Impact of Recovery from Febrile Neutropenia on Intra-Individual Variability in Vancomycin Pharmacokinetics in Pediatric Patients
Abstract
1. Introduction
2. Results
2.1. Patient Characteristics
2.2. Correlation Between CLvcm and Clinical Laboratory Parameters at All Data Points
2.3. Identification of Factors Associated with CLvcm by Multivariate Analysis
2.4. Intra-Individual Comparison of CLvcm Between FN and Non-FN States
2.5. Correlation Between Intra-Individual Changes in CLvcm and in Laboratory Parameters
2.6. Identification of Factors Associated with Intra-Individual Δ CLvcm by Multivariate Analysis
3. Discussion
4. Materials and Methods
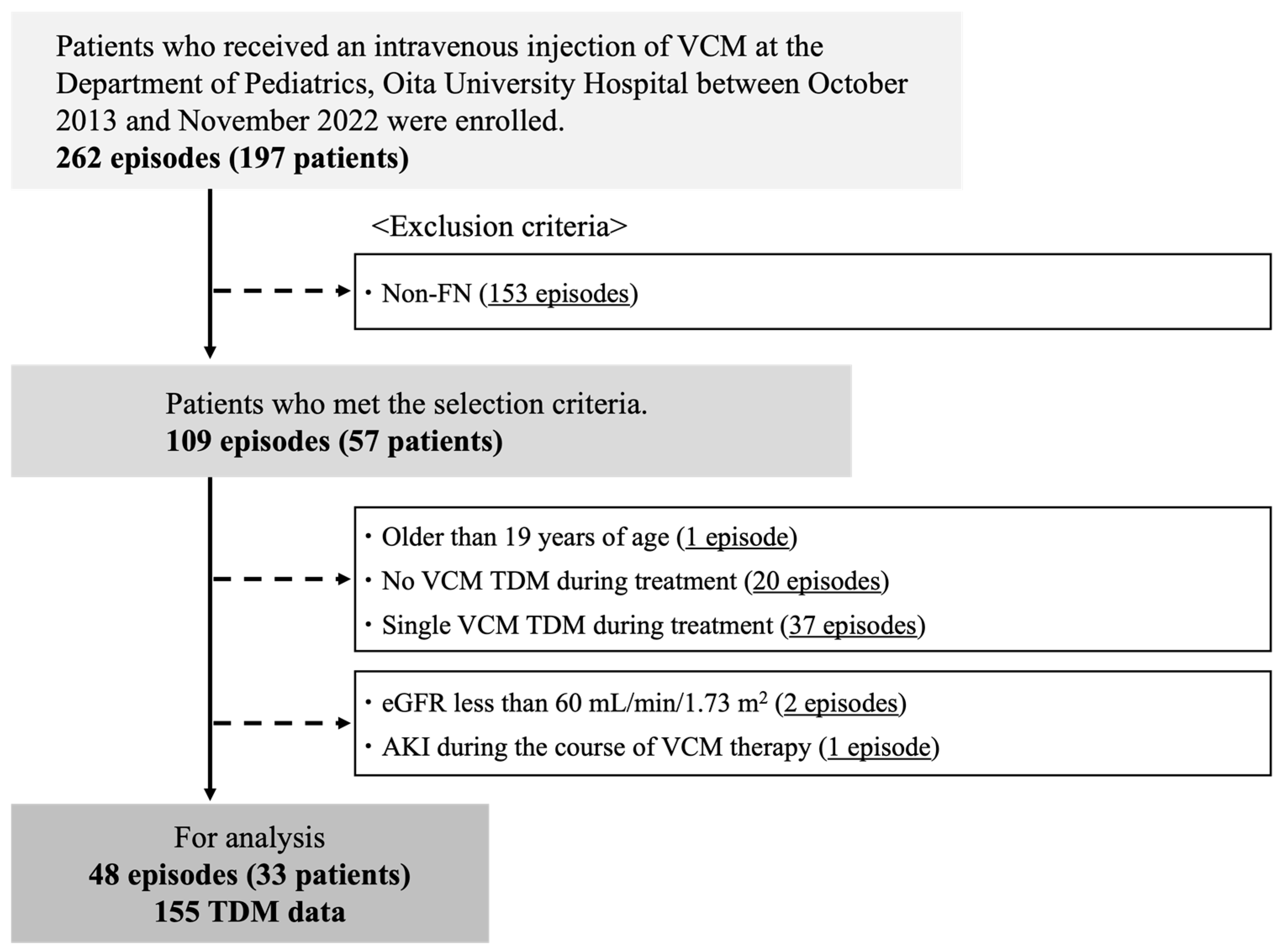
4.1. Subjects
4.2. Data Collection
4.3. Pharmacokinetic Analysis
4.4. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Freifeld, A.G.; Bow, E.J.; Sepkowitz, K.A.; Boeckh, M.J.; Ito, J.I.; Mullen, C.A.; Raad, I.I.; Rolston, K.V.; Young, J.-A.H.; Wingard, J.R.; et al. Clinical Practice Guideline for the Use of Antimicrobial Agents in Neutropenic Patients with Cancer: 2010 Update by the Infectious Diseases Society of America. Clin. Infect. Dis. 2011, 52, e56–e93. [Google Scholar] [CrossRef]
- Japanese Society of Medical Oncology. Clinical Practice Guidelines for Febrile Neutropenia (FN), 2nd ed.; Kanehara & Co., Ltd.: Tokyo, Japan, 2017; pp. 2–3. [Google Scholar]
- National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology: Myeloid Growth Factors. Version 1.2024. Available online: Https://www.nccn.org/guidelines/guidelines-detail?category=3&id=1493 (accessed on 19 May 2025).
