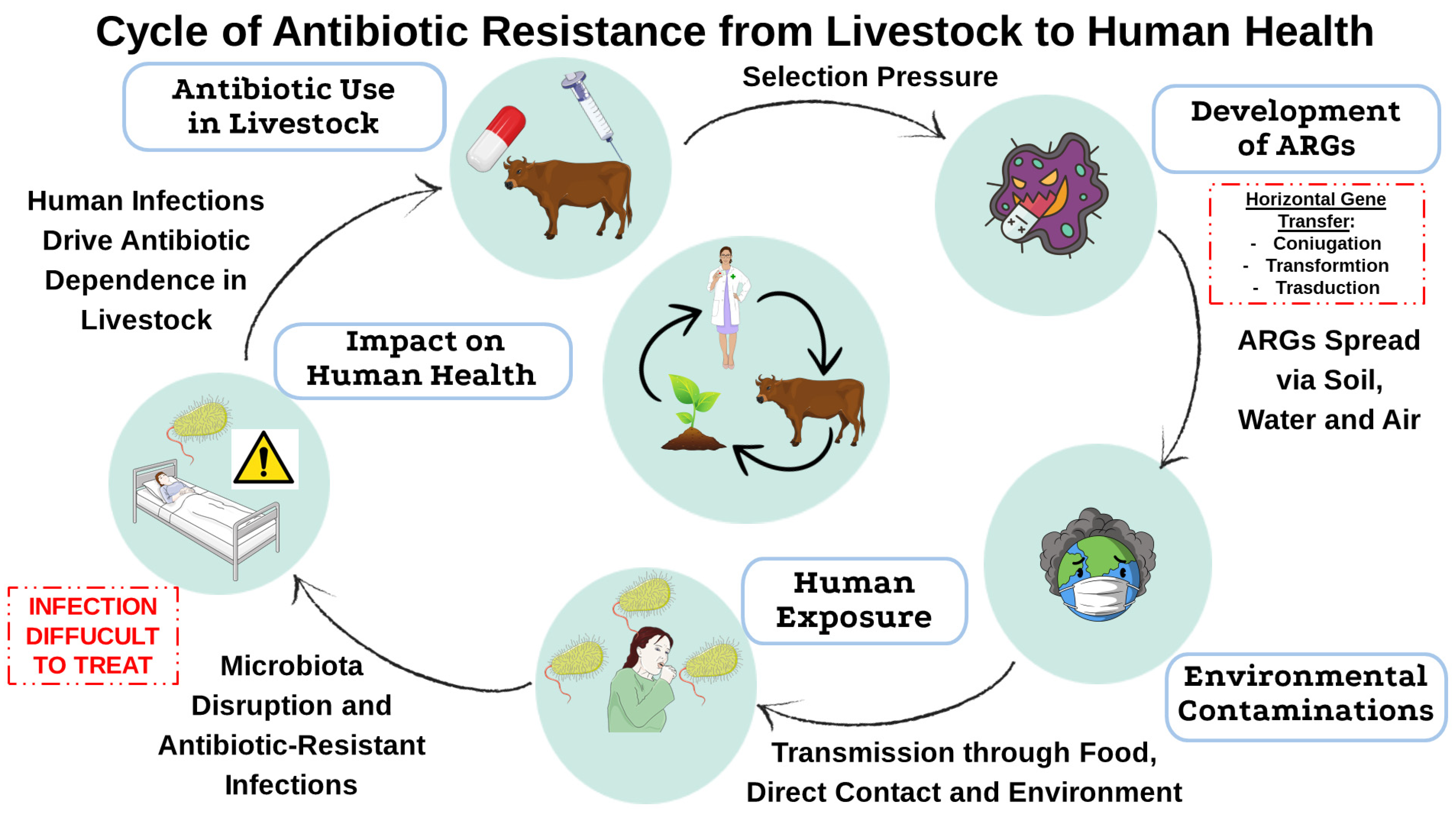
2. Use of Antibiotics in Livestock and Resistance Development
2.1. Mechanisms of Resistance Selection in Animals
2.2. Transmission Pathways of Antimicrobial Resistance to Humans
3. Antibiotic Resistance and Public Health: The Role of Intensive Agriculture
3.1. Environmental Dissemination of Resistance Genes from Intensive Farming
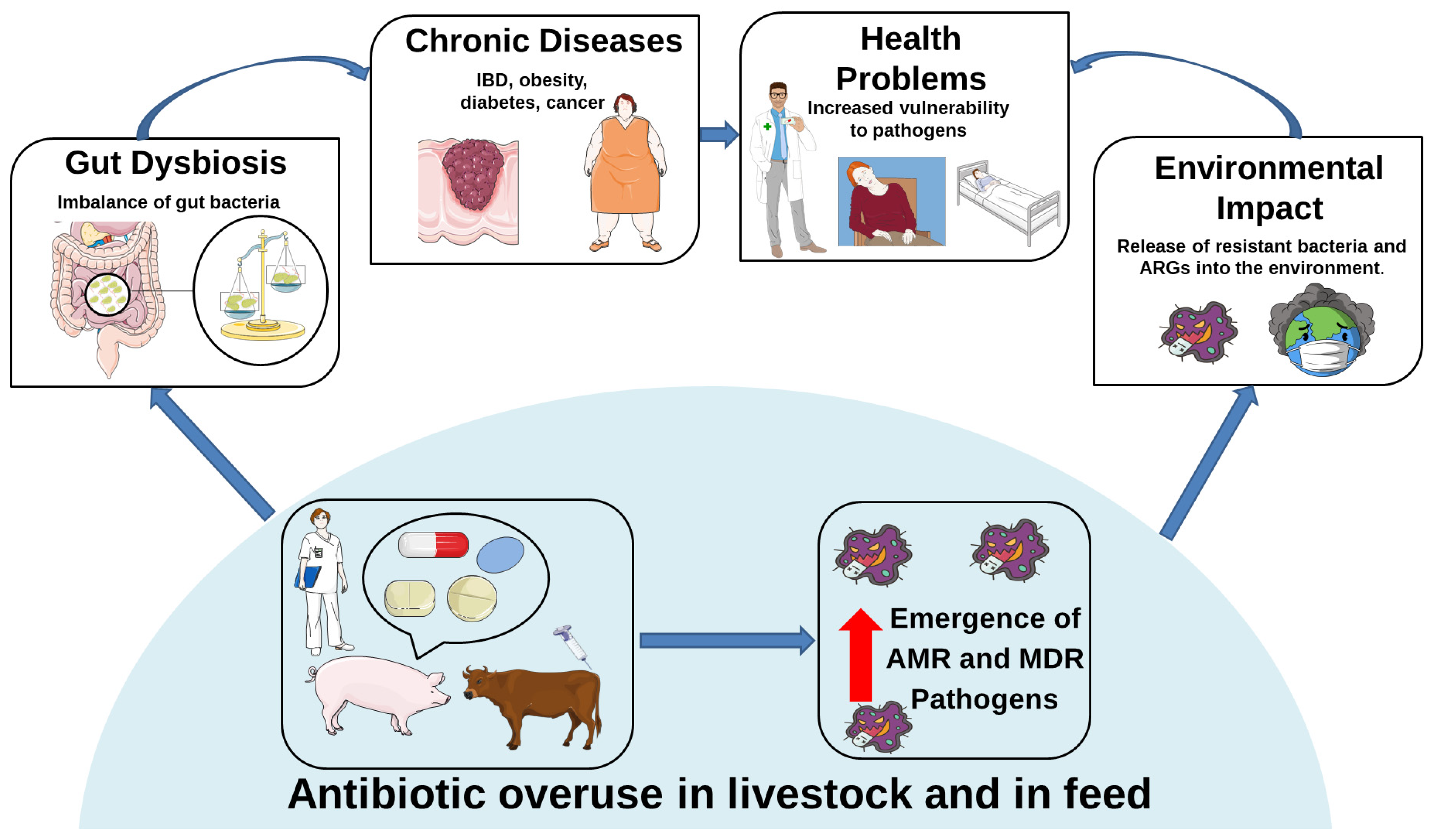
3.2. Antibiotic Resistance in Livestock and Its Effects on Animals and Human Gut Microbiota
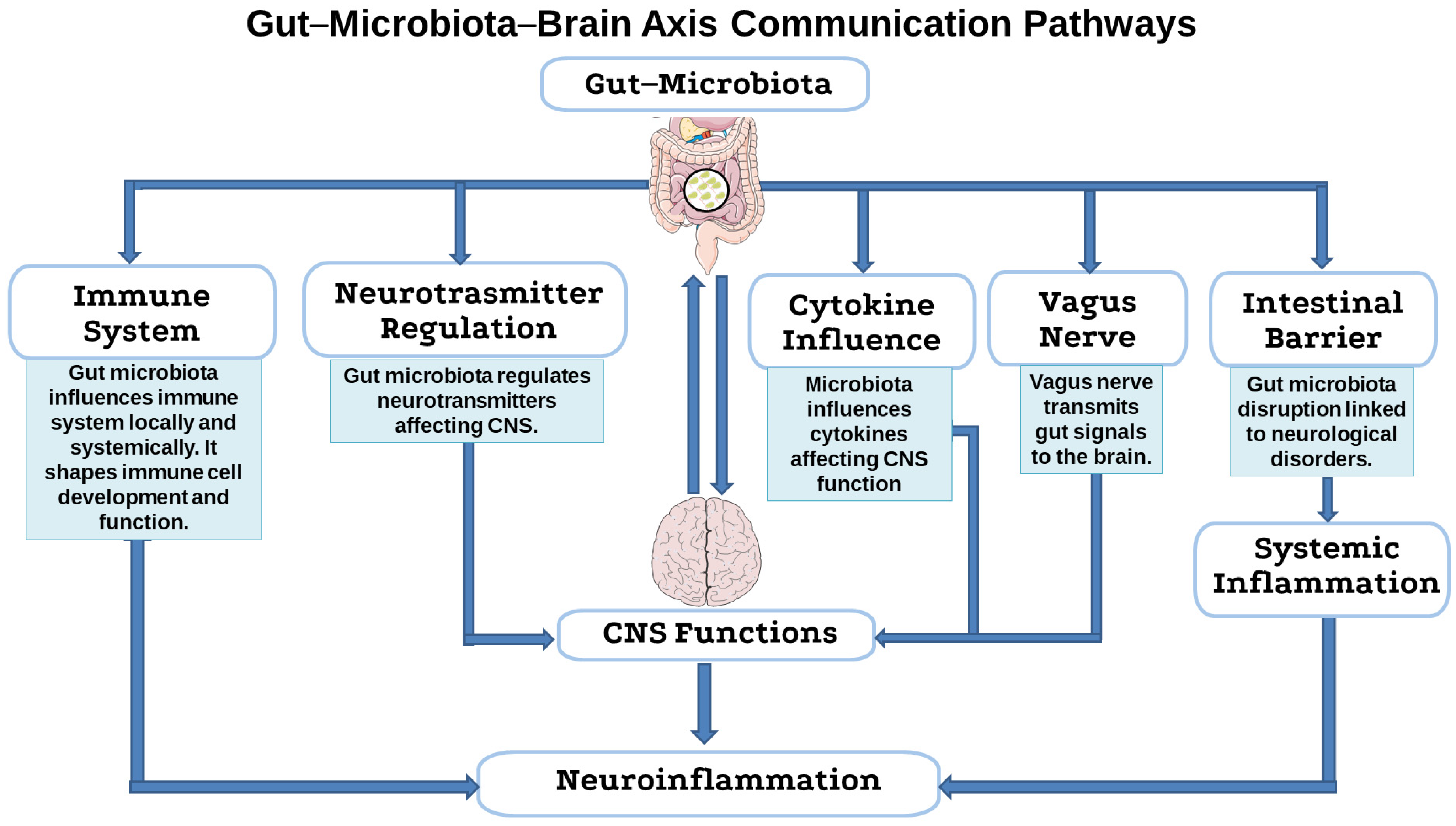
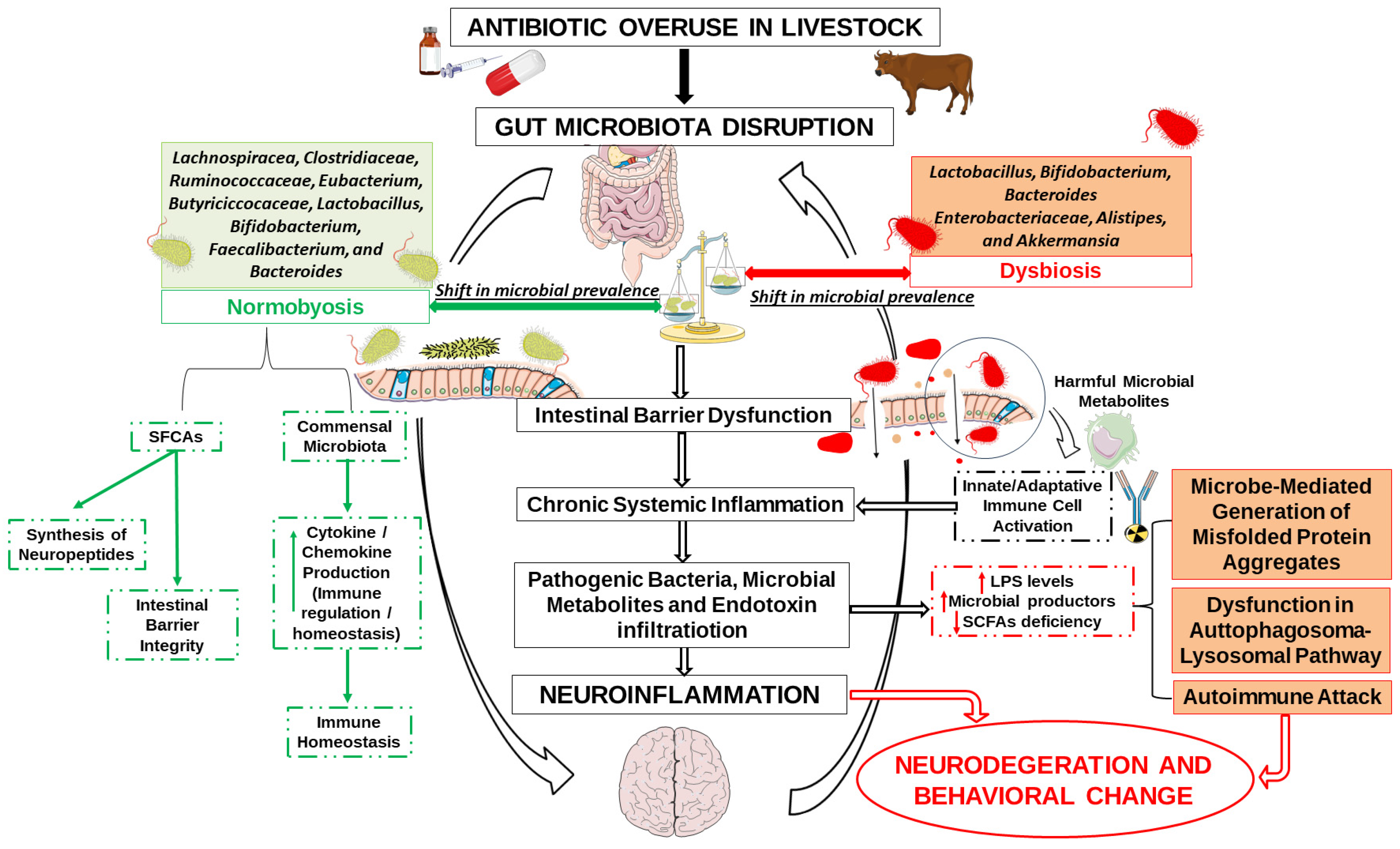
4. Antibiotic Use in Livestock as a Hidden Driver of Neuroinflammatory Risk
4.1. Microbial Balance and the Integrity of the Gut-Microbiota–Brain Axis
4.2. Antibiotic-Driven Dysbiosis as a Trigger for Neuroimmune Activation
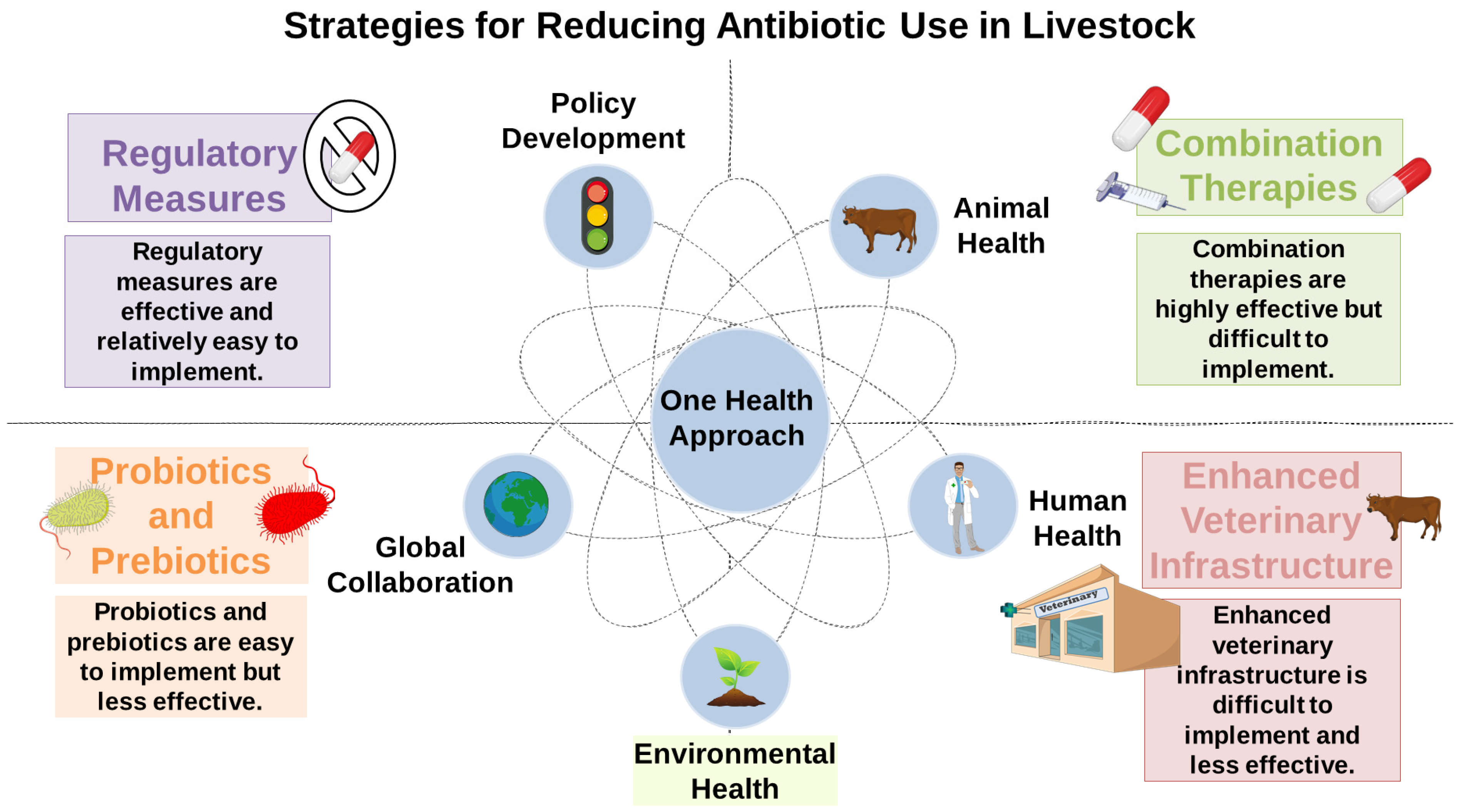
5. Mitigation Strategies
5.1. Reducing Antibiotic Use in Livestock
5.2. Probiotics and Prebiotics
5.3. “One Health” Approach
6. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ARGs | Antibiotic Resistance Genes |
| GyrA | DNA gyrase subunit A |
| ParC | Partition gene C |
| MDR | Multidrug Resistance |
| AcrAB-TolC | Acriflavine Resistance AB channel protein |
| ESBL | Extended-spectrum beta-lactam |
| CTX-M | Cefotaximase-Munich |
| IS | Insertion sequences |
| WHO | World Health Organization |
| CfxA | Cephalosporinase gene A |
| CepA | Cephalosporinase of Bacteroides |
| CblA | Cephalosporinase of Bacteroides Lineage A |
| KPC-2 | Klebsiella pneumoniae carbapenemase-2 |
| NDM-1 | New Delhi metal-β-lactamase-1 |
| MRSA | Methicillin-resistant Staphylococcus aureus |
| MCR-1 | Mobilized colistin resistance-1 |
| MGEs | Mobile Genetic Elements |
| FDA | Food and Drug Administration |
| TetM | Tetracycline resistance gene M |
| TetW | Tetracycline resistance gene W |
| TetQ | Tetracycline Resistance Gene Q |
| IBD | Inflammatory bowel disease |
| CNS | Central Nervous System |
| ENS | Enteric Nervous System |
| PD | Parkinson’s Disease |
| ADHD | Attention-Deficit/Hyperactivity Disorder |
| GABA | γ-aminobutyric acid |
| SCFAs | Short-chain fatty acids |
| LPS | lipopolysaccharides |
| Aβ | beta-amyloid |
| BBB | Blood–brain barrier |
References
- Pandey, S.; Doo, H.; Keum, G.B.; Kim, E.S.; Kwak, J.; Ryu, S.; Choi, Y.; Kang, J.; Kim, S.; Lee, N.R.; et al. Antibiotic resistance in livestock, environment and humans: One Health perspective. J. Anim. Sci. Technol. 2024, 66, 266–278. [Google Scholar] [CrossRef] [PubMed]
- Di Pietro, M.; Filardo, S.; Sessa, R. Editorial for the Special Issue “Antibacterial Activity of Drug-Resistant Strains”. Int. J. Mol. Sci. 2024, 25, 1878. [Google Scholar] [CrossRef] [PubMed]
- Van Boeckel, T.P.; Pires, J.; Silvester, R.; Zhao, C.; Song, J.; Criscuolo, N.G.; Gilbert, M.; Bonhoeffer, S.; Laxminarayan, R. Global trends in antimicrobial resistance in animals in low-and middle-income countries. Science 2019, 365, eaaw1944. [Google Scholar] [CrossRef]
- Aslam, B.; Khurshid, M.; Arshad, M.I.; Muzammil, S.; Rasool, M.; Yasmeen, N.; Shah, T.; Chaudhry, T.H.; Rasool, M.H.; Shahid, A. Antibiotic resistance: One health one world outlook. Front. Cell. Infect. Microbiol. 2021, 11, 771510. [Google Scholar] [CrossRef]
- Burow, E.; Käsbohrer, A. Risk factors for antimicrobial resistance in Escherichia coli in pigs receiving oral antimicrobial treatment: A systematic review. Microb. Drug Resist. 2017, 23, 194–205. [Google Scholar] [CrossRef]
- Marshall, B.M.; Levy, S.B. Food animals and antimicrobials: Impacts on human health. Clin. Microbiol. Rev. 2011, 24, 718–733. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Yang, Y.; Ma, L.; Ju, F.; Guo, F.; Tiedje, J.M.; Zhang, T. Metagenomic and network analysis reveal wide distribution and co-occurrence of environmental antibiotic resistance genes. ISME J. 2015, 9, 2490–2502. [Google Scholar] [CrossRef]
- Xu, C.; Kong, L.; Gao, H.; Cheng, X.; Wang, X. A review of current bacterial resistance to antibiotics in food animals. Front. Microbiol. 2022, 13, 822689. [Google Scholar] [CrossRef]
- Padhi, P.; Worth, C.; Zenitsky, G.; Jin, H.; Sambamurti, K.; Anantharam, V.; Kanthasamy, A.; Kanthasamy, A.G. Mechanistic insights into gut microbiome dysbiosis-mediated neuroimmune dysregulation and protein misfolding and clearance in the pathogenesis of chronic neurodegenerative disorders. Front. Neurosci. 2022, 16, 836605. [Google Scholar] [CrossRef]
- Marć, M.A.; Jastrząb, R.; Mytych, J. Does the gut microbial metabolome really matter? The connection between GUT metabolome and neurological disorders. Nutrients 2022, 14, 3967. [Google Scholar] [CrossRef]
- Çalışkan, G.; French, T.; Lacalle, S.E.; Del Angel, M.; Steffen, J.; Heimesaat, M.M.; Dunay, I.R.; Stork, O. Antibiotic-induced gut dysbiosis leads to activation of microglia and impairment of cholinergic gamma oscillations in the hippocampus. Brain Behav. Immun. 2022, 99, 203–217. [Google Scholar] [CrossRef] [PubMed]
- Reddy, V.P.; Aryal, P.; Robinson, S.; Rafiu, R.; Obrenovich, M.; Perry, G. Polyphenols in Alzheimer’s disease and in the gut–brain axis. Microorganisms 2020, 8, 199. [Google Scholar] [CrossRef]
- Obrenovich, M.; Jaworski, H.; Tadimalla, T.; Mistry, A.; Sykes, L.; Perry, G.; Bonomo, R.A. The role of the microbiota–gut–brain axis and antibiotics in ALS and neurodegenerative diseases. Microorganisms 2020, 8, 784. [Google Scholar] [CrossRef] [PubMed]
- Romano, S.; Savva, G.M.; Bedarf, J.R.; Charles, I.G.; Hildebrand, F.; Narbad, A. Meta-analysis of the Parkinson’s disease gut microbiome suggests alterations linked to intestinal inflammation. npj Park. Dis. 2021, 7, 27. [Google Scholar] [CrossRef]
- Van Boeckel, T.P.; Glennon, E.E.; Chen, D.; Gilbert, M.; Robinson, T.P.; Grenfell, B.T.; Levin, S.A.; Bonhoeffer, S.; Laxminarayan, R. Reducing antimicrobial use in food animals. Science 2017, 357, 1350–1352. [Google Scholar] [CrossRef]
- Catry, B.; Laevens, H.; Devriese, L.; Opsomer, G.; De Kruif, A. Antimicrobial resistance in livestock. J. Vet. Pharmacol. Ther. 2003, 26, 81–93. [Google Scholar] [CrossRef]
- Robinson, T.P.; Bu, D.P.; Carrique-Mas, J.; Fèvre, E.M.; Gilbert, M.; Grace, D.; Hay, S.I.; Jiwakanon, J.; Kakkar, M.; Kariuki, S.; et al. Antibiotic resistance: Mitigation opportunities in livestock sector development. Anim. Int. J. Anim. Biosci. 2017, 11, 1–3. [Google Scholar] [CrossRef] [PubMed]
