Abstract
Background/Objectives: Vancomycin (V) is widely used for catheter lock therapy. However, its ad hoc preparation in pharmacy departments involves discarding most of an intravenous vial and contributes to high workload. We aimed to assess the V concentration and minimum inhibitory biofilm concentration (MIBC) of a frozen V lock solution. Methods: Two V-2 mg/mL solutions were tested: (1) V + heparin 100 IU/mL and (2) V + citrate 2%. Solutions were frozen at −20 °C, followed by 48 h refrigeration, and analyses were performed at baseline and after 2, 4, 8, and 12 weeks (experiment 1). In addition, after the 12-week freezing period, solution 1 was also preserved for 1 and 2 weeks at both 4 °C and room temperature (experiment 2). V concentration was assessed by HPLC-DAD at 205 nm and validated with forced degradation tests. A <10% variation indicated significant change. MBIC was determined by XTT staining of 24 h biofilms exposed to decreasing concentrations of each solution. Microorganisms tested included methicillin-susceptible and -resistant Staphylococcus aureus (MSSA, MRSA), Staphylococcus epidermidis ATCC35984 (SE), and a highly biofilm-forming clinical S. epidermidis strain (SEclin). MIBC was defined as ≥50% reduction in metabolic activity. Results: In experiment 1, while V concentration remained stable over time, MIBC values varied, notably increasing from 8 weeks for all strains. Moreover, in experiment 2, significant reductions in both V concentration and MIBC were detected in the 2-week period. Conclusions: V lock solution appears to be able to be 12-weeks frozen followed by up to 1 week at refrigeration or room temperature. This facilitates the optimization of vial preparation in hospital pharmacy laboratories.
1. Introduction
Guidelines for the management of catheter-related bloodstream infection (C-RBSI) recommend the combination of systemic antibiotics and lock therapy when catheter removal is not possible and the patient is stable and uncomplicated [1]. This approach helps prevent catheter-related infections and maintain patency, but it carries risks such as drug resistance, toxicity, and the potential for systemic side effects. Vancomycin (V) is the most commonly used antibiotic for catheter locks that are infected with Staphylococcus spp., which are the most common microorganisms causing C-RBSI, followed by Gram-negative bacilli and Candida spp. [2]. The ad hoc elaboration of a catheter lock solution’s vials requires individual preparation by the pharmacy department, which not only involves discarding a vial for IV administration of which only a few mL are needed, but such on-demand preparation also creates a high workload. Based on previous studies of our group with V and dalbavancin solutions, it was therefore possible to demonstrate that these solutions can be prepared using the entire vial and divided into single-dose vials to be kept frozen and individually thawed if necessary [3,4,5,6]. However, only the anti-biofilm capacity was assessed by quantification of the reduction in metabolic activity and the V lock solution concentration used was 5 mg/mL. Therefore, it is necessary to determine in a 2 mg/mL V lock solution not only the concentration of V in the frozen solution but also whether there are changes in the minimum inhibitory biofilm concentration (MIBC).
2. Results
2.1. Experiment 1
During freezing for solution 1, no significant oscillation in the MIBC was observed, except for the ATCC strain of S. epidermidis, which increased to 120 µg/mL from week 4 (Figure 1a, Table 1 and Table 2). In solution 2, the MIBC of the S. epidermidis ATCC strain was also slightly increased from week 4 (30 µg/mL) and the MIBC of the three remaining strains also increased from week 8 (Figure 1b, Table 1 and Table 2).
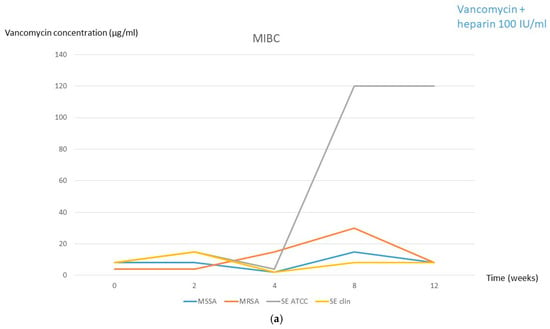
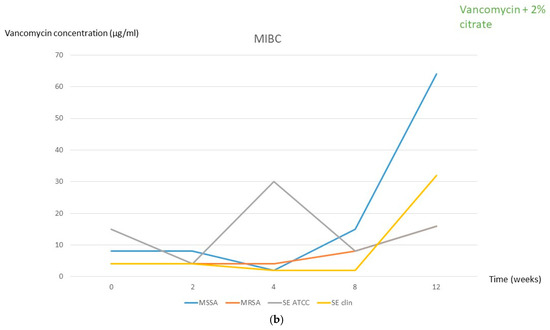
Figure 1.