- Taplitz, R.A.; Kennedy, E.B.; Bow, E.J.; Crews, J.; Gleason, C.; Hawley, D.K.; Langston, A.A.; Nastoupil, L.J.; Rajotte, M.; Rolston, K.; et al. Outpatient Management of Fever and Neutropenia in Adults Treated for Malignancy: American Society of Clinical Oncology and Infectious Diseases Society of America Clinical Practice Guideline Update. J. Clin. Oncol. 2018, 36, 1443–1453. [Google Scholar] [CrossRef]
- Rybak, M.J. The Pharmacokinetic and Pharmacodynamic Properties of Vancomycin. Clin. Infect. Dis. 2006, 42 (Suppl. 1), S35–S39. [Google Scholar] [CrossRef] [PubMed]
- Rybak, M.J.; Le, J.; Lodise, T.P.; Levine, D.P.; Bradley, J.S.; Liu, C.; Mueller, B.A.; Pai, M.P.; Wong-Beringer, A.; Rotschafer, J.C.; et al. Therapeutic Monitoring of Vancomycin for Serious Methicillin-Resistant Staphylococcus Aureus Infections: A Revised Consensus Guideline and Review by the American Society of Health-System Pharmacists, the Infectious Diseases Society of America, the Pediatric Infectious Diseases Society, and the Society of Infectious Diseases Pharmacists. Am. J. Health Syst. Pharm. 2020, 77, 835–864. [Google Scholar] [CrossRef]
- Suzuki, Y.; Kawasaki, K.; Sato, Y.; Tokimatsu, I.; Itoh, H.; Hiramatsu, K.; Takeyama, M.; Kadota, J.-I. Is Peak Concentration Needed in Therapeutic Drug Monitoring of Vancomycin? A Pharmacokinetic-Pharmacodynamic Analysis in Patients with Methicillin-Resistant Staphylococcus Aureus Pneumonia. Chemotherapy 2012, 58, 308–312. [Google Scholar] [CrossRef] [PubMed]
- Blot, S.I.; Pea, F.; Lipman, J. The Effect of Pathophysiology on Pharmacokinetics in the Critically Ill Patient--Concepts Appraised by the Example of Antimicrobial Agents. Adv. Drug Deliv. Rev. 2014, 77, 3–11. [Google Scholar] [CrossRef]
- Baptista, J.P.; Sousa, E.; Martins, P.J.; Pimentel, J.M. Augmented Renal Clearance in Septic Patients and Implications for Vancomycin Optimisation. Int. J. Antimicrob. Agents 2012, 39, 420–423. [Google Scholar] [CrossRef] [PubMed]
- Amano, E.; Tanaka, R.; Ono, H.; Tatsuta, R.; Hashimoto, T.; Hiramatsu, K.; Itoh, H. Association of Vancomycin Trough Concentration and Clearance with Febrile Neutropenia in Pediatric Patients. Ther. Drug Monit. 2022, 44, 543–551. [Google Scholar] [CrossRef]
- Hirai, K.; Ishii, H.; Shimoshikiryo, T.; Shimomura, T.; Tsuji, D.; Inoue, K.; Kadoiri, T.; Itoh, K. Augmented Renal Clearance in Patients with Febrile Neutropenia Is Associated with Increased Risk for Subtherapeutic Concentrations of Vancomycin. Ther. Drug Monit. 2016, 38, 706–710. [Google Scholar] [CrossRef]
- Alzahrani, A.M.; Naeem, A.; AlAzmi, A.; Hakami, A.Y.; Karim, S.; Ali, A.S.; Kamel, F.O.; Alzhrani, R.M.; Alkhaldi, T.S.; Maghrabi, L.A.; et al. Altered Pharmacokinetics Parameters of Vancomycin in Patients with Hematological Malignancy with Febrile Neutropenia, a Bayesian Software Estimation. Antibiotics 2023, 12, 979. [Google Scholar] [CrossRef]
