Integrated Assessment of Antibacterial Activity, Polyphenol Composition, Molecular Docking, and ADME Properties of Romanian Oak and Fir Honeydew Honeys
Abstract
:1. Introduction
2. Results
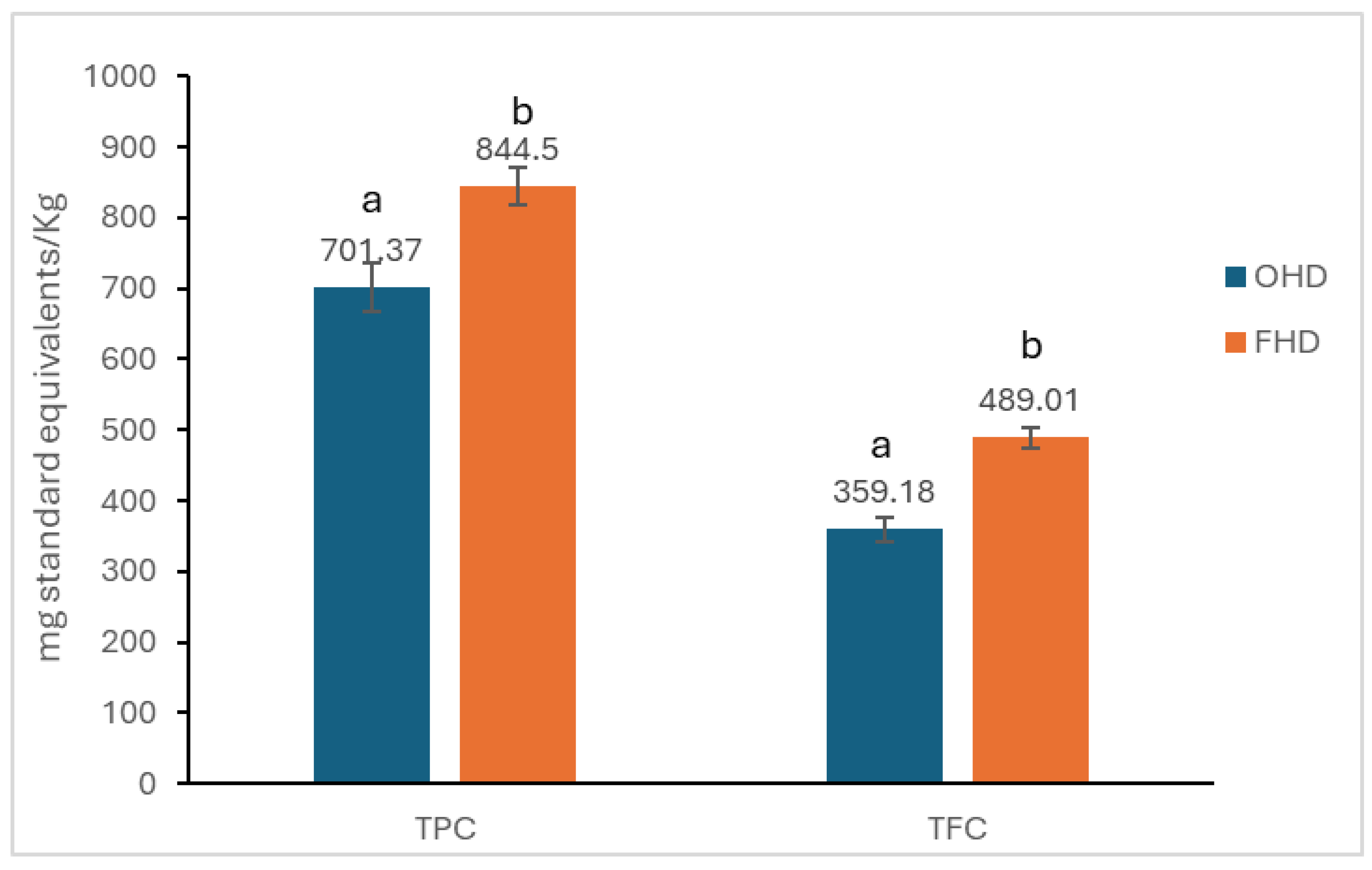
2.1. Determination of Total Phenolic Content (TPC) and Flavonoid Content (TFC)
2.2. Antioxidant Capacity by 1,1-Diphenyl-2-picrylhydrazyl (DPPH) Assay
2.3. High-Performance Liquid Chromatography for the Individual Profiling of Polyphenols
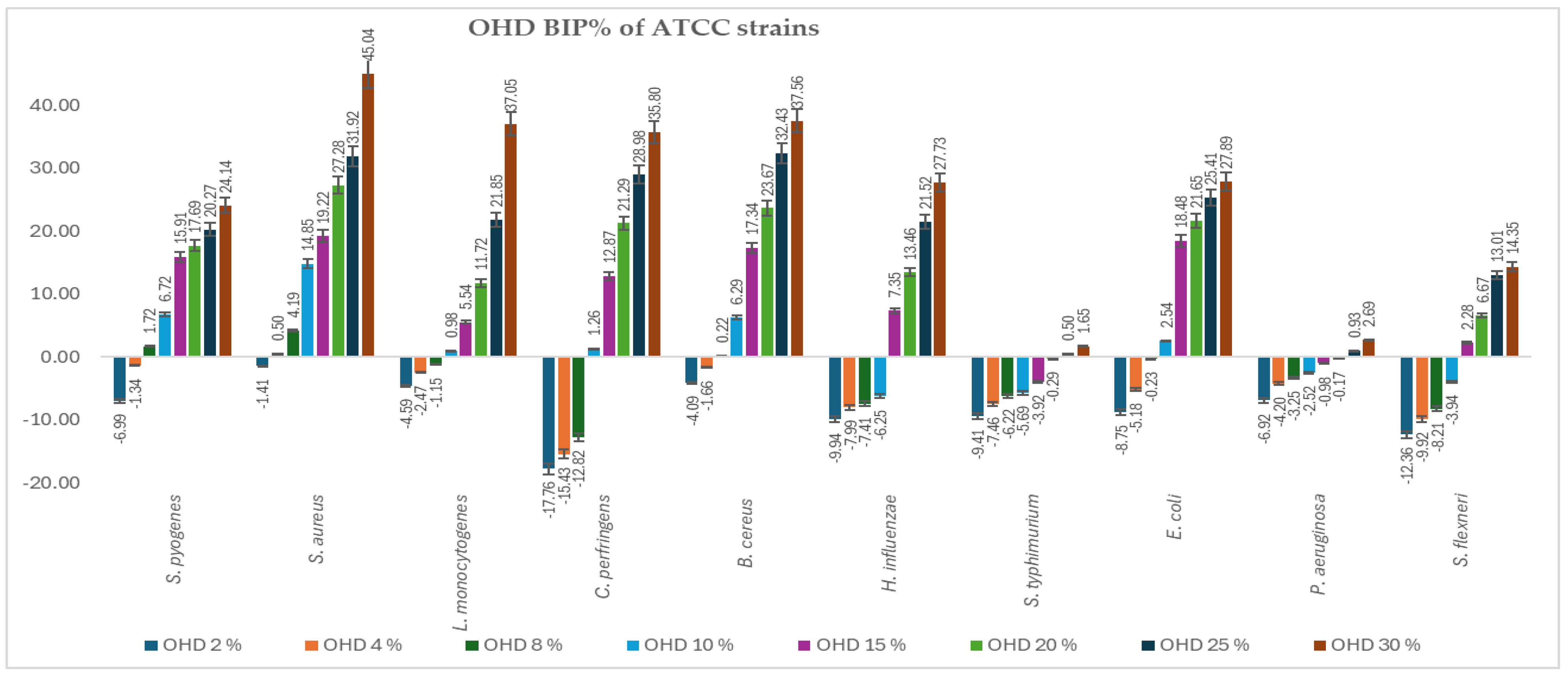
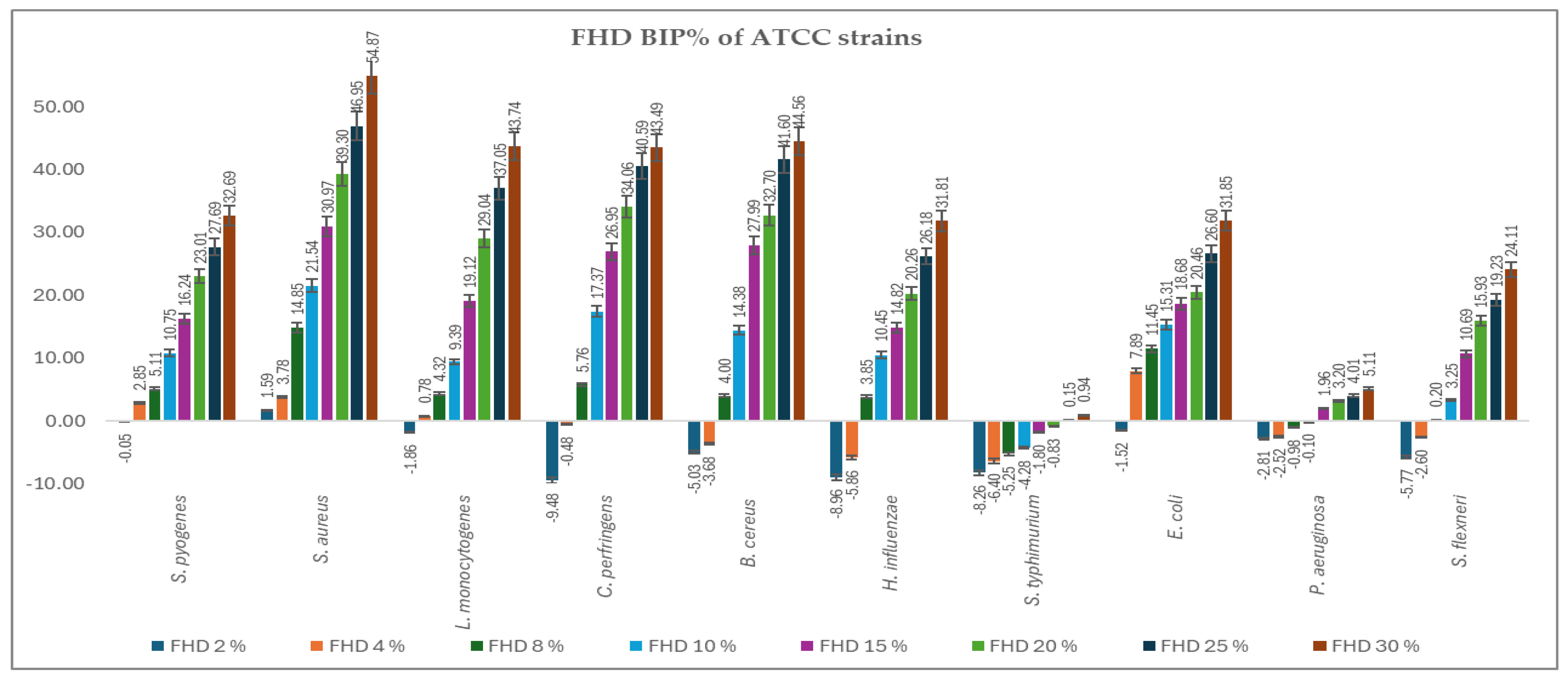
2.4. Antimicrobial Activity
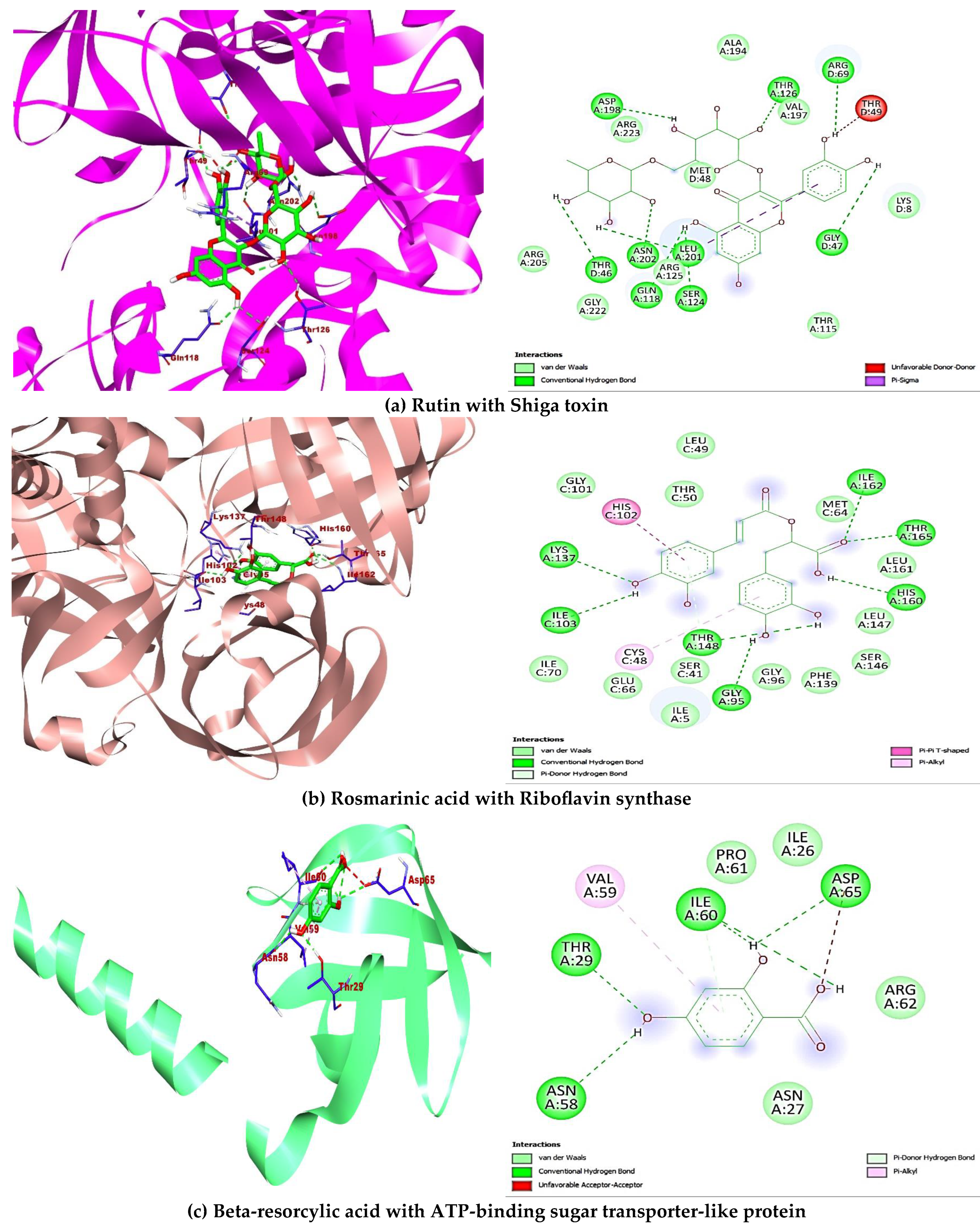
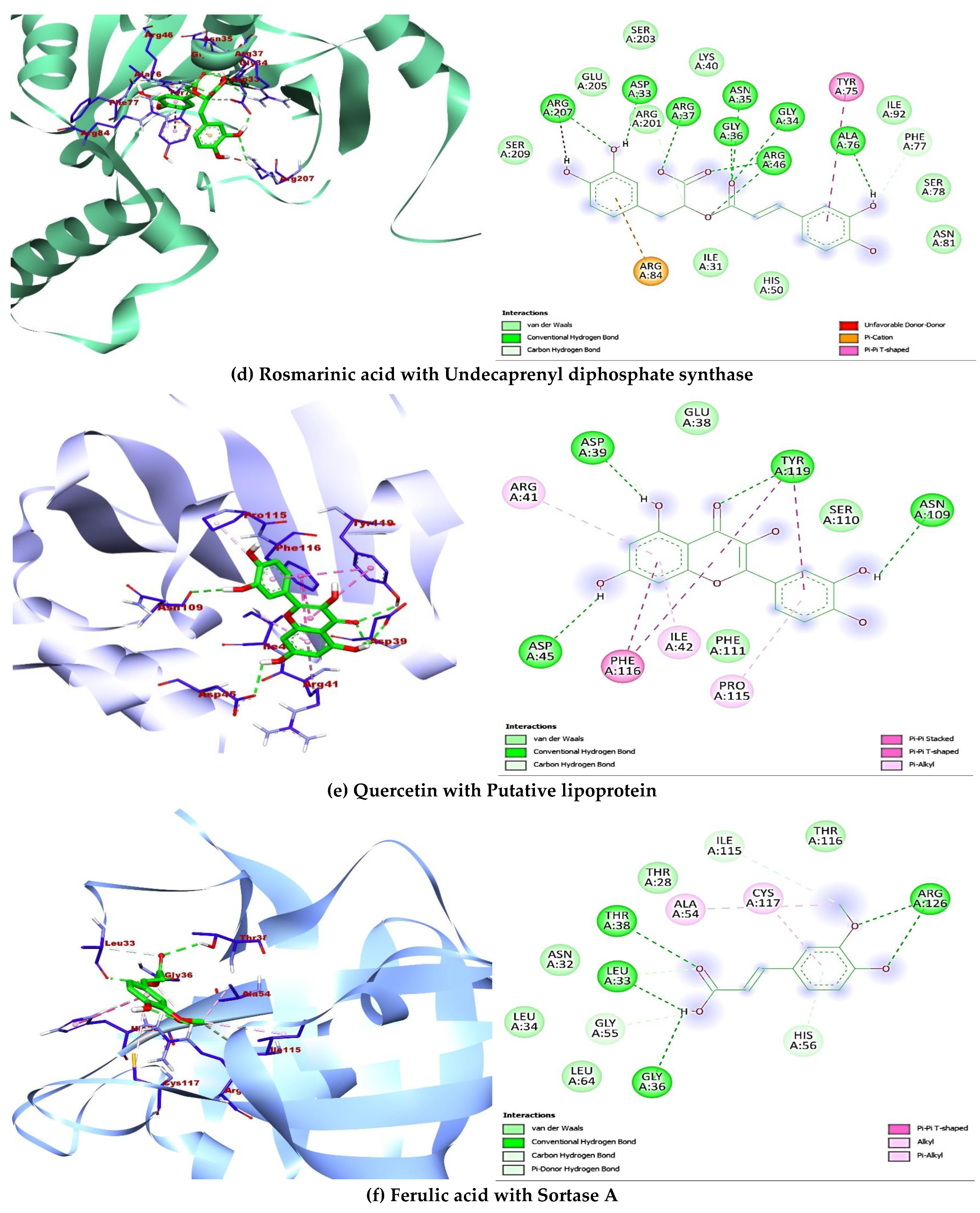
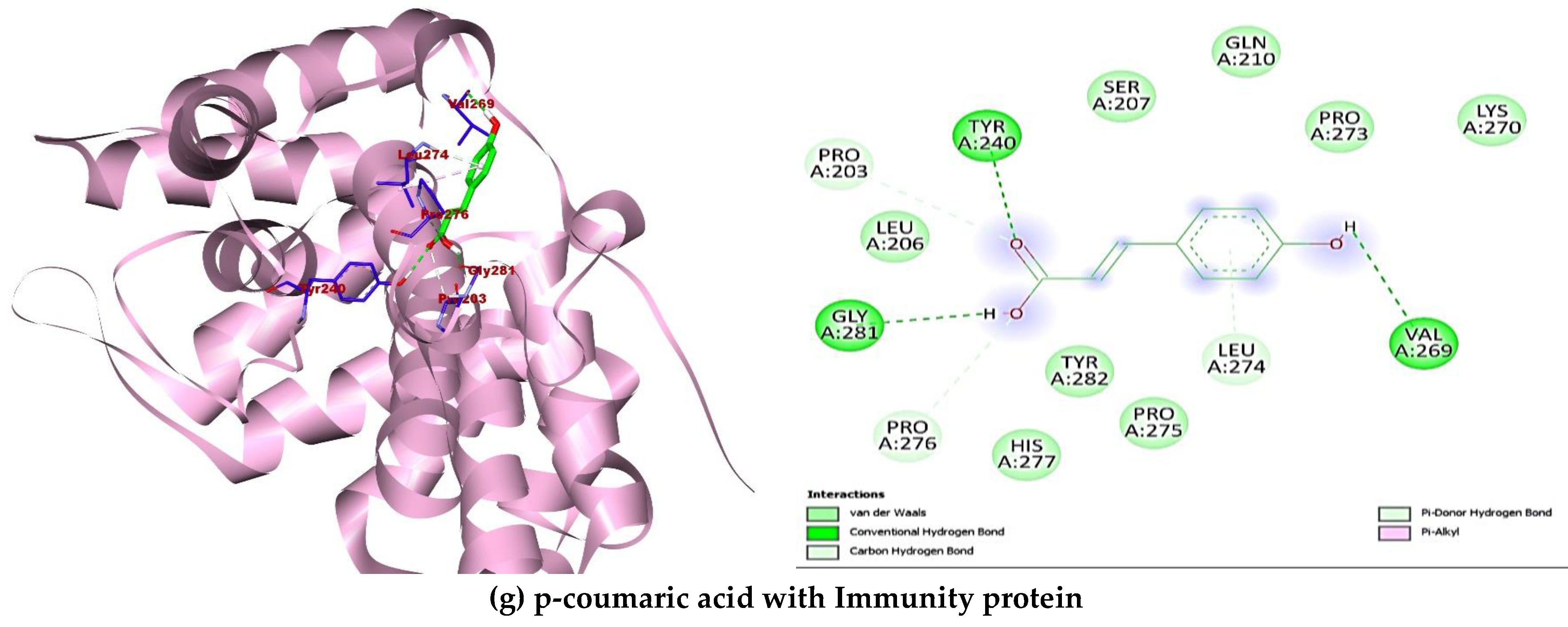
2.5. Molecular Docking
2.6. ADME (Absorption, Distribution, Metabolism, and Excretion) Properties
3. Discussion
4. Materials and Methods
4.1. The Preparation of Extracts
4.2. Chemicals
4.3. Determination of Total Phenolic Content (TPC)
4.4. Determination of Total Flavonoid Content (TFC)
4.5. Individual Identification of Polyphenols by LC-MS Analysis
4.6. DPPH Assay Antioxidant Capacity
4.7. Antimicrobial Activity
4.8. Molecular Docking
4.9. ADMET (Absorption, Distribution, Metabolism, Excretion, Toxicity) Properties
4.10. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ranneh, Y.; Akim, A.M.; Hamid, H.A.; Khazaai, H.; Fadel, A.; Zakaria, Z.A.; Albujja, M.; Bakar, M.F.A. Honey and its nutritional and anti-inflammatory value. BMC Complement. Med. Ther. 2021, 21, 30. [Google Scholar] [CrossRef] [PubMed]
- Stavropoulou, E.; Ieronymaki, E.; Dimitroulia, E.; Constantinidis, T.C.; Vrioni, G.; Tsatsanis, C.; Tsakris, A. Anti-Inflammatory and Antibacterial Effects and Mode of Action of Greek Arbutus, Chestnut, and Fir Honey in Mouse Models of Inflammation and Sepsis. Microorganisms 2022, 10, 2374. [Google Scholar] [CrossRef] [PubMed]
- Hulea, A.; Obiștioiu, D.; Cocan, I.; Alexa, E.; Negrea, M.; Neacșu, A.-G.; Hulea, C.; Pascu, C.; Costinar, L.; Iancu, I.; et al. Diversity of Monofloral Honey Based on the Antimicrobial and Antioxidant Potential. Antibiotics 2022, 11, 595. [Google Scholar] [CrossRef]
- Pătruică, S.; Alexa, E.; Obiștioiu, D.; Cocan, I.; Radulov, I.; Berbecea, A.; Lazăr, R.N.; Simiz, E.; Vicar, N.M.; Hulea, A.; et al. Chemical Composition, Antioxidant and Antimicrobial Activity of Some Types of Honey from Banat Region, Romania. Molecules 2022, 27, 4179. [Google Scholar] [CrossRef]
- Vică, M.L.; Glevitzky, M.; Dumitrel, G.-A.; Bostan, R.; Matei, H.V.; Kartalska, Y.; Popa, M. Qualitative Characterization and Antifungal Activity of Romanian Honey and Propolis. Antibiotics 2022, 11, 1552. [Google Scholar] [CrossRef]
- Al-Hatamleh, M.A.I.; Hatmal, M.M.; Sattar, K.; Ahmad, S.; Mustafa, M.Z.; Bittencourt, M.D.C.; Mohamud, R. Antiviral and Immunomodulatory Effects of Phytochemicals from Honey against COVID-19: Potential Mechanisms of Action and Future Directions. Molecules 2020, 25, 5017. [Google Scholar] [CrossRef]
- Hasam, S.; Qarizada, D.; Azizi, M. A Review: Honey and Its Nutritional Composition. Asian J. Res. Biochem. 2020, 7, 34–43. [Google Scholar] [CrossRef]
- Gemechu, F.G. Embracing nutritional qualities, biological activities and technological properties of coffee byproducts in functional food formulation. Trends Food Sci. Technol. 2020, 104, 235–261. [Google Scholar] [CrossRef]
- Bora, F.D.; Andrecan, A.F.; Călugăr, A.; Bunea, C.I.; Popescu, M.; Petrescu-Mag, I.V.; Bunea, A. Comprehensive Elemental Profiling of Romanian Honey: Exploring Regional Variance, Honey Types, and Analyzed Metals for Sustainable Apicultural and Environmental Practices. Foods 2024, 13, 1253. [Google Scholar] [CrossRef]
- Pop, I.M.; Simeanu, D.; Cucu-Man, S.-M.; Pui, A.; Albu, A. Quality Profile of Several Monofloral Romanian Honeys. Agriculture 2022, 13, 75. [Google Scholar] [CrossRef]
- Gündoğdu, E.; Çakmakçı, S.; Şat, İ.G. An Overview of Honey: Its Composition, Nutritional and Functional Properties. J. Food Sci. Eng. 2019, 9, 10–14. [Google Scholar] [CrossRef]
- Almasaudi, S. The antibacterial activities of honey. Saudi J. Biol. Sci. 2021, 28, 2188–2196. [Google Scholar] [CrossRef] [PubMed]
- Majtan, J.; Bucekova, M.; Kafantaris, I.; Szweda, P.; Hammer, K.; Mossialos, D. Honey antibacterial activity: A neglected aspect of honey quality assurance as functional food. Trends Food Sci. Technol. 2021, 118, 870–886. [Google Scholar] [CrossRef]
- Tafere, D.A. Chemical composition and uses of Honey: A Review. J. Food Sci. Nutr. Res. 2021, 4, 194–201. [Google Scholar] [CrossRef]
- Mohammed, M.E.A. Factors Affecting the Physicochemical Properties and Chemical Composition of Bee’s Honey. Food Rev. Int. 2022, 38, 1330–1341. [Google Scholar] [CrossRef]
