Antibacterial and Synergistic Effects of Terminalia citrina Leaf Extracts Against Gastrointestinal Pathogens: Insights from Metabolomic Analysis
Abstract
1. Introduction
2. Results
2.1. Extraction Yields and Antibacterial Activities
2.2. Disc Diffusion Assay
2.3. Liquid Microdilution Assay
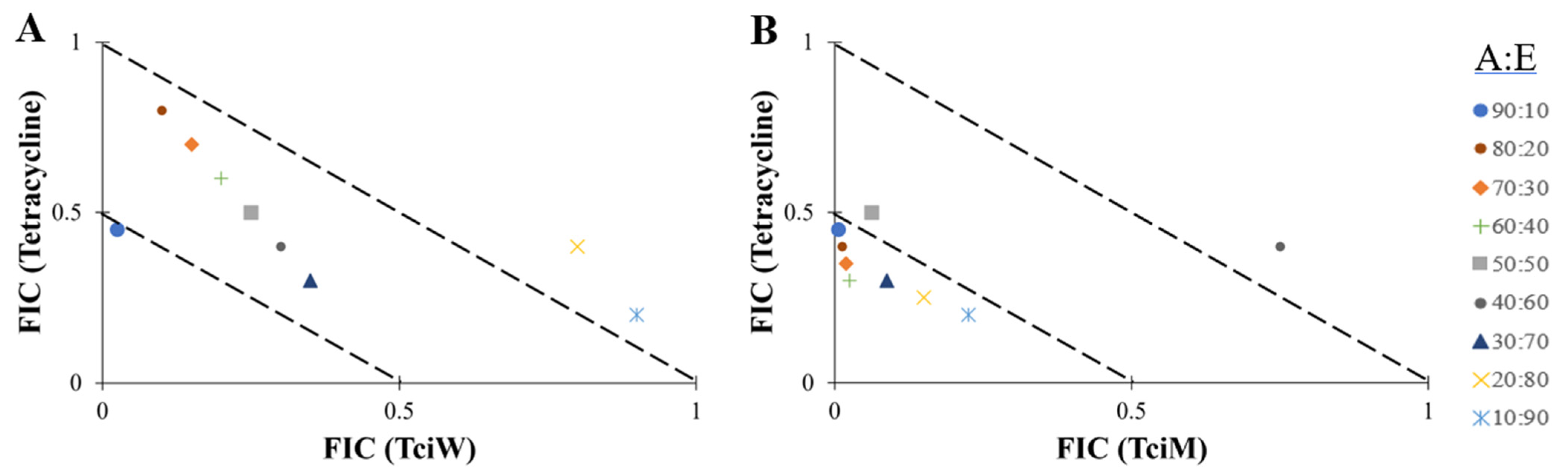
2.4. Fractional Inhibitory Concentration (FIC) Determination
2.5. Isobologram Analysis
2.6. Quantification of Toxicity
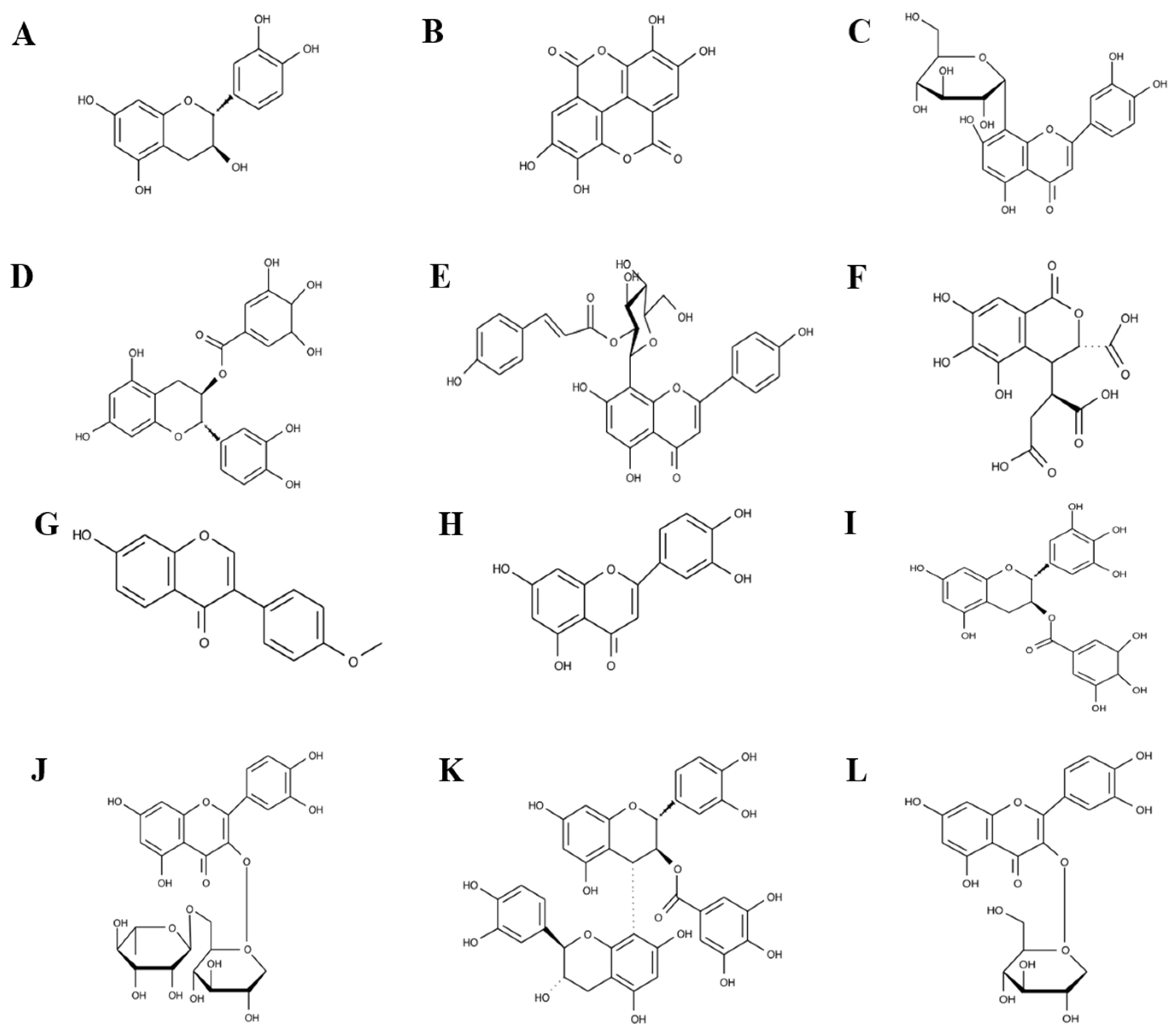
2.7. UPLC-MS Polyphenol Fingerprinting Studies
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Plant Collection and Extraction
4.3. Bacterial Cultures
4.4. Controls for Evaluation of Antibacterial Activity
4.5. Disc Diffusion Assay
4.6. Liquid Microdilution Assay
4.7. Fractional Inhibitory Concentration (FIC) Determination
4.8. Non-Targeted Hheadspace LC-MS Analysis
4.9. Toxicity Studies Using Brine Shrimp
4.10. Toxicity Studies Using Human Dermal Fibroblasts
4.11. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| MDR | Multi-drug resistant |
| UPLC-MS | Ultra-high-performance liquid chromatography–mass spectrometry |
| MRSA | Methicillin-resistant Staphylococcus aureus |
| LD | Linear dichroism |
| MIC | Minimum inhibitory concentration |
| HDF | Human dermal fibroblast |
| SFP | Staph food poisoning |
| DMSO | Dimethyl sulfoxide |
| ATCC | American Type Culture Collection |
| ZOI | Zone of inhibition |
| EGCG | (-)-epigallocatechin gallate |
| CLSI | Clinical and Laboratory Standards Institute |
References
- WHO. WHO Global Strategy for Food Safety 2022–2030: Towards Stronger Food Safety Systems and Global Cooperation: Executive summary; WHO: Geneva, Switzerland, 2022. [Google Scholar]
- Hennekinne, J.A.; De Buyser, M.L.; Dragacci, S. Staphylococcus aureus and its food poisoning toxins: Characterization and outbreak investigation. FEMS Microbiol. Rev. 2012, 36, 815–836. [Google Scholar] [CrossRef] [PubMed]
