Abstract
Background: This study evaluated the polyphenolic composition, antibacterial activity, molecular docking interactions, and pharmacokinetic properties of Romanian oak and fir honeydew honeys. Methods: Spectrophotometric methods quantified total phenolic, flavonoid contents and antioxidant activity, and individual polyphenols were identified via HPLC-MS. Antibacterial efficacy against Gram-positive and Gram-negative bacteria was evaluated by determining the bacterial inhibition percentage and minimum inhibitory concentrations. The bioactive compounds identified via LC-MS analysis were used to further delineate the possible antibacterial activities in silico. Molecular docking was carried out to predict the binding interactions and complex formation of the identified compounds against protein crystal structures of the bacteria used in this study. Additionally, the pharmacokinetic profile of compounds with high inhibitory potential was assessed via ADMET (absorption, Distribution, Metabolism, Excretion, toxicity) predictors to ascertain their value. Results: Fir honeydew honey showed higher total phenolic (844.5 mg GAE/kg) and flavonoid contents (489.01 mg QUE/kg) compared to oak honeydew honey, correlating with more potent antioxidant activity (IC50 = 5.16 mg/mL). In vitro antimicrobial tests indicated a stronger inhibitory effect of fir honeydew honey, especially against Gram-positive strains like S. aureus, S. pyogenes, and L. monocytogenes, alongside certain Gram-negative strains such as E. coli and H. influenzae. Oak honeydew honey displayed selective antimicrobial action, particularly against P. aeruginosa and S. typhimurium. The docking outcomes showed rutin, rosmarinic acid, beta resorcylic acid, quercetin, ferulic acid, and p-coumaric acid have high inhibitory activities characterised by binding affinities and binding interactions against shiga toxin, riboflavin synthase, ATP-binding sugar transporter-like protein, undecaprenyl diphosphate synthase, putative lipoprotein, sortase A, and immunity protein, making them key contributors to the honey’s antimicrobial activity. Moreover, beta-resorcylic acid, quercetin, ferulic acid, and p-coumaric acid revealed interesting ADMET scores that qualify honey to serve as a good antimicrobial agent. Conclusions: These findings support their potential use as natural antibacterial agents and emphasise the value of integrating chemical, biological, and computational approaches for multidisciplinary characterisations.
1. Introduction
Honey, a naturally sweet substance made by Apis mellifera, is among the earliest traditional remedies valued for its ability to treat various ailments, owing to its anti-inflammatory [1,2], antimicrobial [2,3,4], antifungal [5], and antiviral properties [6]. The nutritional and biological properties of honey stem from its various components, including sugars, proteins, free amino acids, organic acids, vitamins, minerals, enzymes, flavonoids, and phenolic acids [7,8,9,10,11,12,13,14]. Several factors influence the levels of these compounds, including geographical origin, botanical source, climate and weather conditions, treatment methods, and importantly, the conditions under which they are harvested, processed, and stored [15,16,17]. The differences in the biological and health-promoting properties of various honeys can be attributed to the varying levels of phytochemical and bee-derived compounds, which are influenced by these factors [18,19,20].
Among the biological characteristics, honey exhibits strong antioxidant activity, being capable of preventing damage caused by oxidants such as OH−, O2, superoxide, and lipid peroxyl radicals. However, the values of antiradical activity, a metric used to quantify honey’s antioxidant qualities, obtained by different methods, are variable depending on various factors, especially floral origin [21,22]. The plants from the Rosaceae, Fabaceae, Asteraceae, and Amaranthaceae families positively impact the quantity of antioxidants in honey [22]. On the other hand, dark honey, such as buckwheat, thyme, dandelion, wildflower, chestnut, meadow, manna, and manuka honey, is characterised by higher antioxidant activity than light honey due to a higher content of polyphenolic compounds [23,24,25]. Honey contains various flavonoids and phenolic compounds, including kaempferol, chrysin, quercetin, pinobanksin, luteolin, pinocembrin, apigenin, naringenin, hesperetin, genistein, p-coumaric acid, ferulic acid, gallic acid, syringic acid, vanillic acid, and caffeic acid [26,27,28,29]. The exact way these compounds affect oxidative stress—by either reducing or inhibiting it—is not completely understood. Nonetheless, it is thought to involve free radical sequestration, metallic ion chelation, hydrogen donation, and the action of flavonoid substrates on hydroxyl and superoxide radicals [21,30].
Besides its antioxidant activity, honey is recognised as an antimicrobial agent due to its high osmolarity, low pH, H2O2 content, and non-peroxide compounds such as bee-defensin one and flavonoids, respectively, and phenolic acid levels [3,12,31,32,33,34]. The mechanism of action has not been fully elucidated. Still, it has been demonstrated that the bactericidal effect is due to membrane permeability alteration, with potassium and protein leakage, inhibition of membrane and intracellular protein synthesis, and bacterial DNA damage [35]. Over time, different types of honey have demonstrated their antibacterial efficacy, both against Gram-positive and Gram-negative bacteria such as methicillin-resistant Staphylococcus aureus (MRSA), Streptococcus pneumoniae, Bacillus subtilis, Listeria monocytogens, Escherichia coli, Pseudomonas aeruginosa, Salmonella spp., Klebsiella pneuomniae [26,32,35,36,37,38]. The values of minimum inhibitory concentrations for each bacterial strain vary from one type of honey to another in different regions of the world, being, in general, lower for Gram-positive than Gram-negative bacteria [26,37,38]. The different cell wall compositions can explain the difference in susceptibility to honey between the two types of bacteria. An outer membrane protecting the peptidoglycan layer characterises Gram-negative bacteria, while Gram-positive bacteria are surrounded only by a thick peptidoglycan layer [39]. Still, several investigations indicated that Gram-positive bacteria are more resistant to various types of honey than Gram-negative bacteria, most likely due to the samples’ increased hydrogen peroxide content and osmolality [40,41]. Although the minimum inhibitory concentrations observed in the literature are variable, depending on the type of honey and its physicochemical characteristics, as well as on the bacterial strains studied, one thing is certain: unlike synthetic antibiotics, microbial resistance to honey has never been reported [42]. This aspect is critical since the increasing emergence of multidrug-resistant bacteria in recent decades represents a significant challenge worldwide for veterinary and public health [21,43,44,45]. However, the biological activity of honey is influenced by the bioavailability of different phytochemical components as well as how they are absorbed and metabolised [46].
Romanian honey production is notable for its high quality and wide variety due to the temperate-continental climate of the country and melliferous plants found across the Carpathian-Danubian-Pontic area [47]. Moreover, it is primarily produced in rural areas, where beekeepers maintain hives in the heart of the countryside, away from industrial pollutants. This aspect contributes to its purity and minimal contamination [48,49]. The diverse relief, different climatic characteristics, and the variable vegetation from one region to another influence honey production and its biological properties throughout the country. The most common types of honey produced in all areas are rapeseed honey, acacia honey, linden honey and polyfloral honey. In addition, honeydew honey—a non-floral honey—is made in mountainous regions of coniferous and deciduous forests. Unlike other kinds of honey, this type is obtained from manna, the sweet secretions produced by aphids and scale insects, which feed on the sap of plants and trees [50]. Few studies have characterised honeydew honey, although it is claimed that the antioxidant and antimicrobial activities are superior to other types of monofloral honey [51], except for manuka honey. Furthermore, to our knowledge, there are no data regarding the biological activity of the varieties of honeydew honey in Romania.
Computer-based virtual screening on the key proteins of the microorganisms has led to the identification and understanding of the mechanism of action by which the inhibition of compounds could match the known ligands of the proteins. Notably, specific compounds responsible for the antimicrobial activities possess a control selectivity tied to the reactive groups’ physicochemical functionality [52]. With the continuous surge in antimicrobial resistance to the available antibiotics, new antibacterial agents from natural origin, which contain bioactive molecules targeting necessary metabolic enzymes of microbial pathogenesis, could be assessed in silico. Thus, the crystal structure of seven bacterial protein targets, shiga toxin, riboflavin synthase, ATP-binding sugar transporter-like protein, undecaprenyl diphosphate synthase, putative lipoprotein, sortase A, and immunity protein are suitable targets for antimicrobial assessment due to their key involvement in the machinery of protein synthesis and bacterial growth ranging from metabolic enzymes, cell wall, virulence factors and key genes necessary for microbial pathogenicity [53].
The samples analysed were sourced from approved local producers from Sibiu, Romania. The Sibiu region in the Southern Carpathians is characterised by extensive mixed forests dominated by fir (Abies alba) and oak (Quercus spp.), providing ideal conditions for honeydew secretion. This area is known for its high-quality honeydew honey, which is traditionally valued for its rich mineral content and distinctive dark colour.
Although various studies have tested the bioactive potential of different monofloral honeys, there is little information available regarding honeydew honeys of different botanical origins, especially fir (FHD) and oak (OHD). Additionally, there have not been many studies linking the polyphenolic profiles of these honeys to their respective quantitative antimicrobial efficacies against a variety of clinically relevant bacterial strains. This study bridges this gap by providing a detailed comparative analysis of the chemical constituents and antimicrobial performance of FHD and OHD using LC-MS phenolic profiling, quantitative antimicrobial metrics (BIP%, MIC) and molecular docking. The main objectives of the research were as follows: (i) to determine the total phenolic (TPC), flavonoid content (TFC) and antioxidant activity; (ii) identify and quantify the major polyphenolic compounds in FHD and OHD using HPLC-MS; (iii) to evaluate the antibacterial activity of OHD and FHD against a panel of clinically relevant Gram-positive and Gram-negative ATCC bacterial strains; (iv) to perform molecular docking simulations of selected polyphenols (e.g., ferulic acid, caffeic acid, resveratrol, quercetin) against key bacterial target proteins such as DNA gyrase and penicillin-binding proteins; (v) to evaluate the ADME properties (absorption, Distribution, Metabolism, and Excretion) of the identified bioactive compounds using in silico pharmacokinetic models. To correlate the antibacterial efficacy with the polyphenolic profile and computational results, identifying which compounds contribute most to antimicrobial potential.