- Ben Amor, S.; Mekious, S.; Allal Benfekih, L.; Abdellattif, M.H.; Boussebaa, W.; Almalki, F.A.; Ben Hadda, T.; Kawsar, S.M.A. Phytochemical Characterization and Bioactivity of Different Honey Samples Collected in the Pre-Saharan Region in Algeria. Life 2022, 12, 927. [Google Scholar] [CrossRef]
- Tomczyk, M.; Tarapatskyy, M.; Dżugan, M. The influence of geographical origin on honey composition studied by Polish and Slovak honeys. Czech J. Food Sci. 2019, 37, 232–238. [Google Scholar] [CrossRef]
- Becerril-Sánchez, A.L.; Quintero-Salazar, B.; Dublán-García, O.; Escalona-Buendía, H.B. Phenolic Compounds in Honey and Their Relationship with Antioxidant Activity, Botanical Origin, and Color. Antioxidants 2021, 10, 1700. [Google Scholar] [CrossRef]
- Bonsignore, G.; Martinotti, S.; Ranzato, E. Honey Bioactive Molecules: There Is a World Beyond the Sugars. BioTech 2024, 13, 47. [Google Scholar] [CrossRef]
- Zammit Young, G.-W.; Blundell, R. A review on the phytochemical composition and health applications of honey. Heliyon 2023, 9, e12507. [Google Scholar] [CrossRef]
- Ahmed, S.K.; Hussein, S.; Qurbani, K.; Ibrahim, R.H.; Fareeq, A.; Mahmood, K.A.; Mohamed, M.G. Antimicrobial resistance: Impacts, challenges, and future prospects. J. Med. Surg. Public Health 2024, 2, 100081. [Google Scholar] [CrossRef]
- Shakoori, Z.; Salaseh, E.; Mehrabian, A.R.; Tehrani, D.M.; Dardashti, N.F.; Salmanpour, F. The amount of antioxidants in honey has a strong relationship with the plants selected by honey bees. Sci. Rep. 2024, 14, 351. [Google Scholar] [CrossRef]
- Majewska, E.; Drużyńska, B.; Derewiaka, D.; Ciecierska, M.; Pakosz, P. Comparison of Antioxidant Properties and Color of Selected Polish Honeys and Manuka Honey. Foods 2024, 13, 2666. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, S.; Sulaiman, S.A.; Baig, A.A.; Ibrahim, M.; Liaqat, S.; Fatima, S.; Jabeen, S.; Shamim, N.; Othman, N.H. Honey as a Potential Natural Antioxidant Medicine: An Insight into Its Molecular Mechanisms of Action. Oxid. Med. Cell. Longev. 2018, 2018, 8367846. [Google Scholar] [CrossRef]
- Bozkuş, T.N. Correlation of Total Polyphenol and Flavonoid Content with Antioxidant Activity of Caucasian Bee Honey. Food Sci. Nutr. 2025, 13, e4611. [Google Scholar] [CrossRef] [PubMed]
- Kačániová, M.; Borotová, P.; Galovičová, L.; Kunová, S.; Štefániková, J.; Kowalczewski, P.Ł.; Šedík, P. Antimicrobial and Antioxidant Activity of Different Honey Samples from Beekeepers and Commercial Producers. Antibiotics 2022, 11, 1163. [Google Scholar] [CrossRef]
- Halagarda, M.; Groth, S.; Popek, S.; Rohn, S.; Pedan, V. Antioxidant Activity and Phenolic Profile of Selected Organic and Conventional Honeys from Poland. Antioxidants 2020, 9, 44. [Google Scholar] [CrossRef] [PubMed]
- Çobanoğlu, D.N. Assessing monofloral bee pollens from Türkiye: Palynological verification, phenolic profile, and antioxidant activity. J. Food Sci. 2024, 89, 1711–1726. [Google Scholar] [CrossRef]
- Qi, N.; Zhao, W.; Xue, C.; Zhang, L.; Hu, H.; Jin, Y.; Xue, X.; Chen, R.; Zhang, J. Phenolic Acid and Flavonoid Content Analysis with Antioxidant Activity Assessment in Chinese C. pi. Shen Honey. Molecules 2025, 30, 370. [Google Scholar] [CrossRef]
- Mushtaq, S.; Imtiyaz, Z.; Wali, A.F.; Khan, A.; Rashid, S.M.; Amin, I.; Ali, A.; Rehman, M.U.; Arafah, A. Honey: A Powerful Natural Antioxidant and Its Possible Mechanism of Action. In Therapeutic Applications of Honey and Its Phytochemicals; Rehman, M.U., Majid, S., Eds.; Springer: Singapore, 2020; pp. 11–29. ISBN 9789811567988. [Google Scholar]
- Morroni, G.; Alvarez-Suarez, J.M.; Brenciani, A.; Simoni, S.; Fioriti, S.; Pugnaloni, A.; Giampieri, F.; Mazzoni, L.; Gasparrini, M.; Marini, E.; et al. Comparison of the Antimicrobial Activities of Four Honeys From Three Countries (New Zealand, Cuba, and Kenya). Front. Microbiol. 2018, 9, 1378. [Google Scholar] [CrossRef]
- Mama, M.; Teshome, T.; Detamo, J. Antibacterial Activity of Honey against Methicillin-Resistant Staphylococcus aureus: A Laboratory-Based Experimental Study. Int. J. Microbiol. 2019, 2019, 7686130. [Google Scholar] [CrossRef] [PubMed]
- Feknous, N.; Boumendjel, M. Natural bioactive compounds of honey and their antimicrobial activity. Czech J. Food Sci. 2022, 40, 163–178. [Google Scholar] [CrossRef]
- Nolan, V.C.; Harrison, J.; Cox, J.A.G. Dissecting the Antimicrobial Composition of Honey. Antibiotics 2019, 8, 251. [Google Scholar] [CrossRef]
- Al-Sayaghi, A.M.; Al-Kabsi, A.M.; Abduh, M.S.; Saghir, S.A.M.; Alshawsh, M.A. Antibacterial Mechanism of Action of Two Types of Honey against Escherichia coli through Interfering with Bacterial Membrane Permeability, Inhibiting Proteins, and Inducing Bacterial DNA Damage. Antibiotics 2022, 11, 1182. [Google Scholar] [CrossRef]