MIBC at each frozen period time followed by 2 days in the fridge for both solutions. (a) Solution 1 (2 mg/mL vancomycin + 100 IU/mL heparin). (b) Solution 2 (2 mg/mL vancomycin + 2% citrate). MSSA, methicillin-susceptible Staphylococcus aureus; MRSA, methicillin-resistant Staphylococcus aureus; SE, Staphylococcus epidermidis; clin, clinical; MIBC, minimum inhibitory biofilm concentration.

Table 1.
Vancomycin concentration and MBEC during the study periods.

Table 2.
MIBC fold dilution changes compared to the basal experiment (t = 0) according to the four tested strains treated with frozen vancomycin solutions during the study periods.
One interesting finding in this experiment was that we observed a paradoxical effect (Eagle effect), especially in S. aureus, when planktonic cells were exposed to increasing concentrations of vancomycin from 8 µg/mL to 8 mg/mL (combined either with heparin or citrate). Figure 2 shows this effect in MSSA and MRSA of both solutions at time 0, as an example. It can be observed that at low concentrations, the metabolic activity was almost absent, whereas from 0.5 to 8 mg/mL it proportionally increased, reaching the same colour (or even higher) than the positive control.
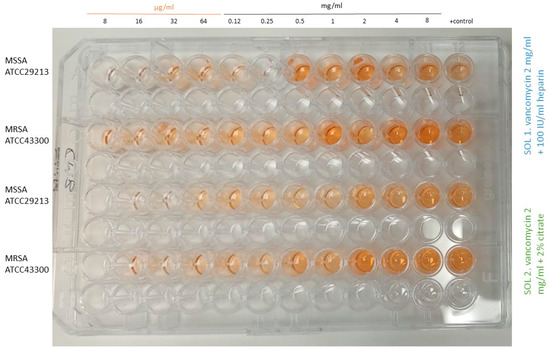
Figure 2.
Paradoxical effect in MSSA and MRSA at time 0 for both tested solutions from 8 µg/mL to 8 mg/mL. MSSA, methicillin-susceptible Staphylococcus aureus; MRSA, methicillin-resistant Staphylococcus aureus; sol, solution.
2.2. Experiment 2
Based on the similar results obtained for both solutions in experiment 1, experiment 2 was only performed with solution 1. As it can be observed in Table 1 and Table 2, significant changes in vancomycin concentration (<limit of significance of 10%) occurred only at the 2-week period both in refrigeration and room temperature (1.38 mg/mL and 1.48 mg/mL, respectively). Moreover, the MIBC was also significantly increased to >2000 mg/mL in room temperature conservation for MSSA and S. epidermidis ATCC and in fridge conservation for MRSA (Table 1 and Table 2, Figure 3).
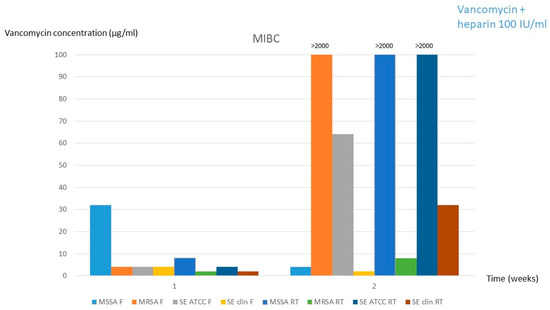
Figure 3.
MIBC for solution 1 (2 mg/mL vancomycin + 100 IU/mL heparin) after the 12-weeks frozen period followed by 1 and 2 weeks at fridge or room temperature. MSSA, methicillin-susceptible Staphylococcus aureus; MRSA, methicillin-resistant Staphylococcus aureus; SE, Staphylococcus epidermidis; clin, clinical; MIBC, minimum inhibitory biofilm concentration; F, fridge; RT, room temperature.