2. Results
2.1. Determination of Total Phenolic Content (TPC) and Flavonoid Content (TFC)
The results for total phenolic content (TPC) and flavonoid content (TFC) are presented in Figure 1.
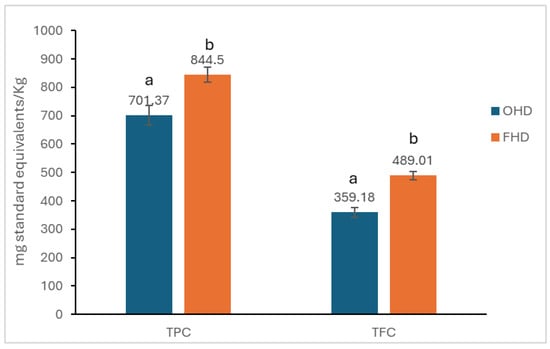
Figure 1.
TPC and TFC of honey samples. The mean of the three determinations ± standard deviation (SD) is used to express the results. Different lowercase letters (a, b) indicate statistically significant differences between samples from TPC and TFC according to one-way ANOVA followed by Tukey’s HSD test (p < 0.05).
The total polyphenol content in the ODH sample was 701.37 mg GAE/Kg, while in the FHD sample, the content was 844.5 mg GAE/Kg. There were statistically significant differences (p < 0.05) in the total polyphenol content between the two samples of analysed honey.
The TFC in the ODH sample was 359.18 mg QUE/Kg, while in the FHD sample, the content was 489.01 mg QUE/Kg. The two samples have statistically significant differences (p < 0.05).
2.2. Antioxidant Capacity by 1,1-Diphenyl-2-picrylhydrazyl (DPPH) Assay
The results of the antioxidant activity determined by the DPPH assay are presented in Table 1.

Table 1.
DPPH radical scavenging activity in the honey samples (% inhibition).
The highest inhibition is observed at the highest studied concentration (100 mg/mL) for both samples. The FHD sample demonstrates a greater radical scavenging activity (52.51%) than the OHD sample (42.93%). Statistically significant differences (p < 0.05) are noted at nearly all concentrations between the two samples, except for the 25 mg/mL concentration in the FHD sample and the 40 mg/mL concentration in the OHD sample, which do not exhibit statistically significant differences (p > 0.05).
2.3. High-Performance Liquid Chromatography for the Individual Profiling of Polyphenols
The retention times, m/z signals and the concentrations of each compound identified are presented in Table 2.

Table 2.
The individual profile of polyphenols detected using the LC-MS method.
The concentrations of epicatechin, rutin, and quercetin seem almost similar in the two samples studied. Differences between the samples were noted in resveratrol concentrations, higher for OHD (16.3 µg/mL) than FHD (11.57 µg/mL). Unlike flavonoids, the concentration of phenolic acids varied between the samples, the only similar concentrations being observed at rosmarinic (6.8 µg/mL) and β-resorcylic acid (25 µg/mL). Gallic, caffeic and ferulic acids were undetectable in the case of OHD, which was characterised by the presence of cumaric acid (4.11 µg/mL). Instead, cumaric acid was absent in the FHD sample, but other phenolic acids were detectable in different concentrations: gallic acid—1.67 µg/mL; caffeic acid—6.3 µg/mL; ferulic acid—12.66 µg/mL.
2.4. Antimicrobial Activity
Figure 2 and Figure 3 present the bacterial inhibition percentage (BIP%) values of OHD (Figure 2) and FHD (Figure 3) against Gram-positive and Gram-negative ATCC bacteria, values calculated using Formulas (2) and (3) given in Section 4.7.
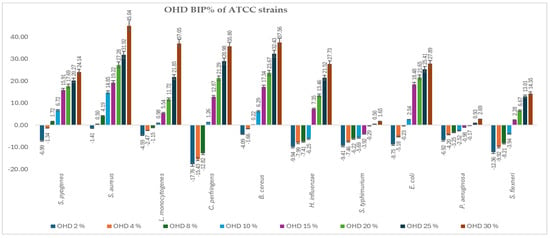
Figure 2.
BIP% of OHD against the tested ATCC strains.
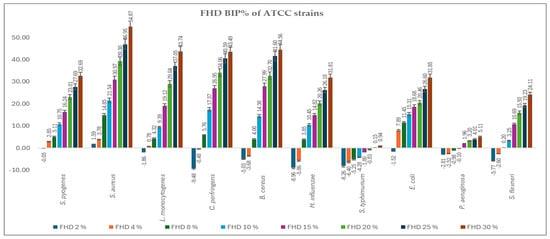
Figure 3.
BIP% of FHD against the tested ATCC strains.
The comparative antimicrobial activity of OHD and FHD honeys showed that there was a difference in the inhibition capacity on a wide array of bacterial strains. The results suggest that FHD showed better antimicrobial activity compared to OHD concerning higher inhibitory values and lower MIC values.
S. aureus and S. pyogenes demonstrated greater sensitivity to both honeys, with notable inhibition observed at concentrations greater than 8%. FHD showed higher inhibition, with BIP% reaching 54.87% (30%) for S. aureus and 31.26% (30%) for S. pyogenes. L. monocytogenes exhibited a dose-dependent response, with FHD demonstrating significantly better efficacy than OHD, starting at 4%. FHD achieved a BIP% of 27.43% at 30%, indicating improved membrane penetration or compound synergy.
An interpretation concerning the type of bacteria involved, the most resilient Gram-positive strains were B. cereus and C. perfringens, which showed negative BIP% at low concentrations, especially for OHD. Only at 8% (5.76%) did FHD start to significantly inhibit C. perfringens, while at 30%, the inhibition increased to 43.96%. With P. aeruginosa and S. flexneri exhibiting low or negative BIP% at lower concentrations, indicating higher resistance because of their outer membrane barrier, the Gram-negative representatives showed a more variable and concentration-dependent trend. At ≥8 mg/mL, E. coli proved highly susceptible to both honeys, particularly FHD, which at 30% showed 31.85% inhibition. FHD significantly inhibited H. influenzae, with the BIP% increasing from 7.93% at 8% to 44.86% at 30%, suggesting that phenolic acids, such as ferulic and caffeic acid, which are abundant in FHD, may have synergistic activity. Although FHD showed more modest results, S. typhimurium responded moderately to both types of honey, clearly favouring OHD, which reached a maximum BIP% of 4.89%. P. aeruginosa exhibited a peak sensitivity of 2.41% (30%) to OHD. FHD’s low effectiveness against this strain was confirmed by its negligible or negative BIP%. All MIC values identified are presented in Table 3.

Table 3.
MIC values (%) for OHD and FHD on selected Gram-positive and Gram-negative strains.
Comparing the two types of honey analysed, FHD inhibited most strains at a low concentration. MIC values ranged from around 2–8% for the most sensitive organisms (S. aureus, S. pyogenes, E. coli, L. monocytogenes, H. influenzae) as compared to 8–15% for the same strains when treated with OHD. Even at the highest concentration tested (30%), FHD showed higher BIP% in almost all species. Moreover, FHD displayed an inhibition percentage of 54.87 for S. aureus and 43.74 for L. monocytogenes, whereas OHD showed inhibition percentages of 45.04 and 37.05 for the same organisms, respectively. The superior performance of FHD is probably because it contains a considerably higher phenolic content than OHD, distributed across a wide range of phenolic compounds, including gallic acid, caffeic acid, and ferulic acid, among others, which are known to cause membrane-disruption and enzyme-inhibiting effects. These compounds could have a synergistic effect in potentiating the ability of honey to breach bacterial defences and inhibit growth.
In contrast, OHD showed activity against bacteria with a narrower spectrum, having positive effects only at higher concentrations. Under such conditions, it displayed selectivity; for instance, being capable of modest inhibition at a rate of 2.69% and 1.65% of P. aeruginosa and S. typhimurium, respectively, at 30%, its MIC values were much higher, at 25%, accounting for less potency. The moderate activity of OHD may be influenced by its specific flavonoid content, such as resveratrol, which may contribute selectively against certain resistant strains, but without broad-spectrum effectiveness.
In addition, both honeys had lower effects on such species that naturally resist them, including S. typhimurium and P. aeruginosa. This confirms much of the introductory literature on the resistance of these strains because of strong efflux and an impermeable outer membrane. Even in such cases, FHD still showed a slight superiority of activity, reinforcing the assertion of its better chemical composition.
2.5. Molecular Docking
A total of ten (10) compounds identified by LC-MS from oak and fir honeydew honeys were successfully docked with seven (7) bacterial target proteins owing to their reported antimicrobial potential. Binding energies and active site binding amino acid residue interactions characterise the result. Thus, hydrophilic bonds (hydrogen bonds, carbon-hydrogen bonds), hydrophobic bonds (π, π-π, -alkyl, -sigma, -sulphur, -T-shaped), electrostatic bonds (π-anion/cation) were involved in the overall binding interactions and complex formation between the ligands (compounds) and receptors (proteins) (Table 4). Additionally, the docking scores revealed interesting binding energies within a concerted range measured in kcal/mol, 1dm0 (−9.1 to −5.9), 1i8d (−9.1 to −5.9), 2pp6 (−6.6 to −4.6), 4h8e (−9.4 to −5.8), 4r7r (−7.2 to −4.9), 5hu4 (−7.8 to −5.0) 5jkp (−8.7 to −5.6), respectively. Interestingly, rutin (−9.1 kcal/mol) gave the nine hydrophilic bonds with Shigella toxin protein (THR46, GLY47, ARG69, GLN118, SER124, THR126, ASP198, LEU201, ASN202), rosmarinic acid (−8.9 kcal/mol) showed seven hydrophilic bonds with riboflavin synthase (GLY95, ILE103, LYS137, THR148, HIS160, ILE162, THR165), beta-resorcylic acid (−4.8 kcal/mol) had four hydrophilic bonds (THR29, ASN58, ILE60, ASP65) with ATP-binding sugar transporter-like protein, again rosmarinic acid (−8.2 kcal/mol) fared better with undecaprenyl diphosphate synthase forming nine hydrogen bonds (ASP33, GLY34, ASN35, GLY36, ARG37, ARG46, ALA76, PHE77, ARG207207), quercetin (−7.0 kcal/mol) formed four hydrogen bonds (ASP39, ILE42, ASP45, ASN109, TYR119) with putative lipoprotein of C. perfringens, ferulic acid (−5.6 kcal/mol) had seven hydrogen bonds with L. monocytogens sortase A protein (LEU33, GLY36, THR38, GLY55, HIS56, ILE115, ARG126), finally p-coumaric acid showed six hydrogen bonds (PRO203, TYR240, VAL269, PRO276, GLY281) with immunity protein of P. aeruginosa (Figure 4). Other compounds interacted fairly with the target proteins and had good binding affinities.