- Velazquez-Meza, M.E.; Galarde-López, M.; Carrillo-Quiróz, B.; Alpuche-Aranda, C.M. Antimicrobial resistance: One health approach. Vet. World 2022, 15, 743. [Google Scholar] [CrossRef]
- Collignon, P.J.; McEwen, S.A. One health—Its importance in helping to better control antimicrobial resistance. Trop. Med. Infect. Dis. 2019, 4, 22. [Google Scholar] [CrossRef]
- O’Neill, J. Tackling Drug-Resistant Infections Globally: Final Report and Recommendations; Government of the United Kingdom: London, UK, 2016.
- Rysz, M.; Alvarez, P.J. Amplification and attenuation of tetracycline resistance in soil bacteria: Aquifer column experiments. Water Res. 2004, 38, 3705–3712. [Google Scholar] [CrossRef]
- Vidovic, N.; Vidovic, S. Antimicrobial Resistance and Food Animals: Influence of Livestock Environment on the Emergence and Dissemination of Antimicrobial Resistance. Antibiotics 2020, 9, 52. [Google Scholar] [CrossRef] [PubMed]
- Redgrave, L.S.; Sutton, S.B.; Webber, M.A.; Piddock, L.J. Fluoroquinolone resistance: Mechanisms, impact on bacteria, and role in evolutionary success. Trends Microbiol. 2014, 22, 438–445. [Google Scholar] [CrossRef] [PubMed]
- Vidovic, S.; An, R.; Rendahl, A. Molecular and physiological characterization of fluoroquinolone-highly resistant Salmonella enteritidis strains. Front. Microbiol. 2019, 10, 729. [Google Scholar] [CrossRef]
- Hartog, E.; Ben-Shalom, L.; Shachar, D.; Matthews, K.R.; Yaron, S. Regulation of marA, soxS, rob, acrAB and micF in Salmonella enterica serovar Typhimurium. Microbiol. Immunol. 2008, 52, 565–574. [Google Scholar] [CrossRef]
- Guo, M.S.; Updegrove, T.B.; Gogol, E.B.; Shabalina, S.A.; Gross, C.A.; Storz, G. MicL, a new σE-dependent sRNA, combats envelope stress by repressing synthesis of Lpp, the major outer membrane lipoprotein. Genes Dev. 2014, 28, 1620–1634. [Google Scholar] [CrossRef] [PubMed]
- Orlek, A.; Anjum, M.F.; Mather, A.E.; Stoesser, N.; Walker, A.S. Factors associated with plasmid antibiotic resistance gene carriage revealed using large-scale multivariable analysis. Sci. Rep. 2023, 13, 2500. [Google Scholar] [CrossRef]
- von Wintersdorff, C.J.; Penders, J.; van Niekerk, J.M.; Mills, N.D.; Majumder, S.; van Alphen, L.B.; Savelkoul, P.H.; Wolffs, P.F. Dissemination of Antimicrobial Resistance in Microbial Ecosystems through Horizontal Gene Transfer. Front. Microbiol. 2016, 7, 173. [Google Scholar] [CrossRef]
- Thakur, S.; Pokhrel, N.; Sharma, M. Prevalence of multidrug resistant enterobacteriaceae and extended spectrum β lactamase producing Escherichia coli in urinary tract infection. Res. J. Pharm. Biol. Chem. Sci. 2013, 4, 1615–1624. [Google Scholar]
- Herindrainy, P.; Randrianirina, F.; Ratovoson, R.; Ratsima Hariniana, E.; Buisson, Y.; Genel, N.; Decre, D.; Arlet, G.; Talarmin, A.; Richard, V. Rectal carriage of extended-spectrum beta-lactamase-producing gram-negative bacilli in community settings in Madagascar. PLoS ONE 2011, 6, e22738. [Google Scholar] [CrossRef]
- Darphorn, T.S.; Bel, K.; Koenders-van Sint Anneland, B.B.; Brul, S.; Ter Kuile, B.H. Antibiotic resistance plasmid composition and architecture in Escherichia coli isolates from meat. Sci. Rep. 2021, 11, 2136. [Google Scholar] [CrossRef]
- Gibson, P.S.; Bexkens, E.; Zuber, S.; Cowley, L.A.; Veening, J.W. The acquisition of clinically relevant amoxicillin resistance in Streptococcus pneumoniae requires ordered horizontal gene transfer of four loci. PLoS Pathog. 2022, 18, e1010727. [Google Scholar] [CrossRef] [PubMed]
- Fani, F.; Leprohon, P.; Zhanel, G.G.; Bergeron, M.G.; Ouellette, M. Genomic analyses of DNA transformation and penicillin resistance in Streptococcus pneumoniae clinical isolates. Antimicrob. Agents Chemother. 2014, 58, 1397–1403. [Google Scholar] [CrossRef] [PubMed]
- Cafini, F.; del Campo, R.; Alou, L.; Sevillano, D.; Morosini, M.I.; Baquero, F.; Prieto, J. Alterations of the penicillin-binding proteins and murM alleles of clinical Streptococcus pneumoniae isolates with high-level resistance to amoxicillin in Spain. J. Antimicrob. Chemother. 2006, 57, 224–229. [Google Scholar] [CrossRef] [PubMed]
- Shkoporov, A.N.; Hill, C. Bacteriophages of the human gut: The “known unknown” of the microbiome. Cell Host Microbe 2019, 25, 195–209. [Google Scholar] [CrossRef]
- Borodovich, T.; Shkoporov, A.N.; Ross, R.P.; Hill, C. Phage-mediated horizontal gene transfer and its implications for the human gut microbiome. Gastroenterol. Rep. 2022, 10, goac012. [Google Scholar] [CrossRef]
- Sagrillo, C.; Changey, F.; Bellanger, X. Bacteriophages vehiculate a high amount of antibiotic resistance determinants of bacterial origin in the Orne River ecosystem. Environ. Microbiol. 2022, 24, 4317–4328. [Google Scholar] [CrossRef]
- Zhang, Y.; Guo, Y.; Qiu, T.; Gao, M.; Wang, X. Bacteriophages: Underestimated vehicles of antibiotic resistance genes in the soil. Front. Microbiol. 2022, 13, 936267. [Google Scholar] [CrossRef]
- Wang, Q.; Wang, M.; Yang, Q.; Feng, L.; Zhang, H.; Wang, R.; Wang, R. The role of bacteriophages in facilitating the horizontal transfer of antibiotic resistance genes in municipal wastewater treatment plants. Water Res. 2025, 268, 122776. [Google Scholar] [CrossRef]
- Livermore, D.M.; Pitt, T.L.; Jones, C.S.; Crees-Morris, J.A.; Williams, R.J. PSE-4 beta lactamase: A serotype-specific enzyme in Pseudomonas aeruginosa. J. Med. Microbiol. 1985, 19, 45–53. [Google Scholar] [CrossRef]
- Blahová, J.; Králiková, K.; Krcméry, V.; Jezek, P. Low-Frequency transduction of imipenem resistance and high-frequency transduction of ceftazidime and aztreonam resistance by the bacteriophage AP-151 isolated from a Pseudomonas aeruginosa strain. J. Chemother. 2000, 12, 482–486. [Google Scholar] [CrossRef]
- Partridge, S.R.; Kwong, S.M.; Firth, N.; Jensen, S.O. Mobile Genetic Elements Associated with Antimicrobial Resistance. Clin. Microbiol. Rev. 2018, 31, e00088-17. [Google Scholar] [CrossRef] [PubMed]
- Hegstad, K.; Mikalsen, T.; Coque, T.M.; Werner, G.; Sundsfjord, A. Mobile genetic elements and their contribution to the emergence of antimicrobial resistant Enterococcus faecalis and Enterococcus faecium. Clin. Microbiol. Infect. Off. Publ. Eur. Soc. Clin. Microbiol. Infect. Dis. 2010, 16, 541–554. [Google Scholar] [CrossRef] [PubMed]
