Does Empirical Antibiotic Use Improve Outcomes in Ventilated Patients with Pandemic Viral Infection? A Multicentre Retrospective Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Setting
2.3. Participants
2.4. Variables
2.5. Data Sources and Measurement
2.6. Bias
2.7. Analysis Plan and Statistical Analysis (Figure 1)

3. Results
3.1. Whole Population
3.2. Patients with Bacterial Coinfection (COI)
3.3. Patients Without Bacterial Coinfection (No-COI)
3.4. Linear Models in Matched Cohort of Patients Without Coinfection
3.4.1. Risk Factors Associated with the Development of Ventilator-Associated Pneumonia (VAP)
3.4.2. Risk Factors Associated with All-Cause ICU Mortality
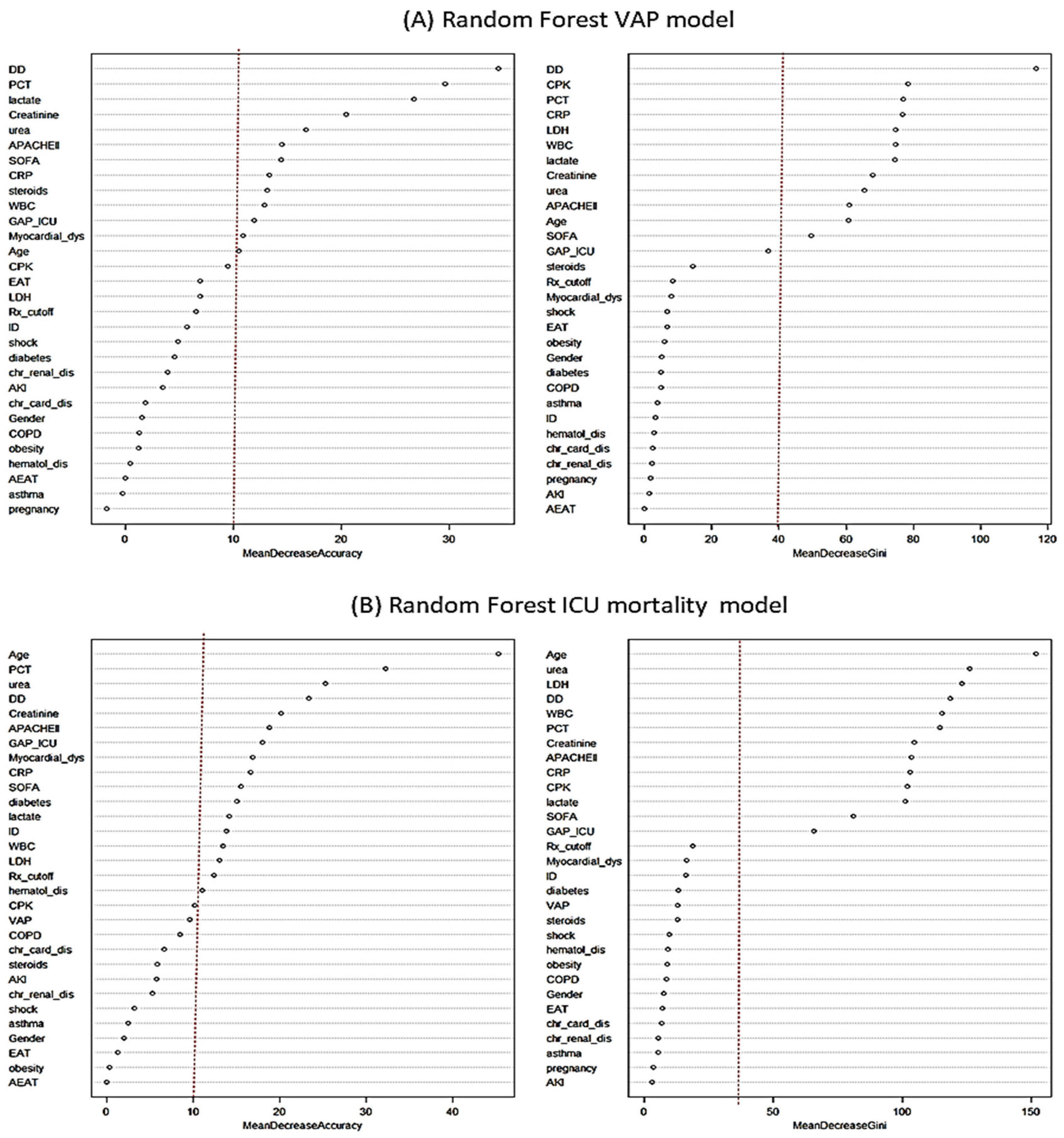
3.5. Non-Linear Analysis–Random Forest Model (RF)
3.5.1. Factors Associated with VAP According to Non-Linear Model
3.5.2. Factors Associated with All-Cause ICU Mortality According to No-Linear Model
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Centers for Disease Control and Prevention (CDC). COVID-19 Case Surveillance Public Use Data. 2021. Available online: https://data.cdc.gov/Case-Surveillance/COVID-19-Case-Surveillance-Public-Use-Data/vbim-akqf (accessed on 24 April 2025).
- Simonsen, L.; Spreeuwenberg, P.; Lustig, R.; Taylor, R.J.; Fleming, D.M.; Kroneman, M.; Van Kerkhove, M.D.; Mounts, A.W.; Paget, W.J.; GLaMOR Collaborating Teams. Global mortality estimates for the 2009 influenza pandemic from the GLaMOR project: A modeling study. PLoS Med. 2013, 10, e1001558. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization (WHO). WHO Coronavirus (COVID-19) Dashboard—Deaths. 2023. Available online: https://data.who.int/dashboards/covid19/deaths (accessed on 24 April 2025).