Table 4.
Molecular docking score between the ten identified compounds and seven bacterial target proteins.
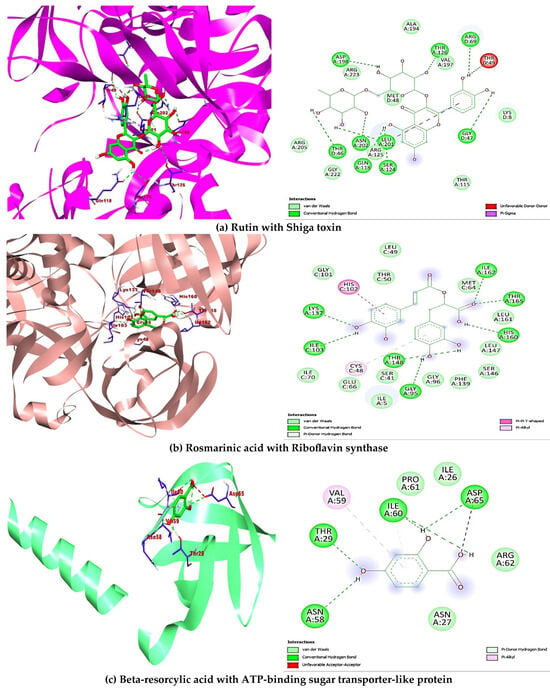
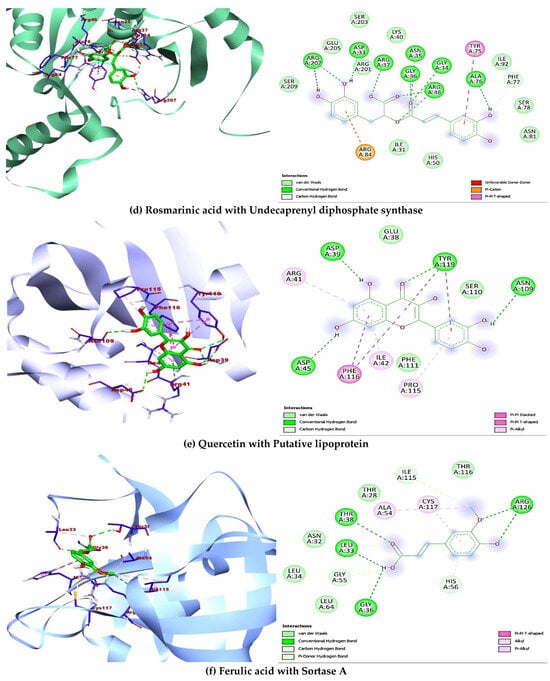
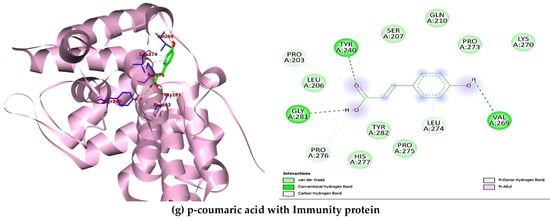
Figure 4.
Showing 3-D and 2-D binding poses of the best docking outcome with each of the seven bacterial target proteins (a–g).
2.6. ADME (Absorption, Distribution, Metabolism, and Excretion) Properties
The ADMET analysis performed on the six (6) bioactive compounds aimed to show any liabilities and possibly depict the safety pattern of utilising them in vivo. In particular, the compounds were assessed for cardiac toxicity, inhibition of cytochrome P450 isoforms, and hepatotoxicity (Table 5). Findings revealed that intestinal absorption was predicted to be moderate for beta-resorcylic acid, quercetin, ferulic acid, and p-coumaric acid. In contrast, rutin and rosmarinic acid had a low potential for intestinal absorption (23.4%, 32.5%), respectively. Additionally, except for quercetin, which showed a positive response to the CYP1A2 isoform, all the other bioactive compounds did not have inhibitory effects on any of the cytochrome proteins. The compounds also harmed hepatotoxicity and skin sensation predictors, although scoring for the blood–brain barrier was insufficiently distributed. More so, Rutin had the numerical violations for Lipinski, Ghose, Verber, Egan, and Muegge’s rules and had the lowest bioavailability score (0.17).

Table 5.
ADMET Predictors of Pharmacokinetic parameters for the six best docked compounds.
3. Discussion
Honeydew honey has gained popularity due to its unique nutritional, sensory, and various biological properties [58,59,60,61,62]. The biological properties are correlated with chemical composition variables, depending mainly on geographical origin, climate, and meteorological conditions, plant type, and insect species [63]. Generally, the results obtained in different studies mention a significant diversity of polyphenolic compounds, with varying values of TPC and TFC [58,64,65]. Can et al. demonstrated a concentration of TPC and TFC of 120.04 mg GAE/100 g and 3.10 mg QUE/100 g in Turkish oak honeydew honey [58]. Comparing three types of honeydew honey from Spain, Fernández-Estellé et al. observed that holm oak varieties had the highest TFC (1.78 mg QUE/g) and FC index (70 mg GAE/g honey), followed by forest and mountain honeydew honey [59]. Instead, Seijo et al. demonstrated that oak honeydew from the same country had a TPC of 134.8 mg GAE/100 g and TFC of 9.7 mg QUE/100 g [66]. In the present study, the values detected in Romanian OHD were higher, respectively, 701.37 mg GAE/kg and 395.18 mg QUE/kg. Similarly, slightly higher TPC and TFC were detected in FHD—844.50 mg GAE/kg and 498.01 mg QUE/kg. In contrast, Kuś et al. highlighted that Polish fir honeydew honey had a moderate total phenolic content (533.2 mg GAE/kg), while Jaśkiewicz et al. showed a concentration of 71.0 mg GAE/100 g, even though the plant origin of this honeydew from Poland is not specified [64,65].
A diversity of polyphenols was identified in different kinds of honeydew from Europe, such as salicylic acid, gallic acid, ferulic acid, coumaric acid, p-hydroxybenzoic acid, chlorogenic acid, vanillic acid, epicatechin, catechin, kaempferol, luteolin, pinocembrin, quercetin, rutin, and chrysin [67]. However, some phenolic compounds correlate with botanical origin. For example, myricetin and genistein were reported only in Thymus vulgare honeydew honey [68], coniferaldehyde, syringaldehyde, and hesperidin in Mimosa scabrella Bentham [69], and kynurenic acid in Salix spp. [70]. The present study highlighted that OHD and FHD contained similar flavonoids, rutin, epicatechin, and quercetin, in the same concentrations. Instead, Oroian et al., by studying five honeydews from the northeast part of Romania with unspecified botanical sources, remarked various concentrations of different flavonoids, such as myricetin (0–0.37 mg/100 g honey), chrysin (0–0.16 mg/100 g honey), pinocembrin (0.27–4.36 mg/100 g honey), quercetin (0.10–2.79 mg/100 g honey), apigenin (0–1.10 mg/100 g honey), kaempferol (0–0.60 mg/100 g honey), isorhamnetin (0–0.12 mg/100 g honey), luteolin (0–0.11 mg/100 g honey), and galangin (0.02–0.49 mg/100 g honey). Regarding the phenolic acids profile, the author found different concentrations of p-coumaric acid (0–4.35 mg/100 g honey), caffeic acid (0–1.92 mg/100 g honey), and gallic acid (0.02–0.26 mg/100 g honey) [71]. The present study showed that the main representative phenolic acid for both honeydew honeys studied was β-resorcylic (25.01 μg/mL). Also, rosmarinic acid was identified in both studied samples at a concentration of 6.8 μg/mL. Other phenolic acids, such as gallic acid, caffeic acid, coumaric acid, and ferulic acid, were the ones that made the difference between OHD and FHD. OHD contained only cumaric acid (4.11 μg/mL), while this compound was lacking in FHD, which was rich in ferulic acid (12.66 μg/mL) but also contained caffeic acid (6.3 μg/mL) and gallic acid (1.67 μg/mL). In contrast to our study, Hernandez et al. demonstrated that most of the Spanish oak honeydew honey tested contained, besides p-coumaric acid (mean = 0.594 mg 100 g−1), caffeic acid (mean = 0.176 mg 100 g−1), and also low concentrations of ferulic acid (mean = 0.071 mg 100 g−1). Like vanillic and chlorogenic acids, gallic acid was more infrequent in the samples [72]. Another study concluded that Polish honeydew is rich in trans-ferulic acid (221.29 µg/100 g), followed by p-coumaric (199.69 µg/100 g) and caffeic acid (143.5 µg/100 g), even though the botanical source is not specified [65].