- Coburn, P.S.; Baghdayan, A.S.; Dolan, G.; Shankar, N. An AraC-type transcriptional regulator encoded on the Enterococcus faecalis pathogenicity island contributes to pathogenesis and intracellular macrophage survival. Infect. Immun. 2008, 76, 5668–5676. [Google Scholar] [CrossRef] [PubMed]
- Périchon, B.; Courvalin, P. VanA-type vancomycin-resistant Staphylococcus aureus. Antimicrob. Agents Chemother. 2009, 53, 4580–4587. [Google Scholar] [CrossRef] [PubMed]
- Souque, C.; Escudero, J.A.; MacLean, R.C. Integron activity accelerates the evolution of antibiotic resistance. eLife 2021, 10, e62474. [Google Scholar] [CrossRef]
- Reyes, A.; Bello, H.; Domínguez, M.; Mella, S.; Zemelman, R.; González, G. Prevalence and types of class 1 integrons in aminoglycoside-resistant Enterobacteriaceae from several Chilean hospitals. J. Antimicrob. Chemother. 2003, 51, 317–321. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Qian, X.; Gu, J.; Sun, W.; Wang, X.J.; Su, J.Q.; Stedfeld, R. Diversity, abundance, and persistence of antibiotic resistance genes in various types of animal manure following industrial composting. J. Hazard. Mater. 2018, 344, 716–722. [Google Scholar] [CrossRef]
- Ebmeyer, S.; Kristiansson, E.; Larsson, D.G.J. A framework for identifying the recent origins of mobile antibiotic resistance genes. Commun. Biol. 2021, 4, 8. [Google Scholar] [CrossRef]
- World Health Organization. Global Antimicrobial Resistance and Use Surveillance System (GLASS) Report 2022; World Health Organization: Geneva, Switzerland, 2022. [Google Scholar]
- Witte, W. Selective pressure by antibiotic use in livestock. Int. J. Antimicrob. Agents 2000, 16 (Suppl. 1), 19–24. [Google Scholar] [CrossRef]
- Verkola, M.; Pietola, E.; Jarvinen, A.; Lindqvist, K.; Kinnunen, P.M.; Heikinheimo, A. Low prevalence of zoonotic multidrug-resistant bacteria in veterinarians in a country with prudent use of antimicrobials in animals. Zoonoses Public Health 2019, 66, 667–678. [Google Scholar] [CrossRef]
- Forsberg, K.; Woodworth, K.; Walters, M.; Berkow, E.L.; Jackson, B.; Chiller, T.; Vallabhaneni, S. Candida auris: The recent emergence of a multidrug-resistant fungal pathogen. Med. Mycol. 2019, 57, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Campagna, R.A.; Kelly, E.A.; Vugia, D.J.; Berman Watson, H.F.; Browne, C.S.; Lau, J.K.; Fritz, C.L. Clinical and Epidemiologic Review of Capnocytophaga Spp. Infections Identified at a Public Health Reference Laboratory-California, 2005–2021. Zoonoses Public Health 2025, 72, 330–336. [Google Scholar] [CrossRef] [PubMed]
- Blair, J.M.; Webber, M.A.; Baylay, A.J.; Ogbolu, D.O.; Piddock, L.J. Molecular mechanisms of antibiotic resistance. Nat. Rev. Microbiol. 2015, 13, 42–51. [Google Scholar] [CrossRef]
- Van Boeckel, T.P.; Brower, C.; Gilbert, M.; Grenfell, B.T.; Levin, S.A.; Robinson, T.P.; Teillant, A.; Laxminarayan, R. Global trends in antimicrobial use in food animals. Proc. Natl. Acad. Sci. USA 2015, 112, 5649–5654. [Google Scholar] [CrossRef]
- Nadimpalli, M.L.; Stewart, J.R.; Pierce, E.; Pisanic, N.; Love, D.C.; Hall, D.; Larsen, J.; Carroll, K.C.; Tekle, T.; Perl, T.M. Face mask use and persistence of livestock-associated Staphylococcus aureus nasal carriage among industrial hog operation workers and household contacts, USA. Environ. Health Perspect. 2018, 126, 127005. [Google Scholar] [CrossRef] [PubMed]
- Nadimpalli, M.; Stewart, J.R.; Pierce, E.; Pisanic, N.; Love, D.C.; Hall, D.; Larsen, J.; Carroll, K.C.; Tekle, T.; Perl, T.M. Livestock-associated, antibiotic-resistant Staphylococcus aureus nasal carriage and recent skin and soft tissue infection among industrial hog operation workers. PLoS ONE 2016, 11, e0165713. [Google Scholar] [CrossRef]
- Jackson, J.; Villarroel, A. A survey of the risk of zoonoses for veterinarians. Zoonoses Public Health 2012, 59, 193–201. [Google Scholar] [CrossRef]
- Thanner, S.; Drissner, D.; Walsh, F. Antimicrobial resistance in agriculture. MBio 2016, 7, e02227-15. [Google Scholar] [CrossRef]
- Cantón, R.; Coque, T.M. The CTX-M β-lactamase pandemic. Curr. Opin. Microbiol. 2006, 9, 466–475. [Google Scholar] [CrossRef]
- Falagas, M.E.; Karageorgopoulos, D.E.; Nordmann, P. Therapeutic options for infections with Enterobacteriaceae producing carbapenem-hydrolyzing enzymes. Future Microbiol. 2011, 6, 653–666. [Google Scholar] [CrossRef]
- Liu, Y.-Y.; Wang, Y.; Walsh, T.R.; Yi, L.-X.; Zhang, R.; Spencer, J.; Doi, Y.; Tian, G.; Dong, B.; Huang, X. Emergence of plasmid-mediated colistin resistance mechanism MCR-1 in animals and human beings in China: A microbiological and molecular biological study. Lancet Infect. Dis. 2016, 16, 161–168. [Google Scholar] [CrossRef]
- Wertheim, H.; Van Nguyen, K.; Hara, G.L.; Gelband, H.; Laxminarayan, R.; Mouton, J.; Cars, O. Global survey of polymyxin use: A call for international guidelines. J. Glob. Antimicrob. Resist. 2013, 1, 131–134. [Google Scholar] [CrossRef]
- Ding, D.; Zhu, J.; Gao, Y.; Yang, F.; Ma, Y.; Cheng, X.; Li, J.; Dong, P.; Yang, H.; Chen, S. Effect of cattle farm exposure on oropharyngeal and gut microbial communities and antibiotic resistance genes in workers. Sci. Total Environ. 2022, 806, 150685. [Google Scholar] [CrossRef] [PubMed]
- Song, L.; Wang, C.; Jiang, G.; Ma, J.; Li, Y.; Chen, H.; Guo, J. Bioaerosol is an important transmission route of antibiotic resistance genes in pig farms. Environ. Int. 2021, 154, 106559. [Google Scholar] [CrossRef]
- Madsen, A.M.; Kurdi, I.; Feld, L.; Tendal, K. Airborne MRSA and total Staphylococcus aureus as associated with particles of different sizes on pig farms. Ann. Work Expo. Health 2018, 62, 966–977. [Google Scholar] [CrossRef]
- Mather, A.; Reid, S.; Maskell, D.; Parkhill, J.; Fookes, M.; Harris, S.; Brown, D.; Coia, J.; Mulvey, M.; Gilmour, M. Distinguishable epidemics of multidrug-resistant Salmonella Typhimurium DT104 in different hosts. Science 2013, 341, 1514–1517. [Google Scholar] [CrossRef] [PubMed]
- Zhou, S.Y.; Zhu, D.; Giles, M.; Daniell, T.; Neilson, R.; Yang, X.R. Does reduced usage of antibiotics in livestock production mitigate the spread of antibiotic resistance in soil, earthworm guts, and the phyllosphere? Environ. Int. 2020, 136, 105359. [Google Scholar] [CrossRef] [PubMed]