3. Discussion
As we previously demonstrated that the efficacy of V was not affected in 5 mg/mL lock solution after a 6-month freezing period [6,7], we have now tested the V concentration and MIBC of a 2 mg/mL V lock solution both with 100 IU/mL of heparin and 2% citrate in different scenarios of conservation and preservation, demonstrating that they can be frozen up 12 weeks followed by a 48 h refrigeration. Moreover, in the vancomycin–heparin solution, it can be extended to a 1-week preservation at 4 °C or room temperature.
We observed that, although the concentration of vancomycin remained unchanged throughout the 12-week freezing + 48 h refrigeration periods in both solutions, the MIBC fluctuated, with a significant increase from week 8 onwards, mainly with solution 2, in both Staphylococci spp. However, none of the MIBC reached the concentration value of the lock solution prepared for clinical administration (2 mg/mL). In contrast, when solution 1 was frozen for 12 weeks followed by 2 weeks of either refrigeration or room temperature, both the solution 1 concentration and the MIBC suffered significant changes. Thus, the best strategy for optimizing a V lock solution is to preserve it under freezing for 12 weeks in pharmacy departments followed by 1 week of either refrigeration or room temperature maintenance in nursing departments.
It is important to highlight the paradoxical effect that we observed during our experiments. This Eagle effect was first described by Eagle in 1948 with penicillin, which was then also observed by Straton et al. in 1987 [8,9] and Stille et al. with Enterococcus in 1973 [10]. Recently, this effect was also reported with echinocandins and Candida [11]. Regarding vancomycin, there is only one study, of Jarrad et al., in which they observed the Eagle effect with C. difficile strains in an in vitro study [12]. Therefore, ours is the first study assessing the vancomycin Eagle effect in Staphylococcal strains, although what remains unclear is its clinical impact. Ericson et al. found no correlation between high doses of ampicillin and worse clinical outcomes in children with bacteremic [13]. In contrast, Griffiths et al. demonstrated clinical failure with penicillin [14]. Price et al. observed a paradoxical relationship between vancomycin MIC and outcomes of S. aureus bacteremia patients [15]. However, in candidemia, Rueda et al. demonstrated that the presence of the paradoxical effect was not associated with a patient’s response to antifungal treatment [16]. Our concern is whether the paradoxical effect of vancomycin can have any negative impact in patient outcomes when used as a catheter lock solution. Guidelines recommend using a vancomycin concentration at least 1,000 times higher than the MIC (5 mg/mL) [1] and clinical studies assessing the clinical impact and efficacy of vancomycin lock therapy (VLT) are somewhat discrepant, mainly because of different criteria being used to define the success of VLT [17,18]. Our group previously described that the success of catheter retention in 76 staphylococcal C-RBSI episodes using VLT was moderate, reaching slightly more than 70% when the catheter was kept in place until the end of use [3]. Worse results have been reported in a recent randomized study (ETHALOCK) of Lesens et al. in which success rates were 46.7% and 58.1% in patients treated with 5 mg/mL VLT and 40% ethanol lock therapy, respectively [19]. In line with the low success rates of VLT, recently, Permuy et al. described three cases of failure in children who were finally successfully treated with daptomycin [20]. This was also observed in a recent study of Blanco Di-Mateo et al. where daptomycin locks achieved the highest eradication rate of coagulase-negative Staphylococci (CoNS) from 21 haemodialysis catheters, with vancomycin being the one showing the worst results [21]. Moreover, in another recent study in 100 cancer patients with totally implantable venous access devices infected with CoNS, the success rate was slightly low after 3 months of VLT (44.0%) [22]. These poor success rates of VLT can be explained in part because of its well-known low anti-biofilm activity, as better results with other lipoglycopeptides have been shown [23,24,25,26,27]. In particular, in the quantitative in vitro model of Kropec et al., the activity of vancomycin and teicoplanin in two concentrations (4× MIBC and 1 µg/mL) against S. aureus and S. epidermidis strains colonizing the internal surface of polyurethane and silicone catheters was studied. They showed that teicoplanin achieved a significantly greater reduction (p < 0.05) in the counts of both strains compared to vancomycin [28]. However, Liang et al. used a lower concentration of heparin-based vancomycin lock solution (25 µg/mL) for 137 very low birth weight infants with peripherally inserted central venous catheters to prevent C-RBSI, showing a statistical difference in the C-RBSI rates between infants treated with vancomycin locks (n = 68) and single heparin locks (n = 69, control) (4.4% vs. 21.7%, p = 0.004) [29]. This high difference in the C-RBSI rates between groups may be explained because they used lower concentrations of vancomycin, which may have avoided the possible negative effect of using high concentrations, based on our described Eagle effect occurring from 30 µg/mL onwards.