- Mirzaei, A.; Karamolah, K.S.; Mahnaie, M.P.; Mousavi, F.; Moghadam, P.M.; Mahmoudi, H. Antibacterial Activity of Honey against Methicillin-Resistant and Sensitive Staphylococcus Aureus Isolated from Patients with Diabetic Foot Ulcer. Open Microbiol. J. 2020, 14, 260–265. [Google Scholar] [CrossRef]
- Luca, L.; Pauliuc, D.; Ursachi, F.; Oroian, M. Physicochemical parameters, microbiological quality, and antibacterial activity of honey from the Bucovina region of Romania. Sci. Rep. 2025, 15, 4358. [Google Scholar] [CrossRef] [PubMed]
- Nagy-Radványi, L.; Balázs, V.L.; Kocsis, B.; Csikós, E.; Ángyán, V.D.; Szabó, P.; Biró, V.; Kocsis, M.; Farkas, Á. Antibacterial activity of Hungarian varietal honeys against respiratory pathogens as a function of storage time. Sci. Rep. 2024, 14, 10200. [Google Scholar] [CrossRef]
- Dörr, T.; Moynihan, P.J.; Mayer, C. Editorial: Bacterial Cell Wall Structure and Dynamics. Front. Microbiol. 2019, 10, 2051. [Google Scholar] [CrossRef]
- Fyfe, L.; Okoro, P.; Paterson, E.; Coyle, S.; McDougall, G.J. Compositional analysis of Scottish honeys with antimicrobial activity against antibiotic-resistant bacteria reveals novel antimicrobial components. LWT Food Sci. Technol. 2017, 79, 52–59. [Google Scholar] [CrossRef]
- Zhang, Y.-Z.; Si, J.-J.; Li, S.-S.; Zhang, G.-Z.; Wang, S.; Zheng, H.-Q.; Hu, F.-L. Chemical Analyses and Antimicrobial Activity of Nine Kinds of Unifloral Chinese Honeys Compared to Manuka Honey (12+ and 20+). Molecules 2021, 26, 2778. [Google Scholar] [CrossRef]
- Combarros-Fuertes, P.; Fresno, J.M.; Estevinho, M.M.; Sousa-Pimenta, M.; Tornadijo, M.E.; Estevinho, L.M. Honey: Another Alternative in the Fight against Antibiotic-Resistant Bacteria? Antibiotics 2020, 9, 774. [Google Scholar] [CrossRef] [PubMed]
- Muteeb, G.; Rehman, M.T.; Shahwan, M.; Aatif, M. Origin of Antibiotics and Antibiotic Resistance, and Their Impacts on Drug Development: A Narrative Review. Pharmaceuticals 2023, 16, 1615. [Google Scholar] [CrossRef]
- Pulingam, T.; Parumasivam, T.; Gazzali, A.M.; Sulaiman, A.M.; Chee, J.Y.; Lakshmanan, M.; Chin, C.F.; Sudesh, K. Antimicrobial resistance: Prevalence, economic burden, mechanisms of resistance and strategies to overcome. Eur. J. Pharm. Sci. 2022, 170, 106103. [Google Scholar] [CrossRef] [PubMed]
- Javed, M.U.; Hayat, M.T.; Mukhtar, H.; Imre, K. CRISPR-Cas9 System: A Prospective Pathway toward Combatting Antibiotic Resistance. Antibiotics 2023, 12, 1075. [Google Scholar] [CrossRef]
- Al-Kafaween, M.A.; Alwahsh, M.; Mohd Hilmi, A.B.; Abulebdah, D.H. Physicochemical Characteristics and Bioactive Compounds of Different Types of Honey and Their Biological and Therapeutic Properties: A Comprehensive Review. Antibiotics 2023, 12, 337. [Google Scholar] [CrossRef]
- Isopescu, R.D.; Josceanu, A.M.; Colta, T.; Spulber, R. Romanian Honey: Characterization and Classification. In Honey Analysis; Toledo, V.D.A.A.D., Ed.; InTech: Rijeka, Croatia, 2017; ISBN 978-953-51-2879-3. [Google Scholar]
- Albu, A.; Radu-Rusu, C.-G.; Pop, I.M.; Frunza, G.; Nacu, G. Quality Assessment of Raw Honey Issued from Eastern Romania. Agriculture 2021, 11, 247. [Google Scholar] [CrossRef]
- Ioniță-Mîndrican, C.-B.; Mititelu, M.; Musuc, A.M.; Oprea, E.; Ziani, K.; Neacșu, S.M.; Grigore, N.D.; Negrei, C.; Dumitrescu, D.-E.; Mireșan, H.; et al. Honey and Other Beekeeping Products Intake among the Romanian Population and Their Therapeutic Use. Appl. Sci. 2022, 12, 9649. [Google Scholar] [CrossRef]
- Rameshk, M.; Khoshbin, E.; Moeinzadeh, M.; Sharififar, K.; Bahrami, D.; Sharififar, F. Mannas, unique products of a dynamic insect-plant interaction: Biodiversity, conservation and ethnopharmacological considerations. Heliyon 2023, 9, e22976. [Google Scholar] [CrossRef] [PubMed]
- Vică, M.L.; Glevitzky, M.; Tit, D.M.; Behl, T.; Heghedűş-Mîndru, R.C.; Zaha, D.C.; Ursu, F.; Popa, M.; Glevitzky, I.; Bungău, S. The antimicrobial activity of honey and propolis extracts from the central region of Romania. Food Biosci. 2021, 41, 101014. [Google Scholar] [CrossRef]
- Barcellos, M.P.; Santos, C.B.R.; Federico, L.B.; Almeida, P.F.D.; Da Silva, C.H.T.D.P.; Taft, C.A. Pharmacophore and structure-based drug design, molecular dynamics and admet/tox studies to design novel potential pad4 inhibitors. J. Biomol. Struct. Dyn. 2019, 37, 966–981. [Google Scholar] [CrossRef]
- Prava, J.; Pranavathiyani, G.; Pan, A. Functional assignment for essential hypothetical proteins of Staphylococcus aureus N315. Int. J. Biol. Macromol. 2018, 108, 765–774. [Google Scholar] [CrossRef] [PubMed]