- Checcucci, A.; Buscaroli, E.; Modesto, M.; Luise, D.; Blasioli, S.; Scarafile, D.; Di Vito, M.; Bugli, F.; Trevisi, P.; Braschi, I.; et al. The swine waste resistome: Spreading and transfer of antibiotic resistance genes in Escherichia coli strains and the associated microbial communities. Ecotoxicol. Environ. Saf. 2024, 283, 116774. [Google Scholar] [CrossRef]
- Zalewska, M.; Blazejewska, A.; Czapko, A.; Popowska, M. Antibiotics and Antibiotic Resistance Genes in Animal Manure—Consequences of Its Application in Agriculture. Front. Microbiol. 2021, 12, 610656. [Google Scholar] [CrossRef]
- Khan, G.A.; Berglund, B.; Khan, K.M.; Lindgren, P.-E.; Fick, J. Occurrence and abundance of antibiotics and resistance genes in rivers, canal and near drug formulation facilities—A study in Pakistan. PLoS ONE 2013, 8, e62712. [Google Scholar] [CrossRef]
- Zhang, Y.J.; Hu, H.W.; Gou, M.; Wang, J.T.; Chen, D.; He, J.Z. Temporal succession of soil antibiotic resistance genes following application of swine, cattle and poultry manures spiked with or without antibiotics. Environ. Pollut. 2017, 231, 1621–1632. [Google Scholar] [CrossRef] [PubMed]
- Alhassan, M.Y.; Kabara, M.K.; Ahmad, A.A.; Abdulsalam, J.; Habib, H.I. Revisiting antibiotic stewardship: Veterinary contributions to combating antimicrobial resistance globally. Bull. Natl. Res. Cent. 2025, 49, 25. [Google Scholar] [CrossRef]
- O’Neill, J. Review on antimicrobial resistance. Antimicrob. Resist. Tackling A Crisis Health Wealth Nations 2014, 2014, 1–16. [Google Scholar]
- Duan, M.; Gu, J.; Wang, X.; Li, Y.; Zhang, R.; Hu, T.; Zhou, B. Factors that affect the occurrence and distribution of antibiotic resistance genes in soils from livestock and poultry farms. Ecotoxicol. Environ. Saf. 2019, 180, 114–122. [Google Scholar] [CrossRef] [PubMed]
- Peng, S.; Zhang, H.; Song, D.; Chen, H.; Lin, X.; Wang, Y.; Ji, L. Distribution of antibiotic, heavy metals and antibiotic resistance genes in livestock and poultry feces from different scale of farms in Ningxia, China. J. Hazard. Mater. 2022, 440, 129719. [Google Scholar] [CrossRef]
- Zhu, Y.G.; Johnson, T.A.; Su, J.Q.; Qiao, M.; Guo, G.X.; Stedtfeld, R.D.; Hashsham, S.A.; Tiedje, J.M. Diverse and abundant antibiotic resistance genes in Chinese swine farms. Proc. Natl. Acad. Sci. USA 2013, 110, 3435–3440. [Google Scholar] [CrossRef]
- Chen, Q.; An, X.; Li, H.; Su, J.; Ma, Y.; Zhu, Y.G. Long-term field application of sewage sludge increases the abundance of antibiotic resistance genes in soil. Environ. Int. 2016, 92–93, 1–10. [Google Scholar] [CrossRef]
- Broom, L.J. The sub-inhibitory theory for antibiotic growth promoters. Poult. Sci. 2017, 96, 3104–3108. [Google Scholar] [CrossRef]
- Mahmoud, M.A.; Abdel-Mohsein, H.S. Hysterical tetracycline in intensive poultry farms accountable for substantial gene resistance, health and ecological risk in Egypt-manure and fish. Environ. Pollut. 2019, 255, 113039. [Google Scholar] [CrossRef]
- Groussin, M.; Mazel, F.; Alm, E.J. Co-evolution and co-speciation of host-gut bacteria systems. Cell Host Microbe 2020, 28, 12–22. [Google Scholar] [CrossRef]
- Schwartz, D.J.; Langdon, A.E.; Dantas, G. Understanding the impact of antibiotic perturbation on the human microbiome. Genome Med. 2020, 12, 82. [Google Scholar] [CrossRef] [PubMed]
- Tsunoda, S.M.; Gonzales, C.; Jarmusch, A.K.; Momper, J.D.; Ma, J.D. Contribution of the gut microbiome to drug disposition, pharmacokinetic and pharmacodynamic variability. Clin. Pharmacokinet. 2021, 60, 971–984. [Google Scholar] [CrossRef] [PubMed]
- Shreiner, A.B.; Kao, J.Y.; Young, V.B. The gut microbiome in health and in disease. Curr. Opin. Gastroenterol. 2015, 31, 69–75. [Google Scholar] [CrossRef]
- Krajmalnik-Brown, R.; Ilhan, Z.E.; Kang, D.W.; DiBaise, J.K. Effects of gut microbes on nutrient absorption and energy regulation. Nutr. Clin. Pract. 2012, 27, 201–214. [Google Scholar] [CrossRef]
- Dobrzanska, D.A.; Lamaudière, M.T.F.; Rollason, J.; Acton, L.; Duncan, M.; Compton, S.; Simms, J.; Weedall, G.D.; Morozov, I.Y. Preventive antibiotic treatment of calves: Emergence of dysbiosis causing propagation of obese state-associated and mobile multidrug resistance-carrying bacteria. Microb. Biotechnol. 2020, 13, 669–682. [Google Scholar] [CrossRef]
- Shin, N.-R.; Whon, T.W.; Bae, J.-W. Proteobacteria: Microbial signature of dysbiosis in gut microbiota. Trends Biotechnol. 2015, 33, 496–503. [Google Scholar] [CrossRef] [PubMed]
- Angelakis, E.; Armougom, F.; Million, M.; Raoult, D. The relationship between gut microbiota and weight gain in humans. Future Microbiol. 2012, 7, 91–109. [Google Scholar] [CrossRef]
- Million, M.; Lagier, J.-C.; Yahav, D.; Paul, M. Gut bacterial microbiota and obesity. Clin. Microbiol. Infect. 2013, 19, 305–313. [Google Scholar] [CrossRef]
- Duboc, H.; Rajca, S.; Rainteau, D.; Benarous, D.; Maubert, M.-A.; Quervain, E.; Thomas, G.; Barbu, V.; Humbert, L.; Despras, G. Connecting dysbiosis, bile-acid dysmetabolism and gut inflammation in inflammatory bowel diseases. Gut 2013, 62, 531–539. [Google Scholar] [CrossRef]
- Zackular, J.P.; Baxter, N.T.; Iverson, K.D.; Sadler, W.D.; Petrosino, J.F.; Chen, G.Y.; Schloss, P.D. The gut microbiome modulates colon tumorigenesis. MBio 2013, 4, e00692-13. [Google Scholar] [CrossRef]
- DeGruttola, A.K.; Low, D.; Mizoguchi, A.; Mizoguchi, E. Current understanding of dysbiosis in disease in human and animal models. Inflamm. Bowel Dis. 2016, 22, 1137–1150. [Google Scholar] [CrossRef] [PubMed]
- Stewart, A.S.; Pratt-Phillips, S.; Gonzalez, L.M. Alterations in intestinal permeability: The role of the “leaky gut” in health and disease. J. Equine Vet. Sci. 2017, 52, 10–22. [Google Scholar] [CrossRef] [PubMed]
- Yi, X.; Cha, M. Gut Dysbiosis has the potential to reduce the sexual attractiveness of mouse female. Front. Microbiol. 2022, 13, 916766. [Google Scholar] [CrossRef] [PubMed]
- Rudolph, K.; Schneider, D.; Fichtel, C.; Daniel, R.; Heistermann, M.; Kappeler, P.M. Drivers of gut microbiome variation within and between groups of a wild Malagasy primate. Microbiome 2022, 10, 28. [Google Scholar] [CrossRef]
- Johnson, K.V.-A.; Watson, K.K.; Dunbar, R.I.; Burnet, P.W. Sociability in a non-captive macaque population is associated with beneficial gut bacteria. Front. Microbiol. 2022, 13, 1032495. [Google Scholar] [CrossRef]
- Ahmed, H.; Leyrolle, Q.; Koistinen, V.; Kärkkäinen, O.; Layé, S.; Delzenne, N.; Hanhineva, K. Microbiota-derived metabolites as drivers of gut-brain communication. Gut Microbes 2022, 14, 2102878. [Google Scholar] [CrossRef]