- Ferrer, R. COVID-19 pandemic: The greatest challenge in the history of critical care. Med. Intensiv. 2020, 44, 323–324. [Google Scholar] [CrossRef] [PubMed]
- Cercenado, E.; Rodríguez-Baño, J.; Alfonso, J.L.; Calbo, E.; Escosa, L.; Fernández-Polo, A.; García-Rodríguez, J.; Garnacho, J.; Gil-Navarro, M.V.; Grau, S.; et al. Antimicrobial stewardship in hospitals: Expert recommendation guidance document for activities in specific populations, syndromes and other aspects (PROA-2) from SEIMC, SEFH, SEMPSPGS, SEMICYUC and SEIP. Enferm. Infecc. Microbiol. Clin. (Engl. Ed.) 2023, 41, 238–242. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez, A.; Gómez, J.; Martín-Loeches, I.; Claverías, L.; Díaz, E.; Zaragoza, R.; Borges-Sa, M.; Gómez-Bertomeu, F.; Franquet, Á.; Trefler, S.; et al. A machine learning approach to determine risk factors for respiratory bacterial/fungal coinfection in critically ill patients with influenza and SARS-CoV-2 infection: A Spanish perspective. Antibiotics 2024, 13, 968. [Google Scholar] [CrossRef]
- Evans, L.; Rhodes, A.; Alhazzani, W.; Antonelli, M.; Coopersmith, C.M.; French, C.; Machado, F.R.; Mcintyre, L.; Ostermann, M.; Prescott, H.C.; et al. Surviving sepsis campaign: International guidelines for management of sepsis and septic shock 2021. Intensive Care Med. 2021, 47, 1181–1247. [Google Scholar] [CrossRef]
- Delhommeau, G.; Buetti, N.; Neuville, M.; Siami, S.; Cohen, Y.; Laurent, V.; Mourvillier, B.; Reignier, J.; Goldgran-Toledano, D.; Schwebel, C.; et al. Bacterial pulmonary co-infections on ICU admission: Comparison in patients with SARS-CoV-2 and influenza acute respiratory failure: A multicentre cohort study. Biomedicines 2022, 10, 2646. [Google Scholar] [CrossRef]
- Galli, F.; Bindo, F.; Motos, A.; Fernández-Barat, L.; Barbeta, E.; Gabarrús, A.; Ceccato, A.; Bermejo-Martin, J.F.; Ferrer, R.; Riera, J.; et al. Procalcitonin and C-reactive protein to rule out early bacterial coinfection in COVID-19 critically ill patients. Intensive Care Med. 2023, 49, 934–945. [Google Scholar] [CrossRef]
- O’Neill, J. Tackling Drug-Resistant Infections Globally: Final Report and Recommendations. Review on Antimicrobial Resistance. 2016. Available online: https://amr-review.org (accessed on 24 April 2025).
- World Health Organization (WHO). Antibiotic Resistance. 2025. Available online: https://www.who.int/news-room/fact-sheets/detail/antimicrobial-resistance (accessed on 24 April 2025).
- World Health Organization (WHO). Global Action Plan on Antimicrobial Resistance. WHO Library Cataloguing-in-Publication Data. 2015. Available online: www.paprika-annecy.com (accessed on 24 April 2025).
- Kariyawasam, R.M.; Julien, D.A.; Jelinski, D.C.; Larose, S.L.; Rennert-May, E.; Conly, J.M.; Dingle, T.C.; Chen, J.Z.; Tyrrell, G.J.; Ronksley, P.E.; et al. Antimicrobial resistance (AMR) in COVID-19 patients: A systematic review and meta-analysis (November 2019–June 2021). Antimicrob. Resist. Infect. Control 2022, 11, 45. [Google Scholar] [CrossRef]
- Carbonell, R.; Moreno, G.; Martín-Loeches, I.; Gomez-Bertomeu, F.; Sarvisé, C.; Gómez, J.; Bodí, M.; Díaz, E.; Papiol, E.; Trefler, S.; et al. Prognostic value of procalcitonin and C-reactive protein in 1608 critically ill patients with severe influenza pneumonia. Antibiotics 2021, 10, 350. [Google Scholar] [CrossRef]
- Carbonell, R.; Urgelés, S.; Salgado, M.; Rodríguez, A.; Reyes, L.F.; Fuentes, Y.V.; Serrano, C.C.; Caceres, E.L.; Bodí, M.; Martín-Loeches, I.; et al. Negative predictive value of procalcitonin to rule out bacterial respiratory co-infection in critical COVID-19 patients. J. Infect. 2022, 85, 374–381. [Google Scholar] [CrossRef]
- Langford, B.J.; So, M.; Raybardhan, S.; Leung, V.; Westwood, D.; MacFadden, D.R.; Soucy, J.R.; Daneman, N. Bacterial co-infection and secondary infection in patients with COVID-19: A living rapid review and meta-analysis. Clin. Microbiol. Infect. 2020, 26, 1622–1629. [Google Scholar] [CrossRef] [PubMed]
- Bartoletti, M.; Azap, O.; Barac, A.; Bussini, L.; Ergonul, O.; Krause, R.; Paño-Pardo, J.R.; Power, N.R.; Sibani, M.; Szabo, B.G.; et al. ESCMID COVID-19 living guidelines: Drug treatment and clinical management. Clin. Microbiol. Infect. 2022, 28, 222–238. [Google Scholar] [CrossRef] [PubMed]
- Wendel-Garcia, P.D.; Ceccato, A.; Motos, A.; Franch-Llasat, D.; Pérez-Moreno, M.O.; Domenech-Spanedda, M.F.; Chamarro-Martí, E.; Ferrer, R.; Fernández-Barat, L.; Riera, J.; et al. Empirical antibiotic therapy improves outcomes in mechanically ventilated patients with COVID-19: An emulated targeted trial within a prospective, multicentre cohort study. J. Infect. 2025, 91, 106411. [Google Scholar] [CrossRef]
- Lansbury, L.; Lim, B.; Baskaran, V.; Lim, W.S. Co-infections in people with COVID-19: A systematic review and meta-analysis. J. Infect. 2020, 81, 266–275. [Google Scholar] [CrossRef]
- Moretto, F.; Sixt, T.; Devilliers, H.; Abdallahoui, M.; Eberl, I.; Rogier, T.; Buisson, M.; Chavanet, P.; Duong, M.; Esteve, C.; et al. Is there a need to widely prescribe antibiotics in patients hospitalized with COVID-19? Int. J. Infect. Dis. 2021, 105, 256–260. [Google Scholar] [CrossRef]
- Reyes, L.F.; Rodriguez, A.; Fuentes, Y.V.; Duque, S.; García-Gallo, E.; Bastidas, A.; Serrano-Mayorga, C.C.; Ibáñez-Prada, E.D.; Moreno, G.; Ramirez-Valbuena, P.C.; et al. Risk factors for developing ventilator-associated lower respiratory tract infection in patients with severe COVID-19: A multinational, multicentre, prospective observational study. Sci. Rep. 2023, 13, 6553. [Google Scholar] [CrossRef]
- Vidal-Cortés, P.; Nieto Del Olmo, J.; Tizón Varela, A.I.; Seoane Fernández, E.; Eiras Abalde, F.; Blanco Chapela, J.; Larrañaga Sigwald, L.; Suárez Fernández, D.E.; Fernández Ugidos, P.; Del Río Carbajo, L. Coinfección bacteriana en el paciente COVID-19 crítico: Incidencia, impacto y necesidad de tratamiento antibiótico. Rev. Esp. Quimioter. 2023, 36, 612–620. [Google Scholar] [CrossRef]
- Pandey, M.; May, A.; Tan, L.; Hughes, H.; Jones, J.P.; Harrison, W.; Bradburn, S.; Tyrrel, S.; Muthuswamy, B.; Berry, N.; et al. Comparative incidence of early and late bloodstream and respiratory tract co-infection in patients admitted to ICU with COVID-19 pneumonia versus Influenza A or B pneumonia versus no viral pneumonia: Wales multicentre ICU cohort study. Crit. Care 2022, 26, 158. [Google Scholar] [CrossRef]
- Wei, S.; Wang, L.; Lin, L.; Liu, X. Predictive values of procalcitonin for coinfections in patients with COVID-19: A systematic review and meta-analysis. Virol. J. 2023, 20, 92. [Google Scholar] [CrossRef]