The various phenolic compounds contribute to the diversity of the biological activities of honeydew honey due to their synergistic and antagonistic interactions. Generally, honey is recognised as an antioxidant natural remedy, capable of scavenging free radicals, reducing oxidative stress, and protecting against cell damage due to the content of resveratrol, epicatechin, rutin, quercetin, kaempferol, p-coumaric acid, caffeic acid, and ferulic acid [59,67,68]. Additionally, enzymes, amino acids, and carotenoids also enhance the antioxidant capacity [67]. Among different types of honey, honeydew honey stands out for its pronounced antioxidant activity, comparable to that of manuka honey [23,26]. Kačániová et al. demonstrated that forest honeydew from Slovakia exhibited antioxidant activity toward DPPH radicals in the 29.84–41.94% range [26]. In contrast, Jaśkiewicz et al. reported higher values for Polish honeydew honey, with a mean of 89.4% [59]. Moreover, the IC50 values for Turkish honeydew honey ranged from 12.56 to 76.20 mg/mL, depending on the botanical origin, with oak honeydew displaying the lowest IC50 (12.56 mg/mL) [52]. Greek oak honeydew appears to be more efficient, with an IC50 value of 7.14 mg/mL [69]. The current study identified an even lower IC50 value for oak honeydew, specifically 6.26 mg/mL. Higher antioxidant activity of FHD was observed compared to OHD, as the IC50 value was 5.16 mg/mL. This aspect is anticipated since the TPC value strongly correlates with antioxidant activity [59]. On the other hand, in addition to the synergistic effects of resveratrol and quercetin, known for their anti-inflammatory and antioxidant properties [70,71,72], FHD contains more phenolic acids, including caffeic acid, ferulic acid, and gallic acid, which contribute to enhanced antioxidant capacity [41,73,74,75].
Building on its chemical composition and antioxidant properties, honey also demonstrates remarkable antimicrobial activity. The ability to combat a wide range of pathogens has been well-documented in scientific studies [3,26,32,35,36,37,38]. However, the antimicrobial activity depends on different honey’s physicochemical properties and bacteria’s characteristics. Generally, Gram-positive bacteria are more susceptible to substances that target peptidoglycan than Gram-negative bacteria due to the lack of an outer lipid membrane [39]. Similarly to this hypothesis, the values of MIC were lower when studying the efficacy of the honey samples against Gram-positive bacteria, respectively, in the range of 2–10%, than against Gram-negative bacteria (4–25%). It is worth mentioning that FHD had lower MIC values than OHD for all strains studied except for S. typhimurium. Analysing the MIC values for each studied strain, it was observed that the most sensitive bacteria from the Gram-positive ones are S. aureus, with MIC values of 2% for FHD and 4% for OHD. For the Gram-negative ones, the lower MIC values were detected against E. coli, 4%, by testing FHD and 10% in the case of OHD, respectively. In contrast, fir honeydew honey from the Podkarpackie Province of Poland seems inefficient against S. aureus and E. coli, but also against L. monocytogenes [73]. This bacterial strain in our study was susceptible to both honeys studied. In contrast, Grabek-Lejko et al. claim that the MIC value of fir honeydew from the same country was 20.5 % for S. aureus [74]. Also, fir honeydew from Croatia is efficient against S. aureus and S. epidermitis, but only at a concentration of 0.25 g/mL [75]. The susceptibility of S. pyogenes to fir honeydew from other European countries was also demonstrated. For example, Dzugan et al. showed the efficacy of Polish fir honeydew honey against this bacterial strain at a concentration between 12.5% and 50% [73]. However, the current study highlighted that even lower concentrations of FHD are necessary to inhibit S. pyogenes growth, respectively, 4%, while the MIC value of OHD for the strain was 10%. Similarly, lower MIC was observed against B. cereus (8%) compared to the values reported in the literature [74].
The antimicrobial activity of honeydew honey studied against Gram-negative bacteria is also higher than that of honey from other countries [74,76]. Still, compared with another study from Bucovina, Romania, FHD and OHD are less efficient against P. aeruginosa and S. typhimurium [37]. Thus, Luca et al. sustained that the MIC values of Romanian honeydew honey are in the range of 3.15–12.50% for P. aeruginosa and 6.25– 12.50% for S. typhimurium, while in the current study, the MIC was 25% against both bacterial strains [37]. However, the author does not mention the botanical origin of honeydew honey. Moreover, the MIC value for E. coli found in the study was 6.25–12.5%, while the FHD from the present study seems to be more efficient against this bacterial strain, since the MIC found was 4% [37].
Given these observations, it is clear that Romanian OHD and FHD exhibit much higher total phenolic content (TPC) and total flavonoid content (TFC) compared to other European honeydew honey varieties, including those from Spain and Poland. Elevated phenolic levels are linked to greater antioxidant and antimicrobial properties in these honeys. This indicates that the unique environmental conditions in Romania may play a key role in shaping honeydew honey’s chemical characteristics and bioactive properties”.
In the attempt to understand and define the possible mechanism of inhibition to which the identified bioactive compounds act as therapeutic agents against certain bacteria of biological importance, impact on chemical alteration or disruption, and evasion of antibacterial resistance are standard mechanisms of inhibition [77]. The ten (10) compounds identified in the oak and fir honeydew honeys all depicted significant comparative binding affinity towards each of the bacterial target proteins. The binding poses, complex formation, strong hydrogen bonds, non-covalent bonds, and high binding energies recorded from each ligand-protein interaction are corroborated by the inhibitory potential exhibited by the bioactive compounds on the target proteins. Although six compounds (Figure 4) displayed better binding affinities and amino acid residue interactions (Table 4), rutin, rosmarinic acid, beta-resorcylic acid, quercetin [78], ferulic acid, and p-coumaric acid all suggested comprehensive potential in combating infections arising from foodborne bacterial infections [79].
The ADMET scores establish a thorough therapeutic framework for the bioactive compounds assessed, potentially enhancing their pharmacological effects. Importantly, their favourable bioavailability, human intestinal absorption, non-hepatotoxicity, and moderate clearance (Table 5) could enhance honey’s overall effectiveness against food-borne bacterial infections. Thus, the beneficial aspects of these in silico properties justify their consideration in calculating honey’s biological activity score. This further emphasises the role of the identified honey compounds in achieving the targeted antimicrobial effectiveness.
FHD exhibited stronger and broader-spectrum antibacterial activity, as reflected by lower MIC values for S. aureus (2%), S. pyogenes (4%), L. monocytogenes (4%), and E. coli (4%), as well as H. influenzae (8%). Its higher concentrations of ferulic, caffeic, and gallic acids, which have been shown in the literature to have bactericidal effects through membrane disruption, DNA damage, and inhibition of enzymatic targets like DNA gyrase and penicillin-binding proteins, are closely associated with this increased activity.
Additionally, the molecular docking studies supported the mechanism by which quercetin and ferulic acid inhibit bacteria, revealing their strong binding affinities to key bacterial enzymes. The therapeutic potential of these phenolic acids was emphasised further by their favourable ADME profiles, which featured high predicted gastrointestinal absorption and acceptable drug-likeness scores.
While FHD was less effective against S. typhimurium (MIC: 25%) and P. aeruginosa (MIC: 25%), OHD demonstrated significant selective efficacy against these two bacteria despite having lower antibacterial activity. This could be because it contains more resveratrol, a stilbenoid compound that inhibits biofilms, modulates the efflux pump, and inhibits quorum sensing in Gram-negative bacteria. This function is further supported by resveratrol’s molecular docking scores against membrane-associated targets. Resveratrol maintains its pharmacological significance despite having a lower oral bioavailability in its ADME profile because it can alter bacterial virulence pathways instead of directly causing cytotoxicity.
Both honeys demonstrated negative or insignificant BIP% values at lower concentrations (e.g., 4–6%), especially against B. cereus, C. perfringens, S. flexneri, and P. aeruginosa. This could reflect not only bacterial resistance mechanisms but also potential antagonistic interactions among honey constituents at subinhibitory doses. It is possible that certain phenolics or sugars may temporarily serve as nutrient sources for some bacteria when present below inhibitory thresholds.
The significant antimicrobial superiority of FHD against most tested strains indicates its potential as a natural antibacterial agent, primarily attributed to its polyphenol-rich composition and positive pharmacokinetic predictions. While OHD shows lower direct inhibition potency, it exhibits selective effectiveness against specific Gram-negative pathogens, suggesting that it may be worthy of further investigation for anti-virulence or supportive applications. These results emphasise the importance of combining phytochemical analysis, microbiological tests, and in silico modelling to better understand the therapeutic potential of complex natural products such as honeydew honeys.
4. Materials and Methods
4.1. The Preparation of Extracts
Two varieties of honeydew were taken into the study: oak honeydew honey (OHD) and fir honeydew honey (FHD). The samples were sourced from approved local producers in Sibiu (SC Apilife Ro SRL, Sibiu, Romania).
The choice of 70% ethanol was based on our previous studies [3], demonstrating its efficacy as a solvent for polyphenolic extraction from natural matrices, including honey and plant-based materials. Ethanol-water mixtures in the range of 60–80% are frequently reported to provide optimal extraction efficiency for a broad spectrum of phenolic compounds, balancing polarity to maximise both flavonoid and phenolic acid solubility.
Therefore, 1 g of each honey was mixed with 10 mL of 70% alcohol and vortexed for 15 min. The extraction was then continued on a hot plate stirrer (IDL, Freising, Germany) for 45 min at room temperature. After extraction, the samples were filtered and stored in a refrigerator at 4 °C for future analyses.
The extracts were filtered using 0.45 µm PTFE syringe filters (Sigma-Aldrich; Merck KGaA, Darmstadt, Germany), each with a diameter of 25 mm.
4.2. Chemicals
Sigma–Aldrich Chemie Gmbh (München, Germany) provided the reagents ethanol, Folin–Ciocalteu, gallic acid and quercetin standard, and 1,1-diphenyl-2-picrylhydrazyl (DPPH), while Geyer Gmbh (Renningen, Germany) provided the sodium carbonate, sodium nitrite, and aluminium chloride. For the chemical analysis, every reagent used was of analytical quality. Ascorbic acid was obtained from Lach-Ner Company (Neratovice, Czech Republic). All HPLC standards (Sigma-Aldrich, Merck KGaA, Darmstadt, Germany) and methanol (Merck KGaA, Darmstadt, Germany) were analytical-grade chemicals.