- Ercoli, L.; Gallina, S.; Nia, Y.; Auvray, F.; Primavilla, S.; Guidi, F.; Pierucci, B.; Graziotti, C.; Decastelli, L.; Scuota, S. Investigation of a staphylococcal food poisoning outbreak from a Chantilly Cream Dessert, in Umbria (Italy). Foodborne Pathog. Dis. 2017, 14, 7. [Google Scholar] [CrossRef] [PubMed]
- Savini, F.; Romano, A.; Giacometti, F.; Indio, V.; Pitti, M.; Decastelli, L.; Devalle, P.L.; Gorrasi, I.S.R.; Miaglia, S.; Serraino, A. Investigation of a Staphylococcus aureus sequence type 72 food poisoning outbreak associated with food-handler contamination in Italy. Zoonoses Public Health 2023, 70, 411–419. [Google Scholar] [CrossRef] [PubMed]
- Le, H.H.T.; Dalsgaard, A.; Andersen, P.S.; Nguyen, H.M.; Ta, Y.T.; Nguyen, T.T. Large-scale Staphylococcus aureus foodborne disease poisoning outbreak among primary school children. Microbiol. Res. 2021, 12, 43–52. [Google Scholar] [CrossRef]
- Othman, B.R.; Kuan, C.H.; Mohammed, A.S.; Cheah, Y.K.; Tan, C.W.; New, C.Y.; Thung, T.Y.; San Chang, W.; Loo, Y.Y.; Nakaguchi, Y.; et al. Occurrence of methicillin-resistant Staphylococcus aureus in raw shellfish at retail markets in Malaysia and antibacterial efficacies of black seed (Nigella sativa) oil against MRSA. Food Control 2018, 90, 324–331. [Google Scholar] [CrossRef]
- Ahmad, N.; Amran, F.; Zamri, H.F.; Liow, Y.L.; Rashid, F.A. National Antibiotic Resistance Surveillance Report 2017. Available online: https://imr.nih.gov.my/images/uploads/NSAR/NSAR_2017/NSAR_report_2017-edited-31.1.2019.pdf (accessed on 24 January 2025).
- WHO. Immunization, Vaccines and Biologicals: Shigella. Available online: https://www.who.int/teams/immunization-vaccines-and-biologicals/diseases/shigella (accessed on 24 January 2025).
- CDC. Increase in Extensively Drug-Resistant Shigellosis in the United States. Available online: https://emergency.cdc.gov/han/2023/han00486.asp (accessed on 4 July 2024).