- Cuschieri, S. The STROBE guidelines. Saudi J. Anaesth. 2019, 13 (Suppl. S1), S31–S34. [Google Scholar] [CrossRef]
- Oliva, I.; Ferré, C.; Daniel, X.; Cartanyà, M.; Villavicencio, C.; Salgado, M.; Vidaur, L.; Papiol, E.; de Molina, F.G.; Bodí, M.; et al. Risk factors and outcome of acute kidney injury in critically ill patients with SARS-CoV-2 pneumonia: A multicenter study. Med. Intensiva (Engl. Ed.) 2024, 48, 3–10. [Google Scholar] [CrossRef]
- Carbonell, R.; Urgelés, S.; Rodríguez, A.; Bodí, M.; Martín-Loeches, I.; Solé-Violán, J.; Díaz, E.; Gómez, J.; Trefler, S.; Vallverdú, M.; et al. Mortality comparison between the first and second/third waves among 3,795 critical COVID-19 patients with pneumonia admitted to the ICU: A multicentre retrospective cohort study. Lancet Reg. Health–Eur. 2021, 11, 100243. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez, A.H.; Ruiz-Botella, M.; Martín-Loeches, I.; Jimenez Herrera, M.; Solé-Violan, J.; Gómez, J.; Bodí, M.; Trefler, S.; Papiol, E.; Díaz, E.; et al. Deploying unsupervised clustering analysis to derive clinical phenotypes and risk factors associated with mortality risk in 2022 critically ill patients with COVID-19 in Spain. Crit. Care 2021, 25, 63. [Google Scholar] [CrossRef] [PubMed]
- Knaus, W.A.; Draper, E.A.; Wagner, D.P.; Zimmerman, J.E. APACHE II: A severity of disease classification system. Crit. Care Med. 1985, 13, 818–829. [Google Scholar] [CrossRef]
- Vincent, J.L.; Moreno, R.; Takala, J.; Willatts, S.; De Mendonça, A.; Bruining, H.; Reinhart, C.K.; Suter, P.M.; Thijs, L.G. The SOFA (Sepsis-related Organ Failure Assessment) score to describe organ dysfunction/failure. Intensive Care Med. 1996, 22, 707–710. [Google Scholar] [CrossRef]
- Torres, A.; Niederman, M.S.; Chastre, J.; Ewig, S.; Fernandez-Vandellos, P.; Hanberger, H.; Kollef, M.; Bassi, G.L.; Luna, C.M.; Martin-Loeches, I.; et al. International ERS/ESICM/ESCMID/ALAT guidelines for the management of hospital-acquired pneumonia and ventilator-associated pneumonia. Eur. Respir. J. 2017, 50, 1700582. [Google Scholar] [CrossRef]
- Boyd, S.; Nseir, S.; Rodriguez, A.; Martin-Loeches, I. Ventilator-associated pneumonia in critically ill patients with COVID-19 infection: A narrative review. ERJ Open Res. 2022, 8, 00241–02021. [Google Scholar] [CrossRef]
- Kellum, J.A.; Lameire, N.; Aspelin, P.; Barsoum, R.S.; Burdmann, E.A.; Goldstein, S.L.; Herzog, C.A.; Joannidis, M.; Kribben, A.; Levey, A.S.; et al. Kidney disease: Improving global outcomes (KDIGO) acute kidney injury work group. KDIGO clinical practice guideline for acute kidney injury. Kidney Int. Suppl. 2012, 2, 1–138. [Google Scholar] [CrossRef]
- Martin-Loeches, I.; Schultz, M.J.; Vincent, J.-L.; Alvarez-Lerma, F.; Bos, L.D.; Solé-Violán, J.; Torres, A.; Rodriguez, A. Increased incidence of co-infection in critically ill patients with influenza. Intensive Care Med. 2017, 43, 48–58. [Google Scholar] [CrossRef]
- Uyeki, T.M.; Bernstein, H.H.; Bradley, J.S.; Englund, J.A.; File, T.M.; Fry, A.M.; Gravenstein, S.; Hayden, F.G.; Harper, S.A.; Hirshon, J.M.; et al. Clinical practice guidelines by the Infectious Diseases Society of America: 2018 update on diagnosis, treatment, chemoprophylaxis, and institutional outbreak management of seasonal influenza. Clin. Infect. Dis. 2019, 68, 895–902. [Google Scholar] [CrossRef]
- World Health Organization. Clinical Management of COVID-19: Interim Guidance, 27 May 2020. WHO-2019-nCoV-clinical-2020.4-eng. Available online: https://policycommons.net/artifacts/538392/clinical-management-of-covid-19/1515086/ (accessed on 20 April 2025).
- Ho, D.E.; Imai, K.; King, G.; Stuart, E.A. MatchIt: Nonparametric preprocessing for parametric causal inference. J. Stat. Softw. 2011, 42, 1–28. Available online: http://www.jstatsoft.org/ (accessed on 20 April 2025). [CrossRef]
- Saseedharan, S. Is empirical antibiotic treatment required in COVID-19 patients? What lessons have we learnt over the past 1 year? Int. J. Res. Med. Sci. 2022, 10, 1470–1474. [Google Scholar] [CrossRef]
- Debes, S.; Haug, J.B.; De Blasio, B.F.; Lindstrøm, J.C.; Jonassen, C.M.; Dudman, S.G. Antibiotic consumption in a cohort of hospitalized adults with viral respiratory tract infection. Antibiotics 2023, 12, 788. [Google Scholar] [CrossRef]
- Hovind, M.J.; Berdal, J.E.; Dalgard, O.; Lyngbakken, M.N. Impact of antibiotic therapy in patients with respiratory viral infections: A retrospective cohort study. Infect. Dis. 2024, 56, 22–30. [Google Scholar] [CrossRef]
- Yin, X.; Xu, X.; Li, H.; Jiang, N.; Wang, J.; Lu, Z.; Xiong, N.; Gong, Y. Evaluation of early antibiotic use in patients with non-severe COVID-19 without bacterial infection. Int. J. Antimicrob. Agents 2022, 59, 106462. [Google Scholar] [CrossRef]
- Buetti, N.; Mazzuchelli, T.; Lo Priore, E.; Balmelli, C.; Llamas, M.; Pallanza, M.; Elzi, L.; Consonni, V.; Trimboli, P.; Forni-Ogna, V.; et al. Early administered antibiotics do not impact mortality in critically ill patients with COVID-19. J. Infect. 2020, 81, e148–e149. [Google Scholar] [CrossRef]
- Rodríguez, A.H.; Avilés-Jurado, F.X.; Díaz, E.; Schuetz, P.; Trefler, S.I.; Solé-Violán, J.; Cordero, L.; Vidaur, L.; Estella, Á.; Pozo Laderas, J.C.; et al. Procalcitonin (PCT) levels for ruling-out bacterial coinfection in ICU patients with influenza: A CHAID decision-tree analysis. J. Infect. 2016, 72, 143–151. [Google Scholar] [CrossRef]
- Zaragoza, R.; Vidal-Cortés, P.; Aguilar, G.; Borges, M.; Diaz, E.; Ferrer, R.; Maseda, E.; Nieto, M.; Nuvials, F.X.; Ramirez, P.; et al. Update of the treatment of nosocomial pneumonia in the ICU. Crit. Care 2020, 24, 383. [Google Scholar] [CrossRef]
- Niederman, M.S.; Torres, A. Severe community-acquired pneumonia. Eur. Respir. Rev. 2022, 31, 220071. [Google Scholar] [CrossRef]
- Tiri, B.; Sensi, E.; Marsiliani, V.; Cantarini, M.; Priante, G.; Vernelli, C.; Martella, L.A.; Costantini, M.; Mariottini, A.; Andreani, P.; et al. Antimicrobial stewardship program, COVID-19, and infection control: Spread of carbapenem-resistant Klebsiella pneumoniae colonization in ICU COVID-19 patients. What did not work? J. Clin. Med. 2020, 9, 2744. [Google Scholar] [CrossRef]
- Li, J.; Wang, J.; Yang, Y.; Cai, P.; Cao, J.; Cai, X.; Zhang, Y. Etiology and antimicrobial resistance of secondary bacterial infections in patients hospitalized with COVID-19 in Wuhan, China: A retrospective analysis. Antimicrob. Resist. Infect. Control 2020, 9, 153. [Google Scholar] [CrossRef] [PubMed]
- Grupo de Trabajo de Medicina Intensiva. Estudio Nacional de Vigilancia de Infección Nosocomial en Servicios ENVIN-COVID. Available online: https://hws.vhebron.net/envin-helics/ (accessed on 20 April 2025).