- Fraser, M.E.; Chernaia, M.M.; Kozlov, Y.V.; James, M.N.G. Crystal structure of the holotoxino from Shigella dysenteriae at 2.5 Å resolution. Nat. Struct. Mol. Biol. 1994, 1, 59–64. [Google Scholar] [CrossRef] [PubMed]
- Liao, D.-I.; Wawrzak, Z.; Calabrese, J.C.; Viitanen, P.V.; Jordan, D.B. Crystal Structure of Riboflavin Synthase. Structure 2001, 9, 399–408. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Chen, Y.; Zhang, B.; Niu, X.; Song, M.; Luo, Z.; Lu, G.; Liu, B.; Zhao, X.; Wang, J.; et al. Inhibition of sortase A by chalcone prevents Listeria monocytogenes infection. Biochem. Pharmacol. 2016, 106, 19–29. [Google Scholar] [CrossRef]
- Yang, X.; Li, Z.; She, Z.; Geng, Z.; Xu, J.; Gao, Z.; Dong, Y. Structural analysis of Pseudomonas aeruginosa H3-T6SS immunity proteins. FEBS Lett. 2016, 590, 2787–2796. [Google Scholar] [CrossRef]
- Can, Z.; Yildiz, O.; Sahin, H.; Akyuz Turumtay, E.; Silici, S.; Kolayli, S. An investigation of Turkish honeys: Their physico-chemical properties, antioxidant capacities and phenolic profiles. Food Chem. 2015, 180, 133–141. [Google Scholar] [CrossRef]
- Fernández-Estellé, M.; Hernández-González, V.; Saurina, J.; Núñez, O.; Sentellas, S. Characterization and Classification of Spanish Honeydew and Blossom Honeys Based on Their Antioxidant Capacity. Antioxidants 2023, 12, 495. [Google Scholar] [CrossRef]
- Halouzka, R.; Tarkowski, P.; Ćavar Zeljković, S. Characterisation of phenolics and other quality parameters of different types of honey. Czech J. Food Sci. 2016, 34, 244–253. [Google Scholar] [CrossRef]
- Koulis, G.A.; Tsagkaris, A.S.; Katsianou, P.A.; Gialouris, P.-L.P.; Martakos, I.; Stergiou, F.; Fiore, A.; Panagopoulou, E.I.; Karabournioti, S.; Baessmann, C.; et al. Thorough Investigation of the Phenolic Profile of Reputable Greek Honey Varieties: Varietal Discrimination and Floral Markers Identification Using Liquid Chromatography–High-Resolution Mass Spectrometry. Molecules 2022, 27, 4444. [Google Scholar] [CrossRef]
- Kováčik, J.; Grúz, J.; Biba, O.; Hedbavny, J. Content of metals and metabolites in honey originated from the vicinity of industrial town Košice (eastern Slovakia). Environ. Sci. Pollut. Res. 2016, 23, 4531–4540. [Google Scholar] [CrossRef]
- Seraglio, S.K.T.; Silva, B.; Bergamo, G.; Brugnerotto, P.; Gonzaga, L.V.; Fett, R.; Costa, A.C.O. An overview of physicochemical characteristics and health-promoting properties of honeydew honey. Food Res. Int. 2019, 119, 44–66. [Google Scholar] [CrossRef] [PubMed]
- Kuś, P.M.; Jerković, I.; Marijanović, Z.; Tuberoso, C.I.G. Screening of Polish Fir Honeydew Honey Using GC/MS, HPLC-DAD, and Physical-Chemical Parameters: Benzene Derivatives and Terpenes as Chemical Markers. Chem. Biodivers. 2017, 14, e1700179. [Google Scholar] [CrossRef] [PubMed]
- Jaśkiewicz, K.; Szczęsna, T.; Jachuła, J. How Phenolic Compounds Profile and Antioxidant Activity Depend on Botanical Origin of Honey—A Case of Polish Varietal Honeys. Molecules 2025, 30, 360. [Google Scholar] [CrossRef]
- Seijo, M.C.; Escuredo, O.; Rodríguez-Flores, M.S. Physicochemical Properties and Pollen Profile of Oak Honeydew and Evergreen Oak Honeydew Honeys from Spain: A Comparative Study. Foods 2019, 8, 126. [Google Scholar] [CrossRef]
- Vasić, V.; Gašić, U.; Stanković, D.; Lušić, D.; Vukić-Lušić, D.; Milojković-Opsenica, D.; Tešić, Ž.; Trifković, J. Towards better quality criteria of European honeydew honey: Phenolic profile and antioxidant capacity. Food Chem. 2019, 274, 629–641. [Google Scholar] [CrossRef]
- Pichichero, E.; Canuti, L.; Canini, A. Characterisation of the phenolic and flavonoid fractions and antioxidant power of Italian honeys of different botanical origin. J. Sci. Food Agric. 2009, 89, 609–616. [Google Scholar] [CrossRef]
- Seraglio, S.K.T.; Valese, A.C.; Daguer, H.; Bergamo, G.; Azevedo, M.S.; Gonzaga, L.V.; Fett, R.; Costa, A.C.O. Development and validation of a LC-ESI-MS/MS method for the determination of phenolic compounds in honeydew honeys with the diluted-and-shoot approach. Food Res. Int. 2016, 87, 60–67. [Google Scholar] [CrossRef]
- Tuberoso, C.I.G.; Jerković, I.; Bifulco, E.; Marijanović, Z. Biodiversity of Salix spp. Honeydew and Nectar Honeys Determined by RP-HPLC and Evaluation of Their Antioxidant Capacity. Chem. Biodivers. 2011, 8, 872–879. [Google Scholar] [CrossRef]
- Oroian, M.; Ropciuc, S.; Buculei, A.; Paduret, S.; Todosi, E. Phenolic Profile of Honeydew Honeys from the North-East Part Of Romania. Bull. Univ. Agric. Sci. Vet. Med. Cluj-Napoca Food Sci. Technol. 2016, 73, 105. [Google Scholar] [CrossRef]