- Thery, M.; Cousin, V.L.; Tissieres, P.; Enault, M.; Morin, L. Multi-organ failure caused by lasagnas: A case report of Bacillus cereus food poisoning. Front. Pediatr. 2022, 10, 978250. [Google Scholar] [CrossRef]
- García-Castro, M.; Sarabia, F.; Díaz-Morilla, A.; López-Romero, J.M. Approved antibacterial drugs in the last 10 years: From the bench to the clinic. Explor. Drug Sci. 2023, 1, 180–209. [Google Scholar] [CrossRef]
- Qiao, W.; Wang, L.; Luo, Y.; Yang, T. Synthetic approaches and therapeutic applications of FDA-approved antibacterial agents: A comprehensive review from 2003 to 2023. Eur. J. Med. Chem. 2025, 285, 117267. [Google Scholar] [CrossRef]
- Chawla, M.; Verma, J.; Gupta, R.; Das, B. Antibiotic potentiators against multidrug-resistant bacteria: Discovery, development, and clinical relevance. Front. Microbiol. 2022, 13. [Google Scholar] [CrossRef]
- Miethke, M.; Pieroni, M.; Weber, T.; Brönstrup, M.; Hammann, P.; Halby, L.; Arimondo, P.B.; Glaser, P.; Aigle, B.; Bode, H.B.; et al. Towards the sustainable discovery and development of new antibiotics. Nat. Rev. Chem. 2021, 5, 726–749. [Google Scholar] [CrossRef]
- Kim, T.H.; Raiz, A.; Unni, A.D.; Murhekar, S.; Donose, B.C.; Floetenmeyer, M.; Cock, I.E.; Brown, C.L. Combating antibiotic-resistant Gram-negative bacteria strains with tetracycline-conjugated carbon nanoparticles. Adv. Biol. 2020, 4, e2000074. [Google Scholar] [CrossRef] [PubMed]
- Cock, I.E. The medicinal properties and phytochemistry of plants of the genus Terminalia (Combretaceae). Inflammopharmacology 2015, 23, 203–229. [Google Scholar] [CrossRef] [PubMed]
- Dhingra, A.K.; Chopra, B.; Grewal, A.S.; Guarve, K. Pharmacological properties of chebulinic acid and related ellagitannins from nature: An emerging contemporary bioactive entity. Pharmacol. Res. Mod. Chin. Med. 2022, 5, 100163. [Google Scholar] [CrossRef]
- Ou, L.; Hao, Y.; Liu, H.; Zhu, Z.; Li, Q.; Chen, Q.; Wei, R.; Feng, Z.; Zhang, G.; Yao, M. Chebulinic acid isolated from aqueous extracts of Terminalia chebula Retz inhibits Helicobacter pylori infection by potential binding to Cag A protein and regulating adhesion. Front. Microbiol. 2024, 15, 1416794. [Google Scholar] [CrossRef]
- Borges, A.; Ferreira, C.; Saavedra, M.J.; Simões, M. Antibacterial activity and mode of action of ferulic and gallic acids against pathogenic bacteria. Microb. Drug Resist. 2013, 19, 256–265. [Google Scholar] [CrossRef]
- Khameneh, B.; Eskin, N.A.M.; Iranshahy, M.; Fazly Bazzaz, B.S. Phytochemicals: A promising weapon in the arsenal against antibiotic-resistant bacteria. Antibiotics 2021, 10, 1044. [Google Scholar] [CrossRef]
- Perry, L.M. Medicinal Plants of East and Southeast Asia: Attributed Properties; Massachusetts Institute of Technology Press: Cambridge, Massachusetts, 1980; p. 361. [Google Scholar]
- Burapadaja, S.; Bunchoo, A. Antimicrobial activity of tannins from Terminalia citrina. Planta Medica 1995, 61, 365–366. [Google Scholar] [CrossRef]
- Tiwana, G.; Cock, I.E.; Cheesman, M.J. Phytochemical analysis and antimicrobial activity of Terminalia bellirica (Gaertn.) Roxb. and Terminalia chebula Retz. fruit extracts against gastrointestinal pathogens: Enhancing antibiotic efficacy. Microorganisms 2024, 12, 2664. [Google Scholar] [CrossRef]
- Tiwana, G.; Cock, I.E.; Cheesman, M.J. Phyllanthus niruri Linn.: Antibacterial activity, phytochemistry, and enhanced antibiotic combinatorial strategies. Antibiotics 2024, 13, 654. [Google Scholar] [CrossRef]
- CLSI Supplement M100; Performance Standards for Antimicrobial Susceptibility Testing. Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2020.
- CLSI Guideline M45; Methods for Antimicrobial Dilution and Disk Susceptibility Testing of Infrequently Isolated or Fastidious Bacteria. Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2015.
- Tiwana, G.; Cock, I.E.; Cheesman, M.J. Combinations of Terminalia bellirica (Gaertn.) Roxb. and Terminalia chebula Retz. extracts with selected antibiotics against antibiotic-resistant bacteria: Bioactivity and phytochemistry. Antibiotics 2024, 13, 994. [Google Scholar] [CrossRef]
- Ali, A.; Bashmil, Y.M.; Cottrell, J.J.; Suleria, H.A.R.; Dunshea, F.R. LC-MS/MS-QTOF Screening and identification of phenolic compounds from Australian grown herbs and their antioxidant potential. Antioxidants 2021, 10, 1770. [Google Scholar] [CrossRef] [PubMed]
- Puig, L.P.; Boque, M.C.; Ferrer, A.V.; Fernandez-Ruano, L.; Blasco, J.L.L.; Cladera, M.A. Advanced mass spectrometry profiling of phenolic and minerals compounds in herbal beverages. Food Chem. 2023, 428, 136767. [Google Scholar] [CrossRef]
- Lopez-Fernandez, O.; Dominguez, R.; Pateiro, M.; Munekata, P.E.S.; Rocchetti, G.; Lorenzo, J.M. Determination of polyphenols using liquid chromatography-tandem mass spectrometry technique (LC-MS/MS): A review. Antioxidants 2020, 9, 479. [Google Scholar] [CrossRef] [PubMed]
- Naznin, M.; Alam, R.; Alam, M.B.; Jung, M.J.; Lee, S.H.; Kim, S. Biological activities, identification, method development, and validation for analysis of polyphenolic compounds in Nymphaea rubra flowers and leaves by UHPLC-Q-cIM-TOF-MS and UHPLC-TQ-MS. Phytochem. Anal. 2024, 35, 799–816. [Google Scholar] [CrossRef]
- Jokar, A.; Masoomi, F.; Sadeghpour, O.; Nassiri-Toosi, M.; Hamedi, S. Potential therapeutic applications for Terminalia chebula in Iranian traditional medicine. J. Tradit. Chin. Med. 2016, 36, 250–254. [Google Scholar] [CrossRef]
- Das, G.; Kim, D.Y.; Fan, C.; Gutierrez-Grijalva, E.P.; Heredia, J.B.; Nissapatorn, V.; Mitsuwan, W.; Pereira, M.L.; Nawaz, M.; Siyadatpanah, A.; et al. Plants of the genus Terminalia: An insight on its biological potentials, pre-clinical and clinical Studies. Front. Pharmacol. 2020, 11, 561248. [Google Scholar] [CrossRef] [PubMed]
- Ozler, E.; Topal, F.; Topal, M.; Ozturk Sarikaya, S.B. LC-HRMS profiling and phenolic content, cholinesterase, and antioxidant activities of Terminalia citrina. Chem. Biodivers. 2023, 20, e202201250. [Google Scholar] [CrossRef]
- Komor, P.; Devi, O.S. Edible Bio-Resources & Livelihoods; Assam State Biodiversity Board: Panjabari, India, 2016.