| Variables # | Whole Population (n = 4197) | Coinfection Patients (n = 654) | No Coinfection Patients (n = 3543) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Total | No EAT (n = 495) | EAT (n = 3702) | Total | No EAT (n = 28) | EAT (n = 626) | Total | No EAT (n = 467) | EAT (n = 3076) | |
| General characteristics | |||||||||
| Age, years | 60 (49–69) | 60 (46–69) | 60(49–69) | 59 (48–70) | 62 (53–69) | 59 (48–70) | 60 (49–69) | 60 (45–69) | 60 (49–69) * |
| Male sex | 2746 (65.4) | 310 (62.6) | 2436 (65.8) | 433 (66.2) | 19 (67.9) | 414 (66.1) | 2313 | 291 (62.3) | 2022 (65.7) |
| APACHE II score | 16 (12.21) | 15(12–18) | 16(12–21) *** | 18 (13–24) | 14 (10–18) | 18 (13–24) *** | 15 (12–20) | 15 (12–18) | 15 (12–21) * |
| SOFA score | 6 (4–8) | 5 (4–7) | 6 (4–8) *** | 7 (5–10) | 5 (3–7) | 7(5–10) *** | 6 (4–8) | 5 (4–7) | 6 (4–8) *** |
| Gap-ICU, days | 1 (1–3) | 2(1–4) | 1(1–43) *** | 1 (0–2) | 1.4 (0–4) | 1.0 (0–2) | 1 (1–3) | 2 (1–4) | 1 (1–3) *** |
| Chest X-ray cutoff | 2646 (63.0) | 317 (74.9) | 2329 (62.9) *** | 360 (55.0) | 23 (82.1) | 337 (53.8) *** | 2340 (66.0) | 348 (74.5) | 1992 (64.8) *** |
| COVID | 2159 (51.4) | 279 (56.4) | 1880 (50.8) ** | 191 (29.2) | 20 (71.4) | 171 (27.3) *** | 1968 (55.5) | 259 (55.5) | 1709 (55.6) |
| Influenza | 2038 (48.5) | 216 (43.6) | 1822 (49.2) ** | 463 (70.8) | 8 (28.6) | 455 (72.7) *** | 1575 (44.4) | 208 (44.5) | 1367 (44.4) |
| Laboratory | |||||||||
| WBC × 103 | 8.7 (5.6–13.0) | 8.6 (6.4–11.6) | 8.8 (5.4–13.1) | 8.5 (4.2–13.6) | 8.3 (5.3–11.7) | 8.6 (4.2–13.7) | 8.8 (5.8–12.8) | 8.6 (6.5–11.5) | 8.8 (5.7–13.0) |
| LDH U/L | 597 (454–763) | 600 (487–722) | 597 (450–768) | 600 (460–745) | 556 (467–628) | 600 (458–749) | 597 (454–766) | 600 (490–725) | 590 (450–770) |
| C-RP mg/mL | 22.7 (11.5–40.0) | 21.1 (9.6–33.0) | 23.0 (11.8–42.9) *** | 30.2 (16.5–80.4) | 13 (6.7–29.0) | 31 (17.7–82.5) *** | 21.3 (10.8–35.8) | 21.4 (10.0–33.1) | 21.4 (11.0–37.0) |
| PCT ng/mL | 1.4 (0.30–8.90) | 0.84 (0.22–3.35) | 1.50 (0.32–10.1) *** | 5.3 (1.0–22.3) | 0.73 (0.21–2.80) | 5.99 (1.2–22.6) *** | 1.08 (0.27–3.38) | 0.87 (0.22–4.48) | 1.14 (0.29–7.11) *** |