Finally, the main advantages of this preservation approach in hospital practice include improved efficiency in compounding processes, allowing the number of syringes per batch and the frequency of preparation to be adjusted according to actual demand. Additionally, it will help to reduce the operational burden on pharmacy staff by extending the interval between preparation cycles from every 3–4 days to every 24–30 days. This strategy also helps avoid waste from expired lock solutions, which was previously common due to their limited shelf life. In contrast, the main limitations of this approach include the “intermediate risk” classification of the preparation, which requires adherence to strict aseptic conditions and quality controls. Additionally, although our study assessed physicochemical and therapeutic stability, it did not evaluate microbiological stability. According to current guidelines, this limits the maximum beyond-use date in frozen storage to 45 days. Extending this period would require microbiological testing to ensure safety. Moreover, freezing and thawing processes must be standardized to avoid variability in the final product [30].
4. Materials and Methods
Two V-2 mg/mL solutions were tested: (1) V + heparin 100 IU/mL (heparina sódica Sala 5000 UI/5 mL, Reig Jofre, Sant Joan Despi, Spain) and (2) V + citrate 2% (Citra-Lock TM 4% 5mL, B. Braun, Melsungen, Germany). We used 2 mg/mL instead of 5 mg/mL because 2 mg/mL is the standard in our institution based on the physical compatibility with heparin. Two experiments were performed: Experiment 1: V solutions were frozen at −20 °C, followed by 48 h refrigeration at 4 °C, and analyses were performed at baseline and after 2, 4, 8, and 12 weeks. In addition, based on the similar results obtained for both solutions in experiment 1 and considering that heparin is the anticoagulant used mostly in clinical practice and in our institution, experiment 2 was only performed with solution 1, which consisted of a 12-week freezing period followed by 1 and 2 weeks of both 4 °C refrigeration and room temperature periods.
4.1. Concentration Test
The pH of the V lock solutions were determined in triplicate. The concentration of V was tested by HPLC-DAD (Agilent 1 Infinity II, Agilent Technologics, Santa Clara, CA, USA). Method validation was performed by forced degradation tests under acidic, alkaline, and oxidizing conditions. The linearity of the calibration line was confirmed at concentrations of 125–250 µg/mL (R2 = 0.989 y = 227.41x + 700). The retention time of V was 4.1 min. The stationary phase was a C18 column and the mobile phase was an acetonitrile–aqueous potassium phosphate mixture (0.05 M, pH = 3.1) at a rate of 0.5 µL/min and a concentration gradient of T° 40 °C. Samples were diluted 1:10, 1 µL was injected in triplicate, and detection was set at 205 nm. Alterations <10% (1.80 mg/mL) were considered to indicate a significant change in sample composition.
4.2. Minimum Inhibitory Biofilm Concentration (MIBC)
MIBC was assessed by the measurement of metabolic activity by tetrazolium salt (XTT) staining (Merck, Spain) in 24 h biofilm in well plates by spectrophotometry (EZ READ 400, Biochrom, Spain) (treated with decreasing concentrations from 8 µg/mL to 8000 µg/mL of sol 1 and sol 2 vs. positive control) of each of the following microorganisms: methicillin-susceptible Staphylococcus aureus (MSSA ATCC29213), methicillin-resistant S. aureus (MRSA ATCC43300), Staphylococcus epidermidis (ATCC35984), and a biofilm-forming clinical strain of S. epidermidis obtained from the blood of a patient with C-RBSI that we had previously used in other experimental studies because it showed high biomass production. MIBC was considered when the reduction in metabolic activity was ≥50% when contrasting the XTT absorbance of treated vs. positive control wells. We considered significant alteration of MIBC change to have occurred when it reached the vancomycin lock solution concentration of 2 mg/mL. No other statistical analysis could be performed comparing MIBC at different times because only one single value of absorbance was obtained and no mean/median could be compared.
5. Conclusions
Despite that vancomycin lock solution can be frozen up 12 weeks, preservation at 4 °C or room temperature can only be carried out up to 1 week when used in combination with heparin. Even so, this strategy can help to optimize the elaboration and preservation of vancomycin lock solutions in pharmacy and nursing departments. This approach may serve to reduce risks due to variability in preparation and regulatory considerations.