- Das, N.; Goshwami, D.; Hasan, M.S.; Mahmud, Z.A.; Raihan, S.Z. Evaluation of antioxidant, antimicrobial and cytotoxic activities of Terminalia citrina leaves. J. Pharm. Res. 2016, 10, 8–15. [Google Scholar]
- Das, N.; Hasan, M.S.; Mahmud, Z.A.; Raihan, S.Z. Evaluation of anti-diarrheal, central nervous system (CNS), hypoglycemic and thrombolytic activities of methanolic extract of Terminalia citrina leaves. World J. Pharm. Res. 2016, 5, 795–805. [Google Scholar] [CrossRef]
- Karim, R.; Begum, M.M.; Jui, Y.; Islam, T.; Billah, M.; Arafat, Y.; Karim, M.; Khan, A.F.; Rahman, M.S. In-vitro cytotoxic and anti-Vibrio cholerae activities of alcoholic extracts of Desmodium triflorum (L.) whole plant and Terminalia citrina (Roxb.) fruits. Clin. Phytosci. 2021, 7, 36. [Google Scholar] [CrossRef]
- Akhtar, M.F.; Saleem, A.; Sharif, A.; Akhtar, B.; Nasim, M.B.; Peerzada, S.; Raza, M.; Ijaz, H.; Ahmed, S.; Shabbir, M.; et al. Genotoxic and cytotoxic action potential of Terminalia citrina, a medicinal plant of ethnopharmacological significance. EXCLI J. 2016, 15, 589–598. [Google Scholar] [CrossRef] [PubMed]
- Zai, M.J.; Cheesman, M.J.; Cock, I.E. Selected Australian Terminalia species extracts inhibit beta-lactam drug-resistant bacteria growth and potentiate the activity of conventional antibiotics: Bioactivities and phytochemistry. Microorganisms 2024, 12, 498. [Google Scholar] [CrossRef] [PubMed]
- Florenca, C.; Tiwana, G.; Grant, G.D.; Cock, I.E.; Cheesman, M.J. Phytochemical analysis and antibacterial properties of Terminalia phanerophlebia and Terminalia sambesiaca leaf extracts. S. Afr. J. Bot. 2024, 174, 9–22. [Google Scholar] [CrossRef]
- Murathan, Z.T.; ErbİL, N.; Arslan, M. Antiradical, antibacterial and mutagenic activity analysis of dried fruits of Terminalia chebula and Terminalia citrina plants used for medical purposes. Aduziraat 2020, 17, 181–187. [Google Scholar] [CrossRef]
- Peacock, S.J.; Paterson, G.K. Mechanisms of methicillin resistance in Staphylococcus aureus. Annu. Rev. Biochem. 2015, 84, 577–601. [Google Scholar] [CrossRef]
- Shariati, A.; Dadashi, M.; Moghadam, M.T.; van Belkum, A.; Yaslianifard, S.; Darban-Sarokhalil, D. Global prevalence and distribution of vancomycin resistant, vancomycin intermediate and heterogeneously vancomycin intermediate Staphylococcus aureus clinical isolates: A systematic review and meta-analysis. Sci. Rep. 2020, 10, 12689. [Google Scholar] [CrossRef]
- Eliopoulos, G.M.; Eliopoulos, G.M.; Roberts, M.C. Tetracycline Therapy: Update. Clin. Infect. Dis. 2003, 36, 462–467. [Google Scholar] [CrossRef]
- Collins, J.A.; Oviatt, A.A.; Chan, P.F.; Osheroff, N. Target-mediated fluoroquinolone resistance in Neisseria gonorrhoeae: Actions of ciprofloxacin against gyrase and topoisomerase IV. ACS Infect. Dis. 2024, 10, 1351–1360. [Google Scholar] [CrossRef]
- Zai, M.J.; Cheesman, M.J.; Cock, I.E. Phytochemical evaluation of Terminalia canescens DC. Radlk. extracts with antibacterial and antibiotic potentiation activities against selected beta-lactam drug-resistant bacteria. Molecules 2024, 29, 1385. [Google Scholar] [CrossRef]
- Shamsudin, N.F.; Ahmed, Q.U.; Mahmood, S.; Ali Shah, S.A.; Khatib, A.; Mukhtar, S.; Alsharif, M.A.; Parveen, H.; Zakaria, Z.A. Antibacterial effects of flavonoids and their structure-activity relationship study: A comparative interpretation. Molecules 2022, 27, 1149. [Google Scholar] [CrossRef]
- Chang, E.H.; Huang, J.; Lin, Z.; Brown, A.C. Catechin-mediated restructuring of a bacterial toxin inhibits activity. Biochim. Biophys. Acta Gen. Subj. 2019, 1863, 191–198. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; Kumar, S.; Kumar, B. LC-MS Identification of proanthocyanidins in bark and fruit of six Terminalia species. Nat. Prod. Commun. 2018, 13, 555–560. [Google Scholar] [CrossRef]
- Friedman, M.; Henika, P.R.; Levin, C.E.; Mandrell, R.E.; Kozukue, N. Antimicrobial activities of tea catechins and theaflavins and tea extracts against Bacillus cereus. J. Food Prot. 2006, 69, 354–361. [Google Scholar] [CrossRef]