| Creatinine mg/dL | 0.92 (0.70–1.32) | 0.95 (0.72–1.25) | 0.91 (0.70–1.34) | 1.1 (0.7–1.8) | 0.92 (0.65–1.41) | 1.11 (0.77–1.81) | 0.90 (0.70–1.26) | 0.95 (0.72–1.25) | 0.90 (0.70–1.27) |
| CPK | 265 (119–485) | 280 (124–487) | 263 (119–485) | 318 (138–589) | 213 (142–358) | 326 (138–602) | 253 (117–473) | 288 (124–497) | 248 (115–469) |
| Lactate mmol/L | 2.2 (1.5–3.6) | 1.9 (1.4–2.8) | 2.3 (1.5–3.7) *** | 3.1 (2.0–4.6) | 2.0 (1.4–2.5) | 3.2 (2.0–4.7) *** | 2.1 (1.4–3.9) | 1.9 (1.4–2.8) | 2.1 (1.4–3.5) ** |
| D-dimer | 4343 (1560–8170) | 3316 (1360–6200) | 4560 (1600–8400) *** | 6400 (3030–11,131) | 2030 (980–6270) | 6585 (3290–11,230) *** | 4000 (1470–7620) | 3327 (1404–6200) | 4111 (1480–7770) *** |
| Comobidities | |||||||||
| COPD | 613 (14.6) | 66 (13.3) | 547 (14.8) | 126 (19.3) | 4 (14.3) | 122 (19.5) | 487 (13.7) | 62 (13.3) | 425 (13.8) |
| Asthma | 302 (7.2) | 37 (7.4) | 265 (7.1) | 41 (6.3) | 2 (7.1) | 39 (6.2) | 261 (7.3) | 35 (7.5) | 226 (7.3) |
| Chr. Heart Dis | 271 (6.4) | 21 (4.2) | 250 (6.7) | 57 (8.7) | 1 (3.5) | 56 (8.9) | 214 (6.0) | 20 (4.3) | 194 (6.3) |
| Chr.Renal Dis. | 260 (6.2) | 32 (6.4) | 228 (6.1) | 52 (7.9) | 1 (3.5) | 51 (8.1) | 208 (5.8) | 31 (6.6) | 177 (5.7) |
| Hematologic Dis. | 215 (5.1) | 27 (5.4) | 188 (5.0) | 42 (6.4) | 2 (7.1) | 40 (6.4) | 173 (4.8) | 25 (5.3) | 148 (4.8) |
| Pregnancy | 200 (4.8) | 14 (2.8) | 186 (5.0) * | 53 (8.1) | 0 (0.0) | 53 (8.4) | 147 (4.1) | 14 (3.0) | 133 (4.3) |
| Obesity | 1471 (35.0) | 195 (39.4) | 1276 (34.5) * | 183 (28.0) | 9 (32.1) | 174 (27.8) | 1288 (36.3) | 186 (39.8) | 1102 (35.8) |
| Diabetes | 493 (11.7) | 83 (16.8) | 410 (11.1) | 57 (8.7) | 5 (17.9) | 52 (8.3) | 436 (12.3) | 78 (16.7) | 358 (11.6) |
| Immunosuppression | 349 (8.3) | 33 (6.6) | 316 (8.5) | 77 (11.8) | 1 (3.5) | 76 (12.1) | 272 (7.6) | 32 (6.8) | 240 (7.8) |
| Treatments and complications | |||||||||
| Bacterial coinfection | 654 (15.6) | 28 (5.6) | 626 (16.9) | NA | NA | NA | NA | NA | NA |
| AEAT | 541 (12.9) | 0 (0.0) | 541(86.4) | 541 (82.7) | 0 (0.0) | 541 (86.4) | NA | NA | NA |
| Corticosteriods | 2424 (57.8) | 237 (47.9) | 2187 (59.1) *** | 400 (61.2) | 20 (32.1) | 380 (60.7) | 2024 (57.1) | 217 (46.5) | 1807 (58.7) *** |
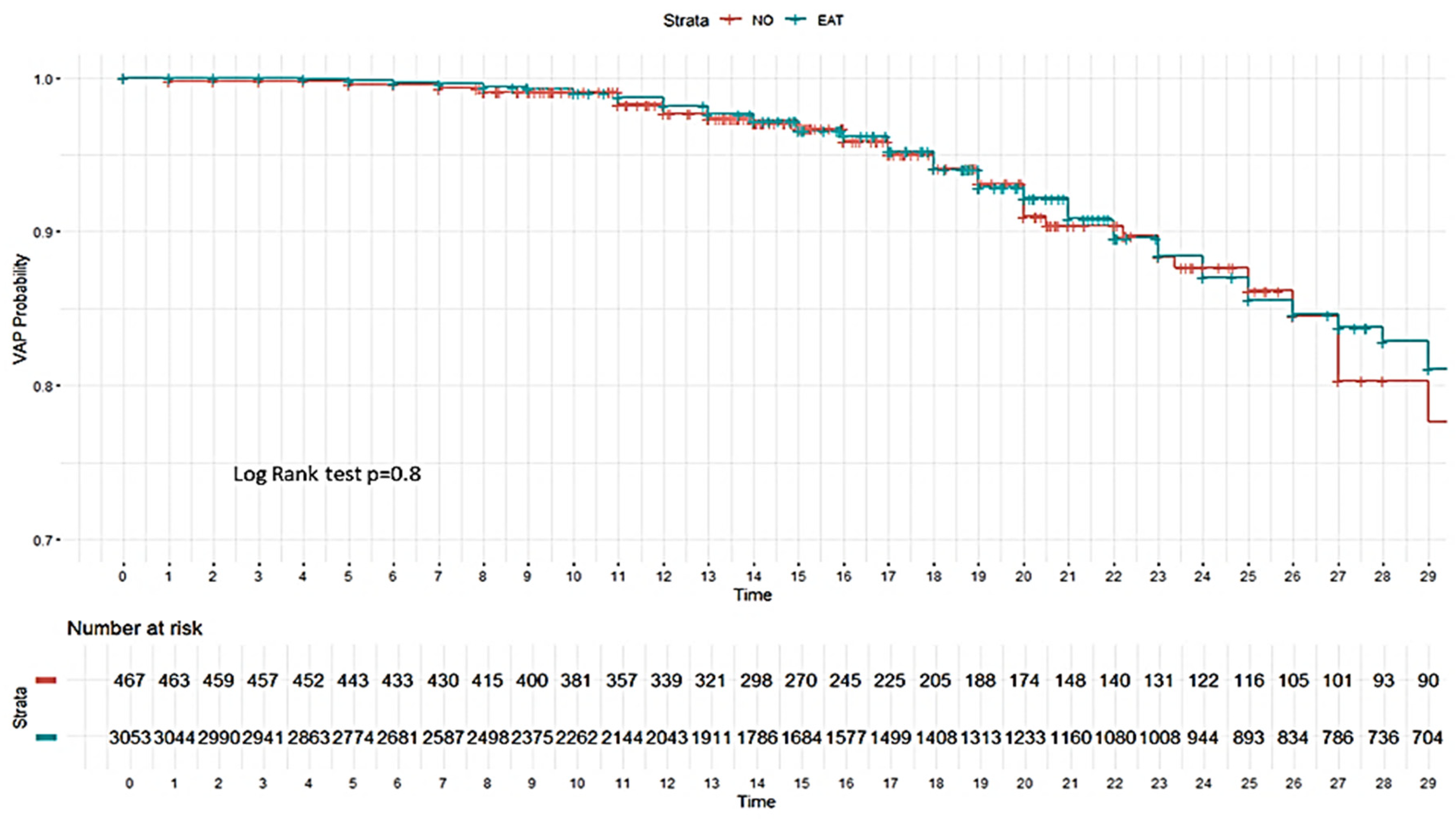