- Zane, N.R.; Reedy, M.D.; Gastonguay, M.R.; Himebauch, A.S.; Ramsey, E.Z.; Topjian, A.A.; Zuppa, A.F. A Population Pharmacokinetic Analysis to Study the Effect of Therapeutic Hypothermia on Vancomycin Disposition in Children Resuscitated from Cardiac Arrest. Pediatr. Crit. Care Med. 2017, 18, e290–e297. [Google Scholar] [CrossRef] [PubMed]
- Shimamoto, Y.; Verstegen, R.H.J.; Mizuno, T.; Schechter, T.; Allen, U.; Ito, S. Population Pharmacokinetics of Vancomycin in Paediatric Patients with Febrile Neutropenia and Augmented Renal Clearance: Development of New Dosing Recommendations. J. Antimicrob. Chemother. 2021, 76, 2932–2940. [Google Scholar] [CrossRef] [PubMed]
- Akunne, O.O.; Mugabo, P.; Argent, A.C. Pharmacokinetics of Vancomycin in Critically Ill Children: A Systematic Review. Eur. J. Drug Metab. Pharmacokinet. 2022, 47, 31–48. [Google Scholar] [CrossRef] [PubMed]
- Hirai, K.; Ihara, S.; Kinae, A.; Ikegaya, K.; Suzuki, M.; Hirano, K.; Itoh, K. Augmented Renal Clearance in Pediatric Patients with Febrile Neutropenia Associated with Vancomycin Clearance. Ther. Drug Monit. 2016, 38, 393–397. [Google Scholar] [CrossRef]
- He, N.; Dong, F.; Liu, W.; Zhai, S. A Systematic Review of Vancomycin Dosing in Patients with Hematologic Malignancies or Neutropenia. Infect. Drug Resist. 2020, 13, 1807–1821. [Google Scholar] [CrossRef]
- Avedissian, S.N.; Bradley, E.; Zhang, D.; Bradley, J.S.; Nazer, L.H.; Tran, T.M.; Nguyen, A.; Le, J. Augmented Renal Clearance Using Population-Based Pharmacokinetic Modeling in Critically Ill Pediatric Patients. Pediatr. Crit. Care Med. 2017, 18, e388–e394. [Google Scholar] [CrossRef]
- Herget-Rosenthal, S.; Marggraf, G.; Hüsing, J.; Göring, F.; Pietruck, F.; Janssen, O.; Philipp, T.; Kribben, A. Early Detection of Acute Renal Failure by Serum Cystatin C. Kidney Int. 2004, 66, 1115–1122. [Google Scholar] [CrossRef]
- Baptista, J.P.; Udy, A.A.; Sousa, E.; Pimentel, J.; Wang, L.; Roberts, J.A.; Lipman, J. A Comparison of Estimates of Glomerular Filtration in Critically Ill Patients with Augmented Renal Clearance. Crit. Care 2011, 15, R139. [Google Scholar] [CrossRef]
- Grootaert, V.; Willems, L.; Debaveye, Y.; Meyfroidt, G.; Spriet, I. Augmented Renal Clearance in the Critically Ill: How to Assess Kidney Function. Ann. Pharmacother. 2012, 46, 952–959. [Google Scholar] [CrossRef]
- Martin, J.H.; Fay, M.F.; Udy, A.; Roberts, J.; Kirkpatrick, C.; Ungerer, J.; Lipman, J. Pitfalls of Using Estimations of Glomerular Filtration Rate in an Intensive Care Population: EGFR Is a Poor Estimate of Renal Function in Intensive Care. Intern. Med. J. 2011, 41, 537–543. [Google Scholar] [CrossRef]
- Rybak, M.; Lomaestro, B.; Rotschafer, J.C.; Moellering, R., Jr.; Craig, W.; Billeter, M.; Dalovisio, J.R.; Levine, D.P. Therapeutic Monitoring of Vancomycin in Adult Patients: A Consensus Review of the American Society of Health-System Pharmacists, the Infectious Diseases Society of America, and the Society of Infectious Diseases Pharmacists. Am. J. Health. Syst. Pharm. 2009, 66, 82–98. [Google Scholar] [CrossRef] [PubMed]