4.3. Determination of Total Phenolic Content (TPC)
The total polyphenol content was determined using the method described by Hulea et al. [3] with minor modifications. Briefly, 0.5 mL of each extract was mixed with 1.25 mL of Folin–Ciocalteu reagent and incubated at room temperature for 5 min; then 1 mL of 6% Na2CO3 was added. The mixture was vigorously stirred and incubated for 2 h at room temperature.
The absorbance values were measured at 750 nm using a Specord 205 UV-VIS spectrophotometer (Analytik Jena AG, Jena, Germany). The calibration curve was established using gallic acid as the standard, with the results expressed in mg GAE/Kg. Each sample was analysed in triplicate, and the results were reported as the mean ± standard deviation.
4.4. Determination of Total Flavonoid Content (TFC)
Our previous studies describe the method used to determine the total flavonoid content [4,79] with minor modifications as follows: a mixture of 1 mL of each extract, 0.3 mL of 10% AlCl3 and 0.3 mL of 5% NaNO2 was incubated at room temperature for 6 min. After this period, 2 mL of 1M NaOH and 6.4 mL of 70% ethanol were added. The mixture was vigorously shaken and incubated at room temperature for 30 min.
Absorbance values were measured at 415 nm using a Specord 205 UV-VIS spectrophotometer (Analytik Jena AG, Jena, Germany). The calibration curve was constructed using quercetin as the standard, with the measurement unit expressed as mg QUE/Kg.
Each sample was analysed in triplicate, and the results were reported as the mean ± standard deviation.
4.5. Individual Identification of Polyphenols by LC-MS Analysis
Determination of individual polyphenols by LC-MS analysis was performed using a Shimadzu chromatograph (Shimadzu 2010 EV, Kyoto, Japan) equipped with an SPD-10A UV detector, and MS detector (Shimadzu, Kyoto, Japan) EC 150/2 NUCLEODUR C18 Gravity SB 150 × 2 mm × 5 μm column (Macherey-Nagel Gmbh & Co., KG, Dueren, Germany).
Chromatographic conditions included mobile phases A—acidified water with formic acid (pH 3) (Merck KGaA, Darmstadt, Germany), and B—acetonitrile (Merck KGaA, Darmstadt, Germany) acidified with formic acid (pH 3). The gradient programme consisted of 0.01–20 min 5% B, 20.01–50 min 5–40% B, 40–55 min 40–95% B, and 55–60 min 95% B. The flow rate of solvent is 0.2 mL/min at a temperature of 20 °C. The wavelengths selected for monitoring were 280 nm and 320 nm. The calibration curves were made between 20 and 50 μg/mL. Calibration curves for each analysed compounds are as follows: f(x) = 6.04661e − 105x − 3.04827 (gallic acid), f(x) = 7.36054e − 106x + 3.55894 (caffeic acid), f(x) = 3.43873e − 105x + 4.99711 (epicatechin), f(x) = 1.45563e − 105x + 3.75512 (Beta − rezorcilic acid), f(x) = 4.34018e − 106x + 3.48454 (cumaric acid), f(x) = 8.1887e − 106x + 3.79724 (ferulic acid), f(x) = 2.21544e − 105x + 1.3627 (rosmarinic acid), f(x) = 4.37174e − 105x + 1.3537 (resveratrol), and f(x) = 1.3719e − 105x + 3.97487 (quercetin). Analytical-grade chemicals were used for all reagents and solvents. Each sample was tested in triplicate. The MS signals and chromatograms are presented in the (Supplementary Material Figure S1).
4.6. DPPH Assay Antioxidant Capacity
The antioxidant activity was determined using the DPPH radical scavenging assay, following a standardised protocol adapted from previously reported methods [80,81,82] optimised for the characteristics of honey extracts. Briefly, to determine the free radical inhibition capacity, a series of dilutions were performed on the samples, obtaining the following final concentrations: 100 mg/mL, 40 mg/mL, 25 mg/mL, 16.67 mg/mL, and 12.5 mg/mL. A total of 1 mL of the diluted samples was mixed with 2.5 mL of 0.3 mM DPPH reagent, shaken, and left in the dark for 30 min. As a control, a sample was prepared in which the extract volume was replaced with 70% ethanol. In parallel, analyses were performed on five ascorbic acid samples (0.02–0.1 mg/mL), serving as a positive control. Absorbance values were measured at 518 nm using a Specord 205 UV-VIS (Analytik Jena AG, Jena, Germany) spectrophotometer. Each sample was analysed in triplicate, and the results were reported as the mean ± standard deviation. The results were obtained using the following formula:
where Ac = absorbance value of the control sample,
As = absorbance value of the extract sample.
The IC50 value, representing the antioxidant capacity, was compared to that of ascorbic acid.
4.7. Antimicrobial Activity
The ATCC strains taken into study consisted of S. pyogenes (ATCC 19615), S. aureus (ATCC 25923), L. monocytogenes (ATCC 19114), C. perfringens (ATCC 13124), B. cereus (ATCC 10876), H. influenzae type B (ATCC 10211), S. typhimurium (ATCC 14028), E. coli (ATCC 25922), P. aeruginosa (ATCC 27853), and S. flexneri (ATCC 12022).
A 10−3 dilution (0.5 McFarland standard) of the fresh culture in Brain Heart Infusion (BHI) broth (Oxoid, CM1135) was used to test the antimicrobial activity of the honey extracts, as our previous study describes [79,83,84]. The extracts were added directly to the bacterial strain suspensions in different concentrations (2%, 4%, 8%, 10%, 15%, 20%, 25%, or 30%). The concentrations tested were chosen based on previous research and a literature review to cover a wide spectrum of concentrations and identify possible MIC values [3,4,32,35,37,38,83].
The MIC, defined as the lowest compound concentration that yields no visible microorganism growth, was determined by the measurement of optical density (OD) using the spectrophotometric method. The concentrations used to determine the minimum inhibitory concentration (MIC) were as follows: 2%, 4%, 8%, 10%, 15%, 20%, 25%, and 30% (v/v) of honey extract in Brain Heart Infusion (BHI) broth. These values were selected based on prior literature and preliminary screening to ensure an appropriate range that would capture the inhibition threshold for each bacterial strain.
Two indicators, BGP (bacterial growth percentage) and BIP (bacterial inhibition percentage), were calculated for interpreting the results, based on the following formulas:
4.8. Molecular Docking
Molecular docking involving the ten (10) identified compounds by LCMS and seven (7) antimicrobial protein targets was performed with PyRx-virtual screening tool version 0.8 which had the AutoDock Vina algorithm and the empirical free energy scoring function. The 3d structure of the compounds was retrieved [85] in .sdf format and prepared as a ligand in .pdbqt saved format. The .pdb format of the bacterial target proteins (PDB ID: 1DM0, 1I8D, 2PP6, 4H8E, 4R7R, 5HU4, 5JKP) was downloaded from the protein data bank [86]. The proteins defined as macromolecules were all prepared for docking in .pdbqt format. The ligands were screened against all the proteins in a blind docking cubic grid coverage. The binding interactions between the ligands and the proteins were visualised in BIOVIA Discovery Studio v21.1.0.20298, while the binding affinities of the lowest energy score were collected in .csv format.
4.9. ADMET (Absorption, Distribution, Metabolism, Excretion, Toxicity) Properties
The drug-likeliness and pharmacokinetic properties of the hit compounds from the docking scores were subjected to the swissADME [87] prognosis to ascertain predictors of in vivo utilisation and for the pharmacokinetic attributes [88]. The canonical SMILES of the hits were recovered from PubChem [85].
4.10. Statistical Analysis
In case of the spectrophotometric analyses, the statistical analysis was performed using JASP version 0.19.3.0. A one-way ANOVA was applied to identify correlations between the evaluated parameters. Differences between the average values of the examined characteristics were tested for significance using the Tukey post hoc test, with a significance level set at p = 0.05.
5. Conclusions
This study offers an extensive analysis of Romanian oak (OHD) and fir (FHD) honeydew honeys, incorporating polyphenolic profiling, antibacterial efficacy testing, molecular docking, and ADME assessment. FHD exhibited notably higher total phenolic and flavonoid content, correlating with improved antioxidant and antibacterial properties. In vitro antimicrobial tests indicated a stronger inhibitory effect of FHD, especially against Gram-positive strains like S. aureus, S. pyogenes, and L. monocytogenes, alongside certain Gram-negative strains such as E. coli and H. influenzae. Fir honeydew honey exhibited superior and broader-spectrum antimicrobial activity, achieving lower MICs and higher BIP% values across most tested bacterial strains. In contrast, oak honeydew honey showed weaker and more variable activity, with limited efficacy, particularly against resistant Gram-negative strains such as P. aeruginosa and S. typhimurium. Molecular docking simulations reinforced the antibacterial results, with significant compounds like ferulic acid, quercetin, rosmarinic acid, and rutin showing strong binding affinities to bacterial target proteins linked to pathogenicity. ADME predictions affirmed the therapeutic advantages of several polyphenols, primarily ferulic acid and quercetin, exhibiting promising drug-likeness and absorption features. These results promote the pharmacological promise of honeydew honeys, notably FHD, as natural antimicrobial agents. The integration of chemical, biological, and computational techniques provides valuable insights into the mechanisms of their bioactivity, supporting further exploration for food preservation, pharmaceutical formulations, or complementary therapies for bacterial infections.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/antibiotics14060592/s1, Figure S1: A. Chromatogram and m/z signal for FHD sample; B. Chromatogram and m/z signal for OHD sample.