| VAP | 743 (17.7) | 88 (17.8) | 655 (17.7) | 135 (20.6) | 10 (35.7) | 125 (20.0) | 608 (17.1) | 78 (16.7) | 530 (17.2) |
| AKI | 821 (19.6) | 63 (12.7) | 758 (20.5) *** | 220 | 6 (21.4) | 214 (34.2) | 601 (16.9) | 57 (12.2) | 544 (17.7) ** |
| Myocardial dysfunction | 216 (5.1) | 19 (3.8) | 197(5.3) | 131 | 1 (17.9) | 130 (20.8) | 201 (5.6) | 18 (3.8) | 183 (5.9) |
| Shock | 2721 (64.8) | 276 (55.8) | 2445 (66.0) *** | 41 | 2 (7.1) | 39 (6.2) | 2223 (62.7) | 263 (56.3) | 1960 (63.7) ** |
| Outcomes | |||||||||
| LOS ICU, days | 16 (9–27) | 16 (11 -25) | 16 (9–27) | 16 (9–29) | 19 (12–37) | 16 (8–29) | 16 (10–27) | 16 (11–24) | 16 (9–27) |
| LOS Hospital, days | 26 (16–40) | 26 (18–35) | 26 (16–40) | 26 (14–44) | 26 (17–47) | 26 (14–44) | 26 (16–39) | 25 (18–35) | 26 (16–40) |
| IMV days | 12 (5–22) | 8 (1–20) | 12 (6.23) *** | 13 (7–25) | 15 (7–35) | 13 (7–25) | 12 (5–22) | 8 (1–20) | 12 (6–22) *** |
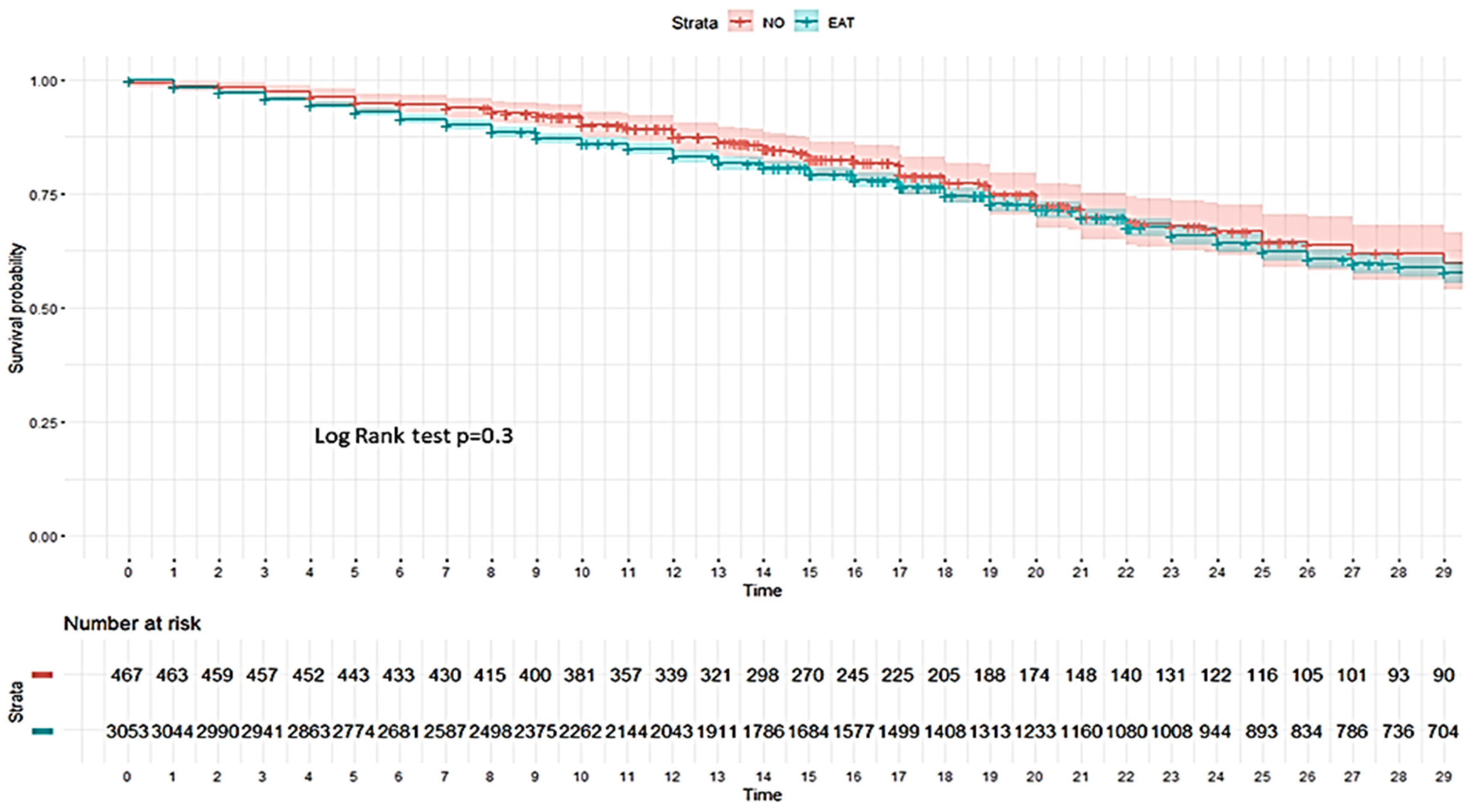
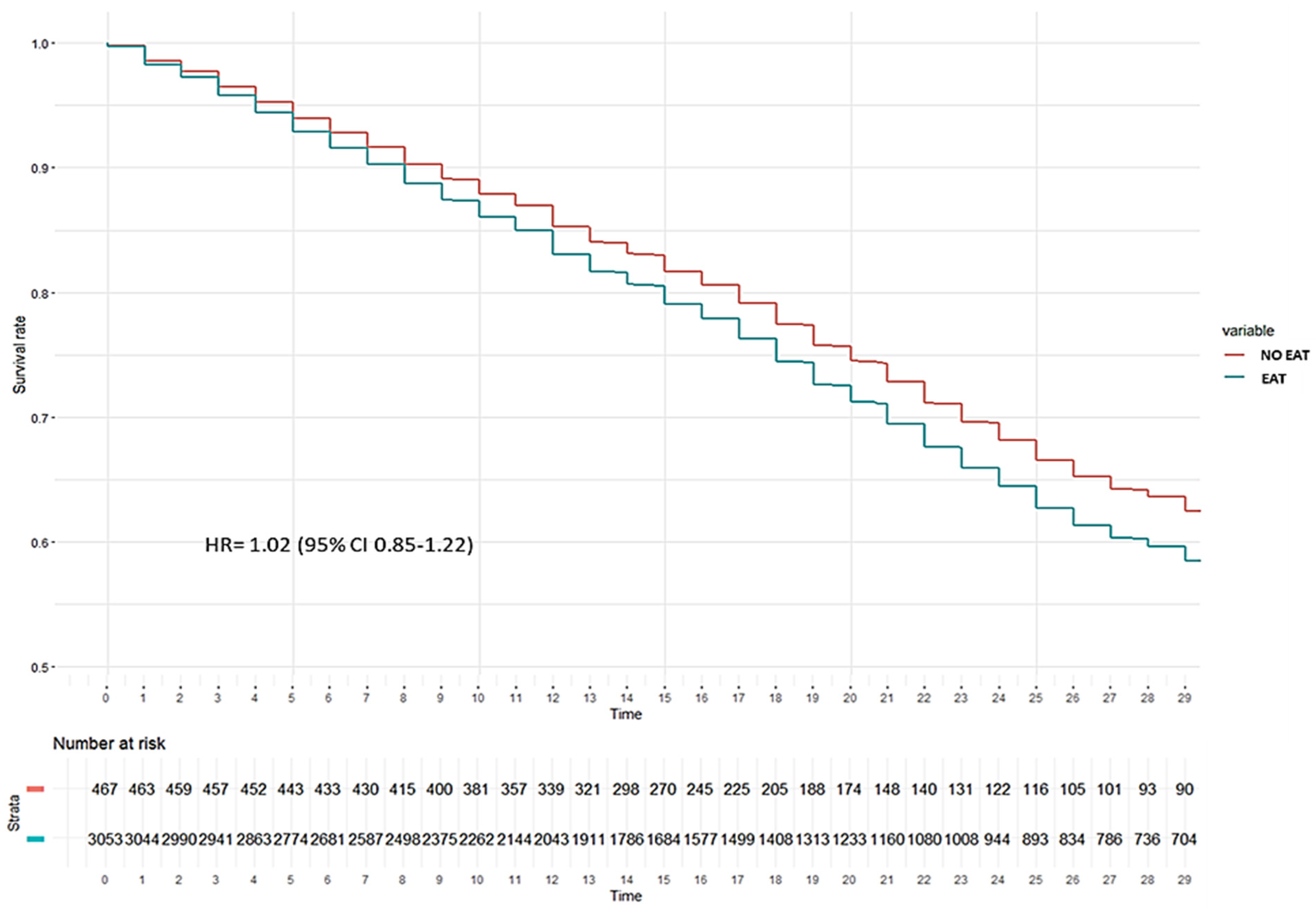
| ICU mortality | 1466 (34.9) | 159 (32.1) | 1307 (35.3) | 264 (40.4) | 16 (57.1) | 248 (39.6) | 1202 (33.9) | 143 (30.6) | 1059 (34.4) |
| Variables # | IEAT (n = 85) | AEAT (n = 541) | p-Value |
|---|---|---|---|
| General Characteristics | |||
| Age, years | 62 (56–72) | 59 (47–70) | 0.009 |
| Male sex | 55 (64.7) | 359 (66.4) | 0.86 |
| APACHE II score | 18 (13–21) | 19 (14–24) | 0.17 |
| SOFA score | 7 (5–9) | 7 (5–10) | 0.04 |
| Gap-ICU, days | 1 (1–2) | 1 (0–2) | 0.18 |
| Chest X-ray cutoff | 51 (60.0) | 286 (52.9) | 0.26 |
| COVID | 48 (56.5) | 123 (22.7) | <0.001 |
| Influenza | 37 (43.5) | 418 (77.3) | <0.001 |
| Laboratory | |||
| WBC × 103 | 8.0 (4.9–11.6) | 8.7 (3.9–13.9) | 0.60 |
| LDH U/L | 630 (473–830) | 600 (458–745) | 0.29 |
| C-RP mg/mL | 22.4 (13.0–33.3) | 33.4 (19.7–91.3) | <0.001 |
| PCT ng/mL | 1.44 (0.24–8.26) | 7.86 (1.55–24.0) | <0.001 |
| Creatinine mg/dL | 0.87 (0.70–1.48) | 1.14 (0.79–1.86) | 0.01 |
| CPK | 218 (119–399) | 338 (151–647) | 0.001 |
| Lactate mmol/L | 2.3 (1.6–3.6) | 2.3 (2.2–4.8) | <0.001 |
| D-dimer | 3940 (1179–7200) | 6800 (3780–11,700) | |
| Comorbidities | |||
| COPD | 11 (12.9) | 111 (20.5) | 0.13 |
| Asthma | 8 (9.4) | 31 (5.7) | 0.28 |
| Chr. Heart Dis | 4 (4.7) | 52 (9.6) | 0.20 |