Author Contributions
Conceptualization, B.T.-S., F.G.-M. and M.G.; methodology, M.D.-N. and D.S.; software, M.G., M.D.-N. and D.S.; validation, B.T.-S. and M.G.; formal analysis, M.D.-N. and D.S.; investigation, M.D.-N., D.S. and F.G.-M.; resources, B.T.-S. and M.G.; data curation, M.D.-N. and D.S.; writing—original draft preparation, M.D.-N. and D.S.; writing—review and editing, B.T.-S. and M.G.; visualization, P.M. and M.S.; supervision, B.T.-S., M.G., P.M. and M.S.; project administration, B.T.-S. and M.G.; funding acquisition, B.T.-S. and M.G. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Fundación MUTUA Madrileña (FMM24/01).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The original contributions presented in this study are included in the article. Further inquiries can be directed to the corresponding author.
Acknowledgments
We thank the experimental medicine and surgery unit for providing us with the use of the facilities and equipment in the microbiology culture room.
Conflicts of Interest
The authors declare no conflicts of interest.
Correction Statement
This article has been republished with a minor correction to an author’s name, existing affiliation information and an author’s ORCID. This change does not affect the scientific content of the article.
Abbreviations
The following abbreviations are used in this manuscript:
| V | Vancomycin |
| VLT | Vancomycin lock therapy |
| C-RBSI | Catheter-related bloodstream infection |
| MIBC | Minimum inhibitory biofilm concentration |
| IQR | Interquartile range |
| XTT | Tetrazolium salt |
| MSSA | Methicillin-susceptible Staphylococcus aureus |
| MRSA | Methicillin-resistant Staphylococcus aureus |
| SE | Staphylococcus epidermidis |
| Ok.SEclin | Staphylococcus epidermidis clinical strain |
| CoNS | Coagulase-negative Staphylococci |
References
- Mermel, L.A.; Allon, M.; Bouza, E.; Craven, D.E.; Flynn, P.; O’Grady, N.P.; Raad, I.I.; Rijnders, B.J.; Sherertz, R.J.; Warren, D.K. Clinical practice guidelines for the diagnosis and management of intravascular catheter-related infection: 2009 Update by the Infectious Diseases Society of America. Clin. Infect. Dis. 2009, 49, 1–45. [Google Scholar] [CrossRef] [PubMed]
- Lafuente Cabrero, E.; Terradas Robledo, R.; Civit Cuñado, A.; García Sardelli, D.; Hidalgo López, C.; Giro Formatger, D.; Lacueva Perez, L. Risk factors of catheter-associated bloodstream infection: Systematic review and meta-analysis. PLoS ONE 2023, 18, e0282290. [Google Scholar] [CrossRef] [PubMed]
- Alonso, B.; Fernández-Cruz, A.; Díaz, M.; Sánchez-Carrillo, C.; Martín-Rabadán, P.; Bouza, E.; Muñoz, P.; Guembe, M. Can vancomycin lock therapy extend the retention time of infected long-term catheters? APMIS Acta Pathol. Microbiol. Immunol. Scand. 2020, 128, 433–439. [Google Scholar] [CrossRef] [PubMed]
- Díaz-Navarro, M.; Hafian, R.; Manzano, I.; Pérez-Granda, M.J.; Cercenado, E.; Pascual, C.; Rodríguez, C.; Muñoz, P.; Guembe, M. A Dalbavancin Lock Solution Can Reduce Enterococcal Biofilms After Freezing. Infect. Dis. Ther. 2022, 11, 743–755. [Google Scholar] [CrossRef]
- Díaz-Navarro, M.; Hafian, R.; Pérez-Granda, M.J.; Cercenado, E.; Muñoz, P.; Guembe, M. Addressing catheter lock therapy: Does heparin reduce the bioactivity of dalbavancin when together in solution during freezing? Enfermedades Infecc. Y Microbiol. Clin. 2024, 42, 435–438. [Google Scholar] [CrossRef]
- Díaz-Ruíz, C.; Alonso, B.; Cercenado, E.; Cruces, R.; Bouza, E.; Muñoz, P.; Guembe, M. Can dalbavancin be used as a catheter lock solution? Antimicrob. Agents Chemother. 2018, 67, 936–944. [Google Scholar] [CrossRef]