- Hernanz, D.; Jara-Palacios, M.J.; Santos, J.L.; Gómez Pajuelo, A.; Heredia, F.J.; Terrab, A. The profile of phenolic compounds by HPLC-MS in Spanish oak (Quercus) honeydew honey and their relationships with color and antioxidant activity. LWT 2023, 180, 114724. [Google Scholar] [CrossRef]
- Dżugan, M.; Ciszkowicz, E.; Tomczyk, M.; Miłek, M.; Lecka-Szlachta, K. Coniferous Honeydew Honey: Antibacterial Activity and Anti-Migration Properties against Breast Cancer Cell Line (MCF-7). Appl. Sci. 2024, 14, 710. [Google Scholar] [CrossRef]
- Grabek-Lejko, D.; Miłek, M.; Dżugan, M. The comparison of the antioxidant, antibacterial and antiviral potential of Polish fir honeydew and Manuka honeys. Sci. Rep. 2024, 14, 31170. [Google Scholar] [CrossRef] [PubMed]
- Broznić, D.; Ratkaj, I.; Malenica Staver, M.; Kraljević Pavelić, S.; Žurga, P.; Bubalo, D.; Gobin, I. Evaluation of the Antioxidant Capacity, Antimicrobial and Antiproliferative Potential of Fir (Abies alba Mill.) Honeydew Honey Collected from Gorski kotar (Croatia). Food Technol. Biotechnol. 2018, 56, 533–545. [Google Scholar] [CrossRef]
- Jokovic, N.; Jovanović, N.; Matejić, J.; Stojanović-Radić, Z.; Vitorović, J. Physicochemical properties, antioxidant and antimicrobial activity of seven honey samples collected in the Nišava district. Biol. Nyssana 2025, 16, 1–10. [Google Scholar] [CrossRef]
- Vaou, N.; Stavropoulou, E.; Voidarou, C.; Tsigalou, C.; Bezirtzoglou, E. Towards Advances in Medicinal Plant Antimicrobial Activity: A Review Study on Challenges and Future Perspectives. Microorganisms 2021, 9, 2041. [Google Scholar] [CrossRef]
- Maalik, A.; Khan, F.; Mumtaz, A.; Mehmood, A.; Azhar, S.; Atif, M.; Karim, S.; Altaf, Y.; Tariq, I. Pharmacological Applications of Quercetin and its Derivatives: A Short Review. Trop. J. Pharm. Res. 2014, 13, 1561. [Google Scholar] [CrossRef]
- Pătruică, S.; Adeiza, S.M.; Hulea, A.; Alexa, E.; Cocan, I.; Moraru, D.; Imbrea, I.; Floares, D.; Pet, I.; Imbrea, F.; et al. Romanian Bee Product Analysis: Chemical Composition, Antimicrobial Activity, and Molecular Docking Insights. Foods 2024, 13, 1455. [Google Scholar] [CrossRef]
- Beicu, R.; Alexa, E.; Obiștioiu, D.; Cocan, I.; Imbrea, F.; Pop, G.; Circioban, D.; Moisa, C.; Lupitu, A.; Copolovici, L.; et al. Antimicrobial Potential and Phytochemical Profile of Wild and Cultivated Populations of Thyme (Thymus sp.) Growing in Western Romania. Plants 2021, 10, 1833. [Google Scholar] [CrossRef] [PubMed]
- Cadariu, A.I.; Cocan, I.; Negrea, M.; Alexa, E.; Obistioiu, D.; Hotea, I.; Radulov, I.; Poiana, M.-A. Exploring the Potential of Tomato Processing Byproduct as a Natural Antioxidant in Reformulated Nitrite-Free Sausages. Sustainability 2022, 14, 11802. [Google Scholar] [CrossRef]
- Dumbrava, D.; Popescu, L.A.; Soica, C.M.; Nicolin, A.; Cocan, I.; Negrea, M.; Alexa, E.; Obistioiu, D.; Radulov, I.; Popescu, S.; et al. Nutritional, Antioxidant, Antimicrobial, and Toxicological Profile of Two Innovative Types of Vegan, Sugar-Free Chocolate. Foods 2020, 9, 1844. [Google Scholar] [CrossRef]
- Dégi, J.; Herman, V.; Igna, V.; Dégi, D.M.; Hulea, A.; Muselin, F.; Cristina, R.T. Antibacterial Activity of Romanian Propolis against Staphylococcus aureus Isolated from Dogs with Superficial Pyoderma: In Vitro Test. Vet. Sci. 2022, 9, 299. [Google Scholar] [CrossRef] [PubMed]
- Bălașoiu (Jigău), R.A.C.; Obistioiu, D.; Hulea, A.; Suleiman, M.A.; Popescu, I.; Floares (Oarga), D.; Imbrea, I.M.; Neacșu, A.-G.; Șmuleac, L.; Pașcalău, R.; et al. Analysing the Antibacterial Synergistic Interactions of Romanian Lavender Essential Oils via Gas Chromatography–Mass Spectrometry: In Vitro and In Silico Approaches. Plants 2024, 13, 2136. [Google Scholar] [CrossRef] [PubMed]
- Available online: https://pubchem.ncbi.nlm.nih.gov/ (accessed on 11 March 2025).
- Available online: www.rcsb.org (accessed on 15 March 2025).
- Available online: www.swissadme.ch (accessed on 21 April 2025).
- Available online: http://biosig.unimelb.edu.au/pkcsm/prediction (accessed on 22 April 2025).
| Concentration (mg/mL) | OHD | FHD | Concentration (mg/mL) | Ascorbic Acid |
|---|---|---|---|---|
| Inhibition % | Inhibition % | |||
| 12.5 | 5.48 ± 0.02 a* | 8.67 ± 0.03 b | 0.02 | 27.02 |
| 16.67 | 11.47 ± 0.04 c | 15.18 ± 0.01 d | 0.04 | 44.94 |
| 25 | 15.82 ± 0.02 e | 26.79 ± 0.40 f | 0.06 | 56.02 |
| 40 | 26.93 ± 0.02 f | 33.00 ± 0.02 g | 0.08 | 71.59 |
| 100 | 42.93 ± 0.01 h | 52.51 ± 0.01 i | 0.10 | 91.75 |
| IC50 (mg/mL) | 6.26 ± 0.30 C | 5.16 ± 0.22 B | 2.47 ± 0.09 A | |
| Individual Polyphenol Compounds | Concentration (µg/mL) | |||
|---|---|---|---|---|
| Flavonoids | Ret. Time (min) | m/z | OHD | FHD |
| Epicatechin | 6.70 | 289 | 7.39 | 7.42 |
| Rutin | 12.99 | 609 | 7.93 | 7.93 |
| Quercetin | 30.74 | 301 | 1.57 | 1.56 |
| Stilbenes | ||||