| Chr.Renal Dis. | 7 (8.2) | 44 (8.1) | 1.0 |
| Hematologic Dis. | 4 (4.7) | 36 (6.6) | 0.65 |
| Pregnancy | 2 (2.3) | 51 (9.4) | 0.04 |
| Obesity | 34 (40.0) | 140 (25.9) | 0.01 |
| Diabetes | 13 (15.3) | 39 (7.2) | 0.02 |
| Immunosuppression | 8 (9.4) | 68 (12.6) | 0.51 |
| Treatment and complications | |||
| Corticosteroids | 57 (67.1) | 323 (59.7) | 0.24 |
| Presence of MDR bacteria | 69 (81.2) | 98 (18.1) | <0.001 |
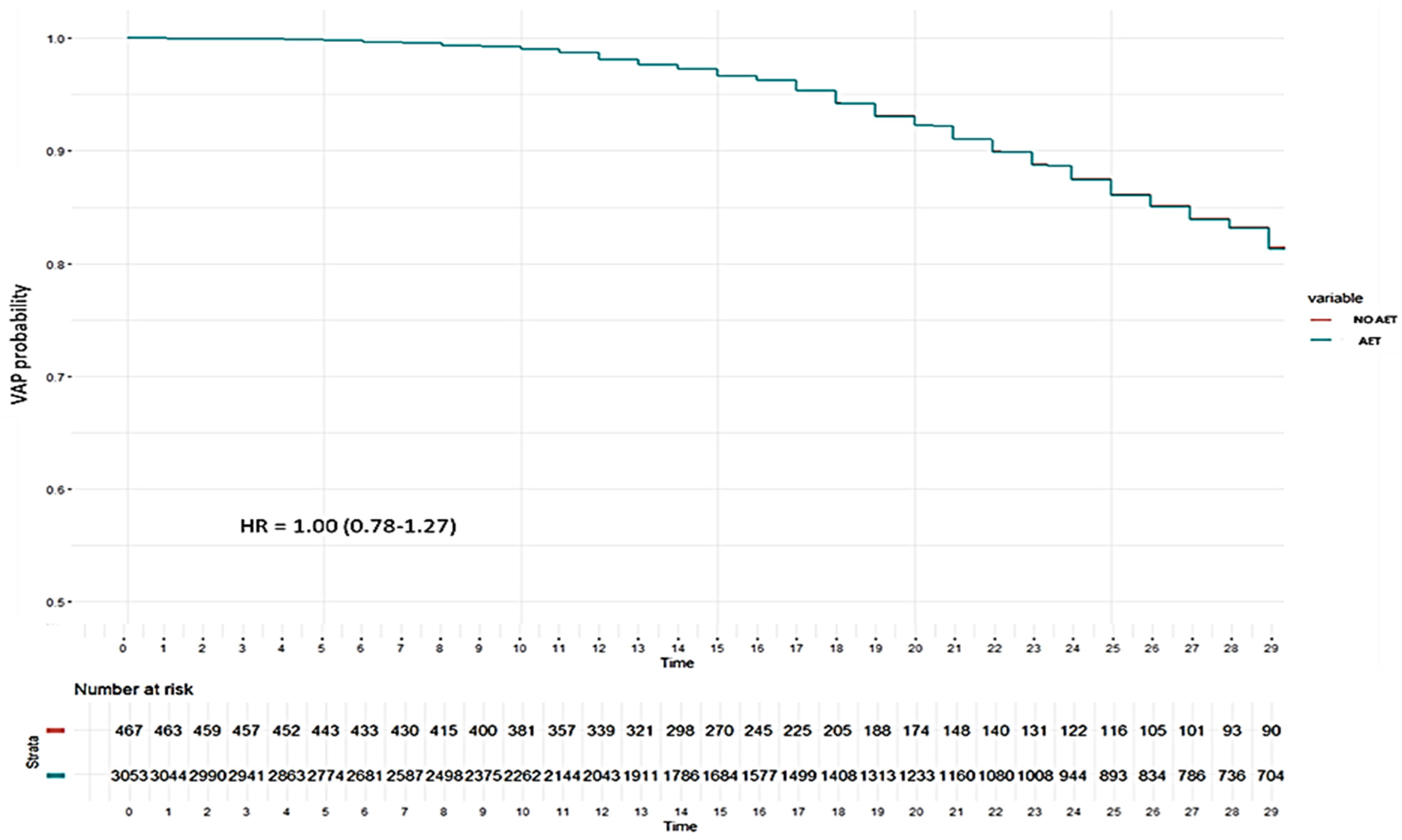
| VAP | 31 (36.5) | 94 (17.4) | <0.001 |
| AKI | 20 (23.5) | 194 (35.9) | 0.03 |
| Myocardial dysfunction | 4 (4.7) | 10 (1.8) | 0.10 |
| Shock | 61 (71.8) | 424 (78.4) | 0.22 |
| Outcomes | |||
| LOS ICU, days | 22 (12–37) | 16 (8–28) | 0.001 |
| LOS Hospital, days | 30 (21–50) | 25 (12–42) | 0.008 |
| IMV days | 15 (10–30) | 12 (6–24) | 0.01 |
| ICU mortality | 40 (47.1) | 208 (38.4) | 0.16 |
| Variables # | No VAP (n = 519) | VAP (n = 135) | p-Value |
|---|---|---|---|
| General Characteristics | |||
| Age, years | 59 (47–70) | 61 (52–71) | 0.09 |
| Male sex | 337 (64.9) | 96 (71.1) | 0.21 |
| APACHE II score | 19 (14–25) | 17 (12–21) | <0.001 |
| SOFA score | 7 (5–10) | 7 (4–10) | 0.44 |
| Gap-ICU, days | 1 (0–2) | 1 (0–2) | 0.55 |
| Chest X-ray cutoff | 268 (51.6) | 92 (68.1) | 0.001 |
| Laboratory | |||
| WBC × 103 | 8.3 (3.7–13.7) | 9.0 (5.0–13.3) | 0.64 |
| LDH U/L | 600 (450–757) | 590 (480–720) | 0.62 |
| C-RP mg/mL | 33.0 (19.1–85.0) | 22.6 (12.6–40.0) | <0.001 |
| PCT ng/mL | 7.0 (1.5–24.0) | 1.5 (0.4–10.1) | <0.001 |
| Creatinine mg/dL | 1.1 (0.7–1.8) | 0.9 (0.7–1.4) | 0.01 |
| CPK | 338 (138–657) | 268 (141–400) | 0.01 |
| Lactate mmol/L | 3.2 (1.2–4.8) | 2.3 (1.6–3.7) | <0.001 |
| D-dimer | 6667 (3900–11,220) | 4000 (1000–9940) | <0.001 |
| Comorbidities | |||
| COPD | 109 (21.0) | 17 (12.6) | 0.03 |
| Asthma | 32 (6.1) | 9 (6.7) | 0.98 |
| Chr. Heart Dis | 47 (9.0) | 10 (7.4) | 0.66 |
| Chr.Renal Dis. | 41 (7.9) | 11 (8.1) | 1.00 |
| Hematologic Dis. | 34 (6.5) | 8 (5.9) | 0.94 |
| Pregnancy | 46 (8.8) | 7 (5.2) | 0.22 |
| Obesity | 134 (25.8) | 49 (36.3) | 0.02 |
| Diabetes | 34 (6.5) | 23 (17.0) | <0.001 |
| Immunosuppression | 65 (12.5) | 12 (8.9) | 0.30 |
| Treatment and complications | |||