- Liu, L.; Huh, J.R.; Shah, K. Microbiota and the gut-brain-axis: Implications for new therapeutic design in the CNS. EBioMedicine 2022, 77, 103908. [Google Scholar] [CrossRef]
- Seethaler, B.; Nguyen, N.K.; Basrai, M.; Kiechle, M.; Walter, J.; Delzenne, N.M.; Bischoff, S.C. Short-chain fatty acids are key mediators of the favorable effects of the Mediterranean diet on intestinal barrier integrity: Data from the randomized controlled LIBRE trial. Am. J. Clin. Nutr. 2022, 116, 928–942. [Google Scholar] [CrossRef]
- Li, D.; Zhou, J.; Wang, L.; Gong, Z.; Le, H.; Huang, Y.; Xu, C.; Tian, C.; Cai, W.; Wu, J. Gut microbial metabolite deoxycholic acid facilitates Th17 differentiation through modulating cholesterol biosynthesis and participates in high-fat diet-associated colonic inflammation. Cell Biosci. 2023, 13, 186. [Google Scholar] [CrossRef]
- Wang, Q.; Yang, Q.; Liu, X. The microbiota–gut–brain axis and neurodevelopmental disorders. Protein Cell 2023, 14, 762–775. [Google Scholar] [CrossRef]
- Needham, B.D.; Funabashi, M.; Adame, M.D.; Wang, Z.; Boktor, J.C.; Haney, J.; Wu, W.-L.; Rabut, C.; Ladinsky, M.S.; Hwang, S.-J. A gut-derived metabolite alters brain activity and anxiety behaviour in mice. Nature 2022, 602, 647–653. [Google Scholar] [CrossRef] [PubMed]
- Loh, J.S.; Mak, W.Q.; Tan, L.K.S.; Ng, C.X.; Chan, H.H.; Yeow, S.H.; Foo, J.B.; Ong, Y.S.; How, C.W.; Khaw, K.Y. Microbiota–gut–brain axis and its therapeutic applications in neurodegenerative diseases. Signal Transduct. Target. Ther. 2024, 9, 37. [Google Scholar] [PubMed]
- Gribble, F.M.; Reimann, F. Enteroendocrine cells: Chemosensors in the intestinal epithelium. Annu. Rev. Physiol. 2016, 78, 277–299. [Google Scholar] [CrossRef]
- Kaelberer, M.M.; Rupprecht, L.E.; Liu, W.W.; Weng, P.; Bohórquez, D.V. Neuropod cells: The emerging biology of gut-brain sensory transduction. Annu. Rev. Neurosci. 2020, 43, 337–353. [Google Scholar] [CrossRef] [PubMed]
- Strandwitz, P.; Kim, K.H.; Terekhova, D.; Liu, J.K.; Sharma, A.; Levering, J.; McDonald, D.; Dietrich, D.; Ramadhar, T.R.; Lekbua, A. GABA-modulating bacteria of the human gut microbiota. Nat. Microbiol. 2019, 4, 396–403. [Google Scholar] [CrossRef]
- Engevik, M.A.; Luck, B.; Visuthranukul, C.; Ihekweazu, F.D.; Engevik, A.C.; Shi, Z.; Danhof, H.A.; Chang-Graham, A.L.; Hall, A.; Endres, B.T. Human-derived Bifidobacterium dentium modulates the mammalian serotonergic system and gut–brain axis. Cell. Mol. Gastroenterol. Hepatol. 2021, 11, 221–248. [Google Scholar] [CrossRef]
- Wang, Y.; Tong, Q.; Ma, S.-R.; Zhao, Z.-X.; Pan, L.-B.; Cong, L.; Han, P.; Peng, R.; Yu, H.; Lin, Y. Oral berberine improves brain dopa/dopamine levels to ameliorate Parkinson’s disease by regulating gut microbiota. Signal Transduct. Target. Ther. 2021, 6, 77. [Google Scholar] [CrossRef] [PubMed]
- Miquel-Rio, L.; Alarcón-Arís, D.; Torres-López, M.; Cóppola-Segovia, V.; Pavia-Collado, R.; Paz, V.; Ruiz-Bronchal, E.; Campa, L.; Casal, C.; Montefeltro, A. Human α-synuclein overexpression in mouse serotonin neurons triggers a depressive-like phenotype. Rescue by oligonucleotide therapy. Transl. Psychiatry 2022, 12, 79. [Google Scholar] [CrossRef]
- Jiang, Z.; Wang, X.; Zhang, H.; Yin, J.; Zhao, P.; Yin, Q.; Wang, Z. Ketogenic diet protects MPTP-induced mouse model of Parkinson’s disease via altering gut microbiota and metabolites. MedComm 2023, 4, e268. [Google Scholar] [CrossRef]
- Bravo, J.A.; Forsythe, P.; Chew, M.V.; Escaravage, E.; Savignac, H.M.; Dinan, T.G.; Bienenstock, J.; Cryan, J.F. Ingestion of Lactobacillus strain regulates emotional behavior and central GABA receptor expression in a mouse via the vagus nerve. Proc. Natl. Acad. Sci. USA 2011, 108, 16050–16055. [Google Scholar] [CrossRef]
- Margolis, K.G.; Cryan, J.F.; Mayer, E.A. The microbiota-gut-brain axis: From motility to mood. Gastroenterology 2021, 160, 1486–1501. [Google Scholar] [CrossRef] [PubMed]
- Sharon, G.; Cruz, N.J.; Kang, D.-W.; Gandal, M.J.; Wang, B.; Kim, Y.-M.; Zink, E.M.; Casey, C.P.; Taylor, B.C.; Lane, C.J. Human gut microbiota from autism spectrum disorder promote behavioral symptoms in mice. Cell 2019, 177, 1600–1618.e17. [Google Scholar] [CrossRef]
- Radjabzadeh, D.; Bosch, J.A.; Uitterlinden, A.G.; Zwinderman, A.H.; Ikram, M.A.; van Meurs, J.B.; Luik, A.I.; Nieuwdorp, M.; Lok, A.; van Duijn, C.M. Gut microbiome-wide association study of depressive symptoms. Nat. Commun. 2022, 13, 7128. [Google Scholar] [CrossRef] [PubMed]
- Dohnalová, L.; Lundgren, P.; Carty, J.R.; Goldstein, N.; Wenski, S.L.; Nanudorn, P.; Thiengmag, S.; Huang, K.-P.; Litichevskiy, L.; Descamps, H.C. A microbiome-dependent gut–brain pathway regulates motivation for exercise. Nature 2022, 612, 739–747. [Google Scholar] [CrossRef]
- Ferreiro, A.L.; Choi, J.; Ryou, J.; Newcomer, E.P.; Thompson, R.; Bollinger, R.M.; Hall-Moore, C.; Ndao, I.M.; Sax, L.; Benzinger, T.L. Gut microbiome composition may be an indicator of preclinical Alzheimer’s disease. Sci. Transl. Med. 2023, 15, eabo2984. [Google Scholar] [CrossRef]
- Huang, B.; Chau, S.W.; Liu, Y.; Chan, J.W.; Wang, J.; Ma, S.L.; Zhang, J.; Chan, P.K.; Yeoh, Y.K.; Chen, Z. Gut microbiome dysbiosis across early Parkinson’s disease, REM sleep behavior disorder and their first-degree relatives. Nat. Commun. 2023, 14, 2501. [Google Scholar] [CrossRef] [PubMed]
- Palacios, N.; Wilkinson, J.; Bjornevik, K.; Schwarzschild, M.A.; McIver, L.; Ascherio, A.; Huttenhower, C. Metagenomics of the gut microbiome in Parkinson’s disease: Prodromal changes. Ann. Neurol. 2023, 94, 486–501. [Google Scholar] [CrossRef]
- Wang, N.; Gao, X.; Zhang, Z.; Yang, L. Composition of the gut microbiota in attention deficit hyperactivity disorder: A systematic review and meta-analysis. Front. Endocrinol. 2022, 13, 838941. [Google Scholar] [CrossRef]
- Sadighara, P.; Rostami, S.; Shafaroodi, H.; Sarshogi, A.; Mazaheri, Y.; Sadighara, M. The effect of residual antibiotics in food on intestinal microbiota: A systematic review. Front. Sustain. Food Syst. 2023, 7, 1163885. [Google Scholar] [CrossRef]
- Sun, J.; Liao, X.P.; D’Souza, A.W.; Boolchandani, M.; Li, S.H.; Cheng, K.; Luis Martinez, J.; Li, L.; Feng, Y.J.; Fang, L.X.; et al. Environmental remodeling of human gut microbiota and antibiotic resistome in livestock farms. Nat. Commun. 2020, 11, 1427. [Google Scholar] [CrossRef]