| Resveratrol | 26.62 | 227 | 16.3 | 11.57 |
| Phenolic acids | ||||
| Gallic | 3.68 | 169 | nd | 1.67 |
| Caffeic | 7.63 | 179 | nd | 6.3 |
| Cumaric | 12.27 | 163 | 4.11 | nd |
| Ferulic | 14.4 | 193 | nd | 12.66 |
| Rosmarinic | 23.93 | 359 | 6.81 | 6.8 |
| β-Resorcylic | 10.15 | 153 | 25.01 | 25 |
| S. pyogenes | S. aureus | L. monocytogenes | C. perfringens | B. cereus | H. influenzae | S. typhimurium | E. coli | P. aeruginosa | S. flexneri | |
|---|---|---|---|---|---|---|---|---|---|---|
| OHD (%) | 10 | 4 | 10 | 10 | 8 | 15 | 25 | 10 | 25 | 15 |
| FHD (%) | 4 | 2 | 4 | 8 | 8 | 8 | 25 | 4 | 15 | 8 |
| Protein Targets (PDB ID) | Ligands | Pubchem CID | Binding Energy (kcal/mol) | Binding Interaction |
|---|---|---|---|---|
| Shiga toxin 1DM0 | Beta-resorcylic acid | 1491 | −6.1 | Hydrophilic bonds: LEU76, SER112, TYR114, ARG170; Hydrophobic bonds: VAL78, VAL162 |
| Caffeic acid | 689043 | −6.4 | Hydrophilic bonds: VAL78, SER112, ARG170, THR200; Hydrophobic bonds: TYR114, VAL162 | |
| Epicatechin | 72276 | −7.9 | Hydrophilic bonds: VAL78, SER112, THR200, LEU201; Hydrophobic bonds: TYR114, VAL162, MET260; Electrostatic bonds: GLU167 | |
| Ferulic acid | 445858 | −6.3 | Hydrophilic bonds: TRP34, ASN35; Hydrophobic bonds: TRP34 | |
| Gallic acid | 370 | −5.9 | Hydrophilic bonds: ASP16, ASN32, THR54, GLY62, SER64; Hydrophobic bonds: NIL | |
| p-coumaric acid | 637542 | −6.2 | Hydrophilic bonds: VAL78, SER112; Hydrophobic bonds: TYR114, VAL162 | |
| Quercetin | 5280343 | −8.8 | Hydrophilic bonds: VAL78, SER112, ARG170, THR200; Hydrophobic bonds: TYR114, VAL162 | |
| Resveratrol | 445154 | −7.1 | Hydrophilic bonds: NIL; Hydrophobic bonds: TRP34 | |
| Rosmarinic acid | 5281792 | −7.8 | Hydrophilic bonds: ASP18, ARG33, ASN35; Hydrophobic bonds: TRP34 | |
| Rutin | 5280805 | −9.1 | Hydrophilic bonds: THR46, GLY47, ARG69, GLN118, SER124, THR126, ASP198, LEU201, ASN202; Hydrophobic bonds: THR49, LEU201 | |
| Riboflavin synthase 1I8D | Beta-resorcylic acid | 1491 | −5.9 | Hydrophilic bonds: GLY4, VAL6, THR50; Hydrophobic bonds: ILE5 |
| Caffeic acid | 689043 | −5.9 | Hydrophilic bonds: THR3, VAL6, GLU93, ARG168; Hydrophobic bonds: ILE5, GLN7 | |
| Epicatechin | 72276 | −9.1 | Hydrophilic bonds: VAL6, SER146; Hydrophobic bonds: ILE5, ILE162 | |
| Ferulic acid | 445858 | −6.8 | Hydrophilic bonds: HIS102, THR148, THR165; Hydrophobic bonds: LEU147 | |
| Gallic acid | 370 | −6.1 | Hydrophilic bonds: VAL6, GLY39, THR50, SER146, ARG168; Hydrophobic bonds: ILE5 | |
| p-coumaric acid | 637542 | −6.1 | Hydrophilic bonds: HIS102; Hydrophobic bonds: THR165 | |
| Quercetin | 5280343 | −7.6 | Hydrophilic bonds: HIS31; Hydrophobic bonds: ILE5, ARG86 | |
| Resveratrol | 445154 | −7.6 | Hydrophilic bonds: CYS48; Hydrophobic bonds: ILE5, ILE162 | |
| Rosmarinic acid | 5281792 | −8.9 | Hydrophilic bonds: GLY95, ILE103, LYS137, THR148, HIS160, ILE162, THR165; Hydrophobic bonds: CYS48, HIS102 | |
| Rutin | 5280805 | −9.1 | Hydrophilic bonds: THR3, VAL6, GLN7, HIS31, GLU93, ARG168; Hydrophobic bonds: NIL | |
| ATP-binding sugar transporter-like protein 2PP6 | Beta-resorcylic acid | 1491 | −4.8 | Hydrophilic bonds: THR29, ASN58, ILE60, ASP65; Hydrophobic bonds: VAL59 |
| Caffeic acid | 689043 | −5.0 | Hydrophilic bonds: GLU18; Hydrophobic bonds: ALA14, ALA17, ILE32, PHE55 | |
| Epicatechin | 72276 | −6.1 | Hydrophilic bonds: ALA17, SER56; Hydrophobic bonds: ALA14, ILE32 | |
| Ferulic acid | 445858 | −4.6 | Hydrophilic bonds: ASP13, LEU39; Hydrophobic bonds: ILE16, PHE82, LYS85 | |
| Gallic acid | 370 | −4.7 | Hydrophilic bonds: ILE60, ARG62, ASP65; Hydrophobic bonds: NIL | |
| p-coumaric acid | 637542 | −4.8 | Hydrophilic bonds: NIL; Hydrophobic bonds: ARG10, ALA14 | |
| Quercetin | 5280343 | −6.6 | Hydrophilic bonds: ARG10, ASP13, GLU18, GLY57; Hydrophobic bonds: ALA14, ALA17, ILE32 | |
| Resveratrol | 445154 | −5.8 | Hydrophilic bonds: ALA17, GLU18; Hydrophobic bonds: ASP13, ALA14, ILE32 | |
| Rosmarinic acid | 5281792 | −6.2 | Hydrophilic bonds: SER56, ASN83, GLY84, PRO86; Hydrophobic bonds: ASP13, ALA14, ALA17, LYS85 | |
| Rutin | 5280805 | −6.6 | Hydrophilic bonds: ARG10, SER56; Hydrophobic bonds: ASP13, ALA14, ALA17, ILE32 | |
| Undecaprenyl diphosphate synthase 4H8E | Beta-resorcylic acid | 1491 | −5.8 | Hydrophilic bonds: PRO196, GLN215, TYR218, SER219; Hydrophobic bonds: TYR218; Electrostatic bonds: ASP195 |
| Caffeic acid | 689043 | −7.1 | Hydrophilic bonds: HIS50; Hydrophobic bonds: ASN35, ALA76, PRO96, PHE99 | |
| Epicatechin | 72276 | −7.8 | Hydrophilic bonds: ASP33, ASN81, ARG84; Hydrophobic bonds: HIS50, ALA76, ILE92; Electrostatic bonds: ARG84 | |
| Ferulic acid | 445858 | −6.6 | Hydrophilic bonds: ALA76; Hydrophobic bonds: MET32, HIS50, LEU95 | |