- Rubia, M.; Cordero, A.; Pérez-Granda, M.J.; Cercenado, E.; Pascual, C.; Muñoz, P. In Vitro Study To Evaluate the Bioactivity of Freezing a Heparin-Based Dalbavancin Lock Solution. Infect. Dis. Ther. 2020, 64, e01495-20. [Google Scholar] [CrossRef]
- Eagle, H. A Paradoxical Zone Phenomenon in the Bactericidal Action of Penicillin in Vitro. Science 1948, 107, 44–45. [Google Scholar] [CrossRef]
- Stratton, C.W.; Liu, C.; Ratner, H.B.; Weeks, L.S. Bactericidal activity of deptomycin (LY146032) compared with those of ciprofloxacin, vancomycin, and ampicillin against enterococci as determined by kill-kinetic studies. Antimicrob. Agents Chemother. 1987, 31, 1014–1016. [Google Scholar] [CrossRef]
- Stille, W.; Uffelmann, H. Paradoxic bactericidal effect of penicillins on enterococci (Eagle effect). Dtsch. Med. Wochenschr. 1973, 98, 611–613. [Google Scholar] [CrossRef]
- Marcos-Zambrano, L.J.; Escribano, P.; Sánchez-Carrillo, C.; Bouza, E.; Guinea, J. Frequency of the Paradoxical Effect Measured Using the EUCAST Procedure with Micafungin, Anidulafungin, and Caspofungin against Candida Species Isolates Causing Candidemia. Antimicrob. Agents Chemother. 2017, 61, e01584-16. [Google Scholar] [CrossRef] [PubMed]
- Jarrad, A.M.; Blaskovich, M.A.T.; Prasetyoputri, A.; Karoli, T.; Hansford, K.A.; Cooper, M.A. Detection and Investigation of Eagle Effect Resistance to Vancomycin in Clostridium difficile With an ATP-Bioluminescence Assay. Front. Microbiol. 2018, 9, 1420. [Google Scholar] [CrossRef] [PubMed]
- Ericson, J.E.; Hornik, C.P.; Greenberg, R.G.; Clark, R.H.; Tremoulet, A.H.; Le, J.; Cohen-Wolkowiez, M.; Smith, P.B.; Benjamin, D.K., Jr. Paradoxical Antibiotic Effect of Ampicillin: Use of a Population Pharmacokinetic Model to Evaluate a Clinical Correlate of the Eagle Effect in Infants With Bacteremia. Pediatr. Infect. Dis. J. 2020, 39, 725–729. [Google Scholar] [CrossRef] [PubMed]
- Griffiths, L.R.; Green, H.T. Paradoxical effect of penicillin in-vivo. J. Antimicrob. Chemother. 1985, 15, 507–508. [Google Scholar] [CrossRef]
- Price, J.; Atkinson, S.; Llewelyn, M.; Paul, J. Paradoxical relationship between the clinical outcome of Staphylococcus aureus bacteremia and the minimum inhibitory concentration of vancomycin. Clin. Infect. Dis. 2009, 48, 997–998. [Google Scholar] [CrossRef]
- Rueda, C.; Puig-Asensio, M.; Guinea, J.; Almirante, B.; Cuenca-Estrella, M.; Zaragoza, O. Evaluation of the possible influence of trailing and paradoxical effects on the clinical outcome of patients with candidemia. Antimicrob. Agents Chemother. 2017, 23, e41–e49. [Google Scholar] [CrossRef]
- Ranch-Lundin, M.; Schedin, A.; Björkhem-Bergman, L. Equal effect of vancomycin lock with or without heparin in treatment of central venous catheter related blood stream infections—An observational study in palliative home care. Infect. Dis. 2021, 53, 719–723. [Google Scholar] [CrossRef]
- Nassiri, A.A.; Ahmadi Koomleh, A.; Sabaghian, T.; Delgosha, M.; Hakemi, M.S. Comparison Between Vancomycin Lock and Taurolock Solution for the Prevention of Catheter- related Infections in Hemodialysis Patients, A Multicenter Study. Iran. J. Kidney Dis. 2023, 17, 215–221. [Google Scholar]
- Lesens, O.; Forestier, E.; Botelho-Nevers, E.; Pavese, P.; David, G.; Nougarede, B.; Corbin, V.; Pereira, B.; Aumeran, C.; Sauvat, L. Comparing ethanol lock therapy versus vancomycin lock in a salvation strategy for totally implantable vascular access device infections due to coagulase-negative staphylococci (the ETHALOCK study): A prospective double-blind randomized clinical trial. Eur. J. Clin. Microbiol. Infect. Dis. 2024, 43, 223–232. [Google Scholar] [CrossRef]