- Osterburg, A.; Gardner, J.; Hyon, S.H.; Neely, A.; Babcock, G. Highly antibiotic-resistant Acinetobacter baumannii clinical isolates are killed by the green tea polyphenol (-)-epigallocatechin-3-gallate (EGCG). Clin. Microbiol. Infect. 2009, 15, 341–346. [Google Scholar] [CrossRef] [PubMed]
- Yoda, Y.; Hu, Z.Q.; Zhao, W.H.; Shimamura, T. Different susceptibilities of Staphylococcus and Gram-negative rods to epigallocatechin gallate. J. Infect. Chemother. 2004, 10, 55–58. [Google Scholar] [CrossRef]
- Buchmann, D.; Schultze, N.; Borchardt, J.; Bottcher, I.; Schaufler, K.; Guenther, S. Synergistic antimicrobial activities of epigallocatechin gallate, myricetin, daidzein, gallic acid, epicatechin, 3-hydroxy-6-methoxyflavone and genistein combined with antibiotics against ESKAPE pathogens. J. Appl. Microbiol. 2022, 132, 949–963. [Google Scholar] [CrossRef]
- Tian, Q.; Wei, S.; Su, H.; Zheng, S.; Xu, S.; Liu, M.; Bo, R.; Li, J. Bactericidal activity of gallic acid against multi-drug resistance Escherichia coli. Microb. Pathog. 2022, 173, 105824. [Google Scholar] [CrossRef] [PubMed]
- Hossain, M.A.; Park, H.C.; Lee, K.J.; Park, S.W.; Park, S.C.; Kang, J. In vitro synergistic potentials of novel antibacterial combination therapies against Salmonella enterica serovar Typhimurium. BMC Microbiol. 2020, 20, 118. [Google Scholar] [CrossRef]
- Girard, M.; Bee, G. Invited review: Tannins as a potential alternative to antibiotics to prevent coliform diarrhea in weaned pigs. Animal 2020, 14, 95–107. [Google Scholar] [CrossRef]
- Engels, C.; Knodler, M.; Zhao, Y.Y.; Carle, R.; Ganzle, M.G.; Schieber, A. Antimicrobial activity of gallotannins isolated from mango (Mangifera indica L.) kernels. J. Agric. Food Chem. 2009, 57, 7712–7718. [Google Scholar] [CrossRef]
- Ou, L.; Zhu, Z.; Hao, Y.; Li, Q.; Liu, H.; Chen, Q.; Peng, C.; Zhang, C.; Zou, Y.; Jia, J.; et al. 1,3,6-Trigalloylglucose: A novel potent anti-Helicobacter pylori adhesion agent derived from aqueous extracts of Terminalia chebula Retz. Molecules 2024, 29, 1161. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, M.; Shiota, S.; Mizushima, T.; Ito, H.; Hatano, T.; Yoshida, T.; Tsuchiya, T. Marked potentiation of activity of beta-lactams against methicillin-resistant Staphylococcus aureus by corilagin. Antimicrob. Agents Chemother. 2001, 45, 3198–3201. [Google Scholar] [CrossRef]
- Shiota, S.; Shimizu, M.; Sugiyama, J.; Morita, Y.; Mizushima, T.; Tsuchiya, T. Mechanisms of action of corilagin and tellimagrandin I that remarkably potentiate the activity of beta-lactams against methicillin-resistant Staphylococcus aureus. Microbiol. Immunol. 2004, 48, 67–73. [Google Scholar] [CrossRef] [PubMed]
- Yao, G.; Miao, X.; Wu, M.; Lv, Z.; Bai, Y.; Chang, Y.; Ouyang, H.; He, J. Pharmacokinetics of active compounds of a Terminalia chebula Retz. ethanolic extract after oral administration rats using UPLC-MS/MS. Front. Pharmacol. 2023, 14, 1067089. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Zhang, H.; Wang, X.; Wang, X.; Li, X.; Li, C.; Wang, Y.; Zhang, M. Comprehensive review on fruit of Terminalia chebula: Traditional uses, phytochemistry, pharmacology, toxicity, and pharmacokinetics. Molecules 2024, 29, 5547. [Google Scholar] [CrossRef]
- Li, K.; Han, X.; Li, R.; Xu, Z.; Pan, T.; Liu, J.; Li, B.; Wang, S.; Diao, Y.; Liu, X. Composition, antivirulence activity, and active property distribution of the fruit of Terminalia chebula Retz. J. Food Sci. 2019, 84, 1721–1729. [Google Scholar] [CrossRef]
- Ratti, A.; Fassi, E.M.A.; Forlani, F.; Mori, M.; Villa, F.; Cappitelli, F.; Sgrignani, J.; Roda, G.; Cavalli, A.; Villa, S.; et al. Mechanistic insights into the antibiofilm mode of action of ellagic acid. Pharmaceutics 2023, 15, 1757. [Google Scholar] [CrossRef]
- Quave, C.L.; Estevez-Carmona, M.; Compadre, C.M.; Hobby, G.; Hendrickson, H.; Beenken, K.E.; Smeltzer, M.S. Ellagic acid derivatives from Rubus ulmifolius inhibit Staphylococcus aureus biofilm formation and improve response to antibiotics. PLoS ONE 2012, 7, e28737. [Google Scholar] [CrossRef]