Author Contributions
Conceptualization, C.H., D.O., A.H., M.A.S., D.F., E.A., I.M.I., A.-G.N., M.P., C.A.P. and F.I.; methodology, C.H., D.O., A.H., M.A.S., D.F., E.A., I.M.I., A.-G.N., M.P., C.A.P. and F.I.; software C.H., D.O., A.H., M.A.S., D.F., E.A. and F.I.; validation, C.H., D.O., A.H., M.A.S., D.F. and F.I.; formal analysis, C.H., D.O., A.H., M.A.S., D.F., E.A., I.M.I., A.-G.N., M.P., C.A.P. and F.I.; investigation, C.H., D.O., A.H., M.A.S., D.F., E.A., I.M.I., A.-G.N., M.P., C.A.P. and F.I. resources, C.A.P. and F.I.; data curation, C.H., D.O., A.H., M.A.S., D.F. and F.I.; writing—original draft preparation, C.H., D.O., A.H., M.A.S., D.F., E.A., I.M.I., A.-G.N., M.P., C.A.P. and F.I.; writing—review and editing, C.H., D.O., A.H., M.A.S., D.F., E.A., I.M.I., A.-G.N., M.P., C.A.P. and F.I.; visualisation, C.H., D.O., A.H., M.A.S., D.F., E.A., I.M.I., A.-G.N., M.P., C.A.P. and F.I.; supervision, C.H., D.O., A.H., M.A.S., D.F. and F.I.; project administration, C.H., D.O., A.H., M.A.S., D.F. and F.I.; funding acquisition, C.A.P. and F.I. All authors have read and agreed to the published version of the manuscript.
Funding
The publication of the present paper was supported by the University of Life Sciences “King Mihai I” from Timisoara, Romania.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors on request.
Acknowledgments
We have carried out this study with the support of the Interdisciplinary Research Platform belonging to the King Michael I University of Life Sciences, Timisoara.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Ranneh, Y.; Akim, A.M.; Hamid, H.A.; Khazaai, H.; Fadel, A.; Zakaria, Z.A.; Albujja, M.; Bakar, M.F.A. Honey and its nutritional and anti-inflammatory value. BMC Complement. Med. Ther. 2021, 21, 30. [Google Scholar] [CrossRef] [PubMed]
- Stavropoulou, E.; Ieronymaki, E.; Dimitroulia, E.; Constantinidis, T.C.; Vrioni, G.; Tsatsanis, C.; Tsakris, A. Anti-Inflammatory and Antibacterial Effects and Mode of Action of Greek Arbutus, Chestnut, and Fir Honey in Mouse Models of Inflammation and Sepsis. Microorganisms 2022, 10, 2374. [Google Scholar] [CrossRef] [PubMed]
- Hulea, A.; Obiștioiu, D.; Cocan, I.; Alexa, E.; Negrea, M.; Neacșu, A.-G.; Hulea, C.; Pascu, C.; Costinar, L.; Iancu, I.; et al. Diversity of Monofloral Honey Based on the Antimicrobial and Antioxidant Potential. Antibiotics 2022, 11, 595. [Google Scholar] [CrossRef]
- Pătruică, S.; Alexa, E.; Obiștioiu, D.; Cocan, I.; Radulov, I.; Berbecea, A.; Lazăr, R.N.; Simiz, E.; Vicar, N.M.; Hulea, A.; et al. Chemical Composition, Antioxidant and Antimicrobial Activity of Some Types of Honey from Banat Region, Romania. Molecules 2022, 27, 4179. [Google Scholar] [CrossRef]
- Vică, M.L.; Glevitzky, M.; Dumitrel, G.-A.; Bostan, R.; Matei, H.V.; Kartalska, Y.; Popa, M. Qualitative Characterization and Antifungal Activity of Romanian Honey and Propolis. Antibiotics 2022, 11, 1552. [Google Scholar] [CrossRef]
- Al-Hatamleh, M.A.I.; Hatmal, M.M.; Sattar, K.; Ahmad, S.; Mustafa, M.Z.; Bittencourt, M.D.C.; Mohamud, R. Antiviral and Immunomodulatory Effects of Phytochemicals from Honey against COVID-19: Potential Mechanisms of Action and Future Directions. Molecules 2020, 25, 5017. [Google Scholar] [CrossRef]
- Hasam, S.; Qarizada, D.; Azizi, M. A Review: Honey and Its Nutritional Composition. Asian J. Res. Biochem. 2020, 7, 34–43. [Google Scholar] [CrossRef]
- Gemechu, F.G. Embracing nutritional qualities, biological activities and technological properties of coffee byproducts in functional food formulation. Trends Food Sci. Technol. 2020, 104, 235–261. [Google Scholar] [CrossRef]
- Bora, F.D.; Andrecan, A.F.; Călugăr, A.; Bunea, C.I.; Popescu, M.; Petrescu-Mag, I.V.; Bunea, A. Comprehensive Elemental Profiling of Romanian Honey: Exploring Regional Variance, Honey Types, and Analyzed Metals for Sustainable Apicultural and Environmental Practices. Foods 2024, 13, 1253. [Google Scholar] [CrossRef]
- Pop, I.M.; Simeanu, D.; Cucu-Man, S.-M.; Pui, A.; Albu, A. Quality Profile of Several Monofloral Romanian Honeys. Agriculture 2022, 13, 75. [Google Scholar] [CrossRef]
- Gündoğdu, E.; Çakmakçı, S.; Şat, İ.G. An Overview of Honey: Its Composition, Nutritional and Functional Properties. J. Food Sci. Eng. 2019, 9, 10–14. [Google Scholar] [CrossRef]
- Almasaudi, S. The antibacterial activities of honey. Saudi J. Biol. Sci. 2021, 28, 2188–2196. [Google Scholar] [CrossRef] [PubMed]
- Majtan, J.; Bucekova, M.; Kafantaris, I.; Szweda, P.; Hammer, K.; Mossialos, D. Honey antibacterial activity: A neglected aspect of honey quality assurance as functional food. Trends Food Sci. Technol. 2021, 118, 870–886. [Google Scholar] [CrossRef]
- Tafere, D.A. Chemical composition and uses of Honey: A Review. J. Food Sci. Nutr. Res. 2021, 4, 194–201. [Google Scholar] [CrossRef]
- Mohammed, M.E.A. Factors Affecting the Physicochemical Properties and Chemical Composition of Bee’s Honey. Food Rev. Int. 2022, 38, 1330–1341. [Google Scholar] [CrossRef]
- Ben Amor, S.; Mekious, S.; Allal Benfekih, L.; Abdellattif, M.H.; Boussebaa, W.; Almalki, F.A.; Ben Hadda, T.; Kawsar, S.M.A. Phytochemical Characterization and Bioactivity of Different Honey Samples Collected in the Pre-Saharan Region in Algeria. Life 2022, 12, 927. [Google Scholar] [CrossRef]
- Tomczyk, M.; Tarapatskyy, M.; Dżugan, M. The influence of geographical origin on honey composition studied by Polish and Slovak honeys. Czech J. Food Sci. 2019, 37, 232–238. [Google Scholar] [CrossRef]
- Becerril-Sánchez, A.L.; Quintero-Salazar, B.; Dublán-García, O.; Escalona-Buendía, H.B. Phenolic Compounds in Honey and Their Relationship with Antioxidant Activity, Botanical Origin, and Color. Antioxidants 2021, 10, 1700. [Google Scholar] [CrossRef]
- Bonsignore, G.; Martinotti, S.; Ranzato, E. Honey Bioactive Molecules: There Is a World Beyond the Sugars. BioTech 2024, 13, 47. [Google Scholar] [CrossRef]
- Zammit Young, G.-W.; Blundell, R. A review on the phytochemical composition and health applications of honey. Heliyon 2023, 9, e12507. [Google Scholar] [CrossRef]
- Ahmed, S.K.; Hussein, S.; Qurbani, K.; Ibrahim, R.H.; Fareeq, A.; Mahmood, K.A.; Mohamed, M.G. Antimicrobial resistance: Impacts, challenges, and future prospects. J. Med. Surg. Public Health 2024, 2, 100081. [Google Scholar] [CrossRef]
- Shakoori, Z.; Salaseh, E.; Mehrabian, A.R.; Tehrani, D.M.; Dardashti, N.F.; Salmanpour, F. The amount of antioxidants in honey has a strong relationship with the plants selected by honey bees. Sci. Rep. 2024, 14, 351. [Google Scholar] [CrossRef]
- Majewska, E.; Drużyńska, B.; Derewiaka, D.; Ciecierska, M.; Pakosz, P. Comparison of Antioxidant Properties and Color of Selected Polish Honeys and Manuka Honey. Foods 2024, 13, 2666. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, S.; Sulaiman, S.A.; Baig, A.A.; Ibrahim, M.; Liaqat, S.; Fatima, S.; Jabeen, S.; Shamim, N.; Othman, N.H. Honey as a Potential Natural Antioxidant Medicine: An Insight into Its Molecular Mechanisms of Action. Oxid. Med. Cell. Longev. 2018, 2018, 8367846. [Google Scholar] [CrossRef]
- Bozkuş, T.N. Correlation of Total Polyphenol and Flavonoid Content with Antioxidant Activity of Caucasian Bee Honey. Food Sci. Nutr. 2025, 13, e4611. [Google Scholar] [CrossRef] [PubMed]
- Kačániová, M.; Borotová, P.; Galovičová, L.; Kunová, S.; Štefániková, J.; Kowalczewski, P.Ł.; Šedík, P. Antimicrobial and Antioxidant Activity of Different Honey Samples from Beekeepers and Commercial Producers. Antibiotics 2022, 11, 1163. [Google Scholar] [CrossRef]
- Halagarda, M.; Groth, S.; Popek, S.; Rohn, S.; Pedan, V. Antioxidant Activity and Phenolic Profile of Selected Organic and Conventional Honeys from Poland. Antioxidants 2020, 9, 44. [Google Scholar] [CrossRef] [PubMed]