- Ritter, K.; Vetter, D.; Wernersbach, I.; Schwanz, T.; Hummel, R.; Schäfer, M.K.E. Pre-traumatic antibiotic-induced microbial depletion reduces neuroinflammation in acute murine traumatic brain injury. Neuropharmacology 2023, 237, 109648. [Google Scholar] [CrossRef] [PubMed]
- Celorrio, M.; Abellanas, M.A.; Rhodes, J.; Goodwin, V.; Moritz, J.; Vadivelu, S.; Wang, L.; Rodgers, R.; Xiao, S.; Anabayan, I.; et al. Gut microbial dysbiosis after traumatic brain injury modulates the immune response and impairs neurogenesis. Acta Neuropathol. Commun. 2021, 9, 40. [Google Scholar] [CrossRef]
- Arzani, M.; Jahromi, S.R.; Ghorbani, Z.; Vahabizad, F.; Martelletti, P.; Ghaemi, A.; Sacco, S.; Togha, M.; School of Advanced Studies of the European Headache Federation (EHF-SAS). Gut-brain axis and migraine headache: A comprehensive review. J. Headache Pain 2020, 21, 15. [Google Scholar] [CrossRef]
- Kraimi, N.; Dawkins, M.; Gebhardt-Henrich, S.G.; Velge, P.; Rychlik, I.; Volf, J.; Creach, P.; Smith, A.; Colles, F.; Leterrier, C. Influence of the microbiota-gut-brain axis on behavior and welfare in farm animals: A review. Physiol. Behav. 2019, 210, 112658. [Google Scholar] [CrossRef] [PubMed]
- Carabotti, M.; Scirocco, A.; Maselli, M.A.; Severi, C. The gut-brain axis: Interactions between enteric microbiota, central and enteric nervous systems. Ann. Gastroenterol. Q. Publ. Hell. Soc. Gastroenterol. 2015, 28, 203. [Google Scholar]
- Lansbury, E.L.; Vana, V.; Lund, M.L.; Ludwig, M.Q.; Mamedova, E.; Gautron, L.; Arnold, M.; Egerod, K.L.; Kuhre, R.E.; Holst, J.J.; et al. Neurons Co-Expressing GLP-1, CCK, and PYY Receptors Particularly in Right Nodose Ganglion and Innervating Entire GI Tract in Mice. Int. J. Mol. Sci. 2025, 26, 2053. [Google Scholar] [CrossRef] [PubMed]
- Sharvin, B.L.; Aburto, M.R.; Cryan, J.F. Decoding the neurocircuitry of gut feelings: Region-specific microbiome-mediated brain alterations. Neurobiol. Dis. 2023, 179, 106033. [Google Scholar] [CrossRef]
- Diaz Heijtz, R.; Wang, S.; Anuar, F.; Qian, Y.; Björkholm, B.; Samuelsson, A.; Hibberd, M.L.; Forssberg, H.; Pettersson, S. Normal gut microbiota modulates brain development and behavior. Proc. Natl. Acad. Sci. USA 2011, 108, 3047–3052. [Google Scholar] [CrossRef]
- Stilling, R.M.; Ryan, F.J.; Hoban, A.E.; Shanahan, F.; Clarke, G.; Claesson, M.J.; Dinan, T.G.; Cryan, J.F. Microbes & neurodevelopment—Absence of microbiota during early life increases activity-related transcriptional pathways in the amygdala. Brain Behav. Immun. 2015, 50, 209–220. [Google Scholar]
- Val-Laillet, D.; Besson, M.; Guérin, S.; Coquery, N.; Randuineau, G.; Kanzari, A.; Quesnel, H.; Bonhomme, N.; Bolhuis, J.E.; Kemp, B.; et al. A maternal Western diet during gestation and lactation modifies offspring’s microbiota activity, blood lipid levels, cognitive responses, and hippocampal neurogenesis in Yucatan pigs. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 2017, 31, 2037–2049. [Google Scholar] [CrossRef]
- Ma, Y.-Y.; Li, X.; Yu, J.-T.; Wang, Y.-J. Therapeutics for neurodegenerative diseases by targeting the gut microbiome: From bench to bedside. Transl. Neurodegener. 2024, 13, 12. [Google Scholar] [CrossRef]
- Fu, Y.; Cheng, H.W. The Influence of Cecal Microbiota Transplantation on Chicken Injurious Behavior: Perspective in Human Neuropsychiatric Research. Biomolecules 2024, 14, 1017. [Google Scholar] [CrossRef] [PubMed]
- Zhai, J.; Li, Y.; Liu, J.; Dai, C. Neuroimmune interactions: The bridge between inflammatory bowel disease and the gut microbiota. Clin. Transl. Med. 2025, 15, e70329. [Google Scholar] [CrossRef]
- Pellegrini, C.; Antonioli, L.; Colucci, R.; Blandizzi, C.; Fornai, M. Interplay among gut microbiota, intestinal mucosal barrier and enteric neuro-immune system: A common path to neurodegenerative diseases? Acta Neuropathol. 2018, 136, 345–361. [Google Scholar] [CrossRef]
- Lukiw, W.J.; Arceneaux, L.; Li, W.; Bond, T.; Zhao, Y. Gastrointestinal (GI)-tract microbiome derived neurotoxins and their potential contribution to inflammatory neurodegeneration in Alzheimer’s disease (AD). J. Alzheimer’s Dis. Park. 2021, 11, 525. [Google Scholar]
- Mayer, E.A. Gut feelings: The emerging biology of gut–brain communication. Nat. Rev. Neurosci. 2011, 12, 453–466. [Google Scholar] [CrossRef] [PubMed]
- Mayer, E.A.; Savidge, T.; Shulman, R.J. Brain–gut microbiome interactions and functional bowel disorders. Gastroenterology 2014, 146, 1500–1512. [Google Scholar] [CrossRef]
- Huang, C.; Yue, Q.; Sun, L.; Di, K.; Yang, D.; Hao, E.; Wang, D.; Chen, Y.; Shi, L.; Zhou, R. Restorative effects of Lactobacillus rhamnosus LR-32 on the gut microbiota, barrier integrity, and 5-HT metabolism in reducing feather-pecking behavior in laying hens with antibiotic-induced dysbiosis. Front. Microbiol. 2023, 14, 1173804. [Google Scholar] [CrossRef]
- Jiang, S.; Hu, J.-Y.; Cheng, H.-W. The impact of probiotic Bacillus subtilis on injurious behavior in laying hens. Animals 2022, 12, 870. [Google Scholar] [CrossRef]
- Amorim Neto, D.P.; Bosque, B.P.; Pereira de Godoy, J.V.; Rodrigues, P.V.; Meneses, D.D.; Tostes, K.; Costa Tonoli, C.C.; González-Billault, C.; de Castro Fonseca, M. Akkermansia muciniphila secretome promotes α-synuclein aggregation in enteroendocrine cells. bioRxiv 2021. bioRxiv: 2012.430931. [Google Scholar]
- Ihekweazu, F.D.; Fofanova, T.Y.; Queliza, K.; Nagy-Szakal, D.; Stewart, C.J.; Engevik, M.A.; Hulten, K.G.; Tatevian, N.; Graham, D.Y.; Versalovic, J.; et al. Bacteroides ovatus ATCC 8483 monotherapy is superior to traditional fecal transplant and multi-strain bacteriotherapy in a murine colitis model. Gut Microbes 2019, 10, 504–520. [Google Scholar] [CrossRef] [PubMed]
- Goya, M.E.; Xue, F.; Sampedro-Torres-Quevedo, C.; Arnaouteli, S.; Riquelme-Dominguez, L.; Romanowski, A.; Brydon, J.; Ball, K.L.; Stanley-Wall, N.R.; Doitsidou, M. Probiotic Bacillus subtilis Protects against alpha-Synuclein Aggregation in C. elegans. Cell Rep. 2020, 30, 367–380.e7. [Google Scholar] [CrossRef] [PubMed]
- Monaco, A.; Fraldi, A. Protein Aggregation and Dysfunction of Autophagy-Lysosomal Pathway: A Vicious Cycle in Lysosomal Storage Diseases. Front. Mol. Neurosci. 2020, 13, 37. [Google Scholar] [CrossRef] [PubMed]