- De Cock, P.A.J.G.; Desmet, S.; De Jaeger, A.; Biarent, D.; Dhont, E.; Herck, I.; Vens, D.; Colman, S.; Stove, V.; Commeyne, S.; et al. Impact of Vancomycin Protein Binding on Target Attainment in Critically Ill Children: Back to the Drawing Board? J. Antimicrob. Chemother. 2017, 72, 801–804. [Google Scholar] [CrossRef]
- Oyaert, M.; Spriet, I.; Allegaert, K.; Smits, A.; Vanstraelen, K.; Peersman, N.; Wauters, J.; Verhaegen, J.; Vermeersch, P.; Pauwels, S. Factors Impacting Unbound Vancomycin Concentrations in Different Patient Populations. Antimicrob. Agents Chemother. 2015, 59, 7073–7079. [Google Scholar] [CrossRef]
- Sridharan, K.; Al Daylami, A.; Ajjawi, R.; Al-Ajooz, H.; Veeramuthu, S. Clinical Pharmacokinetics of Vancomycin in Critically Ill Children. Eur. J. Drug Metab. Pharmacokinet. 2019, 44, 807–816. [Google Scholar] [CrossRef]
- Albrecht, L.M.; Rybak, M.J.; Warbasse, L.H.; Edwards, D.J. Vancomycin Protein Binding in Patients with Infections Caused by Staphylococcus Aureus. DICP 1991, 25, 713–715. [Google Scholar] [CrossRef]
- Giachetto, G.A.; Telechea, H.M.; Speranza, N.; Oyarzun, M.; Nanni, L.; Menchaca, A. Vancomycin Pharmacokinetic-Pharmacodynamic Parameters to Optimize Dosage Administration in Critically Ill Children. Pediatr. Crit. Care Med. 2011, 12, e250–e254. [Google Scholar] [CrossRef]
- Filippone, E.J.; Kraft, W.K.; Farber, J.L. The Nephrotoxicity of Vancomycin. Clin. Pharmacol. Ther. 2017, 102, 459–469. [Google Scholar] [CrossRef] [PubMed]
- Ragab, A.R.; Al-Mazroua, M.K.; Al-Harony, M.A. Incidence and Predisposing Factors of Vancomycin-Induced Nephrotoxicity in Children. Infect. Dis. Ther. 2013, 2, 37–46. [Google Scholar] [CrossRef] [PubMed]
- Guilhaumou, R.; Marsot, A.; Dupouey, J.; Galambrun, C.; Boulamery, A.; Coze, C.; Simon, N.; André, N. Pediatric Patients with Solid or Hematological Tumor Disease: Vancomycin Population Pharmacokinetics and Dosage Optimization. Ther. Drug Monit. 2016, 38, 559–566. [Google Scholar] [CrossRef]
- Schwartz, G.J.; Muñoz, A.; Schneider, M.F.; Mak, R.H.; Kaskel, F.; Warady, B.A.; Furth, S.L. New Equations to Estimate GFR in Children with CKD. J. Am. Soc. Nephrol. 2009, 20, 629–637. [Google Scholar] [CrossRef]
- Kellum, J.A.; Lameire, N.; KDIGO AKI Guideline Work Group. Diagnosis, Evaluation, and Management of Acute Kidney Injury: A KDIGO Summary (Part 1). Crit. Care 2013, 17, 204. [Google Scholar] [CrossRef] [PubMed]
- Maeda, Y.; Tanaka, R.; Tatsuta, R.; Takano, K.; Hashimoto, T.; Ogata, M.; Hiramatsu, K.; Itoh, H. Impact of Inflammation on Intra-Individual Variation in Trough Voriconazole Concentration in Patients with Hematological Malignancies. Biol. Pharm. Bull. 2022, 45, 1084–1090. [Google Scholar] [CrossRef] [PubMed]
- Le, J.; Bradley, J.S.; Murray, W.; Romanowski, G.L.; Tran, T.T.; Nguyen, N.; Cho, S.; Natale, S.; Bui, I.; Tran, T.M.; et al. Improved Vancomycin Dosing in Children Using Area under the Curve Exposure. Pediatr. Infect. Dis. J. 2013, 32, e155–e163. [Google Scholar] [CrossRef]