| Corticosteroids | 310 (59.7) | 90 (66.7) | 0.16 |
| EAT | 501 (96.5) | 125 (92.6) | 0.07 |
| AEAT | 451 (86.9) | 98 (72.6) | <0.001 |
| Global IEAT | 72 (13.9) | 41 (30.4) | <0.001 |
| AKI | 188 (36.2) | 32 (23.7) | 0.008 |
| Myocardial dysfunction | 10 (1.9) | 5 (3.7) | 0.20 |
| Shock | 403 (77.6) | 95 (70.4) | 0.09 |
| Outcomes | |||
| LOS ICU, days | 14 (7–23) | 31 (19–48) | <0.001 |
| LOS Hospital, days | 23 (12–36) | 44 (27–59) | <0.001 |
| IMV days | 10 (6–19) | 27 (17–41) | <0.001 |
| ICU mortality | 208 (40.1) | 56 (41.5) | 0.84 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Papiol, E.; Berrueta, J.; Ruíz-Rodríguez, J.C.; Ferrer, R.; Manrique, S.; Claverias, L.; García-Martínez, A.; Orts, P.; Díaz, E.; Zaragoza, R.; et al. Does Empirical Antibiotic Use Improve Outcomes in Ventilated Patients with Pandemic Viral Infection? A Multicentre Retrospective Study. Antibiotics 2025, 14, 594. https://doi.org/10.3390/antibiotics14060594
Papiol E, Berrueta J, Ruíz-Rodríguez JC, Ferrer R, Manrique S, Claverias L, García-Martínez A, Orts P, Díaz E, Zaragoza R, et al. Does Empirical Antibiotic Use Improve Outcomes in Ventilated Patients with Pandemic Viral Infection? A Multicentre Retrospective Study. Antibiotics. 2025; 14(6):594. https://doi.org/10.3390/antibiotics14060594
Chicago/Turabian StylePapiol, Elisabeth, Julen Berrueta, Juan Carlos Ruíz-Rodríguez, Ricard Ferrer, Sara Manrique, Laura Claverias, Alejandro García-Martínez, Pau Orts, Emili Díaz, Rafael Zaragoza, and et al. 2025. "Does Empirical Antibiotic Use Improve Outcomes in Ventilated Patients with Pandemic Viral Infection? A Multicentre Retrospective Study" Antibiotics 14, no. 6: 594. https://doi.org/10.3390/antibiotics14060594
APA StylePapiol, E., Berrueta, J., Ruíz-Rodríguez, J. C., Ferrer, R., Manrique, S., Claverias, L., García-Martínez, A., Orts, P., Díaz, E., Zaragoza, R., Marotta, M., Bodí, M., Trefler, S., Gómez, J., Martín-Loeches, I., & Rodríguez, A., on behalf of the GETGAG and COVID-19 Working Group from SEMICYUC. (2025). Does Empirical Antibiotic Use Improve Outcomes in Ventilated Patients with Pandemic Viral Infection? A Multicentre Retrospective Study. Antibiotics, 14(6), 594. https://doi.org/10.3390/antibiotics14060594