- Permuy, C.; Ruiz-Azcárate, J.; Sampedro, M.; Jiménez, C.; Baquero-Artigao, F.; Calvo, C.; Méndez-Echevarría, A. Usefulness of daptomycin lock therapy in children with catheter-related bacteremia after failed vancomycin lock therapy. Ann. Clin. Microbiol. Antimicrob. 2023, 22, 48. [Google Scholar] [CrossRef]
- Blanco-Di Matteo, A.; Garcia-Fernandez, N.; Aguinaga Pérez, A.; Carmona-Torre, F.; Oteiza, A.C.; Leiva, J.; Del Pozo, J.L. In Vivo Effectiveness of Several Antimicrobial Locks To Eradicate Intravascular Catheter Coagulase-Negative Staphylococci Biofilms. Antimicrob. Agents Chemother. 2023, 67, e0126422. [Google Scholar] [CrossRef] [PubMed]
- Lafaurie, M.; Montlahuc, C.; Kerneis, S.; de Lastours, V. Efficacy of vancomycin lock therapy for totally implantable venous access port-related infection due to coagulase-negative staphylococci in 100 patients with cancer. Ann. Clin. Microbiol. Antimicrob. 2023, 78, 1253–1258. [Google Scholar] [CrossRef] [PubMed]
- Lebeaux, D.; Leflon-Guibout, V.; Ghigo, J.M.; Beloin, C. In vitro activity of gentamicin, vancomycin or amikacin combined with EDTA or l-arginine as lock therapy against a wide spectrum of biofilm-forming clinical strains isolated from catheter-related infections. J. Antimicrob. Chemother. 2015, 70, 1704–1712. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Hidalgo, N.; Gavaldà, J.; Almirante, B.; Martín, M.T.; Onrubia, P.L.; Gomis, X.; Pahissa, A. Evaluation of linezolid, vancomycin, gentamicin and ciprofloxacin in a rabbit model of antibiotic-lock technique for Staphylococcus aureus catheter-related infection. J. Antimicrob. Chemother. 2010, 65, 525–530. [Google Scholar] [CrossRef]
- Luther, M.K.; Mermel, L.A.; LaPlante, K.L. Comparison of ML8-X10 (a prototype oil-in-water micro-emulsion based on a novel free fatty acid), taurolidine/citrate/heparin and vancomycin/heparin antimicrobial lock solutions in the eradication of biofilm-producing staphylococci from central venous catheters. J. Antimicrob. Chemother. 2014, 69, 3263–3267. [Google Scholar] [CrossRef]
- Luther, M.K.; Mermel, L.A.; LaPlante, K.L. Comparison of telavancin and vancomycin lock solutions in eradication of biofilm-producing staphylococci and enterococci from central venous catheters. Am. J. Health-Syst. Pharm. 2016, 73, 315–321. [Google Scholar] [CrossRef]
- Luther, M.K.; Mermel, L.A.; LaPlante, K.L. Comparison of linezolid and vancomycin lock solutions with and without heparin against biofilm-producing bacteria. Am. J. Health-Syst. Pharm. 2017, 74, e193–e201. [Google Scholar] [CrossRef]
- Kropec, A.; Huebner, J.; Wursthorn, M.; Daschner, F.D. In vitro activity of vancomycin and teicoplanin against Staphylococcus aureus and Staphylococcus epidermidis colonizing catheters. Eur. J. Clin. Microbiol. Infect. Dis. 1993, 12, 545–548. [Google Scholar] [CrossRef]
- Liang, H.; Zhang, L. Vancomycin-lock therapy for prevention of catheter-related bloodstream infection in very low body weight infants. BMC Pediatr. 2021, 21, 3. [Google Scholar] [CrossRef]
- Ministerio de Sanidad. Guía de Buenas Prácticas de Preparación de Medicamentos en Servicios de Farmacia Hospitalaria. 2024. Available online: https://www.sanidad.gob.es/areas/farmacia/publicaciones/GuiaBPMedicamentosServFarmHosp/Docs/04GuiaMedicamentos2024Accesible.pdf (accessed on 10 June 2025).
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).