- Harach, T.; Marungruang, N.; Duthilleul, N.; Cheatham, V.; Mc Coy, K.D.; Frisoni, G.; Neher, J.J.; Fak, F.; Jucker, M.; Lasser, T.; et al. Reduction of Abeta amyloid pathology in APPPS1 transgenic mice in the absence of gut microbiota. Sci. Rep. 2017, 7, 41802. [Google Scholar] [CrossRef]
- Chen, C.; Ahn, E.H.; Kang, S.S.; Liu, X.; Alam, A.; Ye, K. Gut dysbiosis contributes to amyloid pathology, associated with C/EBPbeta/AEP signaling activation in Alzheimer’s disease mouse model. Sci. Adv. 2020, 6, eaba0466. [Google Scholar] [CrossRef]
- Cannon, T.; Sinha, A.; Trudeau, L.E.; Maurice, C.F.; Gruenheid, S. Characterization of the intestinal microbiota during Citrobacter rodentium infection in a mouse model of infection-triggered Parkinson’s disease. Gut Microbes 2020, 12, 1830694. [Google Scholar] [CrossRef]
- Braniste, V.; Al-Asmakh, M.; Kowal, C.; Anuar, F.; Abbaspour, A.; Tóth, M.; Korecka, A.; Bakocevic, N.; Ng, L.G.; Kundu, P. The gut microbiota influences blood-brain barrier permeability in mice. Sci. Transl. Med. 2014, 6, 263ra158. [Google Scholar] [CrossRef]
- Luan, H.; Wang, X.; Cai, Z. Mass spectrometry-based metabolomics: Targeting the crosstalk between gut microbiota and brain in neurodegenerative disorders. Mass Spectrom. Rev. 2019, 38, 22–33. [Google Scholar] [CrossRef]
- Ye, J.; Lv, L.; Wu, W.; Li, Y.; Shi, D.; Fang, D.; Guo, F.; Jiang, H.; Yan, R.; Ye, W. Butyrate protects mice against methionine–choline-deficient diet-induced non-alcoholic steatohepatitis by improving gut barrier function, attenuating inflammation and reducing endotoxin levels. Front. Microbiol. 2018, 9, 1967. [Google Scholar] [CrossRef]
- Mulak, A. Bile acids as key modulators of the brain-gut-microbiota axis in Alzheimer’s disease. J. Alzheimer’s Dis. 2021, 84, 461–477. [Google Scholar] [CrossRef]
- Donaldson, G.P.; Lee, S.M.; Mazmanian, S.K. Gut biogeography of the bacterial microbiota. Nat. Rev. Microbiol. 2016, 14, 20–32. [Google Scholar] [CrossRef] [PubMed]
- Tullo, E.; Finzi, A.; Guarino, M. Review: Environmental impact of livestock farming and Precision Livestock Farming as a mitigation strategy. Sci. Total Environ. 2019, 650, 2751–2760. [Google Scholar] [CrossRef] [PubMed]
- Gilbert, W.; Thomas, L.F.; Coyne, L.; Rushton, J. Review: Mitigating the risks posed by intensification in livestock production: The examples of antimicrobial resistance and zoonoses. Anim. Int. J. Anim. Biosci. 2021, 15, 100123. [Google Scholar] [CrossRef] [PubMed]
- Ma, Z.; Lee, S.; Jeong, K.C. Mitigating Antibiotic Resistance at the Livestock-Environment Interface:A Review. J. Microbiol. Biotechnol. 2019, 29, 1683–1692. [Google Scholar] [CrossRef]
- Amachawadi, R.G.; Nagaraja, T.G. Liver abscesses in cattle: A review of incidence in Holsteins and of bacteriology and vaccine approaches to control in feedlot cattle. J. Anim. Sci. 2016, 94, 1620–1632. [Google Scholar] [CrossRef]
- Markland, S.; Weppelmann, T.A.; Ma, Z.; Lee, S.; Mir, R.A.; Teng, L.; Ginn, A.; Lee, C.; Ukhanova, M.; Galindo, S.; et al. High Prevalence of Cefotaxime Resistant Bacteria in Grazing Beef Cattle: A Cross Sectional Study. Front. Microbiol. 2019, 10, 176. [Google Scholar] [CrossRef]
- Tyers, M.; Wright, G.D. Drug combinations: A strategy to extend the life of antibiotics in the 21st century. Nat. Rev. Microbiol. 2019, 17, 141–155. [Google Scholar] [CrossRef]
- Nichols, M.; Gollarza, L.; Sockett, D.; Aulik, N.; Patton, E.; Francois Watkins, L.K.; Gambino-Shirley, K.J.; Folster, J.P.; Chen, J.C.; Tagg, K.A.; et al. Outbreak of Multidrug-Resistant Salmonella Heidelberg Infections Linked to Dairy Calf Exposure, United States, 2015–2018. Foodborne Pathog. Dis. 2022, 19, 199–208. [Google Scholar] [CrossRef]
- Magnusson, U.; Moodley, A.; Osbjer, K. Antimicrobial resistance at the livestock-human interface: Implications for Veterinary Services. Rev. Sci. Tech. 2021, 40, 511–521. [Google Scholar] [CrossRef]
- Zhang, Z.; Wang, J.; Wang, H.; Zhang, L.; Shang, W.; Li, Z.; Song, L.; Li, T.; Cheng, M.; Zhang, C.; et al. Molecular Surveillance of MRSA in Raw Milk Provides Insight into MRSA Cross Species Evolution. Microbiol. Spectr. 2023, 11, e0031123. [Google Scholar] [CrossRef]
- Zhou, A.; Yuan, Y.; Yang, M.; Huang, Y.; Li, X.; Li, S.; Yang, S.; Tang, B. Crosstalk between the gut microbiota and epithelial cells under physiological and infectious conditions. Front. Cell. Infect. Microbiol. 2022, 12, 832672. [Google Scholar] [CrossRef] [PubMed]
- Recharla, N.; Park, S.; Kim, M.; Kim, B.; Jeong, J.Y. Protective effects of biological feed additives on gut microbiota and the health of pigs exposed to deoxynivalenol: A review. J. Anim. Sci. Technol. 2022, 64, 640. [Google Scholar] [CrossRef] [PubMed]
- Liao, Y.; Peng, Z.; Chen, L.; Nüssler, A.K.; Liu, L.; Yang, W. Deoxynivalenol, gut microbiota and immunotoxicity: A potential approach? Food Chem. Toxicol. 2018, 112, 342–354. [Google Scholar] [CrossRef]
- Fan, J.; Zhang, Y.; Zuo, M.; Ding, S.; Li, J.; Feng, S.; Xiao, Y.; Tao, S. Novel mechanism by which extracellular vesicles derived from Lactobacillus murinus alleviates deoxynivalenol-induced intestinal barrier disruption. Environ. Int. 2024, 185, 108525. [Google Scholar] [CrossRef]
- Arsène, M.M.; Davares, A.K.; Andreevna, S.L.; Vladimirovich, E.A.; Carime, B.Z.; Marouf, R.; Khelifi, I. The use of probiotics in animal feeding for safe production and as potential alternatives to antibiotics. Vet. World 2021, 14, 319. [Google Scholar] [CrossRef]
- Bevilacqua, A.; Campaniello, D.; Speranza, B.; Racioppo, A.; Sinigaglia, M.; Corbo, M.R. An update on prebiotics and on their health effects. Foods 2024, 13, 446. [Google Scholar] [CrossRef] [PubMed]
- Uyeno, Y.; Shigemori, S.; Shimosato, T. Effect of probiotics/prebiotics on cattle health and productivity. Microbes Environ. 2015, 30, 126–132. [Google Scholar] [CrossRef]
- Le Bon, M.; Carvell-Miller, L.; Marshall-Jones, Z.; Watson, P.; Amos, G. A novel prebiotic fibre blend supports the gastrointestinal health of senior dogs. Animals 2023, 13, 3291. [Google Scholar] [CrossRef]
- Csernus, B.; Czeglédi, L. Physiological, antimicrobial, intestine morphological, and immunological effects of fructooligosaccharides in pigs. Arch. Anim. Breed. 2020, 63, 325–335. [Google Scholar] [CrossRef]
- Jin, M.; Osman, M.; Green, B.A.; Yang, Y.; Ahuja, A.; Lu, Z.; Cazer, C.L. Evidence for the transmission of antimicrobial resistant bacteria between humans and companion animals: A scoping review. One Health 2023, 17, 100593. [Google Scholar] [CrossRef]