- Yamaoka, K.; Nakagawa, T.; Tanaka, H.; Yasuhara, M.; Okumura, K.; Hori, R. A Nonlinear Multiple Regression Program, MULTI2 (BAYES), Based on Bayesian Algorithm for Microcomputers. J. Pharmacobio-Dyn. 1985, 8, 246–256. [Google Scholar] [CrossRef]
- Anderson, B.J.; Holford, N.H.G. Mechanism-Based Concepts of Size and Maturity in Pharmacokinetics. Annu. Rev. Pharmacol. Toxicol. 2008, 48, 303–332. [Google Scholar] [CrossRef] [PubMed]
- Kashiwagura, S.; Kamioka, Y.; Seki, M.; Koshika, S.; Okada, K. Analysis of Risk Factors Associated with Reduced Trough Concentrations of Vancomycin in Relation to Renal Function in a Tertiary Hospital in Japan. Infect. Drug Resist. 2021, 14, 4207–4214. [Google Scholar] [CrossRef]
- Chuma, M.; Makishima, M.; Imai, T.; Tochikura, N.; Suzuki, S.; Kuwana, T.; Sawada, N.; Iwabuchi, S.; Sekimoto, M.; Nakayama, T.; et al. Relationship between Hemoglobin Levels and Vancomycin Clearance in Patients with Sepsis. Eur. J. Clin. Pharmacol. 2019, 75, 929–937. [Google Scholar] [CrossRef]

| Characteristics | Value | |
|---|---|---|
| Number of episodes; n | 48 | |
| Sex ratio * (male/female); n (%) | 15/18 | (45/55) |
| Age (years) | 7.98 | [3.71–12.2] |
| Body weight (kg) | 25.5 | [12.6–37.7] |
| Height (cm) | 119.1 | [94–146] |
| Duration (days) | 12 | [10.0–14.3] |
| Vancomycin daily dose (mg/kg) | 45.7 | [40.7–57.1] |
| Time to blood sampling for initial therapeutic drug monitoring (days) | 3 | [2.0–4.0] |
| Maximum body temperature (°C) | 38.4 | [38.0–39.0] |
| White blood cell count (×103/μL) | 0.22 | [0.11–0.40] |
| Neutrophil count (/μL) | 0 | [0–12.6] |
| Hemoglobin (g/dL) | 8.9 | [7.48–10.1] |
| Platelet count (×103/μL) | 29 | [19.3–44.3] |
| C-reactive protein (mg/dL) | 3.71 | [1.03–7.35] |
| Serum albumin (g/dL) | 3.46 | [2.99–3.69] |
| Total bilirubin (mg/dL) | 0.65 | [0.42–1.02] |
| Aspartate aminotransferase (U/L) | 27.9 | [18.9–54.0] |
| Alanine aminotransferase (U/L) | 28.9 | [16.5–66.2] |
| Gamma-glutamyl transpeptidase (U/L) | 41.5 | [26.6–106.4] |
| Serum creatinine (mg/dL) | 0.28 | [0.18–0.35] |
| Estimated glomerular filtration rate (mL/min/1.73 m2) | 175.8 | [157.4–212.6] |
| Blood urea nitrogen (mg/dL) | 9.4 | [6.85–12.4] |
| No bacteria detection; n (%) | 32 | (66.7) |
| Bacteria detection in blood culture; n (%) | 16 | (33.3) |
| Coagulase-negative Staphylococcus spp. | 4 | (25) |
| Enterococcus spp. | 1 | (6.25) |
| Methicillin-resistant Staphylococcus aureus | 1 | (6.25) |
| Methicillin-sensitive Staphylococcus aureus | 1 | (6.25) |
| Streptococcus spp. | 6 | (37.5) |
| Others | 3 | (18.75) |
| Primary disease; n (%) | ||
| Hematological malignancy | 38 | (79.2) |
| Solid tumor | 8 | (16.7) |
| Others | 2 | (4.2) |
| Parameter | Correlation Coefficient | p-Value (Two-Tailed) |
|---|---|---|
| C-reactive protein (mg/dL) | 0.142 | 0.077 |
| Serum albumin (g/dL) | 0.062 | 0.458 |
| Total bilirubin (mg/dL) | 0.287 | <0.001 |
| Aspartate aminotransferase (U/L) | 0.026 | 0.747 |
| Alanine aminotransferase (U/L) | −0.133 | 0.099 |
| Gamma-glutamyl transpeptidase (U/L) | 0.012 | 0.892 |