| Gallic acid | 370 | −6.3 | Hydrophilic bonds: HIS50, ASN81, ARG84; Hydrophobic bonds: ASN35 Electrostatic bonds: ARG84 | |
| p-coumaric acid | 637542 | −7.1 | Hydrophilic bonds: HIS50; Hydrophobic bonds: ILE57, ALA76, PRO96, PHE99, PHE148 | |
| Quercetin | 5280343 | −8.1 | Hydrophilic bonds: MET32, ASP33, ASN35, HIS50, TYR75; Hydrophobic bonds: TYR75, ILE92; Electrostatic bonds: ARG84 | |
| Resveratrol | 445154 | −8.6 | Hydrophilic bonds: HIS50, ALA76; Hydrophobic bonds: ASN35, ILE92, PRO96, PHE99, PHE148 | |
| Rosmarinic acid | 5281792 | −8.2 | Hydrophilic bonds: ASP33, GLY34, ASN35, GLY36, ARG37, ARG46, ALA76, PHE77, ARG207207; Hydrophobic bonds: ARG207, TYR75; Electrostatic bonds: ARG84 | |
| Rutin | 5280805 | −9.4 | Hydrophilic bonds: GLY36, ARG37, ALA76, GLU80, ARG84, ARG201 Hydrophobic bonds: ARG46, SER78, ASN81; Electrostatic bonds: ARG84 | |
| Putative lipoprotein 4R7R | Beta-resorcylic acid | 1491 | −5.1 | Hydrophilic bonds: ARG23, SER75, LYS77; Hydrophobic bonds: LYS77, TRP93 |
| Caffeic acid | 689043 | −5.6 | Hydrophilic bonds: GLU38, ASP39, TYR119; Hydrophobic bonds: PHE116, TYR119 | |
| Epicatechin | 72276 | −6.9 | Hydrophilic bonds: GLU38; Hydrophobic bonds: TYR119; Electrostatic bonds: ASP39 | |
| Ferulic acid | 445858 | −5.7 | Hydrophilic bonds: ASP45, PRO115; Hydrophobic bonds: ARG41, ILE42, PHE111, PHE116, TYR119 | |
| Gallic acid | 370 | −4.9 | Hydrophilic bonds: ASP39, SER110; Hydrophobic bonds: PHE116, TYR119 | |
| p-coumaric acid | 637542 | −5.5 | Hydrophilic bonds: PRO115; Hydrophobic bonds: ILE42, PHE116 | |
| Quercetin | 5280343 | −7.0 | Hydrophilic bonds: ASP39, ASP45, ASN109, TYR119; Hydrophobic bonds: ARG41, ILE42, PRO115, PHE116 | |
| Resveratrol | 445154 | −6.9 | Hydrophilic bonds: ASP39; Hydrophobic bonds: ARG41, ILE42, TYR119 | |
| Rosmarinic acid | 5281792 | −6.1 | Hydrophilic bonds: GLU38, ASP39, ASP45, LYS112; Hydrophobic bonds: NIL; Electrostatic bonds: ASP39 | |
| Rutin | 5280805 | −7.2 | Hydrophilic bonds: GLU38, ASP39, PRO115; Hydrophobic bonds: ILE42, TYR119 | |
| Sortase A 5HU4 | Beta-resorcylic acid | 1491 | −5.0 | Hydrophilic bonds: GLY55, HIS56, CYS117; Hydrophobic bonds: ALA54, ARG126 |
| Caffeic acid | 689043 | −5.3 | Hydrophilic bonds: LEU33, THR38, CYS117, ARG126; Hydrophobic bonds: ALA54 | |
| Epicatechin | 72276 | −6.9 | Hydrophilic bonds: HIS56, GLU98, CYS117, ARG126; Hydrophobic bonds: LEU33, ALA54; Electrostatic bonds: ARG126 | |
| Ferulic acid | 445858 | −5.6 | Hydrophilic bonds: LEU33, GLY36, THR38, GLY55, HIS56, ILE115, ARG126; Hydrophobic bonds: ALA54, CYS117 | |
| Gallic acid | 370 | −5.5 | Hydrophilic bonds: ASN29, GLY36, ALA54, HIS56; Hydrophobic bonds: THR28, LEU33, ALA54 | |
| p-coumaric acid | 637542 | −5.4 | Hydrophilic bonds: LEU33, THR38; Hydrophobic bonds: ALA54 | |
| Quercetin | 5280343 | −7.0 | Hydrophilic bonds: ILE115; Hydrophobic bonds: ALA54, VAL101, CYS117, ARG126 | |
| Resveratrol | 445154 | −6.3 | Hydrophilic bonds: HIS56, THR116; Hydrophobic bonds: ALA54, GLU100, ILE115, ARG126, CYS117 | |
| Rosmarinic acid | 5281792 | −7.8 | Hydrophilic bonds: GLY36, ALA54, GLU98, GLU100, THR116, CYS117; Hydrophobic bonds: THR28, LEU33, ILE115, ARG126 | |
| Rutin | 5280805 | −7.0 | Hydrophilic bonds: HIS56, GLU100, ILE115; Hydrophobic bonds: ALA54, VAL101, CYS117, ARG126 | |
| Immunity protein 5JKP | Beta-resorcylic acid | 1491 | −5.7 | Hydrophilic bonds: PRO203, TYR240; Hydrophobic bonds: LEU274, TYR282 |
| Caffeic acid | 689043 | −5.7 | Hydrophilic bonds: TYR240, VAL269, LEU274, GLY281; Hydrophobic bonds: PRO273 | |
| Epicatechin | 72276 | −6.6 | Hydrophilic bonds: GLN117, PRO261; Hydrophobic bonds: PRO152, ALA185; Electrostatic bonds: ARG264 | |
| Ferulic acid | 445858 | −5.6 | Hydrophilic bonds: GLN210, TYR240, GLY281; Hydrophobic bonds: VAL269, LEU274, HIS277 | |
| Gallic acid | 370 | −6.0 | Hydrophilic bonds: TYR240, LEU274, GLY281, TYR282; Hydrophobic bonds: LEU274, TYR282 | |
| p-coumaric acid | 637542 | −5.5 | Hydrophilic bonds: PRO203, TYR240, VAL269, PRO276, GLY281; Hydrophobic bonds: NIL | |
| Quercetin | 5280343 | −6.9 | Hydrophilic bonds: LYS55, ALA259, ASP269; Hydrophobic bonds: ALA51, ALA54, LYS55, PRO261 | |
| Resveratrol | 445154 | −6.8 | Hydrophilic bonds: LEU274; Hydrophobic bonds: TYR282, TRP289 | |
| Rosmarinic acid | 5281792 | −6.6 | Hydrophilic bonds: LYS55, ARG60, ASN83; Hydrophobic bonds: ALA54, TRP62, LEU87, PRO261 | |
| Rutin | 5280805 | −8.7 | Hydrophilic bonds: HIS82, GLN215, ASP262, ARG264; Hydrophobic bonds: TYR184 |
| Parameters | Rutin | Rosmarinic Acid | Beta-Resorcylic Acid | Quercetin | Ferulic Acid | p-Coumaric Acid | Unit |
|---|---|---|---|---|---|---|---|
| Absorption | |||||||