- Çobanoğlu, D.N. Assessing monofloral bee pollens from Türkiye: Palynological verification, phenolic profile, and antioxidant activity. J. Food Sci. 2024, 89, 1711–1726. [Google Scholar] [CrossRef]
- Qi, N.; Zhao, W.; Xue, C.; Zhang, L.; Hu, H.; Jin, Y.; Xue, X.; Chen, R.; Zhang, J. Phenolic Acid and Flavonoid Content Analysis with Antioxidant Activity Assessment in Chinese C. pi. Shen Honey. Molecules 2025, 30, 370. [Google Scholar] [CrossRef]
- Mushtaq, S.; Imtiyaz, Z.; Wali, A.F.; Khan, A.; Rashid, S.M.; Amin, I.; Ali, A.; Rehman, M.U.; Arafah, A. Honey: A Powerful Natural Antioxidant and Its Possible Mechanism of Action. In Therapeutic Applications of Honey and Its Phytochemicals; Rehman, M.U., Majid, S., Eds.; Springer: Singapore, 2020; pp. 11–29. ISBN 9789811567988. [Google Scholar]
- Morroni, G.; Alvarez-Suarez, J.M.; Brenciani, A.; Simoni, S.; Fioriti, S.; Pugnaloni, A.; Giampieri, F.; Mazzoni, L.; Gasparrini, M.; Marini, E.; et al. Comparison of the Antimicrobial Activities of Four Honeys From Three Countries (New Zealand, Cuba, and Kenya). Front. Microbiol. 2018, 9, 1378. [Google Scholar] [CrossRef]
- Mama, M.; Teshome, T.; Detamo, J. Antibacterial Activity of Honey against Methicillin-Resistant Staphylococcus aureus: A Laboratory-Based Experimental Study. Int. J. Microbiol. 2019, 2019, 7686130. [Google Scholar] [CrossRef] [PubMed]
- Feknous, N.; Boumendjel, M. Natural bioactive compounds of honey and their antimicrobial activity. Czech J. Food Sci. 2022, 40, 163–178. [Google Scholar] [CrossRef]
- Nolan, V.C.; Harrison, J.; Cox, J.A.G. Dissecting the Antimicrobial Composition of Honey. Antibiotics 2019, 8, 251. [Google Scholar] [CrossRef]
- Al-Sayaghi, A.M.; Al-Kabsi, A.M.; Abduh, M.S.; Saghir, S.A.M.; Alshawsh, M.A. Antibacterial Mechanism of Action of Two Types of Honey against Escherichia coli through Interfering with Bacterial Membrane Permeability, Inhibiting Proteins, and Inducing Bacterial DNA Damage. Antibiotics 2022, 11, 1182. [Google Scholar] [CrossRef]
- Mirzaei, A.; Karamolah, K.S.; Mahnaie, M.P.; Mousavi, F.; Moghadam, P.M.; Mahmoudi, H. Antibacterial Activity of Honey against Methicillin-Resistant and Sensitive Staphylococcus Aureus Isolated from Patients with Diabetic Foot Ulcer. Open Microbiol. J. 2020, 14, 260–265. [Google Scholar] [CrossRef]
- Luca, L.; Pauliuc, D.; Ursachi, F.; Oroian, M. Physicochemical parameters, microbiological quality, and antibacterial activity of honey from the Bucovina region of Romania. Sci. Rep. 2025, 15, 4358. [Google Scholar] [CrossRef] [PubMed]
- Nagy-Radványi, L.; Balázs, V.L.; Kocsis, B.; Csikós, E.; Ángyán, V.D.; Szabó, P.; Biró, V.; Kocsis, M.; Farkas, Á. Antibacterial activity of Hungarian varietal honeys against respiratory pathogens as a function of storage time. Sci. Rep. 2024, 14, 10200. [Google Scholar] [CrossRef]
- Dörr, T.; Moynihan, P.J.; Mayer, C. Editorial: Bacterial Cell Wall Structure and Dynamics. Front. Microbiol. 2019, 10, 2051. [Google Scholar] [CrossRef]
- Fyfe, L.; Okoro, P.; Paterson, E.; Coyle, S.; McDougall, G.J. Compositional analysis of Scottish honeys with antimicrobial activity against antibiotic-resistant bacteria reveals novel antimicrobial components. LWT Food Sci. Technol. 2017, 79, 52–59. [Google Scholar] [CrossRef]
- Zhang, Y.-Z.; Si, J.-J.; Li, S.-S.; Zhang, G.-Z.; Wang, S.; Zheng, H.-Q.; Hu, F.-L. Chemical Analyses and Antimicrobial Activity of Nine Kinds of Unifloral Chinese Honeys Compared to Manuka Honey (12+ and 20+). Molecules 2021, 26, 2778. [Google Scholar] [CrossRef]
- Combarros-Fuertes, P.; Fresno, J.M.; Estevinho, M.M.; Sousa-Pimenta, M.; Tornadijo, M.E.; Estevinho, L.M. Honey: Another Alternative in the Fight against Antibiotic-Resistant Bacteria? Antibiotics 2020, 9, 774. [Google Scholar] [CrossRef] [PubMed]
- Muteeb, G.; Rehman, M.T.; Shahwan, M.; Aatif, M. Origin of Antibiotics and Antibiotic Resistance, and Their Impacts on Drug Development: A Narrative Review. Pharmaceuticals 2023, 16, 1615. [Google Scholar] [CrossRef]
- Pulingam, T.; Parumasivam, T.; Gazzali, A.M.; Sulaiman, A.M.; Chee, J.Y.; Lakshmanan, M.; Chin, C.F.; Sudesh, K. Antimicrobial resistance: Prevalence, economic burden, mechanisms of resistance and strategies to overcome. Eur. J. Pharm. Sci. 2022, 170, 106103. [Google Scholar] [CrossRef] [PubMed]
- Javed, M.U.; Hayat, M.T.; Mukhtar, H.; Imre, K. CRISPR-Cas9 System: A Prospective Pathway toward Combatting Antibiotic Resistance. Antibiotics 2023, 12, 1075. [Google Scholar] [CrossRef]
- Al-Kafaween, M.A.; Alwahsh, M.; Mohd Hilmi, A.B.; Abulebdah, D.H. Physicochemical Characteristics and Bioactive Compounds of Different Types of Honey and Their Biological and Therapeutic Properties: A Comprehensive Review. Antibiotics 2023, 12, 337. [Google Scholar] [CrossRef]
- Isopescu, R.D.; Josceanu, A.M.; Colta, T.; Spulber, R. Romanian Honey: Characterization and Classification. In Honey Analysis; Toledo, V.D.A.A.D., Ed.; InTech: Rijeka, Croatia, 2017; ISBN 978-953-51-2879-3. [Google Scholar]
- Albu, A.; Radu-Rusu, C.-G.; Pop, I.M.; Frunza, G.; Nacu, G. Quality Assessment of Raw Honey Issued from Eastern Romania. Agriculture 2021, 11, 247. [Google Scholar] [CrossRef]
- Ioniță-Mîndrican, C.-B.; Mititelu, M.; Musuc, A.M.; Oprea, E.; Ziani, K.; Neacșu, S.M.; Grigore, N.D.; Negrei, C.; Dumitrescu, D.-E.; Mireșan, H.; et al. Honey and Other Beekeeping Products Intake among the Romanian Population and Their Therapeutic Use. Appl. Sci. 2022, 12, 9649. [Google Scholar] [CrossRef]
- Rameshk, M.; Khoshbin, E.; Moeinzadeh, M.; Sharififar, K.; Bahrami, D.; Sharififar, F. Mannas, unique products of a dynamic insect-plant interaction: Biodiversity, conservation and ethnopharmacological considerations. Heliyon 2023, 9, e22976. [Google Scholar] [CrossRef] [PubMed]
- Vică, M.L.; Glevitzky, M.; Tit, D.M.; Behl, T.; Heghedűş-Mîndru, R.C.; Zaha, D.C.; Ursu, F.; Popa, M.; Glevitzky, I.; Bungău, S. The antimicrobial activity of honey and propolis extracts from the central region of Romania. Food Biosci. 2021, 41, 101014. [Google Scholar] [CrossRef]
- Barcellos, M.P.; Santos, C.B.R.; Federico, L.B.; Almeida, P.F.D.; Da Silva, C.H.T.D.P.; Taft, C.A. Pharmacophore and structure-based drug design, molecular dynamics and admet/tox studies to design novel potential pad4 inhibitors. J. Biomol. Struct. Dyn. 2019, 37, 966–981. [Google Scholar] [CrossRef]
- Prava, J.; Pranavathiyani, G.; Pan, A. Functional assignment for essential hypothetical proteins of Staphylococcus aureus N315. Int. J. Biol. Macromol. 2018, 108, 765–774. [Google Scholar] [CrossRef] [PubMed]
- Fraser, M.E.; Chernaia, M.M.; Kozlov, Y.V.; James, M.N.G. Crystal structure of the holotoxino from Shigella dysenteriae at 2.5 Å resolution. Nat. Struct. Mol. Biol. 1994, 1, 59–64. [Google Scholar] [CrossRef] [PubMed]
- Liao, D.-I.; Wawrzak, Z.; Calabrese, J.C.; Viitanen, P.V.; Jordan, D.B. Crystal Structure of Riboflavin Synthase. Structure 2001, 9, 399–408. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Chen, Y.; Zhang, B.; Niu, X.; Song, M.; Luo, Z.; Lu, G.; Liu, B.; Zhao, X.; Wang, J.; et al. Inhibition of sortase A by chalcone prevents Listeria monocytogenes infection. Biochem. Pharmacol. 2016, 106, 19–29. [Google Scholar] [CrossRef]
- Yang, X.; Li, Z.; She, Z.; Geng, Z.; Xu, J.; Gao, Z.; Dong, Y. Structural analysis of Pseudomonas aeruginosa H3-T6SS immunity proteins. FEBS Lett. 2016, 590, 2787–2796. [Google Scholar] [CrossRef]
- Can, Z.; Yildiz, O.; Sahin, H.; Akyuz Turumtay, E.; Silici, S.; Kolayli, S. An investigation of Turkish honeys: Their physico-chemical properties, antioxidant capacities and phenolic profiles. Food Chem. 2015, 180, 133–141. [Google Scholar] [CrossRef]