| Blood urea nitrogen (mg/dL) | −0.115 | 0.154 |
| Serum creatinine (mg/dL) | −0.118 | 0.143 |
| Neutrophil count (/μL) | −0.290 | <0.001 |
| Platelet count (×103/μL) | −0.226 | 0.005 |
| Hemoglobin (g/dL) | −0.071 | 0.378 |
| Maximum daily body temperature (°C) | 0.260 | 0.001 |
| Estimated glomerular filtration rate (mL/min/1.73 m2) | 0.461 | <0.001 |
| Independent Variable | Parameter | ||||
|---|---|---|---|---|---|
| p-Value | B | 95% CI | β | Adjusted R Squared | |
| C-Reactive protein (mg/dL) | 0.911 | 3.01 × 10−3 | −0.050 to 0.056 | 0.010 | 0.047 |
| Neutrophil count (/μL) | 0.746 | −3.83 × 10−5 | −2.72 × 10−4 to 1.95 × 10−4 | −0.026 | |
| Maximum daily body temperature (°C) | 0.008 | 0.442 | 0.12 to 0.764 | 0.244 | |
| Parameter | Correlation Coefficient | p-Value (Two-Tailed) |
|---|---|---|
| Δ C-reactive protein (mg/dL) | 0.194 | 0.046 |
| Δ Serum albumin (g/dL) | −0.084 | 0.411 |
| Δ Total bilirubin (mg/dL) | 0.074 | 0.450 |
| Δ Aspartate aminotransferase (U/L) | 0.003 | 0.976 |
| Δ Alanine aminotransferase (U/L) | 0.025 | 0.799 |
| Δ Gamma-glutamyl transpeptidase (U/L) | −0.075 | 0.471 |
| Δ Blood urea nitrogen (mg/dL) | −0.026 | 0.787 |
| Δ Serum creatinine (mg/dL) | −0.180 | 0.064 |
| Δ Neutrophil count (/μL) | −0.151 | 0.122 |
| Δ Platelet count (×103/μL) | 0.077 | 0.433 |
| Δ Hemoglobin (g/dL) | −0.217 | 0.025 |
| Δ Maximum body temperature (°C) | 0.325 | 0.001 |
| Δ Estimated glomerular filtration rate (mL/min/1.73 m2) | 0.112 | 0.250 |
| Independent Variable | Parameter | ||||
|---|---|---|---|---|---|
| p-Value | B | 95% CI | β | Adjusted R Squared | |
| Δ C-reactive protein (mg/dL) | 0.329 | 0.029 | −0.030 to 0.088 | 0.097 | 0.055 |
| Δ Neutrophil count (/μL) | 0.756 | 3.32 × 10−5 | −1.78 × 10−4 to 2.45 ×10−4 | 0.030 | |
| Δ Maximum daily body temperature (°C) | 0.015 | 0.376 | 0.074 to 0.678 | 0.245 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Takumi, Y.; Tanaka, R.; Iwao, M.; Tatsuta, R.; Itoh, H. Impact of Recovery from Febrile Neutropenia on Intra-Individual Variability in Vancomycin Pharmacokinetics in Pediatric Patients. Antibiotics 2025, 14, 570. https://doi.org/10.3390/antibiotics14060570
Takumi Y, Tanaka R, Iwao M, Tatsuta R, Itoh H. Impact of Recovery from Febrile Neutropenia on Intra-Individual Variability in Vancomycin Pharmacokinetics in Pediatric Patients. Antibiotics. 2025; 14(6):570. https://doi.org/10.3390/antibiotics14060570
Chicago/Turabian StyleTakumi, Yukie, Ryota Tanaka, Motoshi Iwao, Ryosuke Tatsuta, and Hiroki Itoh. 2025. "Impact of Recovery from Febrile Neutropenia on Intra-Individual Variability in Vancomycin Pharmacokinetics in Pediatric Patients" Antibiotics 14, no. 6: 570. https://doi.org/10.3390/antibiotics14060570
APA StyleTakumi, Y., Tanaka, R., Iwao, M., Tatsuta, R., & Itoh, H. (2025). Impact of Recovery from Febrile Neutropenia on Intra-Individual Variability in Vancomycin Pharmacokinetics in Pediatric Patients. Antibiotics, 14(6), 570. https://doi.org/10.3390/antibiotics14060570