- Wang, G.R.; Tang, W.Z.; Yao, Q.Q.; Zhong, H.; Liu, Y.J. New flavonoids with 2BS cell proliferation promoting effect from the seeds of Trigonella foenum-graecum L. J. Nat. Med. 2010, 64, 358–361. [Google Scholar] [CrossRef]
- Chen, S.; Jiang, H.; Wu, X.; Fang, J. Therapeutic effects of quercetin on inflammation, obesity, and type 2 Diabetes. Mediat. Inflamm. 2016, 2016, 9340637. [Google Scholar] [CrossRef]
- Kashyap, D.; Mittal, S.; Sak, K.; Singhal, P.; Tuli, H.S. Molecular mechanisms of action of quercetin in cancer: Recent advances. Tumor Biol. 2016, 37, 12927–12939. [Google Scholar] [CrossRef] [PubMed]
- Zaplatic, E.; Bule, M.; Shah, S.Z.A.; Uddin, M.S.; Niaz, K. Molecular mechanisms underlying protective role of quercetin in attenuating Alzheimer’s disease. Life Sci. 2019, 224, 109–119. [Google Scholar] [CrossRef]
- Zai, M.J.; Cheesman, M.J.; Cock, I.E. Terminalia petiolaris A.Cunn ex Benth. extracts have antibacterial activity and potentiate conventional antibiotics against beta-lactam-drug-resistant bacteria. Antibiotics 2023, 12, 1643. [Google Scholar] [CrossRef]
- Plaper, A.; Golob, M.; Hafner, I.; Oblak, M.; Solmajer, T.; Jerala, R. Characterization of quercetin binding site on DNA gyrase. Biochem. Biophys. Res. Commun. 2003, 306, 530–536. [Google Scholar] [CrossRef] [PubMed]
- Gaudry, A.; Bos, S.; Viranaicken, W.; Roche, M.; Krejbich-Trotot, P.; Gadea, G.; Despres, P.; El-Kalamouni, C. The flavonoid isoquercitrin precludes initiation of Zika virus infection in human cells. Int. J. Mol. Sci. 2018, 19, 1093. [Google Scholar] [CrossRef]
- Shokoohinia, Y.; Rashidi, M.; Hosseinzadeh, L.; Jelodarian, Z. Quercetin-3-O-beta-D-glucopyranoside, a dietary flavonoid, protects PC12 cells from H(2)O(2)-induced cytotoxicity through inhibition of reactive oxygen species. Food Chem. 2015, 167, 162–167. [Google Scholar] [CrossRef] [PubMed]
- Safwat, N.A.; Kashef, M.T.; Aziz, R.K.; Amer, K.F.; Ramadan, M.A. Quercetin 3-O-glucoside recovered from the wild Egyptian Sahara plant, Euphorbia paralias L., inhibits glutamine synthetase and has antimycobacterial activity. Tuberculosis 2018, 108, 106–113. [Google Scholar] [CrossRef]
- Tomas-Menor, L.; Barrajon-Catalan, E.; Segura-Carretero, A.; Marti, N.; Saura, D.; Menendez, J.A.; Joven, J.; Micol, V. The promiscuous and synergic molecular interaction of polyphenols in bactericidal activity: An opportunity to improve the performance of antibiotics? Phytother. Res. 2015, 29, 466–473. [Google Scholar] [CrossRef]
- Wang, Z.; Ding, Z.; Li, Z.; Ding, Y.; Jiang, F.; Liu, J. Antioxidant and antibacterial study of 10 flavonoids revealed rutin as a potential antibiofilm agent in Klebsiella pneumoniae strains isolated from hospitalized patients. Microb. Pathog. 2021, 159, 105121. [Google Scholar] [CrossRef]
- Ivanov, M.; Novovic, K.; Malesevic, M.; Dinic, M.; Stojkovic, D.; Jovcic, B.; Sokovic, M. Polyphenols as inhibitors of antibiotic resistant bacteria-mechanisms underlying rutin interference with bacterial virulence. Pharmaceuticals 2022, 15, 385. [Google Scholar] [CrossRef]
- Wu, T.; He, M.; Zang, X.; Zhou, Y.; Qiu, T.; Pan, S.; Xu, X. A structure-activity relationship study of flavonoids as inhibitors of Escherichia coli by membrane interaction effect. Biochim. Biophys. Acta 2013, 1828, 2751–2756. [Google Scholar] [CrossRef] [PubMed]
- Tripoli, E.; Guardia, M.L.; Giammanco, S.; Majo, D.D.; Giammanco, M. Citrus flavonoids: Molecular structure, biological activity and nutritional properties: A review. Food Chem. 2007, 104, 466–479. [Google Scholar] [CrossRef]
- Guo, Y.; Liu, Y.; Zhang, Z.; Chen, M.; Zhang, D.; Tian, C.; Liu, M.; Jiang, G. The antibacterial activity and mechanism of action of luteolin against Trueperella pyogenes. Infect. Drug Resist. 2020, 13, 1697–1711. [Google Scholar] [CrossRef]
- Liu, M.; Katerere, D.R.; Gray, A.I.; Seidel, V. Phytochemical and antifungal studies on Terminalia mollis and Terminalia brachystemma. Fitoterapia 2009, 80, 369–373. [Google Scholar] [CrossRef]