- Fernández-Estellé, M.; Hernández-González, V.; Saurina, J.; Núñez, O.; Sentellas, S. Characterization and Classification of Spanish Honeydew and Blossom Honeys Based on Their Antioxidant Capacity. Antioxidants 2023, 12, 495. [Google Scholar] [CrossRef]
- Halouzka, R.; Tarkowski, P.; Ćavar Zeljković, S. Characterisation of phenolics and other quality parameters of different types of honey. Czech J. Food Sci. 2016, 34, 244–253. [Google Scholar] [CrossRef]
- Koulis, G.A.; Tsagkaris, A.S.; Katsianou, P.A.; Gialouris, P.-L.P.; Martakos, I.; Stergiou, F.; Fiore, A.; Panagopoulou, E.I.; Karabournioti, S.; Baessmann, C.; et al. Thorough Investigation of the Phenolic Profile of Reputable Greek Honey Varieties: Varietal Discrimination and Floral Markers Identification Using Liquid Chromatography–High-Resolution Mass Spectrometry. Molecules 2022, 27, 4444. [Google Scholar] [CrossRef]
- Kováčik, J.; Grúz, J.; Biba, O.; Hedbavny, J. Content of metals and metabolites in honey originated from the vicinity of industrial town Košice (eastern Slovakia). Environ. Sci. Pollut. Res. 2016, 23, 4531–4540. [Google Scholar] [CrossRef]
- Seraglio, S.K.T.; Silva, B.; Bergamo, G.; Brugnerotto, P.; Gonzaga, L.V.; Fett, R.; Costa, A.C.O. An overview of physicochemical characteristics and health-promoting properties of honeydew honey. Food Res. Int. 2019, 119, 44–66. [Google Scholar] [CrossRef] [PubMed]
- Kuś, P.M.; Jerković, I.; Marijanović, Z.; Tuberoso, C.I.G. Screening of Polish Fir Honeydew Honey Using GC/MS, HPLC-DAD, and Physical-Chemical Parameters: Benzene Derivatives and Terpenes as Chemical Markers. Chem. Biodivers. 2017, 14, e1700179. [Google Scholar] [CrossRef] [PubMed]
- Jaśkiewicz, K.; Szczęsna, T.; Jachuła, J. How Phenolic Compounds Profile and Antioxidant Activity Depend on Botanical Origin of Honey—A Case of Polish Varietal Honeys. Molecules 2025, 30, 360. [Google Scholar] [CrossRef]
- Seijo, M.C.; Escuredo, O.; Rodríguez-Flores, M.S. Physicochemical Properties and Pollen Profile of Oak Honeydew and Evergreen Oak Honeydew Honeys from Spain: A Comparative Study. Foods 2019, 8, 126. [Google Scholar] [CrossRef]
- Vasić, V.; Gašić, U.; Stanković, D.; Lušić, D.; Vukić-Lušić, D.; Milojković-Opsenica, D.; Tešić, Ž.; Trifković, J. Towards better quality criteria of European honeydew honey: Phenolic profile and antioxidant capacity. Food Chem. 2019, 274, 629–641. [Google Scholar] [CrossRef]
- Pichichero, E.; Canuti, L.; Canini, A. Characterisation of the phenolic and flavonoid fractions and antioxidant power of Italian honeys of different botanical origin. J. Sci. Food Agric. 2009, 89, 609–616. [Google Scholar] [CrossRef]
- Seraglio, S.K.T.; Valese, A.C.; Daguer, H.; Bergamo, G.; Azevedo, M.S.; Gonzaga, L.V.; Fett, R.; Costa, A.C.O. Development and validation of a LC-ESI-MS/MS method for the determination of phenolic compounds in honeydew honeys with the diluted-and-shoot approach. Food Res. Int. 2016, 87, 60–67. [Google Scholar] [CrossRef]
- Tuberoso, C.I.G.; Jerković, I.; Bifulco, E.; Marijanović, Z. Biodiversity of Salix spp. Honeydew and Nectar Honeys Determined by RP-HPLC and Evaluation of Their Antioxidant Capacity. Chem. Biodivers. 2011, 8, 872–879. [Google Scholar] [CrossRef]
- Oroian, M.; Ropciuc, S.; Buculei, A.; Paduret, S.; Todosi, E. Phenolic Profile of Honeydew Honeys from the North-East Part Of Romania. Bull. Univ. Agric. Sci. Vet. Med. Cluj-Napoca Food Sci. Technol. 2016, 73, 105. [Google Scholar] [CrossRef][Green Version]
- Hernanz, D.; Jara-Palacios, M.J.; Santos, J.L.; Gómez Pajuelo, A.; Heredia, F.J.; Terrab, A. The profile of phenolic compounds by HPLC-MS in Spanish oak (Quercus) honeydew honey and their relationships with color and antioxidant activity. LWT 2023, 180, 114724. [Google Scholar] [CrossRef]
- Dżugan, M.; Ciszkowicz, E.; Tomczyk, M.; Miłek, M.; Lecka-Szlachta, K. Coniferous Honeydew Honey: Antibacterial Activity and Anti-Migration Properties against Breast Cancer Cell Line (MCF-7). Appl. Sci. 2024, 14, 710. [Google Scholar] [CrossRef]
- Grabek-Lejko, D.; Miłek, M.; Dżugan, M. The comparison of the antioxidant, antibacterial and antiviral potential of Polish fir honeydew and Manuka honeys. Sci. Rep. 2024, 14, 31170. [Google Scholar] [CrossRef] [PubMed]
- Broznić, D.; Ratkaj, I.; Malenica Staver, M.; Kraljević Pavelić, S.; Žurga, P.; Bubalo, D.; Gobin, I. Evaluation of the Antioxidant Capacity, Antimicrobial and Antiproliferative Potential of Fir (Abies alba Mill.) Honeydew Honey Collected from Gorski kotar (Croatia). Food Technol. Biotechnol. 2018, 56, 533–545. [Google Scholar] [CrossRef]
- Jokovic, N.; Jovanović, N.; Matejić, J.; Stojanović-Radić, Z.; Vitorović, J. Physicochemical properties, antioxidant and antimicrobial activity of seven honey samples collected in the Nišava district. Biol. Nyssana 2025, 16, 1–10. [Google Scholar] [CrossRef]
- Vaou, N.; Stavropoulou, E.; Voidarou, C.; Tsigalou, C.; Bezirtzoglou, E. Towards Advances in Medicinal Plant Antimicrobial Activity: A Review Study on Challenges and Future Perspectives. Microorganisms 2021, 9, 2041. [Google Scholar] [CrossRef]
- Maalik, A.; Khan, F.; Mumtaz, A.; Mehmood, A.; Azhar, S.; Atif, M.; Karim, S.; Altaf, Y.; Tariq, I. Pharmacological Applications of Quercetin and its Derivatives: A Short Review. Trop. J. Pharm. Res. 2014, 13, 1561. [Google Scholar] [CrossRef]
- Pătruică, S.; Adeiza, S.M.; Hulea, A.; Alexa, E.; Cocan, I.; Moraru, D.; Imbrea, I.; Floares, D.; Pet, I.; Imbrea, F.; et al. Romanian Bee Product Analysis: Chemical Composition, Antimicrobial Activity, and Molecular Docking Insights. Foods 2024, 13, 1455. [Google Scholar] [CrossRef]
- Beicu, R.; Alexa, E.; Obiștioiu, D.; Cocan, I.; Imbrea, F.; Pop, G.; Circioban, D.; Moisa, C.; Lupitu, A.; Copolovici, L.; et al. Antimicrobial Potential and Phytochemical Profile of Wild and Cultivated Populations of Thyme (Thymus sp.) Growing in Western Romania. Plants 2021, 10, 1833. [Google Scholar] [CrossRef] [PubMed]
- Cadariu, A.I.; Cocan, I.; Negrea, M.; Alexa, E.; Obistioiu, D.; Hotea, I.; Radulov, I.; Poiana, M.-A. Exploring the Potential of Tomato Processing Byproduct as a Natural Antioxidant in Reformulated Nitrite-Free Sausages. Sustainability 2022, 14, 11802. [Google Scholar] [CrossRef]
- Dumbrava, D.; Popescu, L.A.; Soica, C.M.; Nicolin, A.; Cocan, I.; Negrea, M.; Alexa, E.; Obistioiu, D.; Radulov, I.; Popescu, S.; et al. Nutritional, Antioxidant, Antimicrobial, and Toxicological Profile of Two Innovative Types of Vegan, Sugar-Free Chocolate. Foods 2020, 9, 1844. [Google Scholar] [CrossRef]
- Dégi, J.; Herman, V.; Igna, V.; Dégi, D.M.; Hulea, A.; Muselin, F.; Cristina, R.T. Antibacterial Activity of Romanian Propolis against Staphylococcus aureus Isolated from Dogs with Superficial Pyoderma: In Vitro Test. Vet. Sci. 2022, 9, 299. [Google Scholar] [CrossRef] [PubMed]
- Bălașoiu (Jigău), R.A.C.; Obistioiu, D.; Hulea, A.; Suleiman, M.A.; Popescu, I.; Floares (Oarga), D.; Imbrea, I.M.; Neacșu, A.-G.; Șmuleac, L.; Pașcalău, R.; et al. Analysing the Antibacterial Synergistic Interactions of Romanian Lavender Essential Oils via Gas Chromatography–Mass Spectrometry: In Vitro and In Silico Approaches. Plants 2024, 13, 2136. [Google Scholar] [CrossRef] [PubMed]
- Available online: https://pubchem.ncbi.nlm.nih.gov/ (accessed on 11 March 2025).
- Available online: www.rcsb.org (accessed on 15 March 2025).
- Available online: www.swissadme.ch (accessed on 21 April 2025).
- Available online: http://biosig.unimelb.edu.au/pkcsm/prediction (accessed on 22 April 2025).
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).