- Danasekaran, R. One Health: A Holistic Approach to Tackling Global Health Issues. Indian J. Community Med. Off. Publ. Indian Assoc. Prev. Soc. Med. 2024, 49, 260–263. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Integrated Surveillance of Antimicrobial Resistance in Foodborne Bacteria: Application of a One Health Approach: Guidance from the WHO Advisory Group on Integrated Surveillanec of Antimicrobial Resistance (AGISAR); World Health Organization: Geneva, Switzerland, 2017. [Google Scholar]
- He, Y.; Yuan, Q.; Mathieu, J.; Stadler, L.; Senehi, N.; Sun, R.; Alvarez, P.J.J. Antibiotic resistance genes from livestock waste: Occurrence, dissemination, and treatment. NPJ Clean Water 2020, 3, 4. [Google Scholar] [CrossRef]
| Transmission Type | Mechanism | Description | Involved Elements | Example | Effects | Refs. |
|---|---|---|---|---|---|---|
| Vertical | Hereditary Mutations | Random mutations that confer resistance are passed down to bacterial progeny during cell division. | Chromosomal DNA mutations | Mutations in the gyrA and parC genes. | Confer resistance to fluoroquinolones by altering topoisomerases and reducing antibiotic binding affinity. | [23] |
| Efflux pump genes (AcrAB-TolC) | Overexpression of AcrAB-TolC efflux system | Increased efflux of antibiotics, contributing to MDR. | [25] | |||
| Porin genes (OmpF, OmpC) and Regulatory pathways (σE cycle) | Mutations reducing expression of OmpF/OmpC and altering σE stress response system. | Reduced membrane permeability, limiting antibiotic uptake and modulation of resistance and susceptibility profiles. | [26] | |||
| Horizontal | Conjugation | Direct transfer of plasmids containing resistance genes between bacteria via the conjugative pilus. | Plasmids, conjugative pilus | Escherichia coli transferring ESBL resistance plasmids to another strain. | Rapid transfer of resistance traits between bacteria, leading to increased resistance across different species. | [29,30] |
| Transformation | Uptake of free DNA fragments from the environment by competent bacteria. | Free DNA, competent bacteria | Transformation of Streptococcus pneumoniae with DNA from a resistant strain. | Acquisition of resistance genes from the environment, leading to new resistant strains. | [32] | |
| Transduction | Transfer of resistance genes mediated by bacteriophages that infect bacteria. | Bacteriophages, bacterial DNA | Transfer of beta-lactamase genes by bacteriophage infection in Pseudomonas aeruginosa. | Genes from bacteriophages can integrate into bacterial genomes, spreading resistance in previously susceptible strains. | [40,41] | |
| Transposition | Movement of resistance genes within the genome or between plasmids and chromosomes via transposons. | Transposons, chromosomal and plasmid DNA | Transfer resistance genes via transposon in Enterococcus faecalis and Enterococcus faecium. | Facilitates the spread of resistance genes both within the bacterial chromosome and between plasmids. | [43,44] | |
| Integrons | Integration of gene cassettes containing resistance genes into MGEs. | Integrons, gene cassettes | Class 1 integrons in Enterobacteriaceae incorporating aminoglycoside resistance genes. | Enables bacteria to capture and integrate resistance genes from various sources, spreading resistance across species. | [46,47] |
| Antibiotic/ Condition | Model/ Species | Mechanism of Action | Observed Effect (Behavior/Physiology) | Disease Stage | Refs. |
|---|---|---|---|---|---|
| Antibiotic-induced microbiota depletion | Rodents (general) | Increased LPS levels triggering immune activation | Neuroinflammation; behavioral changes | General/Induced dysbiosis | [134] |
| LPS exposure | Rodents (general) | Increased gut permeability via epithelial damage; systemic inflammation | Neuroinflammation; chronic systemic inflammation | General/Induced dysbiosis | [13,136] |
| Antibiotic-induced dysbiosis (hens) | Laying hens | Restoration of serotonin metabolism; microbiota balance | Reduced stress-related behaviors | Antibiotic-induced dysbiosis | [140,141] |
| Secretome of Akkermansia muciniphila | Enteroendocrine cells (in vitro) | Promotion of α-syn aggregation | α-syn aggregation linked to PD pathogenesis | Pathogenesis of PD | [142] |
| Bacteroides ovatus metabolites | Commensal gut bacteria | Inhibition of α-syn aggregation via phenolic acids | Neuroprotective effect via decreased aggregation | Potential therapeutic effect | [143] |
| Bacillus subtilis metabolites | Commensal gut bacteria | Inhibition of protein aggregation via sphingolipid modulation | Neuroprotective effect via decreased aggregation | Potential therapeutic effect | [144] |
| Germ-free state in AD mouse model | Mouse model (AD) | Reduced Aβ load; altered neuroinflammation | Reduced AD pathology and neuroinflammation | AD | [146] |
| Antibiotics in 5XFAD AD model | Mouse model (5XFAD Alzheimer’s) | Reduced pro-inflammatory microglia activation; decreased Aβ aggregation | Improved cognition and decreased neuroinflammation | AD | [147] |
| Infection in Pink1-KO PD model | Mouse model (Pink1-KO Parkinson’s) | Immune activation and recruitment of CD8+ T cells; increased butyric acid | Dopaminergic neuronal dysfunction; motor deficits | PD | [148] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Silvestro, S.; Biondo, C.; Midiri, A.; Lucia, B.; Mancuso, G. The Role of Livestock Antibiotic Use in Microbiota Dysbiosis and Neuroinflammation. Antibiotics 2025, 14, 608. https://doi.org/10.3390/antibiotics14060608
Silvestro S, Biondo C, Midiri A, Lucia B, Mancuso G. The Role of Livestock Antibiotic Use in Microbiota Dysbiosis and Neuroinflammation. Antibiotics. 2025; 14(6):608. https://doi.org/10.3390/antibiotics14060608
Chicago/Turabian StyleSilvestro, Serena, Carmelo Biondo, Angelina Midiri, Borrello Lucia, and Giuseppe Mancuso. 2025. "The Role of Livestock Antibiotic Use in Microbiota Dysbiosis and Neuroinflammation" Antibiotics 14, no. 6: 608. https://doi.org/10.3390/antibiotics14060608
APA StyleSilvestro, S., Biondo, C., Midiri, A., Lucia, B., & Mancuso, G. (2025). The Role of Livestock Antibiotic Use in Microbiota Dysbiosis and Neuroinflammation. Antibiotics, 14(6), 608. https://doi.org/10.3390/antibiotics14060608