- Lam, K.Y.; Ling, A.P.; Koh, R.Y.; Wong, Y.P.; Say, Y.H. A review on medicinal properties of orientin. Adv. Pharmacol. Pharm. Sci. 2016, 2016, 4104595. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Jing, S.; Qu, H.; Wang, K.; Jin, Y.; Ding, Y.; Yang, L.; Yu, H.; Shi, Y.; Li, Q.; et al. Orientin mediates protection against MRSA-induced pneumonia by inhibiting Sortase A. Virulence 2021, 12, 2149–2161. [Google Scholar] [CrossRef]
- Hu, X.; Wang, M.; Pan, Y.; Xie, Y.; Han, J.; Zhang, X.; Niayale, R.; He, H.; Li, Q.; Zhao, T.; et al. Anti-inflammatory effect of astragalin and chlorogenic acid on Escherichia coli-induced inflammation of sheep endometrial epithelium cells. Front. Vet. Sci. 2020, 7, 201. [Google Scholar] [CrossRef]
- Chen, J.; Zhong, K.; Qin, S.; Jing, Y.; Liu, S.; Li, D.; Peng, C. Astragalin: A food-origin flavonoid with therapeutic effect for multiple diseases. Front. Pharmacol. 2023, 14, 1265960. [Google Scholar] [CrossRef]
- Riaz, A.; Rasul, A.; Hussain, G.; Zahoor, M.K.; Jabeen, F.; Subhani, Z.; Younis, T.; Ali, M.; Sarfraz, I.; Selamoglu, Z. Astragalin: A bioactive phytochemical with potential therapeutic activities. Adv. Pharmacol. Pharm. Sci. 2018, 2018, 9794625. [Google Scholar] [CrossRef]
- Ivanov, M.; Kannan, A.; Stojkovic, D.; Glamoclija, J.; Golic Grdadolnik, S.; Sanglard, D.; Sokovic, M. Revealing the astragalin mode of anticandidal action. EXCLI J. 2020, 19, 1436–1445. [Google Scholar] [CrossRef]
- Elbatreek, M.H.; Mahdi, I.; Ouchari, W.; Mahmoud, M.F.; Sobeh, M. Current advances on the therapeutic potential of pinocembrin: An updated review. Biomed. Pharmacother. 2023, 157, 114032. [Google Scholar] [CrossRef] [PubMed]
- Wojtyczka, R.D.; Dziedzic, A.; Idzik, D.; Kepa, M.; Kubina, R.; Kabala-Dzik, A.; Smolen-Dzirba, J.; Stojko, J.; Sajewicz, M.; Wasik, T.J. Susceptibility of Staphylococcus aureus clinical isolates to propolis extract alone or in combination with antimicrobial drugs. Molecules 2013, 18, 9623–9640. [Google Scholar] [CrossRef] [PubMed]
- Park, Y.K.; Koo, M.H.; Abreu, J.A.S.; Ikegaki, M.; Cury, J.A.; Rosalen, P.L. Antimicrobial activity of propolis on oral microorganisms. Curr. Microbiol. 1998, 36, 24–28. [Google Scholar] [CrossRef] [PubMed]
- Hegazi, A.G.; Abd El Hady, F.K.; Abd Allah, F.A.M. Chemical composition and antimicrobial activity of European propolis. Z. Für Naturforschung 2000, 55, 70–75. [Google Scholar] [CrossRef]
- Arkhipov, A.; Sirdaarta, J.; Rayan, P.; Mc Donnell, P.A.; Cock, I.E. An examination of the antibacterial, antifungal, anti-Giardial and anticancer properties of Kigelia africana fruit extracts. Pharmacogn. Commun. 2014, 4, 62–76. [Google Scholar] [CrossRef]
- Hübsch, Z.; Van Zyl, R.L.; Cock, I.E.; Van Vuuren, S.F. Interactive antimicrobial and toxicity profiles of conventional antimicrobials with Southern African medicinal plants. S. Afr. J. Bot. 2014, 93, 185–197. [Google Scholar] [CrossRef]
- Eloff, J.N. A sensitive and quick microplate method to determine the minimal inhibitory concentration of plant extracts for bacteria. Planta Medica 1998, 64, 711–713. [Google Scholar] [CrossRef]
- Rios, J.L.; Recio, M.C. Medicinal plants and antimicrobial activity. J. Ethnopharmacol. 2005, 100, 80–84. [Google Scholar] [CrossRef]
- Kuete, V. Potential of Cameroonian plants and derived products against microbial infections: A review. Planta Medica 2010, 76, 1479–1491. [Google Scholar] [CrossRef]




| Sample | MIC (µg/mL) | |||
|---|---|---|---|---|
| S. aureus | MRSA | B. cereus | S. flexneri | |
| Aqueous | 625 | 625 | 468.8 | 2500 |
| Methanol | 833.3 | 625 | 562.5 | 2500 |
| Cef | 2.5 | >10 | >10 | >10 |
| Cip | 1.3 | 3.3 | 0.6 | 2.5 |
| Met | 10 | >10 | >10 | >10 |
| Tet | 1.9 | 0.5 | 0.3 | 2.5 |
| Van | 3.8 | 3.8 | 1.3 | >10 |
| Species | Extract | ∑Fractional Inhibitory Concentration (FIC) | |||
|---|---|---|---|---|---|
| Cef | Cip | Tet | Van | ||
| S. aureus | Aqueous | 0.58 | 1.5 | 1.13 | 2.0 |
| Methanol | 1.5 | 1.5 | 0.75 | 2.0 | |
| MRSA | Aqueous | - | 0.75 | 1.25 | 2.0 |
| Methanol | - | 1.5 | 0.91 | 2.0 | |
| B. cereus | Aqueous | - | 1.5 | 0.75 | 1.0 |