| Water solubility | −2.892 | −3.059 | −2.183 | −2.925 | −2.817 | −2.37 | Numeric (log mol/L) |
| CaCO2 permeability | −0.949 | −0.937 | 0.653 | −0.229 | 0.176 | 1.61 | Numeric (log Papp in 10−6 cm/s) |
| Intestinal absorption (human) | 23.446 | 32.516 | 70.99 | 77.207 | 93.685 | 88.401 | Numeric (% Absorbed) |
| Skin permeability | −2.735 | −2.735 | −2.735 | −2.735 | −2.72 | −2.735 | Numeric (log Kp) |
| P-glycoprotein substrate | Yes | Yes | No | Yes | No | No | Categorical (Yes/No) |
| P-glycoprotein I inhibitor | No | No | No | No | No | No | Categorical (Yes/No) |
| P-glycoprotein II inhibitor | No | No | No | No | No | No | Categorical (Yes/No) |
| Distribution | |||||||
| VDss (human) | 1.663 | 0.393 | −1.816 | 1.559 | −1.367 | 0.011 | Numeric (log L/kg) |
| Fraction unbound (human) | 0.187 | 0.348 | 0.651 | 0.206 | 0.343 | 0.751 | Numeric (Fu) |
| BBB permeability | −1.899 | −1.378 | −0.757 | −1.098 | −0.239 | 0.181 | Numeric (log BB) |
| CNS permeability | −5.178 | −3.347 | −3.317 | −3.065 | −2.612 | −3.24 | Numeric (log PS) |
| Metabolism | |||||||
| CYP2D6 substrate | No | No | No | No | No | No | Categorical (Yes/No) |
| CYP3A4 substrate | No | No | No | No | No | No | Categorical (Yes/No) |
| CYP1A2 inhibitor | No | No | No | Yes | No | No | Categorical (Yes/No) |
| CYP2C19 inhibitor | No | No | No | No | No | No | Categorical (Yes/No) |
| CYP2C9 inhibitor | No | No | No | No | No | No | Categorical (Yes/No) |
| CYP2D6 inhibitor | No | No | No | No | No | No | Categorical (Yes/No) |
| CYP3A4 inhibitor | No | No | No | No | No | No | Categorical (Yes/No) |
| Excretion | |||||||
| Total Clearance | −0.369 | 0.25 | 0.603 | 0.407 | 0.623 | −6.797 | Numeric (log mL/min/kg) |
| Renal OCT2 substrate | No | No | No | No | No | No | Categorical (Yes/No) |
| Toxicity | |||||||
| AMES toxicity | No | No | No | No | No | Yes | Categorical (Yes/No) |
| Maximum tolerated dose (human) | 0.452 | 0.152 | 0.89 | 0.499 | 1.082 | 0.593 | Numeric (log mg/kg/day) |
| hERG I inhibitor | No | No | No | No | No | No | Categorical (Yes/No) |
| hERG II inhibitor | Yes | No | No | No | No | No | Categorical (Yes/No) |
| Oral Rat Acute Toxicity (LD50) | 2.491 | 2.811 | 2.284 | 2.471 | 2.282 | 2.482 | Numeric (mol/kg) |
| Oral Rat Chronic Toxicity (LOAEL) | 3.673 | 2.907 | 1.857 | 2.612 | 2.065 | 4.375 | Numeric (log mg/kg bw/day) |
| Hepatoxicity | No | No | No | No | No | No | Categorical (Yes/No) |
| Skin Sensitisation | No | No | No | No | No | No | Categorical (Yes/No) |
| Tetrahymena Pyriformis toxicity | 0.285 | 0.302 | 0.285 | 0.288 | 0.271 | 0.285 | Numeric (log ug/L) |
| Minnow toxicity | 7.677 | 2.698 | 2.734 | 3.721 | 1.825 | 5.76 | Numeric (log mM) |
| Oral bioavailability | |||||||
| Lipinski’s rules | 3 | 0 | 0 | 0 | 0 | 0 | Violation (Numeric) |
| Ghose rules | 4 | 3 | Violation (Numeric) | ||||
| Verber’s rules | 1 | 1 | Violation (Numeric) | ||||
| Egan’s rules | 1 | 1 | Violation (Numeric) | ||||
| Muegge rules | 1 | 1 | 1 | 1 | Violation (Numeric) | ||
| Bioavailability score | 0.17 | 0.56 | 0.56 | 0.55 | 0.85 | 0.85 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hulea, C.; Obistioiu, D.; Hulea, A.; Suleiman, M.A.; Floares, D.; Alexa, E.; Imbrea, I.M.; Neacșu, A.-G.; Pentea, M.; Popescu, C.A.; et al. Integrated Assessment of Antibacterial Activity, Polyphenol Composition, Molecular Docking, and ADME Properties of Romanian Oak and Fir Honeydew Honeys. Antibiotics 2025, 14, 592. https://doi.org/10.3390/antibiotics14060592
Hulea C, Obistioiu D, Hulea A, Suleiman MA, Floares D, Alexa E, Imbrea IM, Neacșu A-G, Pentea M, Popescu CA, et al. Integrated Assessment of Antibacterial Activity, Polyphenol Composition, Molecular Docking, and ADME Properties of Romanian Oak and Fir Honeydew Honeys. Antibiotics. 2025; 14(6):592. https://doi.org/10.3390/antibiotics14060592
Chicago/Turabian StyleHulea, Calin, Diana Obistioiu, Anca Hulea, Mukhtar Adeiza Suleiman, Doris Floares (Oarga), Ersilia Alexa, Ilinca Merima Imbrea, Alina-Georgeta Neacșu, Marius Pentea, Cosmin Alin Popescu, and et al. 2025. "Integrated Assessment of Antibacterial Activity, Polyphenol Composition, Molecular Docking, and ADME Properties of Romanian Oak and Fir Honeydew Honeys" Antibiotics 14, no. 6: 592. https://doi.org/10.3390/antibiotics14060592
APA StyleHulea, C., Obistioiu, D., Hulea, A., Suleiman, M. A., Floares, D., Alexa, E., Imbrea, I. M., Neacșu, A.-G., Pentea, M., Popescu, C. A., & Imbrea, F. (2025). Integrated Assessment of Antibacterial Activity, Polyphenol Composition, Molecular Docking, and ADME Properties of Romanian Oak and Fir Honeydew Honeys. Antibiotics, 14(6), 592. https://doi.org/10.3390/antibiotics14060592