| Methanol | - | 1.25 | 0.56 | 1.0 | |
| S. flexneri | Aqueous | - | 0.63 | 0.63 | - |
| Methanol | - | 1.25 | 1.25 | - | |
| Rt (min) | Putative Identification | Empirical Formula | Molecular Weight | Relative Abundance (% Total Area) | ||
|---|---|---|---|---|---|---|
| TciW | TciM | |||||
| F | 2.137 | (-)-Epigallocatechin gallate | C22 H18 O11 | 458.40 | 0.004 | 0.02 |
| 2.137 | Epigallocatechin 3-O-(4-hydroxybenzoate) | C22 H18 O9 | 426.095 | 0.004 | 0.02 | |
| 2.218 | 1,6-Bis-O-(3,4,5-Trihydroxybenzoyl) hexopyranose | C20 H20 O14 | 484.085 | 0.06 | 0.02 | |
| 2.562 | Catechin | C15 H14 O6 | 290.0788 | 9.80 | 7.31 | |
| 4.072 | Vitexin 2″-O-p-coumarate | C30 H26 O12 | 578.142 | 2.31 | 0.55 | |
| 5.508 | 3,5-Dihydroxy-2-(4-hydroxyphenyl)-4-oxo-3,4-dihydro-2H-chromen-7-yl hexopyranoside | C21 H22 O11 | 450.116 | 0.02 | 0.07 | |
| 8.043 | Apigetrin | C21 H20 O10 | 432.105 | 0.02 | 0.01 | |
| 8.464 | Orientin | C21 H20 O11 | 448.1 | 0.18 | 0.2 | |
| 8.845 | Rutin | C27 H30 O16 | 610.153 | 0.10 | 0.06 | |
| 8.853 | (-)-Epicatechin gallate | C22 H18 O10 | 442.09 | 0.02 | 0.23 | |
| 8.924 | Astilbin | C21 H22 O11 | 450.116 | 0.01 | 0.01 | |
| 8.954 | Quercetin-3β-D-glucoside | C21 H20 O12 | 464.095 | 0.08 | 0.09 | |
| 9.036 | Orientin 2″-O-gallate | C28 H24 O15 | 600.112 | 0.04 | - | |
| 9.36 | Quercetin 3-(6″-p-hydroxybenzoylgalactoside) | C28 H24 O14 | 584.116 | 0.22 | 0.28 | |
| 9.446 | Astragalin | C21 H20 O11 | 448.101 | 0.07 | - | |
| 10.485 | Eriodictyol | C15 H12 O6 | 288.063 | 0.02 | 0.04 | |
| 10.627 | Luteolin | C15 H10 O6 | 286.048 | 0.02 | 0.05 | |
| 11.938 | Apigenin | C15 H10 O5 | 270.053 | 0.006 | 0.02 | |
| 12.222 | Formononetin | C16 H12 O4 | 268.073 | 0.02 | 0.21 | |
| 12.392 | Pinocembrin | C15 H12 O4 | 256.074 | - | 0.01 | |
| 18.031 | Strobopinin | C16 H14 O4 | 270.089 | 0.003 | 0.1 | |
| T | 0.415 | Chebulic acid | C14 H12 O11 | 356.038 | 0.03 | 0.03 |
| 0.46 | 6-O-Galloyl-glucose | C13 H16 O10 | 332.074 | 0.02 | 0.16 | |
| 0.568 | Gallic acid | C7 H6 O5 | 170.021 | - | 0.33 | |
| 8.13 | Robinetinidol-(4-α-8)-catechin-(6,4 α)-robinetinidol | C45 H38 O18 | 866.206 | 0.10 | 0.18 | |
| 7.277 | Acertannin | C20 H20 O13 | 468.09 | 0.06 | 0.05 | |
| 7.511 | Ampelopsin 3′-glucoside | C21 H22 O13 | 482.106 | 0.04 | 0.06 | |
| 0.726 | Corilagin | C27 H22 O18 | 634.081 | 0.04 | 0.14 | |
| 7.605 | Cinnamtannin A3 | C75 H62 O30 | 1442.33 | 0.03 | 0.03 | |
| 7.95 | Cinnamtannin A4 | C90 H74 O36 | 1730.39 | 0.18 | 0.08 | |
| 8.027 | 1,3,6-Trigalloyl glucose | C27 H24 O18 | 636.096 | 0.02 | 0.13 | |
| 8.153 | Terminalin | C28 H10 O16 | 601.997 | 0.18 | 0.004 | |
| 8.276 | Procyanidin B3 3-O-gallate | C37 H30 O16 | 730.153 | 0.08 | 0.19 | |
| 8.575 | Ellagic acid | C14 H6 O8 | 302.006 | 1.00 | 0.98 | |
| L | 8.843 | Lariciresinol 4-O-glucoside | C26 H34 O11 | 522.20976 | 0.04 | 0.02 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ang, S.-T.; Kim, T.H.; Cheesman, M.J.; Cock, I.E. Antibacterial and Synergistic Effects of Terminalia citrina Leaf Extracts Against Gastrointestinal Pathogens: Insights from Metabolomic Analysis. Antibiotics 2025, 14, 593. https://doi.org/10.3390/antibiotics14060593
Ang S-T, Kim TH, Cheesman MJ, Cock IE. Antibacterial and Synergistic Effects of Terminalia citrina Leaf Extracts Against Gastrointestinal Pathogens: Insights from Metabolomic Analysis. Antibiotics. 2025; 14(6):593. https://doi.org/10.3390/antibiotics14060593
Chicago/Turabian StyleAng, Sze-Tieng, Tak Hyun Kim, Matthew James Cheesman, and Ian Edwin Cock. 2025. "Antibacterial and Synergistic Effects of Terminalia citrina Leaf Extracts Against Gastrointestinal Pathogens: Insights from Metabolomic Analysis" Antibiotics 14, no. 6: 593. https://doi.org/10.3390/antibiotics14060593
APA StyleAng, S.-T., Kim, T. H., Cheesman, M. J., & Cock, I. E. (2025). Antibacterial and Synergistic Effects of Terminalia citrina Leaf Extracts Against Gastrointestinal Pathogens: Insights from Metabolomic Analysis. Antibiotics, 14(6), 593. https://doi.org/10.3390/antibiotics14060593









